Hyperphosphatemia Contributes to Skeletal Muscle Atrophy in Mice
Abstract
1. Introduction
2. Results
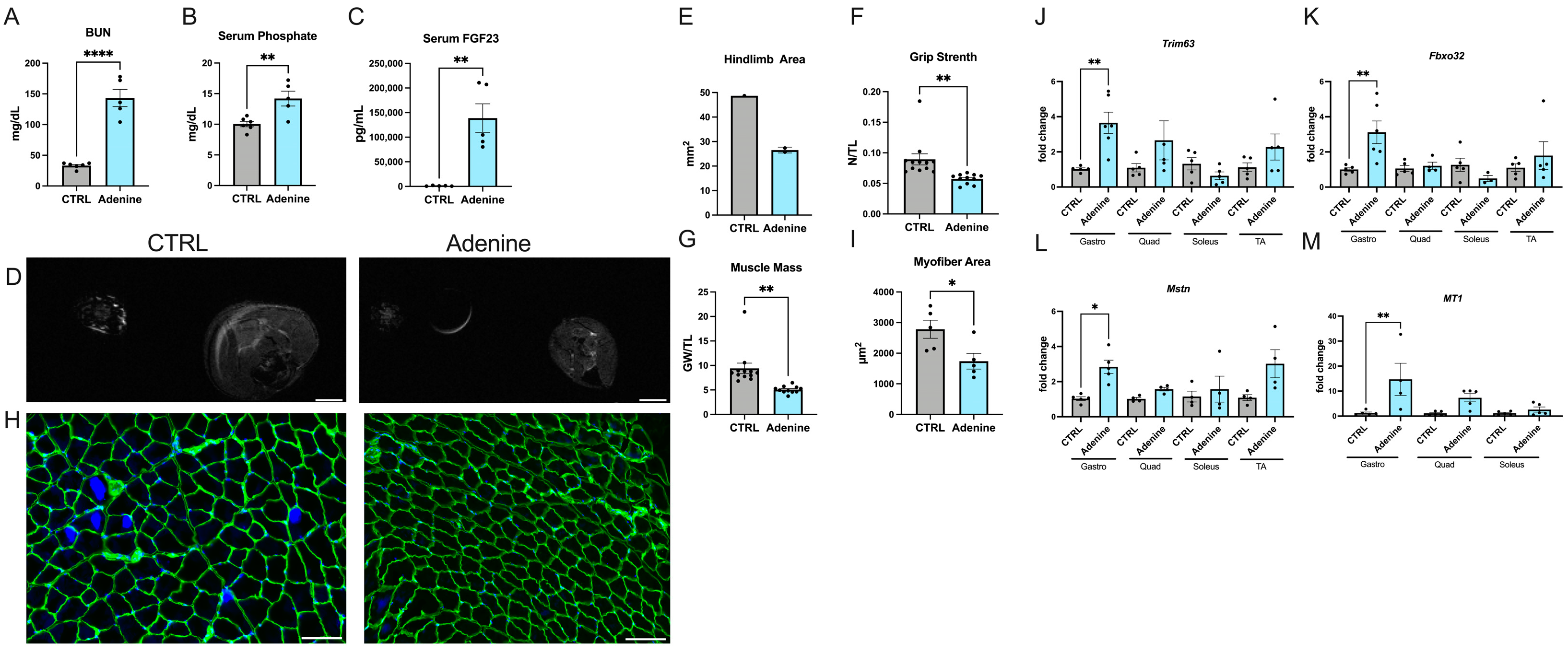
2.1. Mice Fed an Adenine-Rich Diet Develop Skeletal Muscle Atrophy
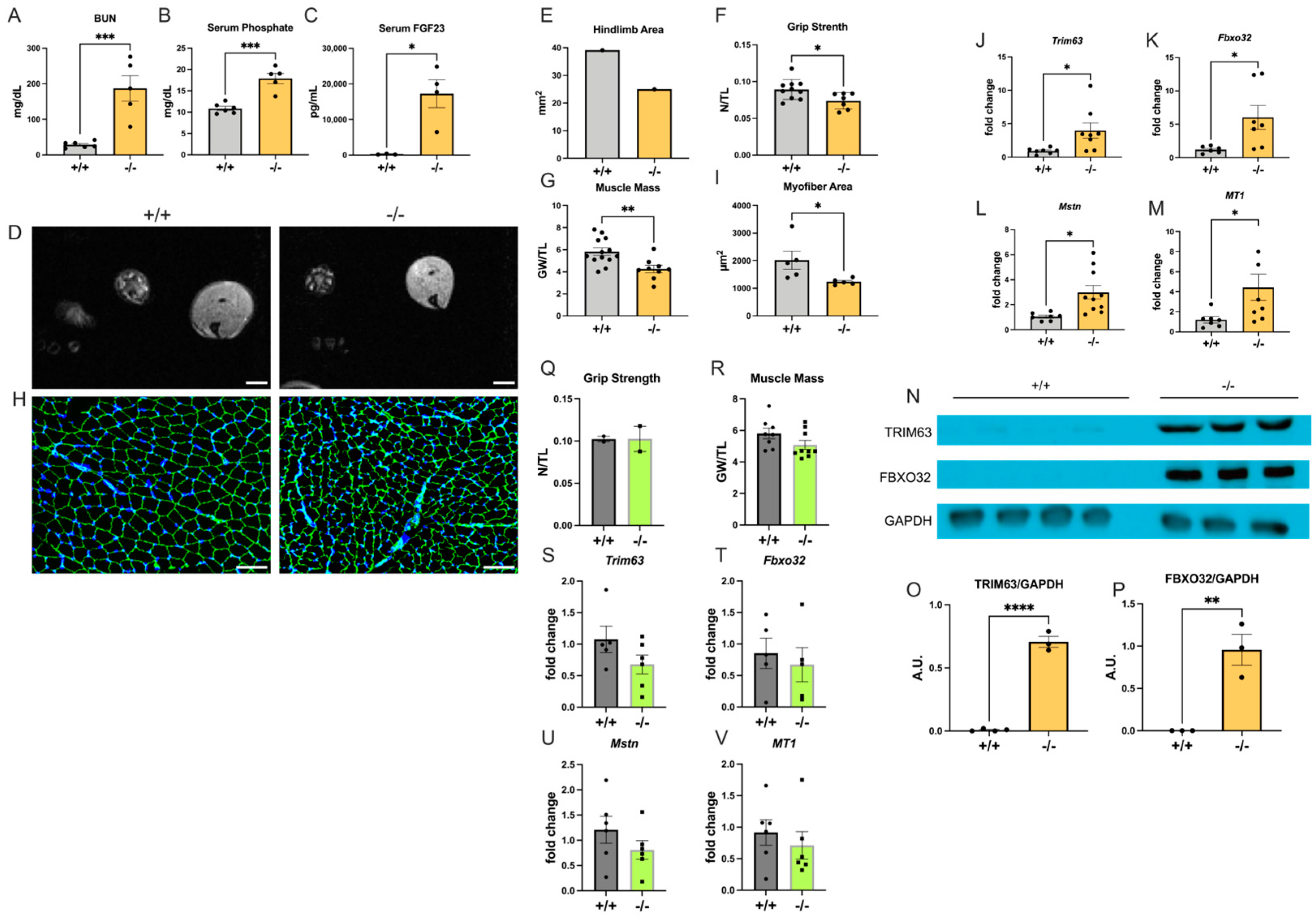
2.2. Col4a3−/− Mice Develop Skeletal Muscle Atrophy
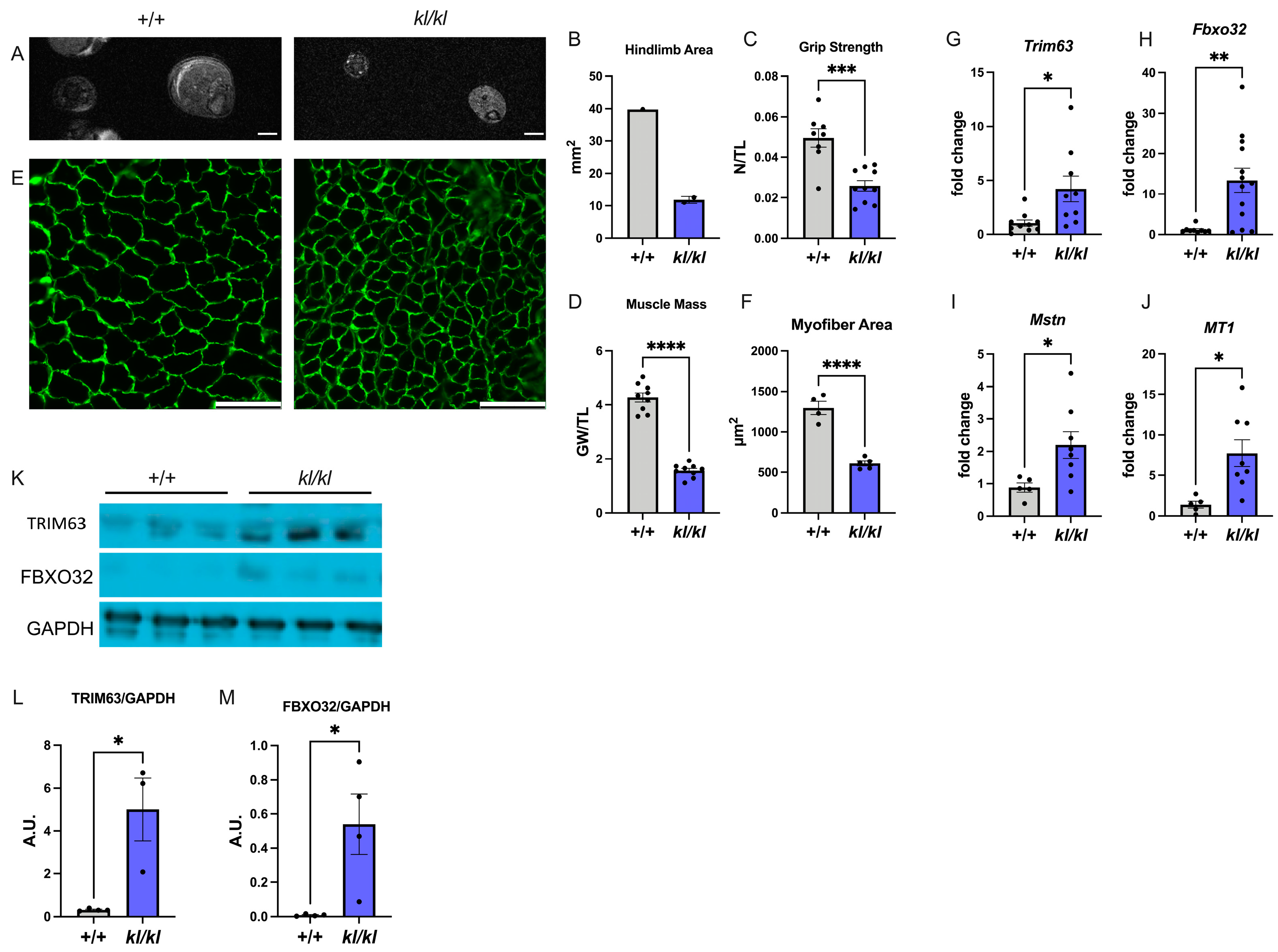
2.3. Klotho-Deficient Mice Develop Skeletal Muscle Atrophy
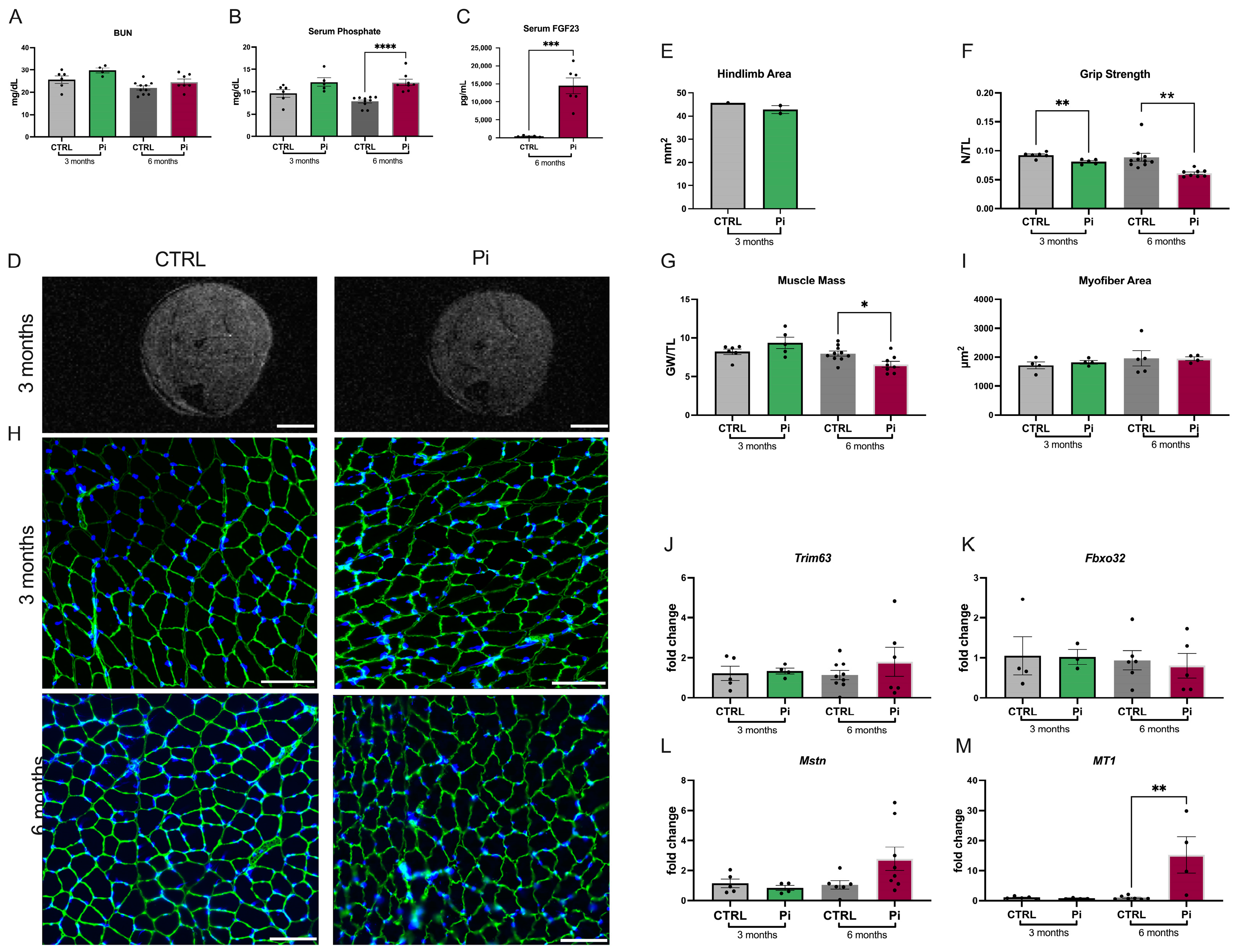
2.4. Mice Fed a High-Phosphate Diet Have Reduced Skeletal Muscle Mass and Strength
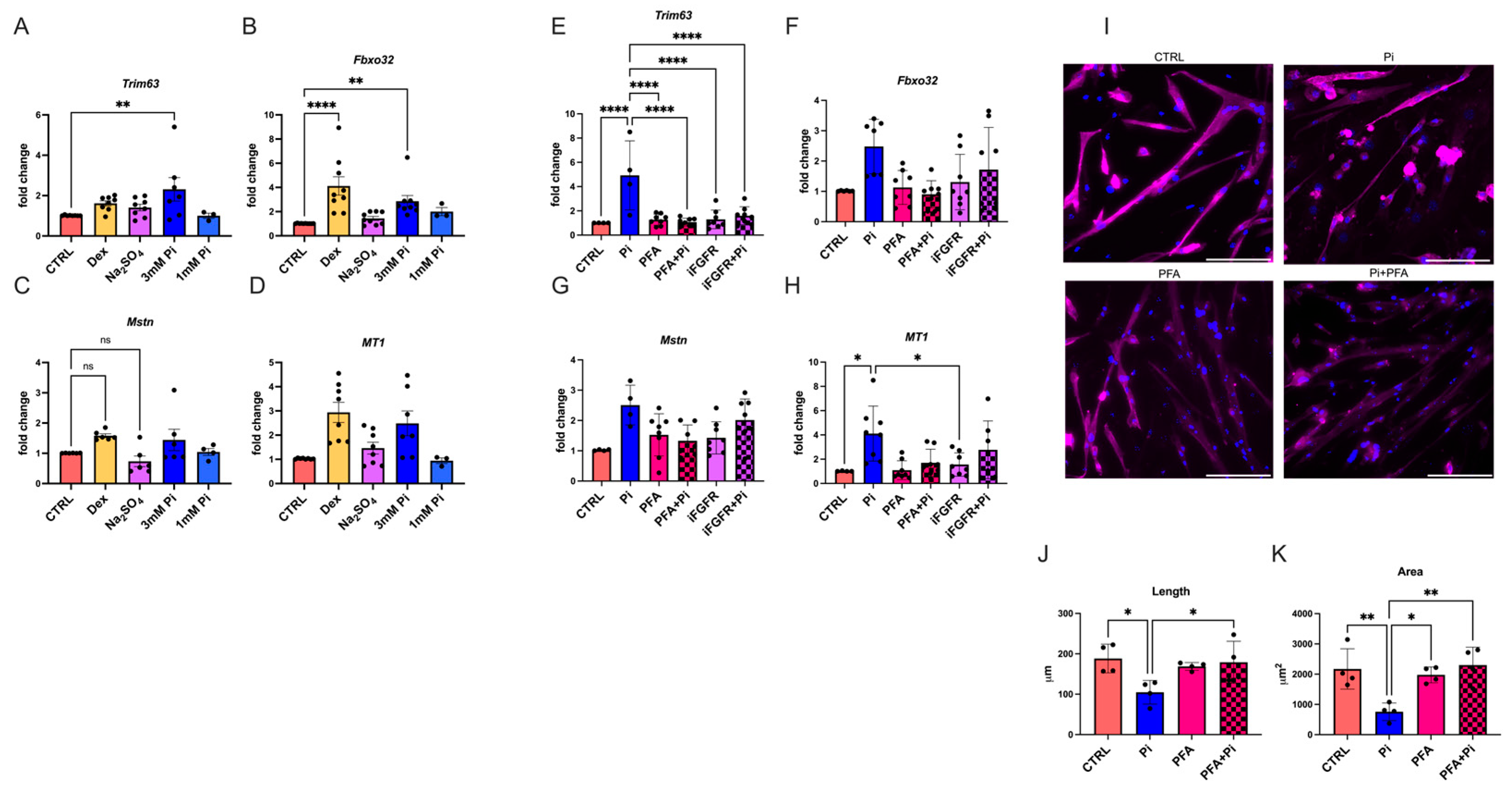
2.5. Phosphate Elevations Induce Atrophy in Cultured Myotubes
2.6. FGF23 Does Not Directly Affect Skeletal Muscle
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Recombinant Proteins, Antibodies, and Small Molecules
4.3. Magnetic Resonance Imaging (MRI)
4.4. Grip Strength
4.5. Tissue Collection
4.6. Serum Chemistry
4.7. Histology
4.8. RNA Isolation from Tissue and Quantitative Real-Time PCR
4.9. Protein Isolation and Western Blotting
4.10. Primary Cell Culture
4.11. C2C12 Cell Culture
4.12. FGF Injections in Mice
4.13. Immunocytochemistry
4.14. Image Analysis
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murphy, D.; McCulloch, C.E.; Lin, F.; Banerjee, T.; Bragg-Gresham, J.L.; Eberhardt, M.S.; Morgenstern, H.; Pavkov, M.E.; Saran, R.; Powe, N.R.; et al. Trends in Prevalence of Chronic Kidney Disease in the United States. Ann. Intern. Med. 2016, 165, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Vart, P.; Powe, N.R.; McCulloch, C.E. Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team. National trends in the prevalence of chronic kidney disease among racial/ethnic and socioeconomic status groups, 1988–2016. JAMA Netw. Open 2020, 3, e20793, Erratum in JAMA Netw. Open 2020, 3, e2019157. [Google Scholar] [CrossRef]
- Faul, C.; Amaral, A.P.; Oskouei, B.; Hu, M.C.; Sloan, A.; Isakova, T.; Gutierrez, O.M.; Aguillon-Prada, R.; Lincoln, J.; Hare, J.M.; et al. FGF23 induces left ventricular hypertrophy. J. Clin. Investig. 2011, 121, 4393–4408. [Google Scholar] [CrossRef]
- Yamada, S.; Tokumoto, M.; Tatsumoto, N.; Taniguchi, M.; Noguschi, H.; Nakano, T.; Masutani, K.; Ooboshi, H.; Tsuruya, K.; Kitazono, T. Phosphate overload directly induces systemic inflammation and malnutrition as well as vascular calcification in uremia. Am. J. Physiol. Renal Physiol. 2014, 306, F1418–F1428. [Google Scholar] [CrossRef]
- Czaya, B.; Heitman, K.; Campos, I.; Yanucil, C.; Kentrup, D.; Westbrook, D.; Gutierrez, O.; Babitt, J.L.; Jung, G.; Salusky, I.B.; et al. Hyperphosphatemia increases inflammation to exacerbate anemia and skeletal muscle wasting independently of FGF23-FGFR4 signaling. eLife 2022, 11, e74782. [Google Scholar] [CrossRef] [PubMed]
