Reconstruction and Analysis of a Genome-Scale Metabolic Model of Acinetobacter lwoffii
Abstract
1. Introduction
2. Results and Discussion
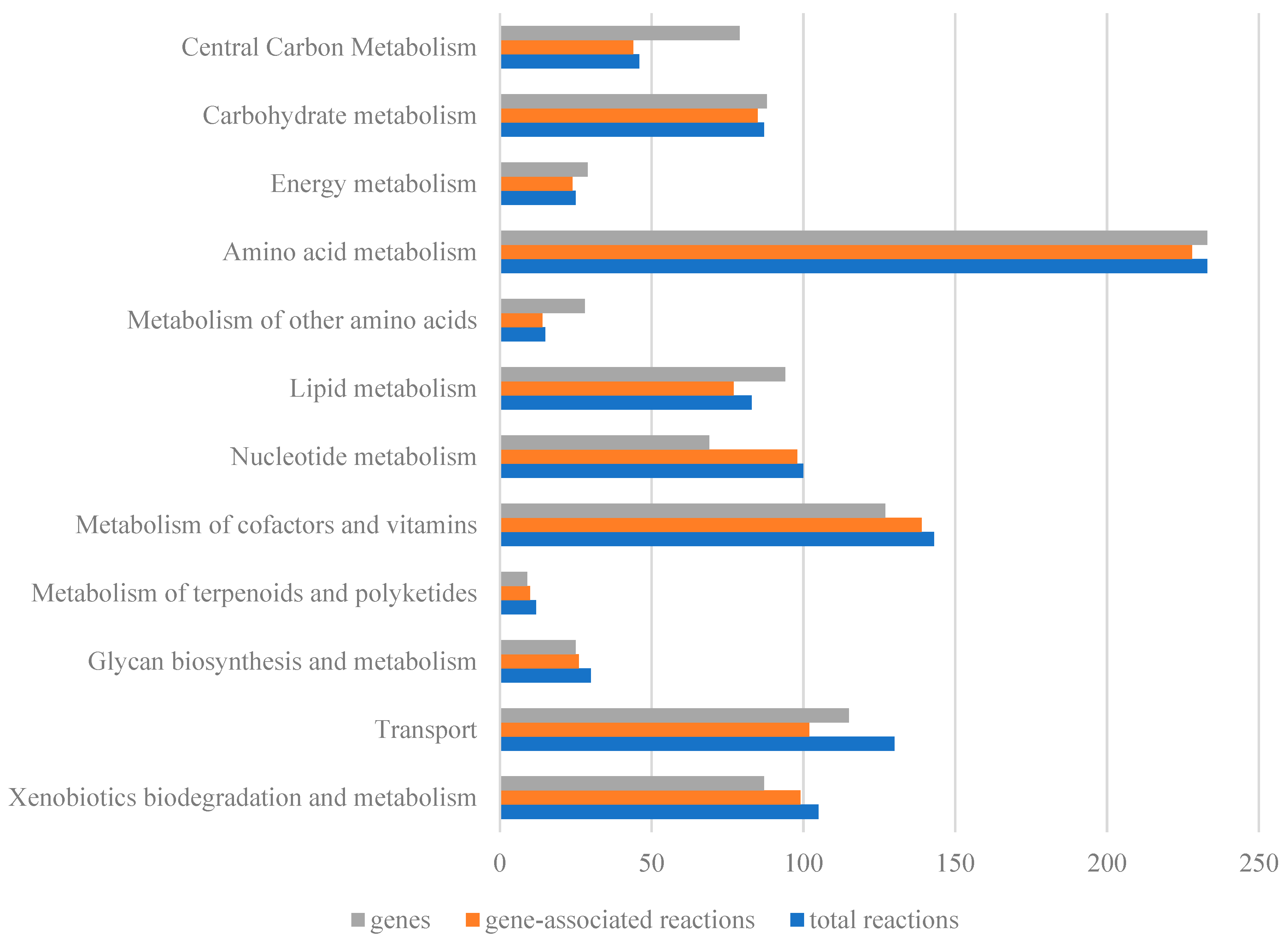
2.1. Characteristics of the Genome-Scale Metabolic Model iNX811
2.2. Model Validation by Cell Growth Phenotypes and Genotypes
2.3. Essential Metabolite Analysis for In Silico Drug Targets
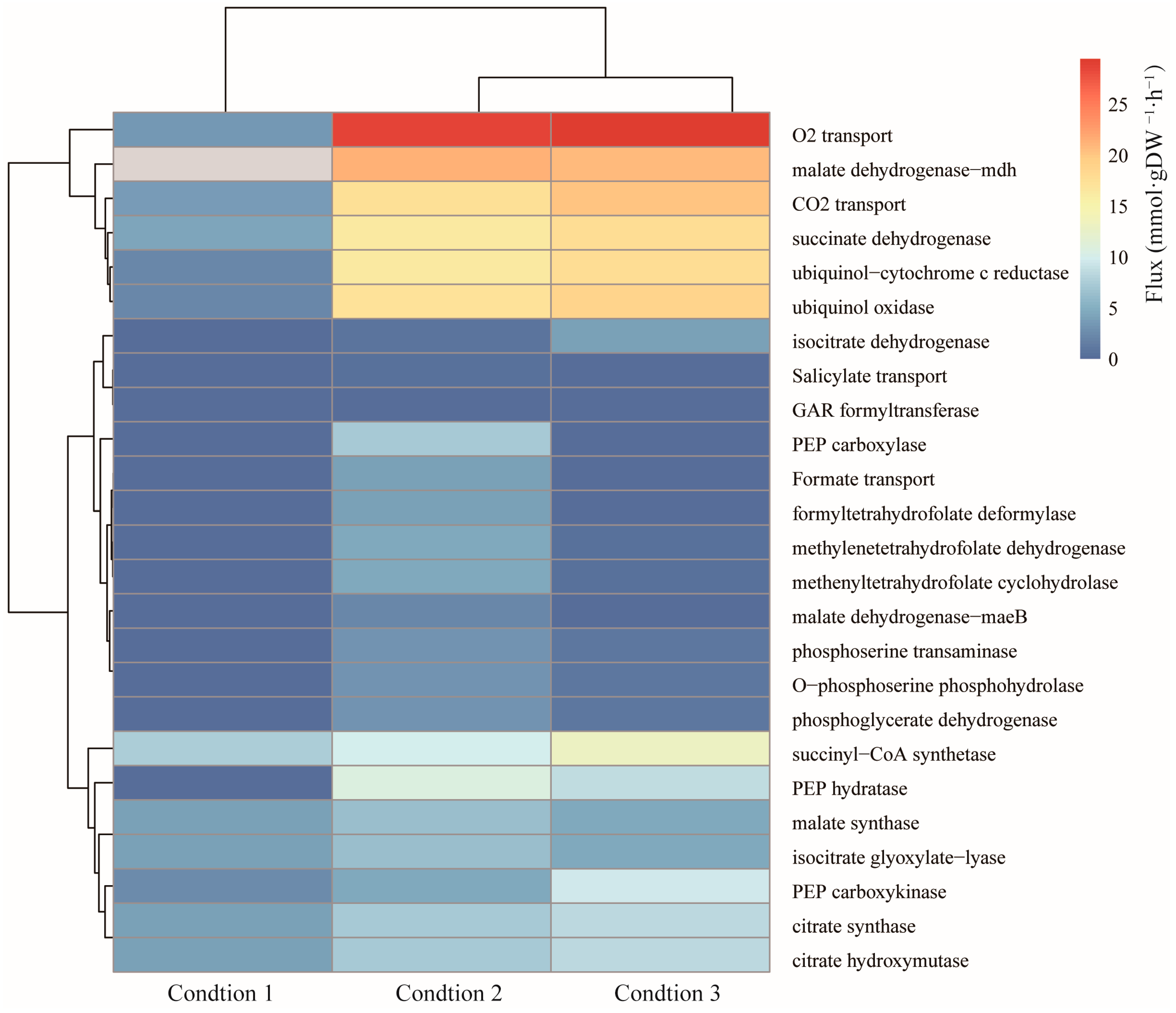
2.4. Integration of Transcriptomic Data in the Genome-Scale Metabolic Model
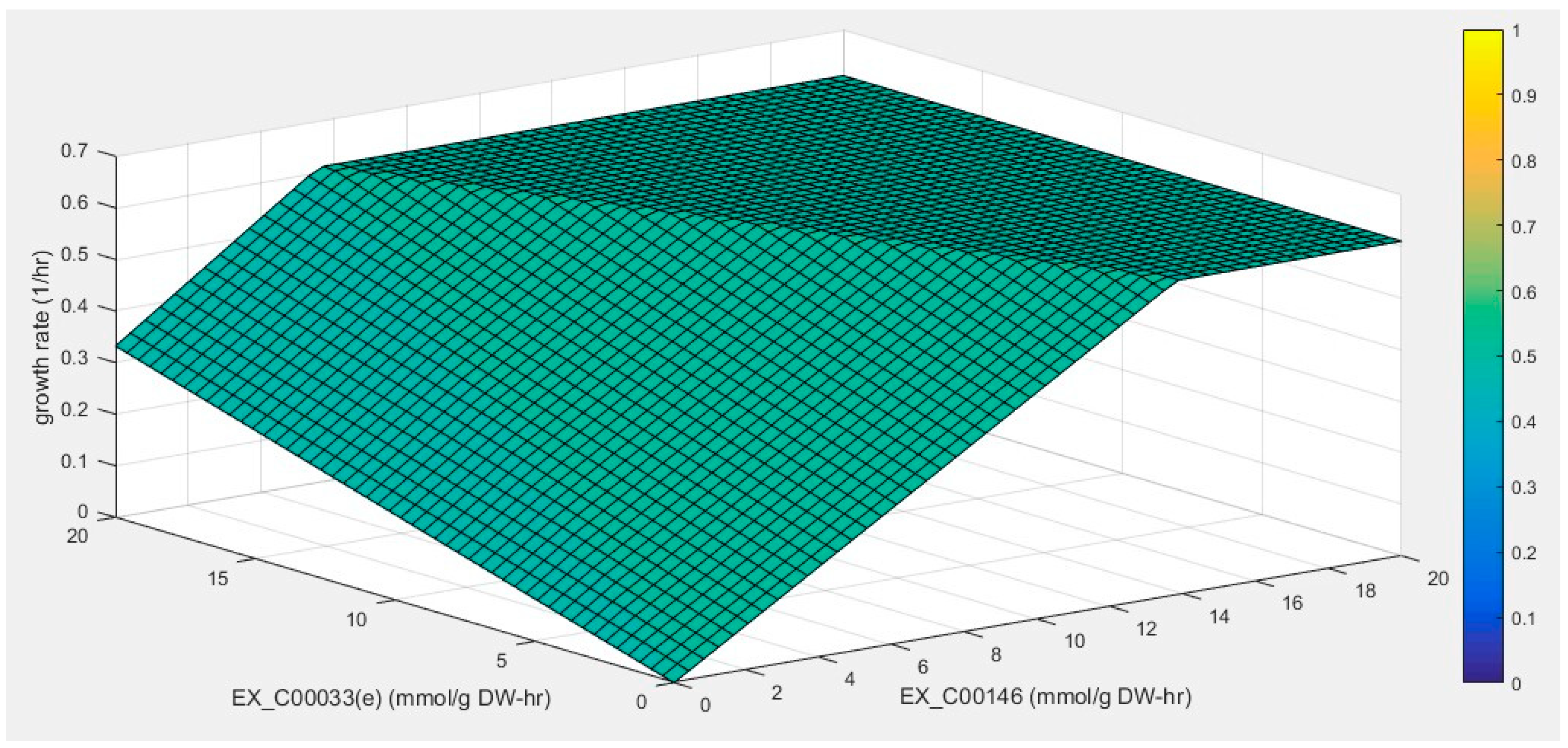
2.5. Prediction of Targets for Increased Phenol Degradation
3. Materials and Methods
3.1. Reconstruction of the Genome-Scale Metabolic Model
3.1.1. Construction of the Draft Model
3.1.2. Biomass Formation
3.2. Model Simulation and Analysis
3.2.1. Cell Growth Simulation
3.2.2. Prediction of Drug Targets
3.2.3. Context-Specific Metabolic Models
3.2.4. Phenotype Phase-Plane (PHPP) Analysis
3.3. Model Validation
3.3.1. Microbial Cultivation
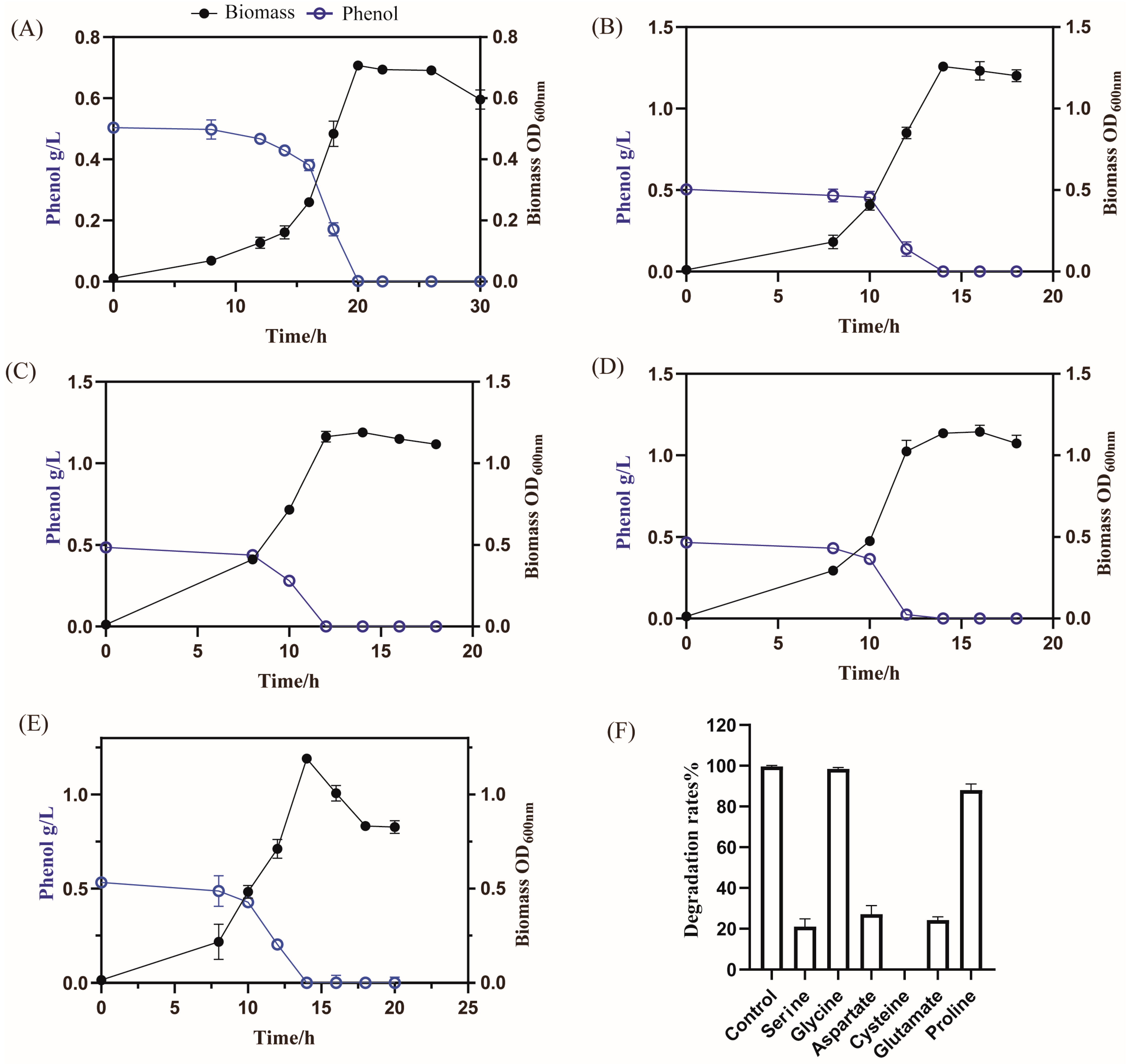
3.3.2. Cell Growth and Phenol Utilization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Visca, P.; Seifert, H.; Towner, K.J. Acinetobacter infection—An emerging threat to human health. IUBMB Life 2011, 63, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Geng, Y.; Yu, Z.; Deng, L.; Gan, W.; Wang, K.; Ou, Y.; Chen, D.; Huang, X.; Zuo, Z.; et al. Acinetobacter lwoffii, an emerging pathogen for fish in Schizothorax genus in China. Transbound. Emerg. Dis. 2018, 65, 1816–1822. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, W.; Liang, H.; Liu, L.; Peng, G.; Pan, Y.; Yang, X.; Zheng, B.; Gao, G.F.; Zhu, B.; et al. Whole-genome sequence of a multidrug-resistant clinical isolate of Acinetobacter lwoffii. J. Bacteriol. 2011, 193, 5549–5550. [Google Scholar] [CrossRef]
- Touchon, M.; Cury, J.; Yoon, E.-J.; Krizova, L.; Cerqueira, G.C.; Murphy, C.; Feldgarden, M.; Wortman, J.; Clermont, D.; Lambert, T.; et al. The Genomic Diversification of the Whole Acinetobacter Genus: Origins, Mechanisms, and Consequences. Genome Biol. Evol. 2014, 6, 2866–2882. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wei, H.M.; Yuan, J.L.; Xu, L.; Sun, J.Q. A comprehensive genomic analysis provides insights on the high environmental adaptability of Acinetobacter strains. Front. Microbiol. 2023, 14, 1177951. [Google Scholar] [CrossRef] [PubMed]
- Almeida, O.G.G.; Furlan, J.P.R.; Stehling, E.G.; De Martinis, E.C.P. Comparative phylo-pangenomics reveals generalist lifestyles in representative Acinetobacter species and proposes candidate gene markers for species identification. Gene 2021, 791, 145707. [Google Scholar] [CrossRef]
- Mindlin, S.; Petrenko, A.; Kurakov, A.; Beletsky, A.; Mardanov, A.; Petrova, M. Resistance of Permafrost and Modern Acinetobacter lwoffii Strains to Heavy Metals and Arsenic Revealed by Genome Analysis. BioMed Res. Int. 2016, 2016, 3970831. [Google Scholar] [CrossRef]
- Alonso-Vásquez, T.; Fondi, M.; Perrin, E. Understanding antimicrobial resistance using genome-scale metabolic modeling. Antibiotics 2023, 12, 896. [Google Scholar] [CrossRef]
- Nazarshodeh, E.; Marashi, S.A.; Gharaghani, S. Structural systems pharmacology: A framework for integrating metabolic network and structure-based virtual screening for drug discovery against bacteria. PLoS ONE 2021, 16, e0261267. [Google Scholar] [CrossRef]
- Han, S.; Tao, Y.; Cui, Y.; Xu, J.; Ju, H.; Fan, L.; Zhang, L.; Zhang, Y. Lanthanum-modified polydopamine loaded Acinetobacter lwoffii DNS32 for phosphate and atrazine removal: Insights into co-adsorption and biodegradation mechanisms. Bioresour. Technol. 2023, 368, 128266. [Google Scholar] [CrossRef]
