Lipodystrophic Laminopathies: From Dunnigan Disease to Progeroid Syndromes
Abstract
1. Introduction
2. Aetiopathogenesis of Adipose Tissue Impairment in Lipodystrophic Laminopathies
3. Dunnigan Disease
4. Hutchinson-Gilford Progeria Syndrome
5. LMNA-Atypical Progeroid Syndrome
6. Other LMNA-Associated Lipodystrophies
6.1. LMNA-Associated Generalised Lipodystrophy
6.2. LMNA-Associated Cardiocutaneous Progeria
7. Mandibuloacral Dysplasia
7.1. Mandibuloacral Dysplasia Type A
7.2. Mandibuloacral Dysplasia Type B
8. Nestor-Guillermo Progeria Syndrome
9. Discussion
10. Research Agenda
- -
- There is a need to establish international cooperation and networks through the International Clinical Working Groups of the European Consortium of Lipodystrophies (ECLip) in order to address the real lipodystrophic laminopathies and create registries that bring together relevant information about these ultra-rare pathologies.
- -
- There is also a need to delve deeper into what should really be considered a progeroid syndrome while trying to gather scientific evidence based on consensus among worldwide experts.
- -
- Increasing knowledge of the molecular basis of lipodystrophic laminopathies will make it possible, in the future, to find new specific therapies to shed light on what until now are life-threatening or fatal disorders.
- -
- Attention should be focused on the field of epigenomics and the possibility of modulating clinical expressivity through reversible epigenetic markers. In this sense, epigenetic studies on whole adipose tissue and preadipocytes, including the already-known miR335 but also HOTAIR involvement and other epigenetic marks, could improve understanding of the big phenotypic differences (when comparing FPLD vs. progeroid syndromes), or mild phenotypic differences in FPLD2 pending the specific LMNA variants.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bidault, G.; Vatier, C.; Capeau, J.; Vigouroux, C.; Béréziat, V. LMNA-Linked Lipodystrophies: From Altered Fat Distribution to Cellular Alterations. Biochem. Soc. Trans. 2011, 39, 1752–1757. [Google Scholar] [CrossRef] [PubMed]
- Perepelina, K.; Klauzen, P.; Kostareva, A.; Malashicheva, A. Tissue-Specific Influence of Lamin A Mutations on Notch Signaling and Osteogenic Phenotype of Primary Human Mesenchymal Cells. Cells 2019, 8, 266. [Google Scholar] [CrossRef] [PubMed]
- Gruenbaum, Y.; Goldman, R.D.; Meyuhas, R.; Mills, E.; Margalit, A.; Fridkin, A.; Dayani, Y.; Prokocimer, M.; Enosh, A. The Nuclear Lamina and Its Functions in the Nucleus. Int. Rev. Cytol. 2003, 226, 1–62. [Google Scholar] [CrossRef]
- Jacob, K.N.; Garg, A. Laminopathies: Multisystem Dystrophy Syndromes. Mol. Genet. Metab. 2006, 87, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Dauer, W.T.; Worman, H.J. The Nuclear Envelope as a Signaling Node in Development and Disease. Dev. Cell. 2009, 17, 626–638. [Google Scholar] [CrossRef]
- Garg, A. Acquired and Inherited Lipodystrophies. N. Engl. J. Med. 2004, 350, 1220–1234. [Google Scholar] [CrossRef]
- Capanni, C.; Cenni, V.; Haraguchi, T.; Squarzoni, S.; Schüchner, S.; Ogris, E.; Novelli, G.; Maraldi, N.; Lattanzi, G. Lamin A Precursor Induces Barrier-to-Autointegration Factor Nuclear Localization. Celli Cycle 2010, 9, 2600–2610. [Google Scholar] [CrossRef]
- Cenni, V.; Capanni, C.; Mattioli, E.; Columbaro, M.; Wehnert, M.; Ortolani, M.; Fini, M.; Novelli, G.; Bertacchini, J.; Maraldi, N.M.; et al. Rapamycin Treatment of Mandibuloacral Dysplasia Cells Rescues Localization of Chromatin-Associated Proteins and Cell Cycle Dynamics. Aging 2014, 6, 755–770. [Google Scholar] [CrossRef]
- Osorio, F.G.; Ugalde, A.P.; Mariño, G.; Puente, X.S.; Freije, J.M.P.; López-Otín, C. Cell Autonomous and Systemic Factors in Progeria Development. Biochem. Soc. Trans. 2011, 39, 1710–1714. [Google Scholar] [CrossRef]
- Broers, J.L.V.; Ramaekers, F.C.S.; Bonne, G.; Yaou, R.B.; Hutchison, C.J. Nuclear Lamins: Laminopathies and Their Role in Premature Ageing. Physiol. Rev. 2006, 86, 967–1008. [Google Scholar] [CrossRef]
- Guillín-Amarelle, C.; Fernández-Pombo, A.; Sánchez-Iglesias, S.; Araújo-Vilar, D. Lipodystrophic Laminopathies: Diagnostic Clues. Nucleus 2018, 9, 249–260. [Google Scholar] [CrossRef]
- Cabanillas, R.; Cadiñanos, J.; Villameytide, J.A.F.; Pérez, M.; Longo, J.; Richard, J.M.; Alvarez, R.; Durán, N.S.; Illán, R.; González, D.J.; et al. Néstor-Guillermo Progeria Syndrome: A Novel Premature Aging Condition with Early Onset and Chronic Development Caused by BANF1 Mutations. Am. J. Med. Genet. A 2011, 155, 2617–2625. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.S.J.; Barnes, R.H.; Tu, Y.; Ren, S.; Andres, D.A.; Spielmann, H.P.; Lammerding, J.; Wang, Y.; Young, S.G.; Fong, L.G. An Accumulation of Non-Farnesylated Prelamin A Causes Cardiomyopathy but Not Progeria. Hum. Mol. Genet. 2010, 19, 2682–2694. [Google Scholar] [CrossRef]