- Bollenbecker, S.; Heitman, K.; Czaya, B.; Easter, M.; Hirsch, M.J.; Vang, S.; Harris, E.; Helton, E.S.; Barnes, J.W.; Faul, C.; et al. Phosphate induces inflammation and exacerbates injury from cigarette smoke in the bronchial epithelium. Sci. Rep. 2023, 13, 4898. [Google Scholar] [CrossRef]
- Heitman, K.; Alexander, M.S.; Faul, C. Skeletal Muscle Injury in Chronic Kidney Disease-From Histologic Changes to Molecular Mechanisms and to Novel Therapies. Int. J. Mol. Sci. 2024, 25, 5117. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Streja, E.; Molnar, M.Z.; Lukowsky, L.R.; Krishnan, M.; Kovesdy, C.P.; Greenland, S. Mortality prediction by surrogates of body composition: An examination of the obesity paradox in hemodialysis patients using composite ranking score analysis. Am. J. Epidemiol. 2012, 175, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Mak, R.H.; Ikizler, A.T.; Kovesdy, C.P.; Raj, D.S.; Stenvinkel, P.; Kalantar-Zadeh, K. Wasting in chronic kidney disease. J. Cachexia Sarcopenia Muscle 2011, 2, 9–25. [Google Scholar] [CrossRef]
- Moorthi, R.N.; Avin, K.G. Clinical relevance of sarcopenia in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2017, 26, 219–228. [Google Scholar] [CrossRef]
- Avesani, C.M.; de Abreu, A.M.; Ribeiro, H.S.; Brismar, T.B.; Stenvinkel, P.; Sabatino, A.; Lindholm, B. Muscle fat infiltration in chronic kidney disease: A marker related to muscle quality, muscle strength and sarcopenia. J. Nephrol. 2023, 36, 895–910. [Google Scholar] [CrossRef]
- Mohanasundaram, S.; Fernando, E. Uremic Sarcopenia. Indian J. Nephrol. 2022, 32, 399–405. [Google Scholar] [CrossRef]
- Johansen, K.L.; Shubert, T.; Doyle, J.; Soher, B.; Sakkas, G.K.; Kent-Braun, J.A. Muscle atrophy in patients receiving hemodialysis: Effects on muscle strength, muscle quality, and physical function. Kidney Int. 2003, 63, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Yajima, T. Skeletal muscle density measured by computed tomography as a predictor of mortality in patients receiving hemodialysis. J. Nephrol. 2022, 35, 1535–1537. [Google Scholar] [CrossRef] [PubMed]
- Keddar, M.; Muylle, T.; Carrie, E.; Trefois, P.; Nachit, M.; Crott, R.; Christiaens, C.; Bammens, B.; Jadoul, M.; Goffin, E.; et al. Non-invasive Quantification of Fat Deposits in Skeletal Muscle Predicts Cardiovascular Outcome in Kidney Failure. Front. Physiol. 2020, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Stenvinkel, P.; Cuppari, L.; Ikizler, T.A.; Kalantar-Zadeh, K.; Kaysen, G.; Mitch, W.E.; Price, S.R.; Wanner, C.; Wang, A.Y.; et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: A consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM). J. Ren. Nutr. 2013, 23, 77–90. [Google Scholar] [CrossRef]
