ZIF-8 as a pH-Responsive Nanoplatform for 5-Fluorouracil Delivery in the Chemotherapy of Oral Squamous Cell Carcinoma
Abstract
:1. Introduction
2. Results
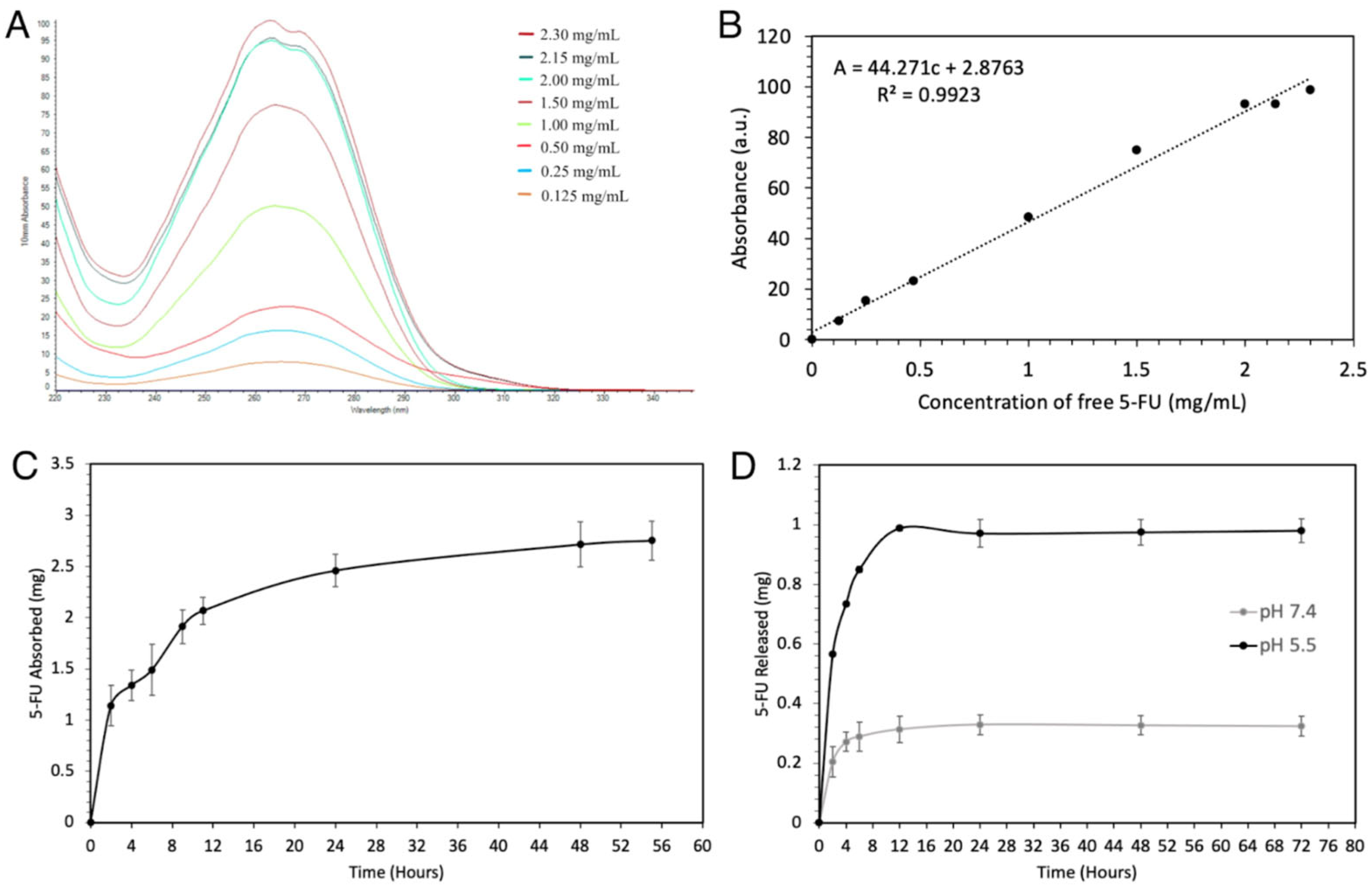
2.1. Loading and Release of 5-FU@ZIF-8
2.2. In Vitro Cell Compatibility against SCC7 Cell Line
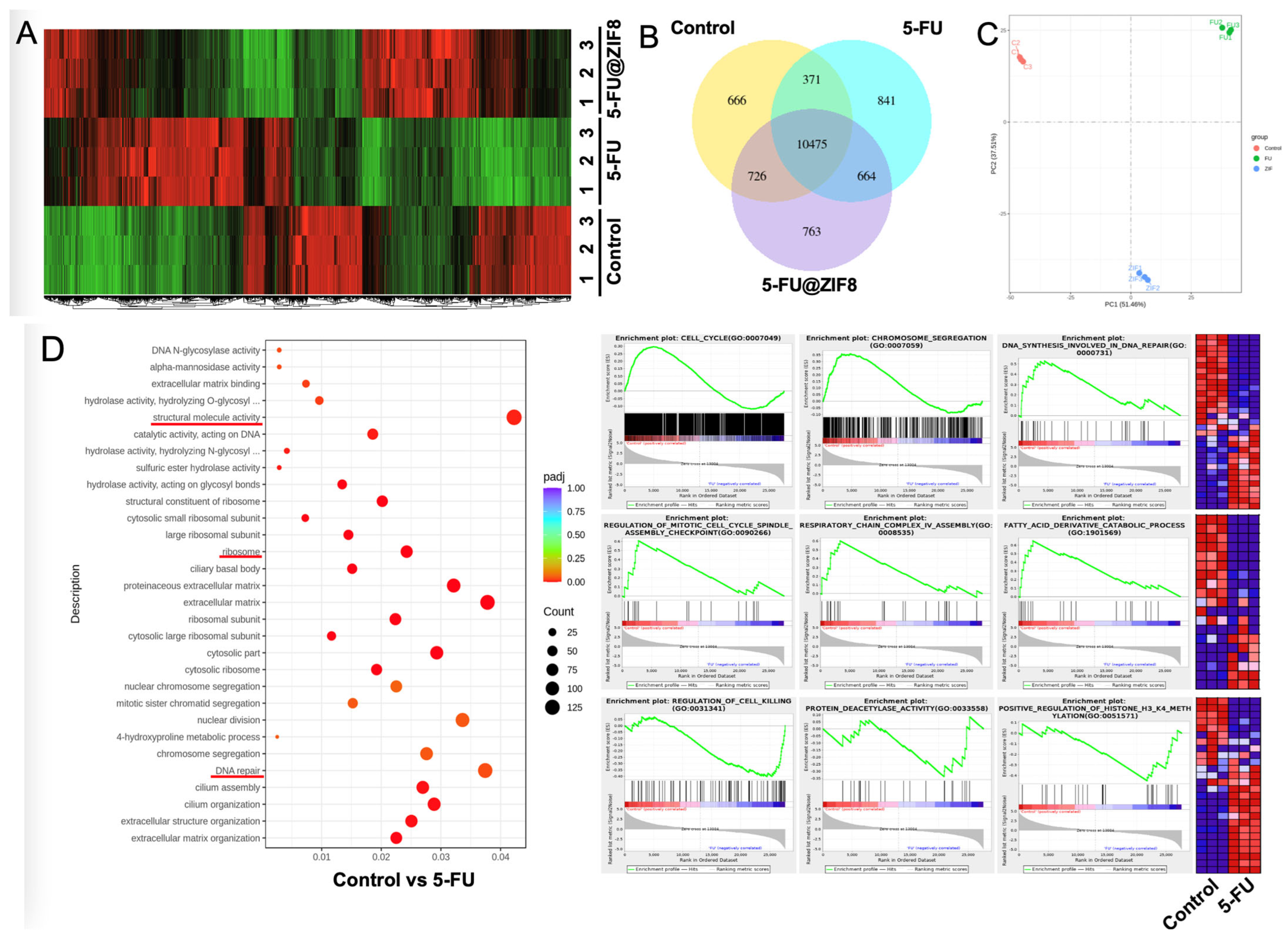
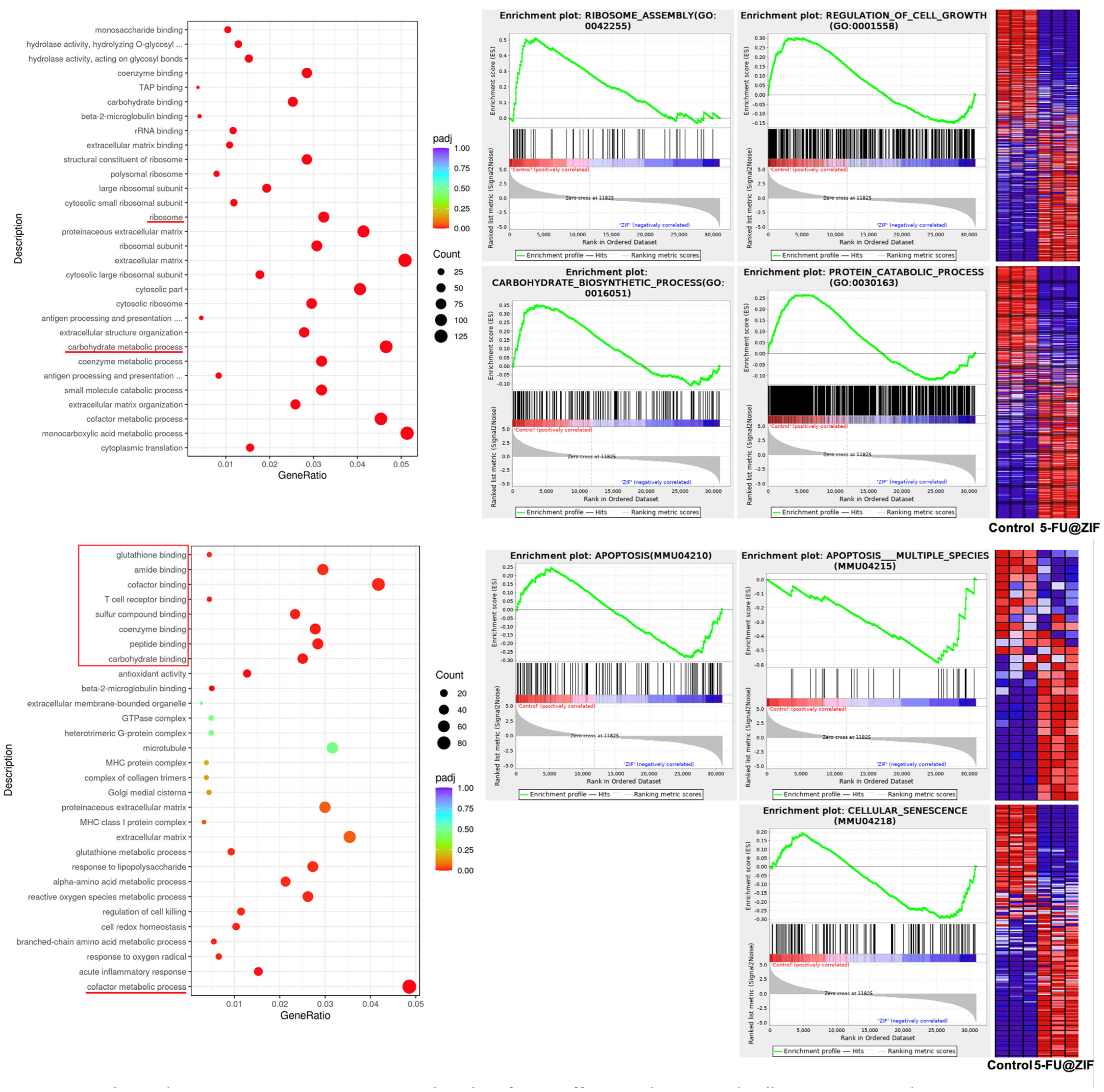
2.3. RNA Sequencing Analysis Revealed Molecular Targets of the DDS
3. Discussion
3.1. The Trial and Error behind the Loading and Release of 5-FU@ZIF-8
3.2. In Vitro Cell Culture Studies and RNA Sequencing Analysis
4. Materials and Methods
4.1. Materials
4.2. Creation of a 5-FU Standard Curve
4.3. Synthesis of 5-FU-Loaded ZIF-8
4.4. In Vitro Quantification of 5-FU Release
4.5. Cell Culturing
4.6. In Vitro Cell Compatibility Studies
4.7. RNA Sequencing Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yete, S.; D’Souza, W.; Saranath, D. High-Risk Human Papillomavirus in Oral Cancer: Clinical Implications. Oncology 2018, 94, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Porcheri, C.; Mitsiadis, T.A. New Scenarios in Pharmacological Treatments of Head and Neck Squamous Cell Carcinomas. Cancers 2021, 13, 5515. [Google Scholar] [CrossRef]
- The American Cancer Society. Treating Oral Cavity and Oropharyngeal Cancer. pp. 1–32. Available online: https://www.cancer.org/content/dam/CRC/PDF/Public/8766.00.pdf (accessed on 14 February 2022).
