Molecular Sex Differences and Clinical Gender Efficacy in Opioid Use Disorders: From Pain Management to Addiction
Abstract
1. Introduction
2. Discussion
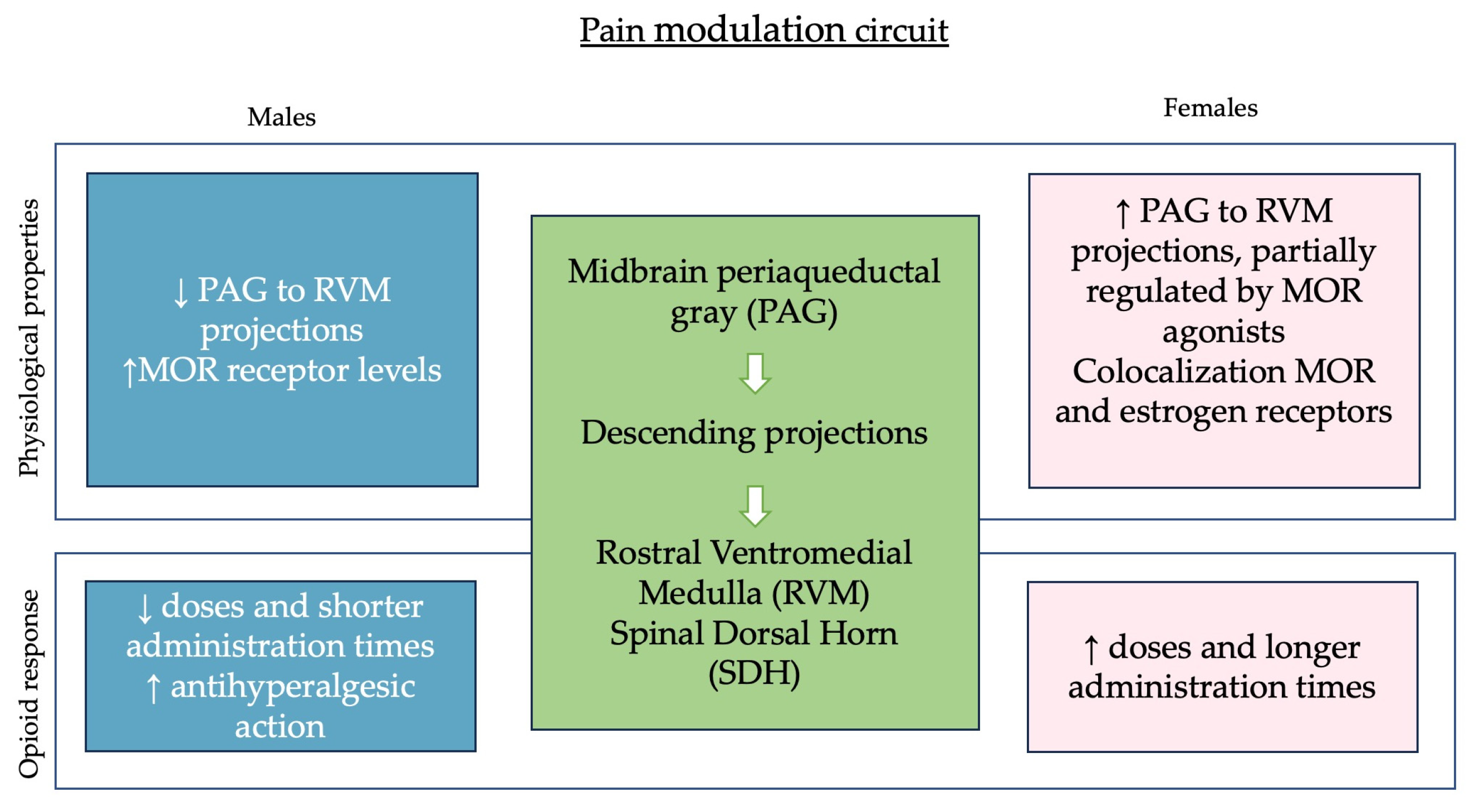
2.1. Pain Modulation and Hormonal Influences
2.2. Addiction and Opioid Withdrawal
3. Materials and Methods
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Gustafsson, M.; Silva, V.; Valeiro, C.; Joaquim, J.; Van Hunsel, F.; Matos, C. Misuse, Abuse and Medication Errors’ Adverse Events Associated with Opioids—A Systematic Review. Pharmaceuticals 2024, 17, 1009. [Google Scholar] [CrossRef]
- Gopalakrishnan, L.; Chatterjee, O.; Ravishankar, N.; Suresh, S.; Raju, R.; Mahadevan, A.; Prasad, T.S.K. Opioid Receptors Signaling Network. J. Cell Commun. Signal 2022, 16, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Papaleo, F.; Contarino, A. Gender- and Morphine Dose-Linked Expression of Spontaneous Somatic Opiate Withdrawal in Mice. Behav. Brain Res. 2006, 170, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Ray, M.H.; Williams, B.R.; Kuppe, M.K.; Bryant, C.D.; Logan, R.W. A Glitch in the Matrix: The Role of Extracellular Matrix Remodeling in Opioid Use Disorder. Front. Integr. Neurosci. 2022, 16, 899637. [Google Scholar] [CrossRef] [PubMed]
- European Monitoring Centre for Drugs and Drug Addiction. European Drug Report; European Monitoring Centre for Drugs and Drug Addiction: Lisbon, Portugal, 2024. [Google Scholar]
- Tisdale, R.K.; Sun, Y.; Park, S.; Ma, S.; Haire, M.; Allocca, G.; Bruchas, M.R.; Morairty, S.R.; Kilduff, T.S. Biological Sex Influences Sleep Phenotype in Mice Experiencing Spontaneous Opioid Withdrawal. J. Sleep Res. 2024, 33, e14037. [Google Scholar] [CrossRef]
- Sharp, B.M.; Fan, X.; Redei, E.E.; Mulligan, M.K.; Chen, H. Sex and Heredity Are Determinants of Drug Intake in a Novel Model of Rat Oral Oxycodone Self-administration. Genes Brain Behav. 2021, 20, e12770. [Google Scholar] [CrossRef]
- Spencer, M.R.; Garnett, M.F.; Miniño, A.M. Drug Overdose Deaths in the United States, 2002–2022; US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics: Washington, DC, USA, 2024.
- The Substance Abuse and Mental Health Services Administration (SAMHSA). Drug Abuse Warning Network (DAWN): Findings from Drug-Related Emergency Department Visits, 2022; The Substance Abuse and Mental Health Services Administration (SAMHSA): Rockville, MD, USA, 2022. [Google Scholar]
- Gustafsson, M.; Matos, C.; Joaquim, J.; Scholl, J.; Van Hunsel, F. Adverse Drug Reactions to Opioids: A Study in a National Pharmacovigilance Database. Drug Saf. 2023, 46, 1133–1148. [Google Scholar] [CrossRef]
- Bartley, E.J.; Fillingim, R.B. Sex Differences in Pain: A Brief Review of Clinical and Experimental Findings. Br. J. Anaesth. 2013, 111, 52–58. [Google Scholar] [CrossRef]