- Han, S.; Tao, Y.; Zhao, L.; Cui, Y.; Zhang, Y. Metabolic insights into how multifunctional microbial consortium enhances atrazine removal and phosphorus uptake at low temperature. J. Hazard. Mater. 2024, 461, 132539. [Google Scholar] [CrossRef]
- Xu, N.; Yang, X.; Yang, Q.; Guo, M. Comparative Genomic and Transcriptomic Analysis of Phenol Degradation and Tolerance in Acinetobacter lwoffii through Adaptive Evolution. Int. J. Mol. Sci. 2023, 24, 16529. [Google Scholar] [CrossRef]
- Uzma, B.; Ali, F.; Qureshi, N.A.; Shakeela, Q.; Asima, B.; Ahmed, S.; Hayat, A.; Rehman, M.U. Isolation and characterization of synthetic pyrethroids-degrading bacterial strains from agricultural soil. Braz. J. Biol. 2023, 83, e271790. [Google Scholar] [CrossRef] [PubMed]
- Walter, T.; Klim, J.; Jurkowski, M.; Gawor, J.; Köhling, I.; Słodownik, M.; Zielenkiewicz, U. Plasmidome of an environmental Acinetobacter lwoffii strain originating from a former gold and arsenic mine. Plasmid 2020, 110, 102505. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Ye, C.; Liu, L.M. Genome-scale biological models for industrial microbial systems. Appl. Microbiol. Biotechnol. 2018, 102, 3439–3451. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhao, J.; Maifiah, M.H.M.; Velkov, T.; Schreiber, F.; Li, J. Metabolic Responses to Polymyxin Treatment in Acinetobacter baumannii ATCC 19606: Integrating Transcriptomics and Metabolomics with Genome-Scale Metabolic Modeling. Msystems 2019, 4, e00157-18. [Google Scholar] [CrossRef]
- Zhao, J.X.; Zhu, Y.; Han, J.R.; Lin, Y.W.; Aichem, M.; Wang, J.P.; Chen, K.; Velkov, T.; Schreiber, F.; Li, J. Genome-Scale Metabolic Modeling Reveals Metabolic Alterations of Multidrug-Resistant Acinetobacter baumannii in a Murine Bloodstream Infection Model. Microorganisms 2020, 8, 18. [Google Scholar] [CrossRef]
- Norsigian, C.J.; Kavvas, E.; Seif, Y.; Palsson, B.O.; Monk, J.M. iCN718, an Updated and Improved Genome-Scale Metabolic Network Reconstruction of Acinetobacter baumannii AYE. Front. Genet. 2018, 9, 121. [Google Scholar] [CrossRef]
- Kim, H.U.; Kim, T.Y.; Lee, S.Y. Genome-scale metabolic network analysis and drug targeting of multi-drug resistant pathogen Acinetobacter baumannii AYE. Mol. Biosyst. 2010, 6, 339–348. [Google Scholar] [CrossRef]
- Zhu, Y.; Lu, J.; Zhao, J.; Zhang, X.; Yu, H.H.; Velkov, T.; Li, J. Complete genome sequence and genome-scale metabolic modelling of Acinetobacter baumannii type strain ATCC 19606. Int. J. Med. Microbiol. 2020, 310, 151412. [Google Scholar] [CrossRef]
- Durot, M.; Le Fèvre, F.; de Berardinis, V.; Kreimeyer, A.; Vallenet, D.; Combe, C.; Smidtas, S.; Salanoubat, M.; Weissenbach, J.; Schachter, V. Iterative reconstruction of a global metabolic model of Acinetobacter baylyi ADP1 using high-throughput growth phenotype and gene essentiality data. BMC Syst. Biol. 2008, 2, 85. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Qiu, C.; Yang, Q.; Zhang, Y.; Wang, M.; Ye, C.; Guo, M. Analysis of phenol biodegradation in antibiotic and heavy metal resistant Acinetobacter lwoffii NL1. Front. Microbiol. 2021, 12, 725755. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef]
- Lieven, C.; Beber, M.E.; Olivier, B.G.; Bergmann, F.T.; Ataman, M.; Babaei, P.; Bartell, J.A.; Blank, L.M.; Chauhan, S.; Correia, K.; et al. MEMOTE for standardized genome-scale metabolic model testing. Nat. Biotechnol. 2020, 38, 504. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, R.U.; Edwards, J.S.; Palsson, B.O. Escherichia coli K-12 undergoes adaptive evolution to achieve predicted optimal growth. Nature 2002, 420, 186–189. [Google Scholar] [CrossRef]
- Imron, M.F.; Titah, H.S. Biodegradation of Diesel by Acinetobacter lwoffii and Vibrio alginolyticus Isolated from Ship Dismantling Facility in Tanjungjati Coast, Madura, Indonesia. J. Appl. Biol. Sci. 2019, 12, 1–8. [Google Scholar]