- Carrero, J.J.; Qureshi, A.R.; Axelsson, J.; Avesani, C.M.; Suliman, M.E.; Kato, S.; Barany, P.; Snaedal-Jonsdottir, S.; Alvestrand, A.; Heimburger, O.; et al. Comparison of nutritional and inflammatory markers in dialysis patients with reduced appetite. Am. J. Clin. Nutr. 2007, 85, 695–701. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Heimburger, O.; Paultre, F.; Diczfalusy, U.; Wang, T.; Berglund, L.; Jogestrand, T. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 1999, 55, 1899–1911. [Google Scholar] [CrossRef]
- Stenvinkel, P. Inflammation in end-stage renal disease: The hidden enemy. Nephrology 2006, 11, 36–41. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Ketteler, M.; Johnson, R.J.; Lindholm, B.; Pecoits-Filho, R.; Riella, M.; Heimburger, O.; Cederholm, T.; Girndt, M. IL-10, IL-6, and TNF-alpha: Central factors in the altered cytokine network of uremia—The good, the bad, and the ugly. Kidney Int. 2005, 67, 1216–1233. [Google Scholar] [CrossRef]
- Wang, X.H.; Mitch, W.E. Mechanisms of muscle wasting in chronic kidney disease. Nat. Rev. Nephrol. 2014, 10, 504–516. [Google Scholar] [CrossRef] [PubMed]
- Simoes, E.S.A.C.; Oliveira, E.A.; Cheung, W.W.; Mak, R.H. Redox Signaling in Chronic Kidney Disease-Associated Cachexia. Antioxidants 2023, 12, 945. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Mitch, W.E.; Price, S.R. Pathophysiological mechanisms leading to muscle loss in chronic kidney disease. Nat. Rev. Nephrol. 2022, 18, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Price, S.R.; Gooch, J.L.; Donaldson, S.K.; Roberts-Wilson, T.K. Muscle atrophy in chronic kidney disease results from abnormalities in insulin signaling. J. Ren. Nutr. 2010, 20, S24–S28. [Google Scholar] [CrossRef][Green Version]
- Robinson, K.A.; Baker, L.A.; Graham-Brown, M.P.M.; Watson, E.L. Skeletal muscle wasting in chronic kidney disease: The emerging role of microRNAs. Nephrol. Dial. Transplant. 2020, 35, 1469–1478. [Google Scholar] [CrossRef]
- Hung, K.C.; Yao, W.C.; Liu, Y.L.; Yang, H.J.; Liao, M.T.; Chong, K.; Peng, C.H.; Lu, K.C. The Potential Influence of Uremic Toxins on the Homeostasis of Bones and Muscles in Chronic Kidney Disease. Biomedicines 2023, 11, 2076. [Google Scholar] [CrossRef]
- Price, S.R.; Mitch, W.E.; Garibotto, G. Muscle Atrophy in CKD: A Historical Perspective of Advancements in Its Understanding. J. Ren. Nutr. 2022, 33, S88–S92. [Google Scholar] [CrossRef]
- Quarles, L.D. Role of FGF23 in vitamin D and phosphate metabolism: Implications in chronic kidney disease. Exp. Cell Res. 2012, 318, 1040–1048. [Google Scholar] [CrossRef]