- Araújo-Vilar, D.; Lattanzi, G.; González-Méndez, B.; Costa-Freitas, A.T.; Prieto, D.; Columbaro, M.; Mattioli, E.; Victoria, B.; Martínez-Sánchez, N.; Ramazanova, A.; et al. Site-Dependent Differences in Both Prelamin A and Adipogenic Genes in Subcutaneous Adipose Tissue of Patients with Type 2 Familial Partial Lipodystrophy. J. Med. Genet. 2009, 46, 40–48. [Google Scholar] [CrossRef]
- Candelario, J.; Borrego, S.; Reddy, S.; Comai, L. Accumulation of Distinct Prelamin A Variants in Human Diploid Fibroblasts Differentially Affects Cell Homeostasis. Exp. Cell. Res. 2011, 317, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.S.; Coffinier, C.; Yang, S.H.; Barnes, R.H.; Jung, H.-J.; Young, S.G.; Fong, L.G. Investigating the Purpose of Prelamin A Processing. Nucleus 2011, 2, 4–9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dechat, T.; Pfleghaar, K.; Sengupta, K.; Shimi, T.; Shumaker, D.K.; Solimando, L.; Goldman, R.D. Nuclear Lamins: Major Factors in the Structural Organization and Function of the Nucleus and Chromatin. Genes. Dev. 2008, 22, 832–853. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Suarez, I.; Gonzalo, S. Nurturing the Genome: A-Type Lamins Preserve Genomic Stability. Nucleus 2010, 1, 129–135. [Google Scholar] [CrossRef]
- Liu, B.; Wang, J.; Chan, K.M.; Tjia, W.M.; Deng, W.; Guan, X.; Huang, J.; Li, K.M.; Chau, P.Y.; Chen, D.J.; et al. Genomic Instability in Laminopathy-Based Premature Aging. Nat. Med. 2005, 11, 780–785. [Google Scholar] [CrossRef]
- Muralikrishna, B.; Dhawan, J.; Rangaraj, N.; Parnaik, V.K. Distinct Changes in Intranuclear Lamin A/C Organization during Myoblast Differentiation. J. Cell. Sci. 2001, 114, 4001–4011. [Google Scholar] [CrossRef]
- Oldenburg, A.; Briand, N.; Sørensen, A.L.; Cahyani, I.; Shah, A.; Moskaug, J.Ø.; Collas, P. A Lipodystrophy-Causing Lamin A Mutant Alters Conformation and Epigenetic Regulation of the Anti-Adipogenic MIR335 Locus. J. Cell. Biol. 2017, 216, 2731–2743. [Google Scholar] [CrossRef] [PubMed]
- Osmanagic-Myers, S.; Dechat, T.; Foisner, R. Lamins at the Crossroads of Mechanosignaling. Genes. Dev. 2015, 29, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, C.; Columbaro, M.; Schena, E.; Prencipe, S.; Andrenacci, D.; Iozzo, P.; Angela Guzzardi, M.; Capanni, C.; Mattioli, E.; Loi, M.; et al. Altered Adipocyte Differentiation and Unbalanced Autophagy in Type 2 Familial Partial Lipodystrophy: An in Vitro and in Vivo Study of Adipose Tissue Browning. Exp. Mol. Med. 2019, 51, 1–17. [Google Scholar] [CrossRef]
- Varela, I.; Cadiñanos, J.; Pendás, A.M.; Gutiérrez-Fernández, A.; Folgueras, A.R.; Sánchez, L.M.; Zhou, Z.; Rodríguez, F.J.; Stewart, C.L.; Vega, J.A.; et al. Accelerated Ageing in Mice Deficient in Zmpste24 Protease Is Linked to P53 Signalling Activation. Nature 2005, 437, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Vigouroux, C.; Auclair, M.; Dubosclard, E.; Pouchelet, M.; Capeau, J.; Courvalin, J.C.; Buendia, B. Nuclear Envelope Disorganization in Fibroblasts from Lipodystrophic Patients with Heterozygous R482Q/W Mutations in the Lamin A/C Gene. J Cell. Sci. 2001, 114, 4459–4468. [Google Scholar] [CrossRef]
- Guénantin, A.C.; Briand, N.; Bidault, G.; Afonso, P.; Béréziat, V.; Vatier, C.; Lascols, O.; Caron-Debarle, M.; Capeau, J.; Vigouroux, C. Nuclear Envelope-Related Lipodystrophies. Semin. Cell. Dev. Biol. 2014, 29, 148–157. [Google Scholar] [CrossRef]
- Lloyd, D.J.; Trembath, R.C.; Shackleton, S. A Novel Interaction between Lamin A and SREBP1: Implications for Partial Lipodystrophy and Other Laminopathies. Hum. Mol. Genet. 2002, 11, 769–777. [Google Scholar] [CrossRef]
- Capanni, C.; Squarzoni, S.; Cenni, V.; D’Apice, M.R.; Gambineri, A.; Novelli, G.; Wehnert, M.; Pasquali, R.; Maraldi, N.M.; Lattanzi, G. Familial Partial Lipodystrophy, Mandibuloacral Dysplasia and Restrictive Dermopathy Feature Barrier-to-Autointegration Factor (BAF) Nuclear Redistribution. Celli Cycle 2012, 11, 3568–3577. [Google Scholar] [CrossRef]
- Johnson, B.R.; Nitta, R.T.; Frock, R.L.; Mounkes, L.; Barbie, D.A.; Stewart, C.L.; Harlow, E.; Kennedy, B.K. A-Type Lamins Regulate Retinoblastoma Protein Function by Promoting Subnuclear Localization and Preventing Proteasomal Degradation. Proc. Natl. Acad. Sci. USA 2004, 101, 9677–9682. [Google Scholar] [CrossRef]
- Araújo-Vilar, D.; Victoria, B.; González-Méndez, B.; Barreiro, F.; Fernández-Rodríguez, B.; Cereijo, R.; Gallego-Escuredo, J.M.; Villarroya, F.; Pañeda-Menéndez, A. Histological and Molecular Features of Lipomatous and Nonlipomatous Adipose Tissue in Familial Partial Lipodystrophy Caused by LMNA Mutations. Clin. Endocrinol. 2012, 76, 816–824. [Google Scholar] [CrossRef]