- Yang, F.; Jiang, Q.; Zhu, M.; Zhao, L.; Zhang, Y. Effects of biochars and MWNTs on biodegradation behavior of atrazine by Acinetobacter lwoffii DNS32. Sci. Total Environ. 2017, 577, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wan, Y.Y.; Wang, C.; Ma, Z.; Liu, X.; Li, S. Biodegradation of n-alkanes in crude oil by three identified bacterial strains. Fuel 2020, 275, 117897. [Google Scholar] [CrossRef]
- Guilhelmelli, F.; Vilela, N.; Albuquerque, P.; Derengowski Lda, S.; Silva-Pereira, I.; Kyaw, C.M. Antibiotic development challenges: The various mechanisms of action of antimicrobial peptides and of bacterial resistance. Front. Microbiol. 2013, 4, 353. [Google Scholar] [CrossRef]
- Dunphy, L.J.; Papin, J.A. Biomedical applications of genome-scale metabolic network reconstructions of human pathogens. Curr. Opin. Biotechnol. 2018, 51, 70–79. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, M.; Lee, Y.Q.; Lee, H.J.; Rho, M.; Kim, Y.; Seo, J.Y.; Youn, S.H.; Hwang, S.J.; Kang, N.G.; et al. Genome-scale metabolic modeling and in silico analysis of opportunistic skin pathogen Cutibacterium acnes. Front. Cell. Infect. Microbiol. 2023, 13, 1099314. [Google Scholar] [CrossRef] [PubMed]
- Díaz Calvo, T.; Tejera, N.; McNamara, I.; Langridge, G.C.; Wain, J.; Poolman, M.; Singh, D. Genome-scale metabolic modelling approach to understand the metabolism of the opportunistic human pathogen Staphylococcus epidermidis RP62A. Metabolites 2022, 12, 136. [Google Scholar] [CrossRef]
- Veith, N.; Solheim, M.; van Grinsven, K.W.; Olivier, B.G.; Levering, J.; Grosseholz, R.; Hugenholtz, J.; Holo, H.; Nes, I.; Teusink, B.; et al. Using a genome-scale metabolic model of Enterococcus faecalis V583 to assess amino acid uptake and its impact on central metabolism. Appl. Environ. Microbiol. 2015, 81, 1622–1633. [Google Scholar] [CrossRef]
- Kim, H.U.; Kim, S.Y.; Jeong, H.; Kim, T.Y.; Kim, J.J.; Choy, H.E.; Yi, K.Y.; Rhee, J.H.; Lee, S.Y. Integrative genome-scale metabolic analysis of Vibrio vulnificus for drug targeting and discovery. Mol. Syst. Biol. 2011, 7, 460. [Google Scholar] [CrossRef] [PubMed]
- Tezcan, E.F.; Demirtas, Y.; Cakar, Z.P.; Ulgen, K.O. Comprehensive genome-scale metabolic model of the human pathogen Cryptococcus neoformans: A platform for understanding pathogen metabolism and identifying new drug targets. Front. Bioinform. 2023, 3, 1121409. [Google Scholar] [CrossRef]
- Hasan, A.; Mazumder, H.H.; Khan, A.; Hossain, M.U.; Chowdhury, H.K. Molecular Characterization of Legionellosis Drug Target Candidate Enzyme Phosphoglucosamine Mutase from Legionella pneumophila (strain Paris): An In Silico Approach. Genom. Inf. 2014, 12, 268–275. [Google Scholar] [CrossRef]
- Sharma, R.; Lambu, M.R.; Jamwal, U.; Rani, C.; Chib, R.; Wazir, P.; Mukherjee, D.; Chaubey, A.; Khan, I.A. Escherichia coli N-Acetylglucosamine-1-Phosphate-Uridyltransferase/Glucosamine-1-Phosphate-Acetyltransferase (GlmU) Inhibitory Activity of Terreic Acid Isolated from Aspergillus terreus. J. Biomol. Screen. 2016, 21, 342–353. [Google Scholar] [CrossRef]
- Stokes, S.S.; Albert, R.; Buurman, E.T.; Andrews, B.; Shapiro, A.B.; Green, O.M.; McKenzie, A.R.; Otterbein, L.R. Inhibitors of the acetyltransferase domain of N-acetylglucosamine-1-phosphate-uridylyltransferase/glucosamine-1-phosphate-acetyltransferase (GlmU). Part 2: Optimization of physical properties leading to antibacterial aryl sulfonamides. Bioorg. Med. Chem. Lett. 2012, 22, 7019–7023. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.J.; Grant, J.C.; Farr, C.L.; Jaroszewski, L.; Knuth, M.W.; Miller, M.D.; Elsliger, M.A.; Deacon, A.M.; Godzik, A.; Lesley, S.A.; et al. Structural analysis of arabinose-5-phosphate isomerase from Bacteroides fragilis and functional implications. Acta Crystallogr. D Biol. Crystallogr. 2014, 70 Pt 10, 2640–2651. [Google Scholar] [CrossRef]