- Jansson, K.P.; Yu, A.S.L.; Stubbs, J.R. Contribution of phosphate and FGF23 to CKD progression. Curr. Opin. Nephrol. Hypertens. 2022, 31, 306–311. [Google Scholar] [CrossRef]
- Richter, B.; Faul, C. FGF23 Actions on Target Tissues-With and Without Klotho. Front. Endocrinol. 2018, 9, 189. [Google Scholar] [CrossRef]
- Barreto, F.C.; Barreto, D.V.; Massy, Z.A.; Drueke, T.B. Strategies for Phosphate Control in Patients With CKD. Kidney Int. Rep. 2019, 4, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Olauson, H.; Lindberg, K.; Amin, R.; Edvardsson, K.; Lindholm, B.; Andersson, G.; Wernerson, A.; Sabbagh, Y.; Schiavi, S.; et al. A novel model of adenine-induced tubulointerstitial nephropathy in mice. BMC Nephrol. 2013, 14, 116. [Google Scholar] [CrossRef]
- Yanucil, C.; Kentrup, D.; Campos, I.; Czaya, B.; Heitman, K.; Westbrook, D.; Osis, G.; Grabner, A.; Wende, A.R.; Vallejo, J.; et al. Soluble alpha-klotho and heparin modulate the pathologic cardiac actions of fibroblast growth factor 23 in chronic kidney disease. Kidney Int. 2022, 102, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Tolle, M.; Henkel, C.; Herrmann, J.; Daniel, C.; Babic, M.; Xia, M.; Schulz, A.M.; Amann, K.; van der Giet, M.; Schuchardt, M. Uremic mouse model to study vascular calcification and “inflamm-aging”. J. Mol. Med. 2022, 100, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Baehr, L.M.; Hughes, D.C.; Waddell, D.S.; Bodine, S.C. SnapShot: Skeletal muscle atrophy. Cell 2022, 185, 1618–1618.e1. [Google Scholar] [CrossRef]
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Na, E.; Dharmarajan, K.; et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef]
- McPherron, A.C.; Lawler, A.M.; Lee, S.J. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef]
- Thomas, M.; Langley, B.; Berry, C.; Sharma, M.; Kirk, S.; Bass, J.; Kambadur, R. Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J. Biol. Chem. 2000, 275, 40235–40243. [Google Scholar] [CrossRef]
- Babula, P.; Masarik, M.; Adam, V.; Eckschlager, T.; Stiborova, M.; Trnkova, L.; Skutkova, H.; Provaznik, I.; Hubalek, J.; Kizek, R. Mammalian metallothioneins: Properties and functions. Metallomics 2012, 4, 739–750. [Google Scholar] [CrossRef]
- Lecker, S.H.; Jagoe, R.T.; Gilbert, A.; Gomes, M.; Baracos, V.; Bailey, J.; Price, S.R.; Mitch, W.E.; Goldberg, A.L. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004, 18, 39–51. [Google Scholar] [CrossRef]