- Jacobs, C.; Lyman, G.; Velez-Garcia, E.; Sridhar, K.S.; Knight, W.; Hochster, H.; Goodnough, L.T.; Mortimer, J.E.; Einhorn, L.H.; Schacter, L.; et al. A phase III randomized study comparing cisplatin and fluorouracil as single agents and in combination for advanced squamous cell carcinoma of the head and neck. J. Clin. Oncol. 1992, 10, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Abak, A.; Tondro Anamag, F.; Shoorei, H.; Fattahi, F.; Javadinia, S.A.; Basiri, A.; Taheri, M. 5-Fluorouracil: A Narrative Review on the Role of Regulatory Mechanisms in Driving Resistance to This Chemotherapeutic Agent. Front. Oncol. 2021, 11, 658636. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Liang, S.; Zhao, Y.; Huang, D.; Xing, B.; Cheng, Z.; Lin, J. Core-shell structured 5-FU@ZIF-90@ZnO as a biodegradable nanoplatform for synergistic cancer therapy. Nanoscale 2020, 12, 3846–3854. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Stavljenic Milasin, I.; Batu Eken, Z.; Mravak-Stipetic, M.; Pavelic, K.; Ozer, F. Effects of zeolite as a drug delivery system on cancer therapy: A systematic review. Molecules 2021, 26, 6196. [Google Scholar] [CrossRef]
- Veselov, V.V.; Nosyrev, A.E.; Jicsinszky, L.; Alyautdin, R.N.; Cravotto, G. Targeted delivery methods for anticancer drugs. Cancers 2022, 14, 622. [Google Scholar] [CrossRef]
- Park, N.S.; Ni, Z.; Cote, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef]
- Martinez, C.; Corma, A. Inorganic molecular sieves: Preparation, modification and industrial application in catalytic processes. Coord. Chem. Rev. 2011, 255, 1558–1580. [Google Scholar] [CrossRef]
- Khodadadi Yazdi, M.; Zarrintaj, P.; Hosseiniamoli, H.; Mashhadzadeh, A.H.; Saeb, M.R.; Ramsey, J.D.; Ganjali, M.R.; Mozafari, M. Zeolites for theranostic applications. J. Mater. Chem. B 2020, 8, 5992–6012. [Google Scholar] [CrossRef]
- Sun, C.Y.; Qin, C.; Wang, X.L.; Yang, G.S.; Shao, K.Z.; Lan, Y.Q.; Su, Z.M.; Huang, P.; Wang, C.G.; Wang, E.B. Zeolitic Imidazolate framework-8 as efficient pH-sensitive drug delivery vehicle. Dalton Trans. 2021, 41, 6906–6909. [Google Scholar] [CrossRef]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Koppenol, W.H.; Bounds, P.L.; Dang, C.V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.; Kimmelman, A.C.; Lyssiotis, C.A.; Hua, S.; Chu, G.C.; Fletcher-Sananikone, E.; Locasale, J.W.; Son, J.; Zhang, H.; Coloff, J.L.; et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 2012, 149, 656–670. [Google Scholar] [CrossRef]