- Airoldi, C.; Sommaruga, S.; Merlo, S.; Sperandeo, P.; Cipolla, L.; Polissi, A.; Nicotra, F. Targeting bacterial membranes: Identification of Pseudomonas aeruginosa D-arabinose-5P isomerase and NMR characterisation of its substrate recognition and binding properties. Chembiochem 2011, 12, 719–727. [Google Scholar] [CrossRef]
- Jenkins, C.H.; Scott, A.E.; O’Neill, P.A.; Norville, I.H.; Prior, J.L.; Ireland, P.M. The Arabinose 5-Phosphate Isomerase KdsD Is Required for Virulence in Burkholderia pseudomallei. J. Bacteriol. 2023, 205, e0003423. [Google Scholar] [CrossRef]
- Tillery, L.M.; Barrett, K.F.; Dranow, D.M.; Craig, J.; Shek, R.; Chun, I.; Barrett, L.K.; Phan, I.Q.; Subramanian, S.; Abendroth, J.; et al. Toward a structome of Acinetobacter baumannii drug targets. Protein Sci. 2020, 29, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Sha, S.; Zhou, Y.; Xin, Y.; Ma, Y. Development of a colorimetric assay and kinetic analysis for Mycobacterium tuberculosis D-glucose-1-phosphate thymidylyltransferase. J. Biomol. Screen. 2012, 17, 252–257. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alphey, M.S.; Pirrie, L.; Torrie, L.S.; Boulkeroua, W.A.; Gardiner, M.; Sarkar, A.; Maringer, M.; Oehlmann, W.; Brenk, R.; Scherman, M.S.; et al. Allosteric competitive inhibitors of the glucose-1-phosphate thymidylyltransferase (RmlA) from Pseudomonas aeruginosa. ACS Chem. Biol. 2013, 8, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.; Shah, B.; Rakshit, S.; Singh, V.; Padmanabhan, B.; Ponnusamy, M.; Pari, K.; Vishwakarma, R.; Nandi, D.; Sadhale, P.P. UDP-glucose 4, 6-dehydratase activity plays an important role in maintaining cell wall integrity and virulence of Candida albicans. PLoS Pathog. 2011, 7, e1002384. [Google Scholar] [CrossRef] [PubMed]
- Vanaporn, M.; Titball, R.W. Trehalose and bacterial virulence. Virulence 2020, 11, 1192–1202. [Google Scholar] [CrossRef]
- Miao, Y.; Tenor, J.L.; Toffaletti, D.L.; Maskarinec, S.A.; Liu, J.; Lee, R.E.; Perfect, J.R.; Brennan, R.G. Structural and In Vivo Studies on Trehalose-6-Phosphate Synthase from Pathogenic Fungi Provide Insights into Its Catalytic Mechanism, Biological Necessity, and Potential for Novel Antifungal Drug Design. mBio 2017, 8, e00643-17. [Google Scholar] [CrossRef]
- Washington, E.J.; Zhou, Y.; Hsu, A.L.; Petrovich, M.; Tenor, J.L.; Toffaletti, D.L.; Guan, Z.; Perfect, J.R.; Borgnia, M.J.; Bartesaghi, A.; et al. Structures of trehalose-6-phosphate synthase, Tps1, from the fungal pathogen Cryptococcus neoformans: A target for antifungals. Proc. Natl. Acad. Sci. USA 2024, 121, e2314087121. [Google Scholar] [CrossRef]
- Kale, M.; Mohd Sayeed, S. Drug discovery of newer analogs of anti-microbials through enzyme-inhibition: A review. Int. J. Pharm. Pharm. Sci. 2014, 6, 27–35. [Google Scholar]
- Mitsakos, V.; Dobson, R.C.; Pearce, F.G.; Devenish, S.R.; Evans, G.L.; Burgess, B.R.; Perugini, M.A.; Gerrard, J.A.; Hutton, C.A. Inhibiting dihydrodipicolinate synthase across species: Towards specificity for pathogens? Bioorg. Med. Chem. Lett. 2008, 18, 842–844. [Google Scholar] [CrossRef]
- Skovpen, Y.V.; Conly, C.J.; Sanders, D.A.; Palmer, D.R. Biomimetic Design Results in a Potent Allosteric Inhibitor of Dihydrodipicolinate Synthase from Campylobacter jejuni. J. Am. Chem. Soc. 2016, 138, 2014–2020. [Google Scholar] [CrossRef] [PubMed]