- Jimenez-Gutierrez, G.E.; Martinez-Gomez, L.E.; Martinez-Armenta, C.; Pineda, C.; Martinez-Nava, G.A.; Lopez-Reyes, A. Molecular Mechanisms of Inflammation in Sarcopenia: Diagnosis and Therapeutic Update. Cells 2022, 11, 2359. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.; Meehan, D.T.; Grunkemeyer, J.A.; Kornak, J.M.; Sayers, R.; Hunter, W.J.; Samuelson, G.C. Collagen COL4A3 knockout: A mouse model for autosomal Alport syndrome. Genes. Dev. 1996, 10, 2981–2992. [Google Scholar] [CrossRef]
- Stubbs, J.R.; He, N.; Idiculla, A.; Gillihan, R.; Liu, S.; David, V.; Hong, Y.; Quarles, L.D. Longitudinal evaluation of FGF23 changes and mineral metabolism abnormalities in a mouse model of chronic kidney disease. J. Bone Miner. Res. 2012, 27, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Kuro-o, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef]
- Grabner, A.; Schramm, K.; Silswal, N.; Hendrix, M.; Yanucil, C.; Czaya, B.; Singh, S.; Wolf, M.; Hermann, S.; Stypmann, J.; et al. FGF23/FGFR4-mediated left ventricular hypertrophy is reversible. Sci. Rep. 2017, 7, 1993. [Google Scholar] [CrossRef]
- Kim, R.; Kim, H.; Im, M.; Park, S.K.; Han, H.J.; An, S.; Kang, J.S.; Lee, S.J.; Bae, G.U. BST204 Protects Dexamethasone-Induced Myotube Atrophy through the Upregulation of Myotube Formation and Mitochondrial Function. Int. J. Environ. Res. Public Health 2021, 18, 2367. [Google Scholar] [CrossRef] [PubMed]
- Zhiyin, L.; Jinliang, C.; Qiunan, C.; Yunfei, Y.; Qian, X. Fucoxanthin rescues dexamethasone induced C2C12 myotubes atrophy. Biomed. Pharmacother. 2021, 139, 111590. [Google Scholar] [CrossRef]
- Villa-Bellosta, R.; Sorribas, V. Phosphonoformic acid prevents vascular smooth muscle cell calcification by inhibiting calcium-phosphate deposition. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 761–766. [Google Scholar] [CrossRef]
- Takashi, Y.; Kosako, H.; Sawatsubashi, S.; Kinoshita, Y.; Ito, N.; Tsoumpra, M.K.; Nangaku, M.; Abe, M.; Matsuhisa, M.; Kato, S.; et al. Activation of unliganded FGF receptor by extracellular phosphate potentiates proteolytic protection of FGF23 by its O-glycosylation. Proc. Natl. Acad. Sci. USA 2019, 116, 11418–11427. [Google Scholar] [CrossRef]
- Grabner, A.; Amaral, A.P.; Schramm, K.; Singh, S.; Sloan, A.; Yanucil, C.; Li, J.; Shehadeh, L.A.; Hare, J.M.; David, V.; et al. Activation of Cardiac Fibroblast Growth Factor Receptor 4 Causes Left Ventricular Hypertrophy. Cell Metab. 2015, 22, 1020–1032. [Google Scholar] [CrossRef]
- Avin, K.G.; Vallejo, J.A.; Chen, N.X.; Wang, K.; Touchberry, C.D.; Brotto, M.; Dallas, S.L.; Moe, S.M.; Wacker, M.J. Fibroblast Growth Factor 23 Does Not Directly Influence Skeletal Muscle Cell Proliferation and Differentiation or Ex Vivo Muscle Contractility. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E594–E604. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, K.; Shiflett, L.A.; Brotto, L.; Bonewald, L.F.; Wacker, M.J.; Dallas, S.L.; Brotto, M. Fibroblast growth factor 9 (FGF9) inhibits myogenic differentiation of C2C12 and human muscle cells. Cell Cycle 2019, 18, 3562–3580. [Google Scholar] [CrossRef] [PubMed]