- Mateos, J.; Landeira-Abia, A.; Fafián-Labora, J.A.; Fernández-Pernas, P.; Lesende-Rodríguez, I.; Fernández-Puente, P.; Fernández-Moreno, M.; Delmiro, A.; Martín, M.A.; Blanco, F.J.; et al. iTRAQ-Based Analysis of Progerin Expression Reveals Mitochondrial Dysfunction, Reactive Oxygen Species Accumulation and Altered Proteostasis. Stem. Cell. Res. Ther. 2015, 6, 119. [Google Scholar] [CrossRef] [PubMed]
- Afonso, P.; Auclair, M.; Boccara, F.; Vantyghem, M.-C.; Katlama, C.; Capeau, J.; Vigouroux, C.; Caron-Debarle, M. LMNA Mutations Resulting in Lipodystrophy and HIV Protease Inhibitors Trigger Vascular Smooth Muscle Cell Senescence and Calcification: Role of ZMPSTE24 Downregulation. Atherosclerosis 2016, 245, 200–211. [Google Scholar] [CrossRef]
- Gabriel, D.; Roedl, D.; Gordon, L.B.; Djabali, K. Sulforaphane Enhances Progerin Clearance in Hutchinson-Gilford Progeria Fibroblasts. Aging. Cell. 2015, 14, 78–91. [Google Scholar] [CrossRef]
- Gruenbaum, Y.; Foisner, R. Lamins: Nuclear Intermediate Filament Proteins with Fundamental Functions in Nuclear Mechanics and Genome Regulation. Annu. Rev. Biochem. 2015, 84, 131–164. [Google Scholar] [CrossRef]
- Dittmer, T.A.; Misteli, T. The Lamin Protein Family. Genome. Biol. 2011, 12, 222. [Google Scholar] [CrossRef]
- Håkelien, A.-M.; Delbarre, E.; Gaustad, K.G.; Buendia, B.; Collas, P. Expression of the Myodystrophic R453W Mutation of Lamin A in C2C12 Myoblasts Causes Promoter-Specific and Global Epigenetic Defects. Exp. Cell. Res. 2008, 314, 1869–1880. [Google Scholar] [CrossRef]
- Arancio, W.; Pizzolanti, G.; Genovese, S.I.; Pitrone, M.; Giordano, C. Epigenetic Involvement in Hutchinson-Gilford Progeria Syndrome: A Mini-Review. Gerontology 2014, 60, 197–203. [Google Scholar] [CrossRef]
- Davidson, M.B.; Young, R.T. Metabolic Studies in Familial Partial Lipodystrophy of the Lower Trunk and Extremities. Diabetologia 1975, 11, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Drac, H.; Madej-Pilarczyk, A.; Gospodarczyk-Szot, K.; Gaweł, M.; Kwieciński, H.; Hausmanowa-Petrusewicz, I. Familial Partial Lipodystrophy Associated with the Heterozygous LMNA Mutation 1445G>A (Arg482Gln) in a Polish Family. Neurol. Neurochir. Pol. 2010, 44, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Köbberling, J.; Dunnigan, M.G. Familial Partial Lipodystrophy: Two Types of an X Linked Dominant Syndrome, Lethal in the Hemizygous State. J. Med. Genet. 1986, 23, 120–127. [Google Scholar] [CrossRef]
- Vigouroux, C.; Magré, J.; Vantyghem, M.C.; Bourut, C.; Lascols, O.; Shackleton, S.; Lloyd, D.J.; Guerci, B.; Padova, G.; Valensi, P.; et al. Lamin A/C Gene: Sex-Determined Expression of Mutations in Dunnigan-Type Familial Partial Lipodystrophy and Absence of Coding Mutations in Congenital and Acquired Generalized Lipoatrophy. Diabetes 2000, 49, 1958–1962. [Google Scholar] [CrossRef] [PubMed]
- Akinci, B.; Onay, H.; Demir, T.; Savas-Erdeve, Ş.; Gen, R.; Simsir, I.Y.; Keskin, F.E.; Erturk, M.S.; Uzum, A.K.; Yaylali, G.F.; et al. Clinical Presentations, Metabolic Abnormalities and End-Organ Complications in Patients with Familial Partial Lipodystrophy. Metabolism 2017, 72, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Araújo-Vilar, D.; Fernández-Pombo, A.; Victoria, B.; Mosquera-Orgueira, A.; Cobelo-Gómez, S.; Castro-Pais, A.; Hermida-Ameijeiras, Á.; Loidi, L.; Sánchez-Iglesias, S. Variable Expressivity and Allelic Heterogeneity in Type 2 Familial Partial Lipodystrophy: The p.(Thr528Met) LMNA Variant. J. Clin. Med. 2021, 10, 1497. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, N.X.S.; Adiyaman, S.C.; Yuksel, B.D.; Ferrari, C.T.; Eldin, A.J.; Saydam, B.O.; Altay, C.; Sharma, P.; Bhave, N.; Little, A.; et al. Unusual presentations of LMNA-associated lipodystrophy with complex phenotypes and generalized fat loss: When the genetic diagnosis uncovers novel features. AACE Clin. Case. Rep. 2020, 6, e79–e85. [Google Scholar] [CrossRef]
- Decaudain, A.; Vantyghem, M.-C.; Guerci, B.; Hécart, A.-C.; Auclair, M.; Reznik, Y.; Narbonne, H.; Ducluzeau, P.-H.; Donadille, B.; Lebbé, C.; et al. New Metabolic Phenotypes in Laminopathies: LMNA Mutations in Patients with Severe Metabolic Syndrome. J. Clin. Endocrinol. Metab. 2007, 92, 4835–4844. [Google Scholar] [CrossRef]
- Garg, A. Gender Differences in the Prevalence of Metabolic Complications in Familial Partial Lipodystrophy (Dunnigan Variety). J. Clin. Endocrinol. Metab. 2000, 85, 1776–1782. [Google Scholar] [CrossRef]
- Montenegro, R.M.; Costa-Riquetto, A.D.; Fernandes, V.O.; Montenegro, A.P.D.R.; de Santana, L.S.; de Lima Jorge, A.A.; de Azevedo Souza Karbage, L.B.; Aguiar, L.B.; Carvalho, F.H.C.; Teles, M.G.; et al. Homozygous and Heterozygous Nuclear Lamin A p.R582C Mutation: Different Lipodystrophic Phenotypes in the Same Kindred. Front. Endocrinol. 2018, 9, 458. [Google Scholar] [CrossRef]