- Angrish, N.; Lalwani, N.; Khare, G. In silico virtual screening for the identification of novel inhibitors against dihydrodipicolinate reductase (DapB) of Mycobacterium tuberculosis, a key enzyme of diaminopimelate pathway. Microbiol. Spectr. 2023, 11, e01359-23. [Google Scholar] [CrossRef] [PubMed]
- Dommaraju, S.R.; Dogovski, C.; Czabotar, P.E.; Hor, L.; Smith, B.J.; Perugini, M.A. Catalytic mechanism and cofactor preference of dihydrodipicolinate reductase from methicillin-resistant Staphylococcus aureus. Arch. Biochem. Biophys. 2011, 512, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Praveen, A.; Khanna, S.M. Computational modelling, functional characterization and molecular docking to lead compounds of Bordetella Pertussis diaminopimelate epimerase. Appl. Biochem. Biotech. 2023, 195, 6675–6693. [Google Scholar] [CrossRef]
- Chaudhary, J.; Singh, N.; Srivastava, V.K.; Jyoti, A.; Kaushik, S. Exploring the significance of diaminopimelate epimerase as a drug target in multidrug resistant Enterococcus faecalis. Vegetos 2023, 36, 1–9. [Google Scholar] [CrossRef]
- Weyand, S.; Kefala, G.; Weiss, M.S. The three-dimensional structure of N-succinyldiaminopimelate aminotransferase from Mycobacterium tuberculosis. J. Mol. Biol. 2007, 367, 825–838. [Google Scholar] [CrossRef]
- Nocek, B.P.; Gillner, D.M.; Fan, Y.; Holz, R.C.; Joachimiak, A. Structural basis for catalysis by the mono- and dimetalated forms of the dapE-encoded N-succinyl-L,L-diaminopimelic acid desuccinylase. J. Mol. Biol. 2010, 397, 617–626. [Google Scholar] [CrossRef]
- Kelley, E.H.; Minasov, G.; Konczak, K.; Shuvalova, L.; Brunzelle, J.S.; Shukla, S.; Beulke, M.; Thabthimthong, T.; Olsen, K.W.; Inniss, N.L.; et al. Biochemical and Structural Analysis of the Bacterial Enzyme Succinyl-Diaminopimelate Desuccinylase (DapE) from Acinetobacter baumannii. ACS Omega 2024, 9, 3905–3915. [Google Scholar] [CrossRef]
- Terrazas-López, M.; Lobo-Galo, N.; Aguirre-Reyes, L.G.; Cuen-Andrade, J.L.; de la Rosa, L.A.; Alvarez-Parrilla, E.; Martínez-Martínez, A.; Díaz-Sánchez, Á.G. Interaction of N-succinyl-diaminopimelate desuccinylase with flavonoids. Biochimie 2020, 177, 198–212. [Google Scholar] [CrossRef]
- Amera, G.M.; Khan, R.J.; Pathak, A.; Jha, R.K.; Muthukumaran, J.; Singh, A.K. Computer aided ligand based screening for identification of promising molecules against enzymes involved in peptidoglycan biosynthetic pathway from Acinetobacter baumannii. Microb. Pathog. 2020, 147, 104205. [Google Scholar] [CrossRef]
- Kumar, R.; Rajkumar, R.; Diwakar, V.; Khan, N.; Kumar Meghwanshi, G.; Garg, P. Structural-functional analysis of drug target aspartate semialdehyde dehydrogenase. Drug Discov. Today 2024, 29, 103908. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Akhtar, S.; Siddiqui, M.H.; Sayeed, U.; Ahmad, S.S.; Arif, J.M.; Khan, M.K. Identification of potential leads against 4-hydroxytetrahydrodipicolinate synthase from Mycobacterium tuberculosis. Bioinformation 2016, 12, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Impey, R.E.; Panjikar, S.; Hall, C.J.; Bock, L.J.; Sutton, J.M.; Perugini, M.A.; Soares da Costa, T.P. Identification of two dihydrodipicolinate synthase isoforms from Pseudomonas aeruginosa that differ in allosteric regulation. FEBS J. 2020, 287, 386–400. [Google Scholar] [CrossRef]
- Girish, T.S.; Sharma, E.; Gopal, B. Structural and functional characterization of Staphylococcus aureus dihydrodipicolinate synthase. FEBS Lett. 2008, 582, 2923–2930. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Roy, P.K.; Mosnaz, A.T.; Shakil, S.K.; Hasan, M.M.; Prodhan, S.H. Structural analysis and molecular docking of potential ligands with chorismate synthase of Listeria monocytogenes: A novel antibacterial drug target. Indian J. Biochem. Biophys. 2015, 52, 45–59. [Google Scholar] [PubMed]