- Beloribi-Djefaflia, S.; Vasseur, S.; Guillaumond, F. Lipid metabolic reprogramming in cancer cells. Oncogenesis 2016, 5, e189. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Lee, I.H.; Lee, E.; Park, J.; Jon, S. pH-sensitive polymer nanospheres for use as a potential drug delivery vehicle. Biomacromolecules 2007, 8, 3401–3407. [Google Scholar] [CrossRef] [PubMed]
- Justus, C.R.; Dong, L.; Yang, L.V. Acidic tumor microenvironment and pH-sensing G protein-coupled receptors. Front. Physiol. 2013, 4, 354. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, Y.; Wei, Z.; Yuan, B.; Wang, Y.; Akakuru, O.U.; Li, Y.; Li, J.; Wu, A. Pressure-induced amorphous zeolitic imidazole frameworks with reduced toxicity and increased tumor accumulation improves therapeutic efficacy in vivo. Bioact. Mater. 2021, 6, 740–748. [Google Scholar] [CrossRef]
- Kulkarni, S.; Pandey, A.; Nikam, A.N.; Nannuri, S.H.; George, S.D.; Fayaz, S.; Vincent, A.P.; Mutalik, S. ZIF-8 nano confined protein-titanocene complex core-shell MOFs for efficient therapy of Neuroblastoma: Optimization, molecular dynamics and toxicity studies. Int. J. Biol. Macromol. 2021, 178, 444–463. [Google Scholar] [CrossRef]
- Pandey, A.; Kulkarni, S.; Vincent, A.P.; Nannuri, S.H.; George, S.D.; Mutalik, S. Hyaluronic acid-drug conjugate modified core-shell MOFs as pH responsive nanoplatform for multimodal therapy of glioblastoma. Int. J. Pharm. 2020, 588, 119735. [Google Scholar] [CrossRef] [PubMed]
- Proenza, Y.G.; Longo, R.L. Simulation of the Adsorption and Release of Large Drugs by ZIF-8. J. Chem. Inf. Model. 2020, 60, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.Y.R.; Proenza, Y.G.; da Luz, L.L.; de Sousa Araujo, S.; Filho, M.A.G.; Junior, S.A.; Soares, T.A.; Longo, R.L. A thermo-responsive adsorbent-heater-thermometer nanomaterial for controlled drug release: (ZIF-8,EuxTby)@AuNP core-shell. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 578–588. [Google Scholar] [CrossRef]
