Single-Cell Transcriptomics Reveals Early Effects of Ionizing Radiation on Bone Marrow Mononuclear Cells in Mice
Abstract
:1. Introduction
2. Results
2.1. Radiosensitivity Analysis of Multiple Cellular Populations in Bone Marrow (BM)
2.2. Single-Cell Transcriptomes of Murine BM Mononuclear Cells after IR
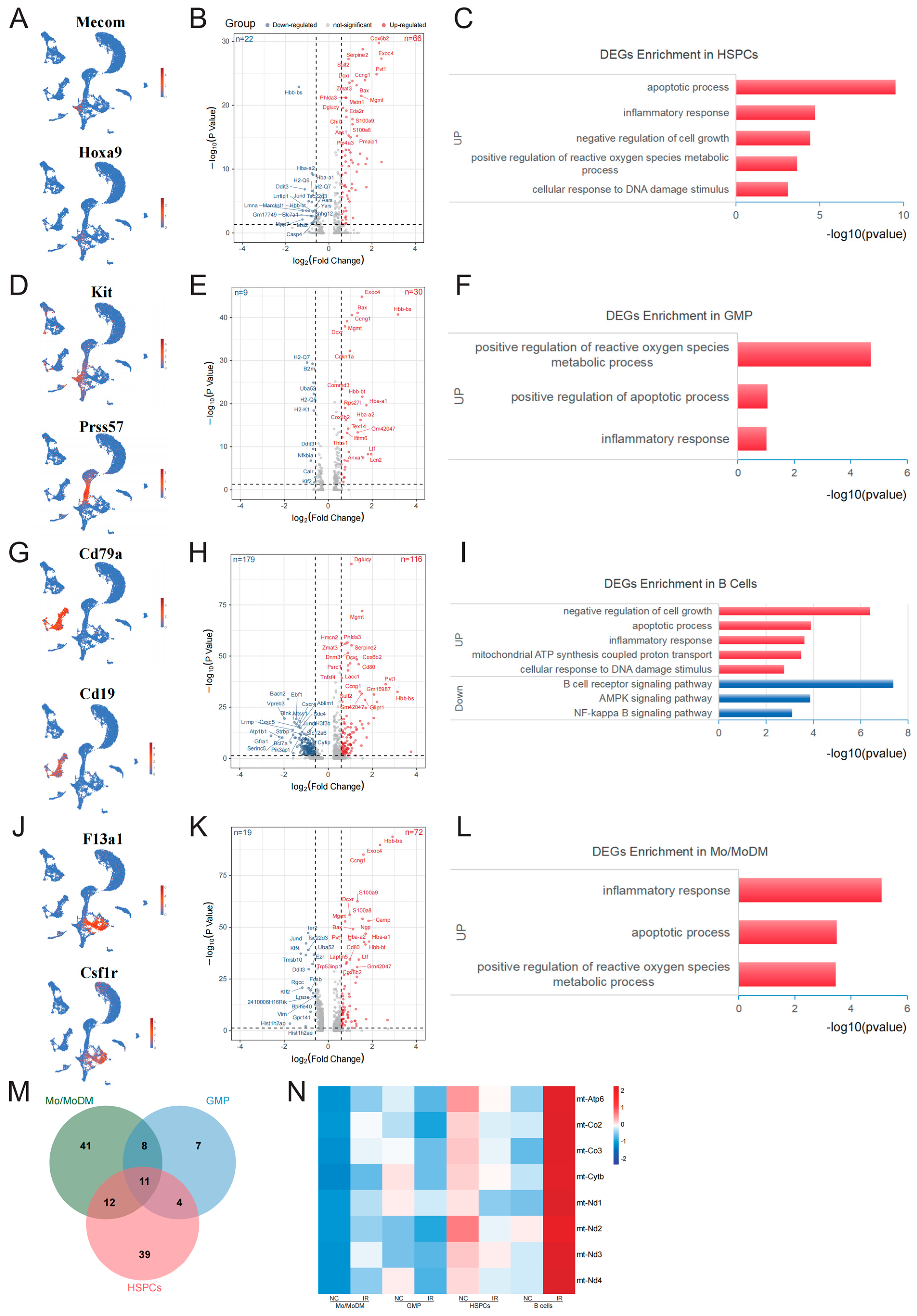
2.3. Early Response Genes in Radiosensitive Cells
2.4. Early Response Genes in Radioresistant Cells
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Isolation of Bone Marrow Mononuclear Cells
4.3. Flow Cytometry
4.4. Single-Cell Transcriptomic Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mavragani, I.V.; Laskaratou, D.A.; Frey, B.; Candéias, S.M.; Gaipl, U.S.; Lumniczky, K.; Georgakilas, A.G. Key mechanisms involved in ionizing radiation-induced systemic effects. A current review. Toxicol. Res. 2016, 5, 12–33. [Google Scholar] [CrossRef]
- Su, L.; Dong, Y.; Wang, Y.; Wang, Y.; Guan, B.; Lu, Y.; Wu, J.; Wang, X.; Li, D.; Meng, A.; et al. Potential role of senescent macrophages in radiation-induced pulmonary fibrosis. Cell Death Dis. 2021, 12, 527. [Google Scholar] [CrossRef] [PubMed]
- Li, H.H.; Wang, Y.W.; Chen, R.; Zhou, B.; Ashwell, J.D.; Fornace, A.J., Jr. Ionizing Radiation Impairs T Cell Activation by Affecting Metabolic Reprogramming. Int. J. Biol. Sci. 2015, 11, 726–736. [Google Scholar] [CrossRef]
- Guo, J.J.; Liu, N.; Ma, Z.; Gong, Z.J.; Liang, Y.L.; Cheng, Q.; Zhong, X.G.; Yao, Z.J. Dose-Response Effects of Low-Dose Ionizing Radiation on Blood Parameters in Industrial Irradiation Workers. Dose Response 2022, 20, 2. [Google Scholar] [CrossRef] [PubMed]
- Heylmann, D.; Ponath, V.; Kindler, T.; Kaina, B. Comparison of DNA repair and radiosensitivity of different blood cell populations. Sci. Rep. 2021, 11, 2478. [Google Scholar] [CrossRef]
- Hidalgo, A.; Chilvers, E.R.; Summers, C.; Koenderman, L. The Neutrophil Life Cycle. Trends Immunol. 2019, 40, 584–597. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Shi, Q.; Zhang, Y.; Liang, G.; Kang, Z.; Huang, B.; Ma, D.; Wang, L.; Jiao, J.; Fang, X.; et al. Identification of HSC/MPP expansion units in fetal liver by single-cell spatiotemporal transcriptomics. Cell Res. 2022, 32, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, L.; Wang, Y.C.; Xu, Z.R.; Feng, Y.; Zhang, J.; Wang, Y.; Xu, C.R. Comparative analysis of cell lineage differentiation during hepatogenesis in humans and mice at the single-cell transcriptome level. Cell Res. 2020, 30, 1109–1126. [Google Scholar] [CrossRef]