- Andrukhova, O.; Zeitz, U.; Goetz, R.; Mohammadi, M.; Lanske, B.; Erben, R.G. FGF23 acts directly on renal proximal tubules to induce phosphaturia through activation of the ERK1/2-SGK1 signaling pathway. Bone 2012, 51, 621–628. [Google Scholar] [CrossRef]
- Urakawa, I.; Yamazaki, Y.; Shimada, T.; Iijima, K.; Hasegawa, H.; Okawa, K.; Fujita, T.; Fukumoto, S.; Yamashita, T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 2006, 444, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Berru, F.N.; Gray, S.E.; Thome, T.; Kumar, R.A.; Salyers, Z.R.; Coleman, M.; Dennis, L.; O'Malley, K.; Ferreira, L.F.; Berceli, S.A.; et al. Chronic kidney disease exacerbates ischemic limb myopathy in mice via altered mitochondrial energetics. Sci. Rep. 2019, 9, 15547. [Google Scholar] [CrossRef]
- Uchiyama, K.; Wakino, S.; Irie, J.; Miyamoto, J.; Matsui, A.; Tajima, T.; Itoh, T.; Oshima, Y.; Yoshifuji, A.; Kimura, I.; et al. Contribution of uremic dysbiosis to insulin resistance and sarcopenia. Nephrol. Dial. Transplant. 2020, 35, 1501–1517. [Google Scholar] [CrossRef] [PubMed]
- Momb, B.A.; Patino, E.; Akchurin, O.M.; Miller, M.S. Iron Supplementation Improves Skeletal Muscle Contractile Properties in Mice with CKD. Kidney360 2022, 3, 843–858. [Google Scholar] [CrossRef]
- Thome, T.; Kumar, R.A.; Burke, S.K.; Khattri, R.B.; Salyers, Z.R.; Kelley, R.C.; Coleman, M.D.; Christou, D.D.; Hepple, R.T.; Scali, S.T.; et al. Impaired muscle mitochondrial energetics is associated with uremic metabolite accumulation in chronic kidney disease. JCI Insight 2020, 6, e139826. [Google Scholar] [CrossRef]
- Lair, B.; Lac, M.; Frassin, L.; Brunet, M.; Buleon, M.; Feuillet, G.; Maslo, C.; Marques, M.; Monbrun, L.; Bourlier, V.; et al. Common mouse models of chronic kidney disease are not associated with cachexia. Commun. Biol. 2024, 7, 346. [Google Scholar] [CrossRef]
- Acevedo, L.M.; Lopez, I.; Peralta-Ramirez, A.; Pineda, C.; Chamizo, V.E.; Rodriguez, M.; Aguilera-Tejero, E.; Rivero, J.L. High-phosphorus diet maximizes and low-dose calcitriol attenuates skeletal muscle changes in long-term uremic rats. J. Appl. Physiol. (1985) 2016, 120, 1059–1069. [Google Scholar] [CrossRef]
- Chung, L.H.; Liu, S.T.; Huang, S.M.; Salter, D.M.; Lee, H.S.; Hsu, Y.J. High phosphate induces skeletal muscle atrophy and suppresses myogenic differentiation by increasing oxidative stress and activating Nrf2 signaling. Aging 2020, 12, 21446–21468. [Google Scholar] [CrossRef] [PubMed]