- Packiasabapathy, S.; Sadhasivam, S. Gender, Genetics, and Analgesia: Understanding the Differences in Response to Pain Relief. JPR 2018, 11, 2729–2739. [Google Scholar] [CrossRef]
- Choo, E.K.; Douriez, C.; Green, T. Gender and Prescription Opioid Misuse in the Emergency Department. Acad. Emerg. Med. 2014, 21, 1493–1498. [Google Scholar] [CrossRef]
- Evans, E.; Kelleghan, A.; Li, L.; Min, J.; Huang, D.; Urada, D.; Hser, Y.I.; Nosyk, B. Gender Differences in Mortality among Treated Opioid Dependent Patients. Drug Alcohol Depend. 2015, 155, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Serdarevic, M.; Striley, C.W.; Cottler, L.B. Sex Differences in Prescription Opioid Use. Curr. Opin. Psychiatry 2017, 30, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Craft, R.M.; Bernal, S.A. Sex Differences in Opioid Antinociception: And ‘Mixed Action’ Agonists. Drug Alcohol Depend. 2001, 63, 215–228. [Google Scholar] [CrossRef]
- Tabanelli, R.; Brogi, S.; Calderone, V. Targeting Opioid Receptors in Addiction and Drug Withdrawal: Where Are We Going? IJMS 2023, 24, 10888. [Google Scholar] [CrossRef] [PubMed]
- Yam, M.F.; Loh, Y.C.; Tan, C.S.; Adam, S.K.; Manan, N.A.; Basir, R. General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation. Int. J. Mol. Sci. 2018, 19, 2164. [Google Scholar] [CrossRef]
- Averitt, D.L.; Eidson, L.N.; Doyle, H.H.; Murphy, A.Z. Neuronal and Glial Factors Contributing to Sex Differences in Opioid Modulation of Pain. Neuropsychopharmacology 2019, 44, 155–165. [Google Scholar] [CrossRef]
- Loyd, D.R.; Murphy, A.Z. The Role of the Periaqueductal Gray in the Modulation of Pain in Males and Females: Are the Anatomy and Physiology Really That Different? Neural Plast. 2009, 2009, 462879. [Google Scholar] [CrossRef]
- Lopresti, N.M.; Esguerra, M.; Mermelstein, P.G. Sex Differences in Animal Models of Opioid Reward. Curr. Sex Health Rep. 2020, 12, 186–194. [Google Scholar] [CrossRef]
- Falconnier, C.; Caparros-Roissard, A.; Decraene, C.; Lutz, P.-E. Functional Genomic Mechanisms of Opioid Action and Opioid Use Disorder: A Systematic Review of Animal Models and Human Studies. Mol. Psychiatry 2023, 28, 4568–4584. [Google Scholar] [CrossRef]
- Cepeda, M.S.; Carr, D.B. Women Experience More Pain and Require More Morphine Than Men to Achieve a Similar Degree of Analgesia. Anesth. Analg. 2003, 97, 1464–1468. [Google Scholar] [CrossRef]
- Wang, X.; Traub, R.J.; Murphy, A.Z. Persistent Pain Model Reveals Sex Difference in Morphine Potency. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2006, 291, R300–R306. [Google Scholar] [CrossRef]