- Dias, M.V.; Ely, F.; Palma, M.S.; de Azevedo, W.F., Jr.; Basso, L.A.; Santos, D.S. Chorismate synthase: An attractive target for drug development against orphan diseases. Curr. Drug Targets 2007, 8, 437–444. [Google Scholar] [CrossRef]
- Ball, H.S.; Girma, M.B.; Zainab, M.; Soojhawon, I.; Couch, R.D.; Noble, S.M. Characterization and Inhibition of 1-Deoxy-d-Xylulose 5-Phosphate Reductoisomerase: A Promising Drug Target in Acinetobacter baumannii and Klebsiella pneumoniae. ACS Infect. Dis. 2021, 7, 2987–2998. [Google Scholar] [CrossRef]
- Parveez Zia, M.; Singh, E.; Jain, M.; Muthukumaran, J.; Singh, A.K. Structural and functional characterization of 1-deoxy-D-xylulose-5-phosphate synthase (DXS) from Acinetobacter baumannii: Identification of promising lead molecules from virtual screening, molecular docking and molecular dynamics simulations. J. Biomol. Struct. Dyn. 2023, 41, 11598–11611. [Google Scholar] [CrossRef]
- Ahmad, S.; Raza, S.; Qurat-ul-Ain; Uddin, R.; Rungrotmongkol, T.; Azam, S.S. From phylogeny to protein dynamics: A computational hierarchical quest for potent drug identification against an emerging enteropathogen “Yersinia enterocolitica”. J. Mol. Liq. 2018, 265, 372–389. [Google Scholar] [CrossRef]
- Bordel, S.; Martín-González, D.; Börner, T.; Muñoz, R.; Santos-Beneit, F. Genome-scale metabolic model of the versatile bacterium Paracoccus denitrificans Pd1222. mSystems 2024, 9, e01077-23. [Google Scholar] [CrossRef]
- Sohn, S.B.; Kim, T.Y.; Park, J.M.; Lee, S.Y. In silico genome-scale metabolic analysis of Pseudomonas putida KT2440 for polyhydroxyalkanoate synthesis, degradation of aromatics and anaerobic survival. Biotechnol. J. 2010, 5, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Dhakar, K.; Zarecki, R.; van Bommel, D.; Knossow, N.; Medina, S.; Öztürk, B.; Aly, R.; Eizenberg, H.; Ronen, Z.; Freilich, S. Strategies for Enhancing in vitro Degradation of Linuron by Variovorax sp. Strain SRS 16 Under the Guidance of Metabolic Modeling. Front. Bioeng. Biotechnol. 2021, 9, 602464. [Google Scholar] [CrossRef] [PubMed]
- Ofaim, S.; Zarecki, R.; Porob, S.; Gat, D.; Lahav, T.; Kashi, Y.; Aly, R.; Eizenberg, H.; Ronen, Z.; Freilich, S. Genome-scale reconstruction of Paenarthrobacter aurescens TC1 metabolic model towards the study of atrazine bioremediation. Sci. Rep. 2020, 10, 13019. [Google Scholar] [CrossRef] [PubMed]
- Scheibe, T.D.; Mahadevan, R.; Fang, Y.; Garg, S.; Long, P.E.; Lovley, D.R. Coupling a genome-scale metabolic model with a reactive transport model to describe in situ uranium bioremediation. Microb. Biotechnol. 2009, 2, 274–286. [Google Scholar] [CrossRef]
- Zhuang, K.; Izallalen, M.; Mouser, P.; Richter, H.; Risso, C.; Mahadevan, R.; Lovley, D.R. Genome-scale dynamic modeling of the competition between Rhodoferax and Geobacter in anoxic subsurface environments. ISME J. 2011, 5, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Saito, K. Omics data input for metabolic modeling. Curr. Opin. Biotechnol. 2016, 37, 127–134. [Google Scholar] [CrossRef]
- Rau, M.H.; Gaspar, P.; Jensen, M.L.; Geppel, A.; Neves, A.R.; Zeidan, A.A. Genome-Scale Metabolic Modeling Combined with Transcriptome Profiling Provides Mechanistic Understanding of Streptococcus thermophilus CH8 Metabolism. Appl. Environ. Microb. 2022, 88, e00780-22. [Google Scholar] [CrossRef]
- Becker, S.A.; Palsson, B.O. Context-specific metabolic networks are consistent with experiments. PLoS Comput. Biol. 2008, 4, e1000082. [Google Scholar] [CrossRef]
- Jenior, M.L.; Moutinho, T.J., Jr.; Dougherty, B.V.; Papin, J.A. Transcriptome-guided parsimonious flux analysis improves predictions with metabolic networks in complex environments. PLoS Comput. Biol. 2020, 16, e1007099. [Google Scholar] [CrossRef]