- Peri-Okonny, P.; Baskin, K.K.; Iwamoto, G.; Mitchell, J.H.; Smith, S.A.; Kim, H.K.; Szweda, L.I.; Bassel-Duby, R.; Fujikawa, T.; Castorena, C.M.; et al. High-Phosphate Diet Induces Exercise Intolerance and Impairs Fatty Acid Metabolism in Mice. Circulation 2019, 139, 1422–1434. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, M.; Razzaque, M.S. Dietary and genetic evidence for phosphate toxicity accelerating mammalian aging. FASEB J. 2010, 24, 3562–3571. [Google Scholar] [CrossRef]
- Ohnishi, M.; Nakatani, T.; Lanske, B.; Razzaque, M.S. Reversal of mineral ion homeostasis and soft-tissue calcification of klotho knockout mice by deletion of vitamin D 1alpha-hydroxylase. Kidney Int. 2009, 75, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, Y.; Ohtsubo, H.; Munekane, A.; Ohkubo, K.; Murakami, T.; Fujino, M.; Nishimatsu, S.I.; Hagiwara, H.; Nishimura, H.; Kaneko, R.; et al. Circulating alpha-Klotho Counteracts Transforming Growth Factor-beta-Induced Sarcopenia. Am. J. Pathol. 2023, 193, 591–607. [Google Scholar] [CrossRef]
- Ahrens, H.E.; Huettemeister, J.; Schmidt, M.; Kaether, C.; von Maltzahn, J. Klotho expression is a prerequisite for proper muscle stem cell function and regeneration of skeletal muscle. Skelet. Muscle 2018, 8, 20. [Google Scholar] [CrossRef]
- Amitani, H.; Chiba, S.; Amitani, M.; Michihara, S.; Takemoto, R.; Han, L.; Fujita, N.; Takahashi, R.; Inui, A. Impact of Ninjin'yoeito on frailty and short life in klotho-hypomorphic (kl/kl) mice. Front. Pharmacol. 2022, 13, 973897. [Google Scholar] [CrossRef]
- Fujitsuka, N.; Asakawa, A.; Morinaga, A.; Amitani, M.S.; Amitani, H.; Katsuura, G.; Sawada, Y.; Sudo, Y.; Uezono, Y.; Mochiki, E.; et al. Increased ghrelin signaling prolongs survival in mouse models of human aging through activation of sirtuin1. Mol. Psychiatry 2016, 21, 1613–1623. [Google Scholar] [CrossRef]
- Nakatani, T.; Sarraj, B.; Ohnishi, M.; Densmore, M.J.; Taguchi, T.; Goetz, R.; Mohammadi, M.; Lanske, B.; Razzaque, M.S. In vivo genetic evidence for klotho-dependent, fibroblast growth factor 23 (Fgf23)-mediated regulation of systemic phosphate homeostasis. FASEB J. 2009, 23, 433–441. [Google Scholar] [CrossRef]
- Erem, S.; Razzaque, M.S. Dietary phosphate toxicity: An emerging global health concern. Histochem. Cell Biol. 2018, 150, 711–719. [Google Scholar] [CrossRef]
- Chande, S.; Caballero, D.; Ho, B.B.; Fetene, J.; Serna, J.; Pesta, D.; Nasiri, A.; Jurczak, M.; Chavkin, N.W.; Hernando, N.; et al. Slc20a1/Pit1 and Slc20a2/Pit2 are essential for normal skeletal myofiber function and survival. Sci. Rep. 2020, 10, 3069. [Google Scholar] [CrossRef] [PubMed]