- Zeng, X.; Li, X.; Li, X.; Wei, C.; Shi, C.; Hu, K.; Kong, D.; Luo, Q.; Xu, Y.; Shan, W.; et al. Fecal microbiota transplantation from young mice rejuvenates aged hematopoietic stem cells by suppressing inflammation. Blood 2023, 141, 1691–1707. [Google Scholar] [CrossRef]
- Heylmann, D.; Rödel, F.; Kindler, T.; Kaina, B. Radiation sensitivity of human and murine peripheral blood lymphocytes, stem and progenitor cells. Biochim. Biophys. Acta 2014, 1846, 121–129. [Google Scholar] [CrossRef]
- Singh, V.; Gupta, D.; Arora, R. NF-kB as a key player in regulation of cellular radiation responses and identification of radiation countermeasures. Discoveries 2015, 3, e35. [Google Scholar] [CrossRef]
- Yin, L.; Hu, X.; Pei, G.; Tang, M.; Zhou, Y.; Zhang, H.; Huang, M.; Li, S.; Zhang, J.; Citu, C.; et al. Genome-wide CRISPR screen reveals the synthetic lethality between BCL2L1 inhibition and radiotherapy. Life Sci. Alliance 2024, 7, e202302353. [Google Scholar] [CrossRef]
- Gustafsson, N.M.S.; Färnegårdh, K.; Bonagas, N.; Ninou, A.H.; Groth, P.; Wiita, E.; Jönsson, M.; Hallberg, K.; Lehto, J.; Pennisi, R.; et al. Targeting PFKFB3 radiosensitizes cancer cells and suppresses homologous recombination. Nat. Commun. 2018, 9, 3872. [Google Scholar] [CrossRef]
- Zhu, H.; Song, H.; Chen, G.; Yang, X.; Liu, J.; Ge, Y.; Lu, J.; Qin, Q.; Zhang, C.; Xu, L.; et al. eEF2K promotes progression and radioresistance of esophageal squamous cell carcinoma. Radiother. Oncol. 2017, 124, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, M.A.; Omaruddin, R.A. Mitochondrial gene expression in directly irradiated and nonirradiated bystander cells. Cancer Biother. Radiopharm. 2011, 26, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Roudkenar, M.H.; Seydi, E.; Sadeghi, L.; Mohseni, A.; Pirahmadi, N.; Pourahmad, J. Chrysin as an Anti-Cancer Agent Exerts Selective Toxicity by Directly Inhibiting Mitochondrial Complex II and V in CLL B-lymphocytes. Cancer Investig. 2017, 35, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Summers, C.; Rankin, S.M.; Condliffe, A.M.; Singh, N.; Peters, A.M.; Chilvers, E.R. Neutrophil kinetics in health and disease. Trends Immunol. 2010, 31, 318–324. [Google Scholar] [CrossRef]
- Pillay, J.; den Braber, I.; Vrisekoop, N.; Kwast, L.M.; de Boer, R.J.; Borghans, J.A.; Tesselaar, K.; Koenderman, L. In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood 2010, 116, 625–627. [Google Scholar] [CrossRef]