- Mory, P.B.; Crispim, F.; Freire, M.B.S.; Salles, J.E.N.; Valério, C.M.; Godoy-Matos, A.F.; Dib, S.A.; Moisés, R.S. Phenotypic Diversity in Patients with Lipodystrophy Associated with LMNA Mutations. Eur. J. Endocrinol. 2012, 167, 423–431. [Google Scholar] [CrossRef]
- Muschke, P.; Kölsch, U.; Jakubiczka, S.; Wieland, I.; Brune, T.; Wieacker, P. The Heterozygous LMNA Mutation p.R471G Causes a Variable Phenotype with Features of Two Types of Familial Partial Lipodystrophy. Am. J. Med. Genet. A 2007, 143, 2810–2814. [Google Scholar] [CrossRef]
- Resende, A.T.P.; Martins, C.S.; Bueno, A.C.; Moreira, A.C.; Foss-Freitas, M.C.; de Castro, M. Phenotypic Diversity and Glucocorticoid Sensitivity in Patients with Familial Partial Lipodystrophy Type 2. Clin. Endocrinol. 2019, 91, 94–103. [Google Scholar] [CrossRef]
- Sorkina, E.L.; Kalashnikova, M.F.; Melnichenko, G.A.; Tyulpakov, A.N. Familial partial lipodystrophy (Dunnigan syndrome) due to LMNA gene mutation: The first description of its clinical case in Russia. Ter. Arkhiv 2015, 87, 83–87. [Google Scholar] [CrossRef]
- Gonzaga-Jauregui, C.; Ge, W.; Staples, J.; Van Hout, C.; Yadav, A.; Colonie, R.; Leader, J.B.; Kirchner, H.L.; Murray, M.F.; Reid, J.G.; et al. Clinical and Molecular Prevalence of Lipodystrophy in an Unascertained Large Clinical Care Cohort. Diabetes 2020, 69, 249–258. [Google Scholar] [CrossRef]
- Spuler, S.; Kalbhenn, T.; Zabojszcza, J.; van Landeghem, F.K.H.; Ludtke, A.; Wenzel, K.; Koehnlein, M.; Schuelke, M.; Lüdemann, L.; Schmidt, H.H. Muscle and Nerve Pathology in Dunnigan Familial Partial Lipodystrophy. Neurology 2007, 68, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Akinci, G.; Topaloglu, H.; Demir, T.; Danyeli, A.E.; Talim, B.; Keskin, F.E.; Kadioglu, P.; Talip, E.; Altay, C.; Yaylali, G.F.; et al. Clinical Spectra of Neuromuscular Manifestations in Patients with Lipodystrophy: A Multicenter Study. Neuromuscul. Disord. 2017, 27, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Burn, J.; Baraitser, M. Partial Lipoatrophy with Insulin Resistant Diabetes and Hyperlipidaemia (Dunnigan Syndrome). J. Med. Genet. 1986, 23, 128–130. [Google Scholar] [CrossRef]
- Vantyghem, M.C.; Pigny, P.; Maurage, C.A.; Rouaix-Emery, N.; Stojkovic, T.; Cuisset, J.M.; Millaire, A.; Lascols, O.; Vermersch, P.; Wemeau, J.L.; et al. Patients with Familial Partial Lipodystrophy of the Dunnigan Type Due to a LMNA R482W Mutation Show Muscular and Cardiac Abnormalities. J. Clin. Endocrinol. Metab. 2004, 89, 5337–5346. [Google Scholar] [CrossRef]
- Morel, C.F.; Thomas, M.A.; Cao, H.; O’Neil, C.H.; Pickering, J.G.; Foulkes, W.D.; Hegele, R.A. A LMNA Splicing Mutation in Two Sisters with Severe Dunnigan-Type Familial Partial Lipodystrophy Type 2. J. Clin. Endocrinol. Metab. 2006, 91, 2689–2695. [Google Scholar] [CrossRef][Green Version]
- Treiber, G.; Flaus Furmaniuk, A.; Guilleux, A.; Medjane, S.; Bonfanti, O.; Schneebeli, S.; Bernard, C.; Le-Moullec, N.; Bakiri, F.; Pholsena, M.; et al. A Recurrent Familial Partial Lipodystrophy Due to a Monoallelic or Biallelic LMNA Founder Variant Highlights the Multifaceted Cardiac Manifestations of Metabolic Laminopathies. Eur. J. Endocrinol. 2021, 185, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, R.A.; Vaidya, A.D.B.; Talwalkar, S.C.; Mehtalia, S.D.; Shringi, M.S.; Pandey, S.N.; Shah, S.J.; Godse, C.; Joshi, J.V.; Sheth, J.; et al. Clinical, Endocrine and Metabolic Studies in the Kindred of Familial Partial Lipodystrophy--a Syndrome of Insulin Resistance. J. Assoc. Physicians India 2002, 50, 773–776. [Google Scholar]
- Jeru, I.; Vatier, C.; Vantyghem, M.-C.; Lascols, O.; Vigouroux, C. LMNA-Associated Partial Lipodystrophy: Anticipation of Metabolic Complications. J. Med. Genet. 2017, 54, 413–416. [Google Scholar] [CrossRef]
- Patni, N.; Li, X.; Adams-Huet, B.; Vasandani, C.; Gomez-Diaz, R.A.; Garg, A. Regional Body Fat Changes and Metabolic Complications in Children With Dunnigan Lipodystrophy-Causing LMNA Variants. J. Clin. Endocrinol. Metab. 2019, 104, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.X.; Harris, J.; Wilber, E.; Gorman, S.; Savage, D.B.; O’Rahilly, S.; Stears, A.; Williams, R.M. Describing the Natural History of Clinical, Biochemical and Radiological Outcomes of Children with Familial Partial Lipodystrophy Type 2 (FPLD2) from the United Kingdom: A Retrospective Case Series. Clin. Endocrinol. 2022, 97, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Huang-Doran, I.; Sleigh, A.; Rochford, J.J.; O’Rahilly, S.; Savage, D.B. Lipodystrophy: Metabolic Insights from a Rare Disorder. J. Endocrinol. 2010, 207, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Hegele, R.A. Nuclear Lamin A/C R482Q Mutation in Canadian Kindreds with Dunnigan-Type Familial Partial Lipodystrophy. Hum. Mol. Genet. 2000, 9, 109–112. [Google Scholar] [CrossRef]
- Haque, W.A.; Oral, E.A.; Dietz, K.; Bowcock, A.M.; Agarwal, A.K.; Garg, A. Risk Factors for Diabetes in Familial Partial Lipodystrophy, Dunnigan Variety. Diabetes Care 2003, 26, 1350–1355. [Google Scholar] [CrossRef]