- Bobeck, E.N.; McNeal, A.L.; Morgan, M.M. Drug Dependent Sex-Differences in Periaqueducatal Gray Mediated Antinociception in the Rat. Pain 2009, 147, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Zhang, X.; Li, Y.; Lu, L.; Li, B.; He, X. Sex Differences in Peripheral Mu-Opioid Receptor Mediated Analgesia in Rat Orofacial Persistent Pain Model. PLoS ONE 2015, 10, e0122924. [Google Scholar] [CrossRef][Green Version]
- Terner, J.M.; Barrett, A.C.; Lomas, L.M.; Negus, S.S.; Picker, M.J. Influence of Low Doses of Naltrexone on Morphine Antinociception and Morphine Tolerance in Male and Female Rats of Four Strains. Pain 2006, 122, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.M.; Whittier, K.L.; Hegarty, D.M.; Aicher, S.A. Periaqueductal Gray Neurons Project to Spinally Projecting GABAergic Neurons in the Rostral Ventromedial Medulla. Pain 2009, 140, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Sarton, E.; Olofsen, E.; Romberg, R.; Hartigh, J.D.; Kest, B.; Nieuwenhuijs, D.; Burm, A.; Teppema, L.; Dahan, A. Sex Differences in Morphine Analgesia. Anesthesiology 2000, 93, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.C.; Smith, E.S.; Picker, M.J. Capsaicin-Induced Hyperalgesia and μ-Opioid-Induced Antihyperalgesia in Male and Female Fischer 344 Rats. J. Pharmacol. Exp. Ther. 2003, 307, 237–245. [Google Scholar] [CrossRef]
- Mogil, J.S. Sex Differences in Pain and Pain Inhibition: Multiple Explanations of a Controversial Phenomenon. Nat. Rev. Neurosci. 2012, 13, 859–866. [Google Scholar] [CrossRef]
- Fillingim, R.B.; King, C.D.; Ribeiro-Dasilva, M.C.; Rahim, B.; Iii, J.L.R. Sex, Gender, and Pain: A Review of Recent Clinical and Experimental Findings. J. Pain 2009, 10, 447–485. [Google Scholar] [CrossRef]
- Claiborne, J.; Nag, S.; Mokha, S.S. Activation of Opioid Receptor Like-1 Receptor in the Spinal Cord Produces Sex-Specific Antinociception in the Rat: Estrogen Attenuates Antinociception in the Female, Whereas Testosterone Is Required for the Expression of Antinociception in the Male. J. Neurosci. 2006, 26, 13048–13053. [Google Scholar] [CrossRef]
- Galaj, E.; Xi, Z.-X. Progress in Opioid Reward Research: From a Canonical Two-Neuron Hypothesis to Two Neural Circuits. Pharmacol. Biochem. Behav. 2022, 200, 173072. [Google Scholar] [CrossRef] [PubMed]
- American Society of Addition Medicine. Opioid Addiction 2016 Facts & Figures; American Society of Addition Medicine: Rockville, MD, USA, 2016. [Google Scholar]
- Hölscher, F.; Reissner, V.; Di Furia, L.; Room, R.; Schifano, F.; Stohler, R.; Yotsidi, V.; Scherbaum, N. Differences between Men and Women in the Course of Opiate Dependence: Is There a Telescoping Effect? Eur. Arch. Psychiatry Clin. Neurosci. 2010, 260, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Cicero, T.J.; Aylward, S.C.; Meyer, E.R. Gender Differences in the Intravenous Self-Administration of Mu Opiate Agonists. Pharmacol. Biochem. Behav. 2002, 74, 541–549. [Google Scholar] [CrossRef]