- Kapley, A.; Tolmare, A.; Purohit, H.J. Role of oxygen in the utilization of phenol by CF600 in continuous culture. World J. Microbiol. Biotechnol. 2001, 17, 801–804. [Google Scholar] [CrossRef]
- Sánchez-Del-Campo, L.; Sáez-Ayala, M.; Chazarra, S.; Cabezas-Herrera, J.; Rodríguez-López, J.N. Binding of natural and synthetic polyphenols to human dihydrofolate reductase. Int. J. Mol. Sci. 2009, 10, 5398–5410. [Google Scholar] [CrossRef] [PubMed]
- Seaver, S.M.D.; Liu, F.; Zhang, Q.; Jeffryes, J.; Faria, J.P.; Edirisinghe, J.N.; Mundy, M.; Chia, N.; Noor, E.; Beber, M.E.; et al. The ModelSEED Biochemistry Database for the integration of metabolic annotations and the reconstruction, comparison and analysis of metabolic models for plants, fungi and microbes. Nucleic Acids Res. 2021, 49, D575–D588. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Saier, M.H., Jr.; Tran, C.V.; Barabote, R.D. TCDB: The Transporter Classification Database for membrane transport protein analyses and information. Nucleic Acids Res. 2006, 34, D181–D186. [Google Scholar] [CrossRef] [PubMed]
- Heirendt, L.; Arreckx, S.; Pfau, T.; Mendoza, S.N.; Richelle, A.; Heinken, A.; Haraldsdóttir, H.S.; Wachowiak, J.; Keating, S.M.; Vlasov, V.; et al. Creation and analysis of biochemical constraint-based models using the COBRA Toolbox v.3.0. Nat. Protoc. 2019, 14, 639–702. [Google Scholar] [CrossRef]
- Boutet, E.; Lieberherr, D.; Tognolli, M.; Schneider, M.; Bairoch, A. UniProtKB/Swiss-Prot. Methods Mol. Biol. 2007, 406, 89–112. [Google Scholar]
- Orth, J.D.; Thiele, I.; Palsson, B.Ø. What is flux balance analysis? Nat. Biotechnol. 2010, 28, 245–248. [Google Scholar] [CrossRef]
- Gurobi Optimization. Gurobi Optimizer Reference Manual; Gurobi Optimization, LLC: Beaverton, OR, USA, 2014. [Google Scholar]
- Gallagher, L.A.; Ramage, E.; Weiss, E.J.; Radey, M.; Hayden, H.S.; Held, K.G.; Huse, H.K.; Zurawski, D.V.; Brittnacher, M.J.; Manoil, C.; et al. Resources for Genetic and Genomic Analysis of Emerging Pathogen Acinetobacter baumannii. J. Bacteriol. 2015, 197, 2027–2035. [Google Scholar] [CrossRef]
- de Berardinis, V.; Vallenet, D.; Castelli, V.; Besnard, M.; Pinet, A.; Cruaud, C.; Samair, S.; Lechaplais, C.; Gyapay, G.; Richez, C.; et al. A complete collection of single-gene deletion mutants of Acinetobacter baylyi ADP1. Mol. Syst. Biol. 2008, 4, 174. [Google Scholar] [CrossRef]
- Luo, H.; Lin, Y.; Liu, T.; Lai, F.L.; Zhang, C.T.; Gao, F.; Zhang, R. DEG 15, an update of the Database of Essential Genes that includes built-in analysis tools. Nucleic Acids Res. 2021, 49, D677–D686. [Google Scholar] [CrossRef]
- Brunk, E.; Sahoo, S.; Zielinski, D.C.; Altunkaya, A.; Dräger, A.; Mih, N.; Gatto, F.; Nilsson, A.; Preciat Gonzalez, G.A.; Aurich, M.K.; et al. Recon3D enables a three-dimensional view of gene variation in human metabolism. Nat. Biotechnol. 2018, 36, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Nogales, J.; Agudo, L. A Practical Protocol for Integration of Transcriptomics Data into Genome-Scale Metabolic Reconstructions. In Hydrocarbon and Lipid Microbiology Protocols; Springer: Berlin/Heidelberg, Germany, 2015; pp. 135–152. [Google Scholar]
- Duarte, N.C.; Palsson, B.O.; Fu, P. Integrated analysis of metabolic phenotypes in Saccharomyces cerevisiae. BMC Genom. 2004, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Lacoste, R.J.; Venable, S.H.; Stone, J.C. Modified 4-Aminoantipyrine Colorimetric Method for Phenols. Application to Acrylic Monomer. Anal. Chem. 1959, 31, 1246–1249. [Google Scholar] [CrossRef]


| Substrates | In Silico | In Vivo | |
|---|---|---|---|
| Saccharides | Glucose | - | - |
| Fructose | + | + | |
| Xylose | - | - | |