- Joy, T.; Kennedy, B.A.; Al-Attar, S.; Rutt, B.K.; Hegele, R.A. Predicting Abdominal Adipose Tissue among Women with Familial Partial Lipodystrophy. Metabolism 2009, 58, 828–834. [Google Scholar] [CrossRef]
- Lazarte, J.; Wang, J.; McIntyre, A.D.; Hegele, R.A. Prevalence of Severe Hypertriglyceridemia and Pancreatitis in Familial Partial Lipodystrophy Type 2. J. Clin. Lipidol. 2021, 15, 653–657. [Google Scholar] [CrossRef]
- Treiber, G.; Guilleux, A.; Huynh, K.; Bonfanti, O.; Flaus-Furmaniuk, A.; Couret, D.; Mellet, N.; Bernard, C.; Le-Moullec, N.; Doray, B.; et al. Lipoatrophic Diabetes in Familial Partial Lipodystrophy Type 2: From Insulin Resistance to Diabetes. Diabetes. Metab. 2023, 49, 101409. [Google Scholar] [CrossRef]
- Haque, W.A.; Vuitch, F.; Garg, A. Post-Mortem Findings in Familial Partial Lipodystrophy, Dunnigan Variety. Diabet. Med. 2002, 19, 1022–1025. [Google Scholar] [CrossRef]
- Foss-Freitas, M.C.; Ferraz, R.C.; Monteiro, L.Z.; Gomes, P.M.; Iwakura, R.; de Freitas, L.C.C.; Foss, M.C. Endoplasmic Reticulum Stress Activation in Adipose Tissue Induces Metabolic Syndrome in Individuals with Familial Partial Lipodystrophy of the Dunnigan Type. Diabetol. Metab. Syndr. 2018, 10, 6. [Google Scholar] [CrossRef]
- Hegele, R.A.; Kraw, M.E.; Ban, M.R.; Miskie, B.A.; Huff, M.W.; Cao, H. Elevated Serum C-Reactive Protein and Free Fatty Acids among Nondiabetic Carriers of Missense Mutations in the Gene Encoding Lamin A/C (LMNA) with Partial Lipodystrophy. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 111–116. [Google Scholar] [CrossRef][Green Version]
- Kutbay, N.O.; Yurekli, B.S.; Onay, H.; Altay, C.T.; Atik, T.; Hekimsoy, Z.; Saygili, F.; Akinci, B. A Case of Familial Partial Lipodystrophy Caused by a Novel Lamin A/C (LMNA) Mutation in Exon 1 (D47N). Eur. J. Intern. Med. 2016, 29, 37–39. [Google Scholar] [CrossRef] [PubMed]
- Speckman, R.A.; Garg, A.; Du, F.; Bennett, L.; Veile, R.; Arioglu, E.; Taylor, S.I.; Lovett, M.; Bowcock, A.M. Mutational and Haplotype Analyses of Families with Familial Partial Lipodystrophy (Dunnigan Variety) Reveal Recurrent Missense Mutations in the Globular C-Terminal Domain of Lamin A/C. Am. J. Hum. Genet. 2000, 66, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Vasandani, C.; Li, X.; Sekizkardes, H.; Brown, R.J.; Garg, A. Phenotypic Differences Among Familial Partial Lipodystrophy Due to LMNA or PPARG Variants. J. Endocr. Soc. 2022, 6, bvac155. [Google Scholar] [CrossRef] [PubMed]
- Akinci, B.; Unlu, S.M.; Celik, A.; Simsir, I.Y.; Sen, S.; Nur, B.; Keskin, F.E.; Ozgen Saydam, B.; Kutbay Ozdemir, N.; Sarer Yurekli, B.; et al. Renal Complications of Lipodystrophy: A Closer Look at the Natural History of Kidney Disease. Clin. Endocrinol. 2018, 89, 65–75. [Google Scholar] [CrossRef]
- Ajluni, N.; Meral, R.; Neidert, A.H.; Brady, G.F.; Buras, E.; McKenna, B.; DiPaola, F.; Chenevert, T.L.; Horowitz, J.F.; Buggs-Saxton, C.; et al. Spectrum of Disease Associated with Partial Lipodystrophy: Lessons from a Trial Cohort. Clin. Endocrinol. 2017, 86, 698–707. [Google Scholar] [CrossRef]
- Araújo-Vilar, D.; Lado-Abeal, J.; Palos-Paz, F.; Lattanzi, G.; Bandín, M.A.; Bellido, D.; Domínguez-Gerpe, L.; Calvo, C.; Pérez, O.; Ramazanova, A.; et al. A Novel Phenotypic Expression Associated with a New Mutation in LMNA Gene, Characterized by Partial Lipodystrophy, Insulin Resistance, Aortic Stenosis and Hypertrophic Cardiomyopathy. Clin. Endocrinol. 2008, 69, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Speckman, R.A.; Bowcock, A.M. Multisystem Dystrophy Syndrome Due to Novel Missense Mutations in the Amino-Terminal Head and Alpha-Helical Rod Domains of the Lamin A/C Gene. Am. J. Med. 2002, 112, 549–555. [Google Scholar] [CrossRef]
- Subramanyam, L.; Simha, V.; Garg, A. Overlapping Syndrome with Familial Partial Lipodystrophy, Dunnigan Variety and Cardiomyopathy Due to Amino-Terminal Heterozygous Missense Lamin A/C Mutations. Clin. Genet. 2010, 78, 66–73. [Google Scholar] [CrossRef]
- Bidault, G.; Garcia, M.; Vantyghem, M.-C.; Ducluzeau, P.-H.; Morichon, R.; Thiyagarajah, K.; Moritz, S.; Capeau, J.; Vigouroux, C.; Béréziat, V. Lipodystrophy-Linked LMNA p.R482W Mutation Induces Clinical Early Atherosclerosis and In Vitro Endothelial Dysfunction. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2162–2171. [Google Scholar] [CrossRef]
- Hegele, R.A. Premature Atherosclerosis Associated with Monogenic Insulin Resistance. Circulation 2001, 103, 2225–2229. [Google Scholar] [CrossRef] [PubMed]
- Weterings, A.A.W.; van Rijsingen, I.A.W.; Plomp, A.S.; Zwinderman, A.H.; Lekanne Deprez, R.H.; Mannens, M.M.; van den Bergh Weerman, M.A.; van der Wal, A.C.; Pinto-Sietsma, S.J. A Novel Lamin A/C Mutation in a Dutch Family with Premature Atherosclerosis. Atherosclerosis 2013, 229, 169–173. [Google Scholar] [CrossRef]