- Sosa, P.; Alcalde-Estevez, E.; Plaza, P.; Troyano, N.; Alonso, C.; Martinez-Arias, L.; Evelem de Melo Aroeira, A.; Rodriguez-Puyol, D.; Olmos, G.; Lopez-Ongil, S.; et al. Hyperphosphatemia Promotes Senescence of Myoblasts by Impairing Autophagy Through Ilk Overexpression, A Possible Mechanism Involved in Sarcopenia. Aging Dis. 2018, 9, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Sosa, P.; Alcalde-Estevez, E.; Asenjo-Bueno, A.; Plaza, P.; Carrillo-Lopez, N.; Olmos, G.; Lopez-Ongil, S.; Ruiz-Torres, M.P. Aging-related hyperphosphatemia impairs myogenic differentiation and enhances fibrosis in skeletal muscle. J. Cachexia Sarcopenia Muscle 2021, 12, 1266–1279. [Google Scholar] [CrossRef]
- Alcalde-Estevez, E.; Sosa, P.; Asenjo-Bueno, A.; Plaza, P.; Valenzuela, P.L.; Naves-Diaz, M.; Olmos, G.; Lopez-Ongil, S.; Ruiz-Torres, M.P. Dietary phosphate restriction prevents the appearance of sarcopenia signs in old mice. J. Cachexia Sarcopenia Muscle 2023, 14, 1060–1074. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Froum, S.; Hamby, J.M.; Schroeder, M.C.; Panek, R.L.; Lu, G.H.; Eliseenkova, A.V.; Green, D.; Schlessinger, J.; Hubbard, S.R. Crystal structure of an angiogenesis inhibitor bound to the FGF receptor tyrosine kinase domain. EMBO J. 1998, 17, 5896–5904. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heitman, K.; Bollenbecker, S.; Bradley, J.; Czaya, B.; Fajol, A.; Thomas, S.M.; Li, Q.; Komarova, S.; Krick, S.; Rowe, G.C.; et al. Hyperphosphatemia Contributes to Skeletal Muscle Atrophy in Mice. Int. J. Mol. Sci. 2024, 25, 9308. https://doi.org/10.3390/ijms25179308
Heitman K, Bollenbecker S, Bradley J, Czaya B, Fajol A, Thomas SM, Li Q, Komarova S, Krick S, Rowe GC, et al. Hyperphosphatemia Contributes to Skeletal Muscle Atrophy in Mice. International Journal of Molecular Sciences. 2024; 25(17):9308. https://doi.org/10.3390/ijms25179308
Chicago/Turabian StyleHeitman, Kylie, Seth Bollenbecker, Jordan Bradley, Brian Czaya, Abul Fajol, Sarah Madison Thomas, Qing Li, Svetlana Komarova, Stefanie Krick, Glenn C. Rowe, and et al. 2024. "Hyperphosphatemia Contributes to Skeletal Muscle Atrophy in Mice" International Journal of Molecular Sciences 25, no. 17: 9308. https://doi.org/10.3390/ijms25179308
APA StyleHeitman, K., Bollenbecker, S., Bradley, J., Czaya, B., Fajol, A., Thomas, S. M., Li, Q., Komarova, S., Krick, S., Rowe, G. C., Alexander, M. S., & Faul, C. (2024). Hyperphosphatemia Contributes to Skeletal Muscle Atrophy in Mice. International Journal of Molecular Sciences, 25(17), 9308. https://doi.org/10.3390/ijms25179308