- Mastinu, A.; Kumar, A.; Maccarinelli, G.; Bonini, S.A.; Premoli, M.; Aria, F.; Gianoncelli, A.; Memo, M. Zeolite Clinoptilolite: Therapeutic Virtues of an Ancient Mineral. Molecules 2019, 24, 1517. [Google Scholar] [CrossRef] [PubMed]
- Sagir, T.; Huysal, M.; Durmus, Z.; Kurt, B.Z.; Senel, M.; Isik, S. Preparation and in vitro evaluation of 5-flourouracil loaded magnetite-zeolite nanocomposite (5-FU-MZNC) for cancer drug delivery applications. Biomed. Pharmacother. 2016, 77, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, A.; Subramaniam, R.M.; Raderer, M.; Langsteger, W.; Beheshti, M. PET/CT in Cancer: An Interdisciplinary Approach to Individualized Imaging; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Howard, B.V.; Howard, W.J. Lipids in normal and tumor cells in culture. Prog. Biochem. Pharmacol. 1975, 10, 135–166. [Google Scholar]
- Butler, L.M.; Perone, Y.; Dehairs, J.; Lupien, L.E.; de Laat, V.; Talebi, A.; Loda, M.; Kinlaw, W.B.; Swinnen, J.V. Lipids and cancer: Emerging roles in pathogenesis, diagnosis and therapeutic intervention. Adv. Drug Deliv. Rev. 2020, 159, 245–293. [Google Scholar]
- Carracedo, A.; Cantley, L.C.; Pandolfi, P.P. Cancer metabolism: Fatty acid oxidation in the limelight. Nat. Rev. Cancer 2013, 13, 227–232. [Google Scholar] [CrossRef]
- Su, Y.W.; Wu, P.S.; Lin, S.H.; Huang, W.Y.; Kuo, Y.S.; Lin, H.P. Prognostic Value of the Overexpression of Fatty Acid Metabolism-Related Enzymes in Squamous Cell Carcinoma of the Head and Neck. Int. J. Mol. Sci. 2020, 21, 6851. [Google Scholar] [CrossRef]
- Guo, C.B.; Cui, L.H.; Yu, G.Y.; Liu, D.X.; Meng, S.C.; Song, Q. Endogenous fatty acid synthesis in squamous cell carcinomas of the oral cavity. Br. J. Oral Maxillofac. Surg. 2000, 38, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Halczy-Kowalik, L.; Drozd, A.; Stachowska, E.; Drozd, R.; Zabski, T.; Domagala, W. Fatty acids distribution and content in oral squamous cell carcinoma tissue and its adjacent microenvironment. PLoS ONE 2019, 14, e0218246. [Google Scholar] [CrossRef] [PubMed]
- Agostini, M.; Silva, S.D.; Zecchin, K.G.; Coletta, R.D.; Jorge, J.; Loda, M.; Graner, E. Fatty acid synthase is required for the proliferation of human oral squamous carcinoma cells. Oral. Oncol. 2007, 40, 728–735. [Google Scholar] [CrossRef]