- Eldin, A.J.; Akinci, B.; da Rocha, A.M.; Meral, R.; Simsir, I.Y.; Adiyaman, S.C.; Ozpelit, E.; Bhave, N.; Gen, R.; Yurekli, B.; et al. Cardiac Phenotype in Familial Partial Lipodystrophy. Clin. Endocrinol. 2021, 94, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Gambineri, A.; Semple, R.K.; Forlani, G.; Genghini, S.; Grassi, I.; Hyden, C.S.S.; Pagotto, U.; O’Rahilly, S.; Pasquali, R. Monogenic Polycystic Ovary Syndrome Due to a Mutation in the Lamin A/C Gene Is Sensitive to Thiazolidinediones but Not to Metformin. Eur. J. Endocrinol. 2008, 159, 347–353. [Google Scholar] [CrossRef]
- Hegele, R.A. Lessons from Human Mutations in PPARgamma. Int. J. Obes. 2005, 29 (Suppl. S1), S31–S35. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Joy, T.R.; Hegele, R.A. Prevalence of Reproductive Abnormalities among Women with Familial Partial Lipodystrophy. Endocr. Pract. 2008, 14, 1126–1132. [Google Scholar] [CrossRef]
- Vantyghem, M.C.; Vincent-Desplanques, D.; Defrance-Faivre, F.; Capeau, J.; Fermon, C.; Valat, A.S.; Lascols, O.; Hecart, A.C.; Pigny, P.; Delemer, B.; et al. Fertility and Obstetrical Complications in Women with LMNA-Related Familial Partial Lipodystrophy. J. Clin. Endocrinol. Metab. 2008, 93, 2223–2229. [Google Scholar] [CrossRef]
- Al-Shali, K.; Cao, H.; Knoers, N.; Hermus, A.R.; Tack, C.J.; Hegele, R.A. A Single-Base Mutation in the Peroxisome Proliferator-Activated Receptor Gamma4 Promoter Associated with Altered in Vitro Expression and Partial Lipodystrophy. J. Clin. Endocrinol. Metab. 2004, 89, 5655–5660. [Google Scholar] [CrossRef]
- Auclair, M.; Vigouroux, C.; Boccara, F.; Capel, E.; Vigeral, C.; Guerci, B.; Lascols, O.; Capeau, J.; Caron-Debarle, M. Peroxisome Proliferator-Activated Receptor-γ Mutations Responsible for Lipodystrophy with Severe Hypertension Activate the Cellular Renin-Angiotensin System. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 829–838. [Google Scholar] [CrossRef]
- Berger, J.R.; Oral, E.A.; Taylor, S.I. Familial Lipodystrophy Associated with Neurodegeneration and Congenital Cataracts. Neurology 2002, 58, 43–47. [Google Scholar] [CrossRef]
- Carboni, N.; Brancati, F.; Cocco, E.; Solla, E.; D’Apice, M.R.; Mateddu, A.; McIntyre, A.; Fadda, E.; Mura, M.; Lattanzi, G.; et al. Partial Lipodystrophy Associated with Muscular Dystrophy of Unknown Genetic Origin. Muscle Nerve 2014, 49, 928–930. [Google Scholar] [CrossRef]
- Farhan, S.M.K.; Robinson, J.F.; McIntyre, A.D.; Marrosu, M.G.; Ticca, A.F.; Loddo, S.; Carboni, N.; Brancati, F.; Hegele, R.A. A Novel LIPE Nonsense Mutation Found Using Exome Sequencing in Siblings with Late-Onset Familial Partial Lipodystrophy. Can. J. Cardiol. 2014, 30, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.A.; Li, G.; Casey, R.; Wang, J.; Cao, H.; Leff, T.; Hegele, R.A. Peroxisomal Proliferator Activated Receptor-Gamma Deficiency in a Canadian Kindred with Familial Partial Lipodystrophy Type 3 (FPLD3). BMC Med. Genet. 2006, 7, 3. [Google Scholar] [CrossRef]
- Gandotra, S.; Le Dour, C.; Bottomley, W.; Cervera, P.; Giral, P.; Reznik, Y.; Charpentier, G.; Auclair, M.; Delépine, M.; Barroso, I.; et al. Perilipin Deficiency and Autosomal Dominant Partial Lipodystrophy. N. Engl. J. Med. 2011, 364, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Kircher, M.; Del Campo, M.; Amato, R.S.; Agarwal, A.K.; University of Washington Center for Mendelian Genomics. Whole Exome Sequencing Identifies de Novo Heterozygous CAV1 Mutations Associated with a Novel Neonatal Onset Lipodystrophy Syndrome. Am. J. Med. Genet. A 2015, 167, 1796–1806. [Google Scholar] [CrossRef]
- Guidorizzi, N.R.; Valerio, C.M.; Viola, L.F.; Veras, V.R.; Fernandes, V.O.; da Cruz Paiva Lima, G.E.; Flor, A.C.; Araújo, J.S.; Gonçalves Muniz, R.B.; Moreira, R.O.; et al. Comprehensive Analysis of Morbidity and Mortality Patterns in Familial Partial Lipodystrophy Patients: Insights from a Population Study. Front. Endocrinol. 2024, 15, 1359211. [Google Scholar] [CrossRef]
- Guillín-Amarelle, C.; Sánchez-Iglesias, S.; Castro-Pais, A.; Rodriguez-Cañete, L.; Ordóñez-Mayán, L.; Pazos, M.; González-Méndez, B.; Rodríguez-García, S.; Casanueva, F.F.; Fernández-Marmiesse, A.; et al. Type 1 Familial Partial Lipodystrophy: Understanding the Köbberling Syndrome. Endocrine 2016, 54, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Hegele, R.A.; Ur, E.; Ransom, T.P.; Cao, H. A Frameshift Mutation in Peroxisome-Proliferator-Activated Receptor-Gamma in Familial Partial Lipodystrophy Subtype 3 (FPLD3; MIM 604367). Clin. Genet. 2006, 70, 360–362. [Google Scholar] [CrossRef]
- Herbst, K.L.; Tannock, L.R.; Deeb, S.S.; Purnell, J.Q.; Brunzell, J.D.; Chait, A. Köbberling Type of Familial Partial Lipodystrophy: An Underrecognized Syndrome. Diabetes Care 2003, 26, 1819–1824. [Google Scholar] [CrossRef]