- Mohammadian, J.; Miladi-Gorji, H. Age- and Sex-Related Changes in the Severity of Physical and Psychological Dependence in Morphine-Dependent Rats. Pharmacol. Biochem. Behav. 2019, 187, 172793. [Google Scholar] [CrossRef] [PubMed]
- Iyer, V.; Woodward, T.; Pacheco, R.; Hohmann, A.G. A Limited Access Oral Oxycodone Paradigm Produces Physical Dependence and Mesocorticolimbic Region-Dependent Increases in DeltaFosB Expression without Preference. Neuropharmacology 2022, 205, 108925. [Google Scholar] [CrossRef]
- Santoro, G.C.; Carrion, J.; Patel, K.; Vilchez, C.; Veith, J.; Brodie, J.D.; Dewey, S.L. Sex Differences in Regional Brain Glucose Metabolism Following Opioid Withdrawal and Replacement. Neuropsychopharmacology 2017, 42, 1841–1849. [Google Scholar] [CrossRef]
- Borrelli, K.N.; Yao, E.J.; Yen, W.W.; Phadke, R.A.; Ruan, Q.T.; Chen, M.M.; Kelliher, J.C.; Langan, C.R.; Scotellaro, J.L.; Babbs, R.K.; et al. Sex Differences in Behavioral and Brainstem Transcriptomic Neuroadaptations Following Neonatal Opioid Exposure in Outbred Mice. eNeuro 2021, 8, 0143. [Google Scholar] [CrossRef]
- Lipari, R.N. Key Substance Use and Mental Health Indicators in the United States: Results from the 2018 National Survey on Drug Use and Health; Substance Abuse and Mental Health Services Administration: Rockville, MD, USA, 2019. [Google Scholar]
- Raffa, R.B.; Pergolizzi, J.V., Jr.; Muniz, E.; Taylor, R., Jr.; Pergolizzi, J. Designing Opioids That Deter Abuse. Pain Res. Treat. 2012, 2012, 282981. [Google Scholar] [CrossRef]

| Articles | Research Subjects | Experimental Model | Results |
|---|---|---|---|
| Papaleo et al., 2006 [3] | C57BL/6J mouse | Spontaneous opiate withdrawal somatic signs —morphine | Sex- and dose-dependent (↑ female) |
| Tisdale at al., 2024 [6] | C57BL/6J mouse | Spontaneous opioid withdrawal —morphine | Sex-dependent effects on sleep (↑ female) |
| Sharp et al., 2021 [7] | Multiple rat strains | Self-administration protocol —oxycodone | Different self-administration behavior Strain-dependent |
| Choo et al., 2014 [13] | Human | DAWN data collection | Gender differences among opioid users seeking emergency care |
| Craft et al., 2001 [16] | Rat | Investigation of the antinociceptive effects of µ opioid agonists | Sex differences not related to µ opioid agonists, κ opioid antinociception may be primarily spinal rather than supraspinal |
| Wang et al., 2006 [24] | Rat | Thermal hyperalgesia —morphine | Sex differences in the anti-hyperalgesic action of morphine |