- Athens, J.W.; Haab, O.P.; Raab, S.O.; Mauer, A.M.; Ashenbrucker, H.; Cartwright, G.E.; Wintrobe, M.M. Leukokinetic studies. IV. The total blood, circulating and marginal granulocyte pools and the granulocyte turnover rate in normal subjects. J. Clin. Investig. 1961, 40, 989–995. [Google Scholar] [CrossRef]
- Ussov, W.Y.; Aktolun, C.; Myers, M.J.; Jamar, F.; Peters, A.M. Granulocyte margination in bone marrow: Comparison with margination in the spleen and liver. Scand. J. Clin. Lab. Investig. 1995, 55, 87–96. [Google Scholar] [CrossRef]
- Ponath, V.; Heylmann, D.; Haak, T.; Woods, K.; Becker, H.; Kaina, B. Compromised DNA Repair and Signalling in Human Granulocytes. J. Innate Immun. 2019, 11, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Barton, S.M.; Grabham, P.W.; Rumeld, A.L.; Okochi, S.; Street, C.; Kadenhe-Chiweshe, A.; Boboila, S.; Yamashiro, D.J.; Connolly, E.P. High-Dose Radiation Increases Notch1 in Tumor Vasculature. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 857–866. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, H.; Chen, L.; Liu, J.; Hu, X.; Zhang, H. Inhibition of Notch1/Hes1 signaling pathway improves radiosensitivity of colorectal cancer cells. Eur. J. Pharmacol. 2018, 818, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, Q.; Zhu, L.; Xie, S.; Tu, L.; Yang, Y.; Wu, K.; Zhao, Y.; Wang, Y.; Xu, Y.; et al. SERPINE2/PN-1 regulates the DNA damage response and radioresistance by activating ATM in lung cancer. Cancer Lett. 2022, 524, 268–283. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-Q.; Ding, K.-X.; Lv, Z.-C.; Cao, Z.-Y.; Zhao, K.; Gao, H.-Y.; Sun, H.-Y.; Li, J.-J.; Li, S.-Y.; Zhao, X.-W.; et al. Single-Cell Transcriptomics Reveals Early Effects of Ionizing Radiation on Bone Marrow Mononuclear Cells in Mice. Int. J. Mol. Sci. 2024, 25, 9287. https://doi.org/10.3390/ijms25179287
Wu Y-Q, Ding K-X, Lv Z-C, Cao Z-Y, Zhao K, Gao H-Y, Sun H-Y, Li J-J, Li S-Y, Zhao X-W, et al. Single-Cell Transcriptomics Reveals Early Effects of Ionizing Radiation on Bone Marrow Mononuclear Cells in Mice. International Journal of Molecular Sciences. 2024; 25(17):9287. https://doi.org/10.3390/ijms25179287
Chicago/Turabian StyleWu, Yun-Qiang, Ke-Xin Ding, Zhi-Chun Lv, Zheng-Yue Cao, Ke Zhao, Hui-Ying Gao, Hui-Ying Sun, Jing-Jing Li, Si-Yu Li, Xiong-Wei Zhao, and et al. 2024. "Single-Cell Transcriptomics Reveals Early Effects of Ionizing Radiation on Bone Marrow Mononuclear Cells in Mice" International Journal of Molecular Sciences 25, no. 17: 9287. https://doi.org/10.3390/ijms25179287