- Jéru, I.; Vantyghem, M.-C.; Bismuth, E.; Cervera, P.; Barraud, S.; PLIN1-Study Group; Auclair, M.; Vatier, C.; Lascols, O.; Savage, D.B.; et al. Diagnostic Challenge in PLIN1-Associated Familial Partial Lipodystrophy. J. Clin. Endocrinol. Metab. 2019, 104, 6025–6032. [Google Scholar] [CrossRef]
- Kozusko, K.; Tsang, V.; Bottomley, W.; Cho, Y.H.; Gandotra, S.; Mimmack, M.L.; Lim, K.; Isaac, I.; Patel, S.; Saudek, V.; et al. Clinical and Molecular Characterization of a Novel PLIN1 Frameshift Mutation Identified in Patients with Familial Partial Lipodystrophy. Diabetes 2015, 64, 299–310. [Google Scholar] [CrossRef]
- Laver, T.W.; Patel, K.A.; Colclough, K.; Curran, J.; Dale, J.; Davis, N.; Savage, D.B.; Flanagan, S.E.; Ellard, S.; Hattersley, A.T.; et al. PLIN1 Haploinsufficiency Is Not Associated With Lipodystrophy. J. Clin. Endocrinol. Metab. 2018, 103, 3225–3230. [Google Scholar] [CrossRef] [PubMed]
- Payne, F.; Lim, K.; Girousse, A.; Brown, R.J.; Kory, N.; Robbins, A.; Xue, Y.; Sleigh, A.; Cochran, E.; Adams, C.; et al. Mutations Disrupting the Kennedy Phosphatidylcholine Pathway in Humans with Congenital Lipodystrophy and Fatty Liver Disease. Proc Natl. Acad. Sci. USA 2014, 111, 8901–8906. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Cabezas, O.; Puri, V.; Murano, I.; Saudek, V.; Semple, R.K.; Dash, S.; Hyden, C.S.S.; Bottomley, W.; Vigouroux, C.; Magré, J.; et al. Partial Lipodystrophy and Insulin Resistant Diabetes in a Patient with a Homozygous Nonsense Mutation in CIDEC. EMBO Mol. Med. 2009, 1, 280–287. [Google Scholar] [CrossRef]
- Schuermans, N.; El Chehadeh, S.; Hemelsoet, D.; Gautheron, J.; Vantyghem, M.-C.; Nouioua, S.; Tazir, M.; Vigouroux, C.; Auclair, M.; Bogaert, E.; et al. Loss of Phospholipase PLAAT3 Causes a Mixed Lipodystrophic and Neurological Syndrome Due to Impaired PPARγ Signaling. Nat. Genet. 2023, 55, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Semple, R.K.; Chatterjee, V.K.K.; O’Rahilly, S. PPAR Gamma and Human Metabolic Disease. J. Clin. Investig. 2006, 116, 581–589. [Google Scholar] [CrossRef]
- Shearin, A.L.; Monks, B.R.; Seale, P.; Birnbaum, M.J. Lack of AKT in Adipocytes Causes Severe Lipodystrophy. Mol. Metab. 2016, 5, 472–479. [Google Scholar] [CrossRef]
- Sollier, C.; Capel, E.; Aguilhon, C.; Smirnov, V.; Auclair, M.; Douillard, C.; Ladsous, M.; Defoort-Dhellemmes, S.; Gorwood, J.; Braud, L.; et al. LIPE-Related Lipodystrophic Syndrome: Clinical Features and Disease Modeling Using Adipose Stem Cells. Eur. J. Endocrinol. 2021, 184, 155–168. [Google Scholar] [CrossRef]
- Garg, A.; Sankella, S.; Xing, C.; Agarwal, A.K. Whole-Exome Sequencing Identifies ADRA2A Mutation in Atypical Familial Partial Lipodystrophy. JCI Insight 2016, 1, e86870. [Google Scholar] [CrossRef]
- Zolotov, S.; Xing, C.; Mahamid, R.; Shalata, A.; Sheikh-Ahmad, M.; Garg, A. Homozygous LIPE Mutation in Siblings with Multiple Symmetric Lipomatosis, Partial Lipodystrophy, and Myopathy. Am. J. Med. Genet. A 2017, 173, 190–194. [Google Scholar] [CrossRef]
- Mosbah, H.; Vantyghem, M.-C.; Nobécourt, E.; Andreelli, F.; Archambeaud, F.; Bismuth, E.; Briet, C.; Cartigny, M.; Chevalier, B.; Donadille, B.; et al. Therapeutic Indications and Metabolic Effects of Metreleptin in Patients with Lipodystrophy Syndromes: Real-Life Experience from a National Reference Network. Diabetes Obes. Metab. 2022, 24, 1565–1577. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Javor, E.D.; Cochran, E.K.; DePaoli, A.M.; Gorden, P. Long-Term Efficacy of Leptin Replacement in Patients with Dunnigan-Type Familial Partial Lipodystrophy. Metabolism 2007, 56, 508–516. [Google Scholar] [CrossRef]
- Simha, V.; Subramanyam, L.; Szczepaniak, L.; Quittner, C.; Adams-Huet, B.; Snell, P.; Garg, A. Comparison of Efficacy and Safety of Leptin Replacement Therapy in Moderately and Severely Hypoleptinemic Patients with Familial Partial Lipodystrophy of the Dunnigan Variety. J. Clin. Endocrinol. Metab. 2012, 97, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Javor, E.D.; Ghany, M.G.; Cochran, E.K.; Oral, E.A.; DePaoli, A.M.; Premkumar, A.; Kleiner, D.E.; Gorden, P. Leptin Reverses Nonalcoholic Steatohepatitis in Patients with Severe Lipodystrophy. Hepatology 2005, 41, 753–760. [Google Scholar] [CrossRef]
- Oral, E.A.; Garg, A.; Tami, J.; Huang, E.A.; O’Dea, L.S.L.; Schmidt, H.; Tiulpakov, A.; Mertens, A.; Alexander, V.J.; Watts, L.; et al. Assessment of Efficacy and Safety of Volanesorsen for Treatment of Metabolic Complications in Patients with Familial Partial Lipodystrophy: Results of the BROADEN Study: Volanesorsen in FPLD; The BROADEN Study. J. Clin. Lipidol. 2022, 16, 833–849. [Google Scholar] [CrossRef]
- Akinci, B.; Swaidan, M.; Foss-Freitas, M.C.; Luo, Y.; Neidert, A.H.; Hench, R.P.; Chenevert, T.L.; Longcore, A.; Bakker-Arkema, R.; Bisgaier, C.L.; et al. 2214-PUB: An Open-Label Study of Gemcabene in Adults with Familial Partial Lipodystrophy. Diabetes 2020, 69 (Suppl. S1), 2214-PUB. [Google Scholar] [CrossRef]