- Menendez, J.A.; Lupu, R. Oncogenic properties of the endogenous fatty acid metabolism: Molecular pathology of fatty acid synthase in cancer cells. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Bergfeld, S.A.; DeClerck, Y.A. Bone marrow-derived mesenchymal stem cells and the tumor microenvironment. Cancer Metastasis Rev. 2010, 29, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Finn, O.J. Immuno-oncology: Understanding the function and dysfunction of the immune system in cancer. Ann. Oncol. 2012, 23 (Suppl. S8), viii6–viii9. [Google Scholar] [CrossRef]
- Liotta, F.; Querci, V.; Mannelli, G.; Santarlasci, V.; Maggi, L.; Capone, M.; Rossi, M.C.; Mazzoni, A.; Cosmi, L.; Romagnani, S.; et al. Mesenchymal stem cells are enriched in head neck squamous cell carcinoma, correlates with tumour size and inhibit T-cell proliferation. Br. J. Cancer. 2015, 112, 745–754. [Google Scholar] [CrossRef]
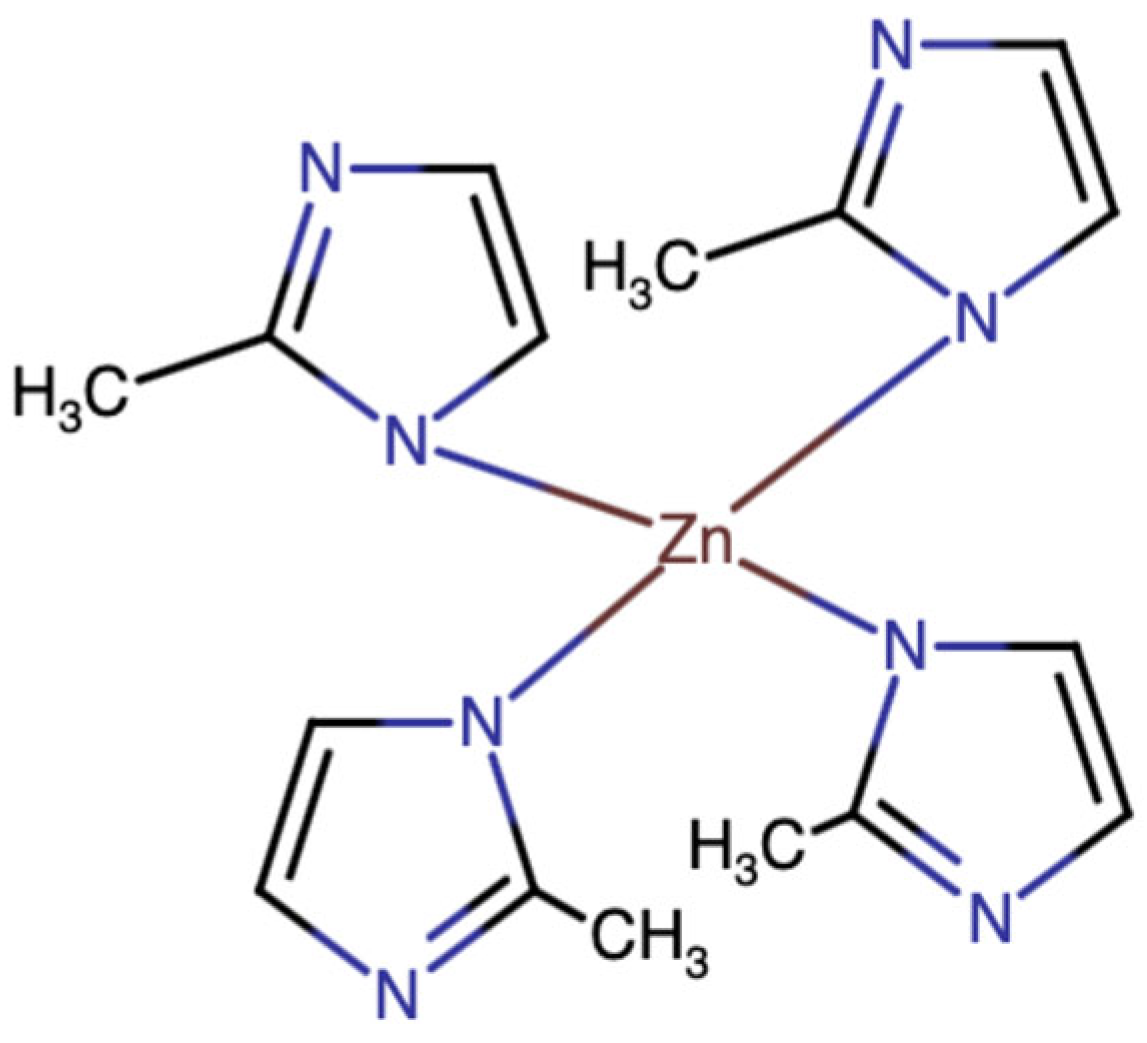

| Variables of Absorption | Loaded Drug Capacity (mg/g) | |
|---|---|---|
| Type of heat-activated carrier | Clinoptilolite | No change |
| ZIF-8 | 271.41 ± 21.94 | |
| ZIF-90 | No change | |
| Heat activation of ZIF-8 | 160 °C for 24 h | 271.41 ± 21.94 |
| None | No change | |
| 5-FU/ZIF-8 ratio | 1/3 | 101.42 ± 10.24 |
| 1/2 | 150.99 ± 2.11 | |
| 1/1 | 271.41 ± 19.07 | |
| 4/1 | 248.38 ± 9.84 | |
| Solvent | dH2O | 200.00 ± 1.95 |
| PBS | 179.68 ± 21.73 | |
| Methanol | No change | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, J.; Chen, C.; Pavelic, K.; Ozer, F. ZIF-8 as a pH-Responsive Nanoplatform for 5-Fluorouracil Delivery in the Chemotherapy of Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2024, 25, 9292. https://doi.org/10.3390/ijms25179292
Hao J, Chen C, Pavelic K, Ozer F. ZIF-8 as a pH-Responsive Nanoplatform for 5-Fluorouracil Delivery in the Chemotherapy of Oral Squamous Cell Carcinoma. International Journal of Molecular Sciences. 2024; 25(17):9292. https://doi.org/10.3390/ijms25179292
Chicago/Turabian StyleHao, Jessica, Chider Chen, Kresimir Pavelic, and Fusun Ozer. 2024. "ZIF-8 as a pH-Responsive Nanoplatform for 5-Fluorouracil Delivery in the Chemotherapy of Oral Squamous Cell Carcinoma" International Journal of Molecular Sciences 25, no. 17: 9292. https://doi.org/10.3390/ijms25179292