| Bobeck et al., 2009 [25] | Rat | Comparison of the antinociceptive actions of various opioids | GABAergic neuron inhibition produces greater antinociception in males |
| Bai et al., 2015 [26] | Rat | Orofacial persistent pain model | Sex differences in the effects of peripheral MOR agonists in long-lasting pain condition |
| Terner et al., 2006 [27] | Rat | Excitatory action of morphine —morphine and naltrexone | No sex or strain differences in opioid sensitivity with low doses of naltrexone |
| Sarton et al., 2000 [29] | Human | Pain threshold and tolerance —morphine | Sex differences in morphine potency and onset–offset speed |
| Barret et al., 2003 [30] | Rat | Capsaicin-induced hyperalgesia | Comparable effect of morphine |
| Mohammadian et al., 2019 [38] | Rat | Chronic morphine administration | Sex differences in the duration of morphine withdrawal |
| Iyer et al., 2022 [39] | C57BL/6J mouse | Voluntary consumption of oxycodone | No sex-dependent differences |
| Santoro et al., 2017 [40] | Rat | Simulation of opioid abuse and opioid replacement | Sex-dependent changes in selected areas of the CNS |
| Borrelli et al., 2021 [41] | Neonatal outbred mouse | Simulation of neonatal withdrawal syndrome | Sex-dependent differences, vocalization, transcriptional alterations |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Concato, M.; Giacomello, E.; Al-Habash, I.; Alempijevic, D.; Kolev, Y.G.; Buffon, M.; Radaelli, D.; D’Errico, S. Molecular Sex Differences and Clinical Gender Efficacy in Opioid Use Disorders: From Pain Management to Addiction. Int. J. Mol. Sci. 2024, 25, 9314. https://doi.org/10.3390/ijms25179314
Concato M, Giacomello E, Al-Habash I, Alempijevic D, Kolev YG, Buffon M, Radaelli D, D’Errico S. Molecular Sex Differences and Clinical Gender Efficacy in Opioid Use Disorders: From Pain Management to Addiction. International Journal of Molecular Sciences. 2024; 25(17):9314. https://doi.org/10.3390/ijms25179314
Chicago/Turabian StyleConcato, Monica, Emiliana Giacomello, Ibrahim Al-Habash, Djordje Alempijevic, Yanko Georgiev Kolev, Maria Buffon, Davide Radaelli, and Stefano D’Errico. 2024. "Molecular Sex Differences and Clinical Gender Efficacy in Opioid Use Disorders: From Pain Management to Addiction" International Journal of Molecular Sciences 25, no. 17: 9314. https://doi.org/10.3390/ijms25179314
APA StyleConcato, M., Giacomello, E., Al-Habash, I., Alempijevic, D., Kolev, Y. G., Buffon, M., Radaelli, D., & D’Errico, S. (2024). Molecular Sex Differences and Clinical Gender Efficacy in Opioid Use Disorders: From Pain Management to Addiction. International Journal of Molecular Sciences, 25(17), 9314. https://doi.org/10.3390/ijms25179314