| Arabinose | - | - | |
| Alcohol | Mannitol | - | - |
| Glycerol | + | - | |
| Ethanol | + | + | |
| Carboxylic acids | Acetate | + | + |
| Citrate | + | + | |
| Succinate | + | + | |
| Malate | + | + | |
| Aromatic xenobiotics | Phenol | + | + |
| 4-Hydroxybenzoic acid | + | + | |
| Salicylate | + | + | |
| Benzoic acid | + | + | |
| Toluene | + | + | |
| Mandelate | + | + | |
| Benzene | + | + | |
| Phthalate | + | + | |
| Amino acids | L-Alanine | + | + |
| Glycine | + | + | |
| Proline | + | + | |
| Serine | + | + | |
| Arginine | + | + | |
| Glutamate | + | + | |
| Glutamine | + | + | |
| Aspartate | + | + | |
| Threonine | + | + | |
| Valine | + | + | |
| Cysteine | + | + | |
| Tryptophan | - | - | |
| Methionine | - | - | |
| Leucine | + | + | |
| Phenylalanine | + | + | |
| Histidine | - | - | |
| Lysine | - | - | |
| Nitrogen sources | NaNO3 | - | - |
| NH4Cl | + | + | |
| Urea | + | + | |
| Pathway | Essential Metabolites | Enzymes | Genes |
|---|---|---|---|
| Amino sugar and nucleotide sugar metabolism | alpha-D-Glucosamine 1-phosphate | D-Glucosamine 1,6-phosphomutase, glucosamine-1-phosphate-acetyltransferase | LNSL_0126 LNSL_2808 |
| Lipopolysaccharide biosynthesis | D-arabinose-5-phosphate | D-Arabinose-5-phosphate isomerase, 3-deoxy-8-phosphooctulonate synthase | LNSL_ 1272 LNSL_ 1680 |
| Polysaccharide biosynthesis | dTDP-glucose | dTDP-Glucose 4,6-hydro-lyase, dTDP-glucose 4-epimerase, dTDP-glucose synthase | LNSL_2851 LNSL_2820 LNSL_2850 |
| Starch and sucrose metabolism | Trehalose 6-phosphate | Trehalose 6-phosphate synthase | LNSL_0763 |
| Lysine biosynthesis | Dehydrodipicolinate | Dehydrodipicolinate synthase, dihydrodipicolinate reductase | LNSL_ 0056 LNSL_ 2865 |
| N-Succinyl-LL-2,6-diaminoheptanedioate | Succinyldiaminopimelate transaminase, succinyl-diaminopimelate desuccinylase | LNSL_ 1115 LNSL_ 2239 | |
| meso-2,6-Diaminoheptanedioate | Diaminopimelate epimerase, UDP-N-acetylmuramoylalanyl-D-glutamate-2,6-diaminopimelate ligase | LNSL_ 2102 LNSL_ 2621 | |
| L-Aspartate 4-semialdehyde | Aspartate-semialdehyde dehydrogenase, 4-hydroxy-tetrahydrodipicolinate synthase | LNSL_0359 LNSL_ 0056 | |
| Folate biosynthesis | 4-Aminobenzoate | 4-Amino-4-deoxychorismate pyruvate-lyase, dihydropteroate synthase | LNSL_2017 LNSL_2140 |
| 2-Amino-4-hydroxy-6-hydroxymethyl-7,8-dihydropteridine | 7,8-Dihydroneopterin aldolase, dihydropteroate synthase | LNSL_1717 LNSL_2140 | |
| Chorismate | Aminodeoxychorismate synthase | LNSL_0563 | |
| Thiamine metabolism | 1-Deoxy-D-xylulose 5-phosphate | 1-Deoxy-D-xylulose-5-phosphate synthase | LNSL_2536 |
| Vitamin B6 metabolism | 1-Deoxy-D-xylulose 5-phosphate | Pyridoxine 5′-phosphate synthase | LNSL_2003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, N.; Zuo, J.; Li, C.; Gao, C.; Guo, M. Reconstruction and Analysis of a Genome-Scale Metabolic Model of Acinetobacter lwoffii. Int. J. Mol. Sci. 2024, 25, 9321. https://doi.org/10.3390/ijms25179321
Xu N, Zuo J, Li C, Gao C, Guo M. Reconstruction and Analysis of a Genome-Scale Metabolic Model of Acinetobacter lwoffii. International Journal of Molecular Sciences. 2024; 25(17):9321. https://doi.org/10.3390/ijms25179321
Chicago/Turabian StyleXu, Nan, Jiaojiao Zuo, Chenghao Li, Cong Gao, and Minliang Guo. 2024. "Reconstruction and Analysis of a Genome-Scale Metabolic Model of Acinetobacter lwoffii" International Journal of Molecular Sciences 25, no. 17: 9321. https://doi.org/10.3390/ijms25179321
APA StyleXu, N., Zuo, J., Li, C., Gao, C., & Guo, M. (2024). Reconstruction and Analysis of a Genome-Scale Metabolic Model of Acinetobacter lwoffii. International Journal of Molecular Sciences, 25(17), 9321. https://doi.org/10.3390/ijms25179321