- Foss-Freitas, M.C.; Akinci, B.; Neidert, A.; Bartlett, V.J.; Hurh, E.; Karwatowska-Prokopczuk, E.; Oral, E.A. Selective Targeting of Angiopoietin-like 3 (ANGPTL3) with Vupanorsen for the Treatment of Patients with Familial Partial Lipodystrophy (FPLD): Results of a Proof-of-Concept Study. Lipids Health Dis. 2021, 20, 174. [Google Scholar] [CrossRef]
- De Sandre-Giovannoli, A.; Bernard, R.; Cau, P.; Navarro, C.; Amiel, J.; Boccaccio, I.; Lyonnet, S.; Stewart, C.L.; Munnich, A.; Le Merrer, M.; et al. Lamin a Truncation in Hutchinson-Gilford Progeria. Science 2003, 300, 2055. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, M.; Brown, W.T.; Gordon, L.B.; Glynn, M.W.; Singer, J.; Scott, L.; Erdos, M.R.; Robbins, C.M.; Moses, T.Y.; Berglund, P.; et al. Recurrent de Novo Point Mutations in Lamin A Cause Hutchinson-Gilford Progeria Syndrome. Nature 2003, 423, 293–298. [Google Scholar] [CrossRef]
- Hennekam, R.C.M. Hutchinson-Gilford Progeria Syndrome: Review of the Phenotype. Am. J. Med. Genet. A 2006, 140, 2603–2624. [Google Scholar] [CrossRef]
- Mazereeuw-Hautier, J.; Wilson, L.C.; Mohammed, S.; Smallwood, D.; Shackleton, S.; Atherton, D.J.; Harper, J.I. Hutchinson-Gilford Progeria Syndrome: Clinical Findings in Three Patients Carrying the G608G Mutation in LMNA and Review of the Literature. Br. J. Dermatol. 2007, 156, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Merideth, M.A.; Gordon, L.B.; Clauss, S.; Sachdev, V.; Smith, A.C.M.; Perry, M.B.; Brewer, C.C.; Zalewski, C.; Kim, H.J.; Solomon, B.; et al. Phenotype and Course of Hutchinson-Gilford Progeria Syndrome. N. Engl. J. Med. 2008, 358, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Gordon, L.B.; Brown, W.T.; Collins, F.S. Hutchinson-Gilford Progeria Syndrome. 2003 Dec 12 [Updated 2023 Oct 19]; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.G.H., Gripp, K.W., Amemiya, A., Eds.; GeneReviews® [Internet] 1993–2024; University of Washington: Seattle, WA, USA, 2003. [Google Scholar]
- Stehbens, W.E.; Wakefield, S.J.; Gilbert-Barness, E.; Olson, R.E.; Ackerman, J. Histological and Ultrastructural Features of Atherosclerosis in Progeria. Cardiovasc. Pathol. 1999, 8, 29–39. [Google Scholar] [CrossRef]
- Gordon, L.B.; Harten, I.A.; Patti, M.E.; Lichtenstein, A.H. Reduced Adiponectin and HDL Cholesterol without Elevated C-Reactive Protein: Clues to the Biology of Premature Atherosclerosis in Hutchinson-Gilford Progeria Syndrome. J. Pediatr. 2005, 146, 336–341. [Google Scholar] [CrossRef]
- Olive, M.; Harten, I.; Mitchell, R.; Beers, J.K.; Djabali, K.; Cao, K.; Erdos, M.R.; Blair, C.; Funke, B.; Smoot, L.; et al. Cardiovascular Pathology in Hutchinson-Gilford Progeria: Correlation with the Vascular Pathology of Aging. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2301–2309. [Google Scholar] [CrossRef]
- Gerhard-Herman, M.; Smoot, L.B.; Wake, N.; Kieran, M.W.; Kleinman, M.E.; Miller, D.T.; Schwartzman, A.; Giobbie-Hurder, A.; Neuberg, D.; Gordon, L.B. Mechanisms of Premature Vascular Aging in Children with Hutchinson-Gilford Progeria Syndrome. Hypertension 2012, 59, 92–97. [Google Scholar] [CrossRef]
- Osorio, F.G.; Navarro, C.L.; Cadiñanos, J.; López-Mejía, I.C.; Quirós, P.M.; Bartoli, C.; Rivera, J.; Tazi, J.; Guzmán, G.; Varela, I.; et al. Splicing-Directed Therapy in a New Mouse Model of Human Accelerated Aging. Sci. Transl. Med. 2011, 3, 106ra107. [Google Scholar] [CrossRef] [PubMed]
- Varga, R.; Eriksson, M.; Erdos, M.R.; Olive, M.; Harten, I.; Kolodgie, F.; Capell, B.C.; Cheng, J.; Faddah, D.; Perkins, S.; et al. Progressive Vascular Smooth Muscle Cell Defects in a Mouse Model of Hutchinson-Gilford Progeria Syndrome. Proc. Natl. Acad. Sci. USA 2006, 103, 3250–3255. [Google Scholar] [CrossRef]
- Villa-Bellosta, R.; Rivera-Torres, J.; Osorio, F.G.; Acín-Pérez, R.; Enriquez, J.A.; López-Otín, C.; Andrés, V. Defective Extracellular Pyrophosphate Metabolism Promotes Vascular Calcification in a Mouse Model of Hutchinson-Gilford Progeria Syndrome That Is Ameliorated on Pyrophosphate Treatment. Circulation 2013, 127, 2442–2451. [Google Scholar] [CrossRef]
- Fong, L.G.; Frost, D.; Meta, M.; Qiao, X.; Yang, S.H.; Coffinier, C.; Young, S.G. A Protein Farnesyltransferase Inhibitor Ameliorates Disease in a Mouse Model of Progeria. Science 2006, 311, 1621–1623. [Google Scholar] [CrossRef]
- Gordon, L.B.; Kleinman, M.E.; Miller, D.T.; Neuberg, D.S.; Giobbie-Hurder, A.; Gerhard-Herman, M.; Smoot, L.B.; Gordon, C.M.; Cleveland, R.; Snyder, B.D.; et al. Clinical Trial of a Farnesyltransferase Inhibitor in Children with Hutchinson-Gilford Progeria Syndrome. Proc. Natl. Acad. Sci. USA 2012, 109, 16666–16671. [Google Scholar] [CrossRef]
- Yang, S.H.; Meta, M.; Qiao, X.; Frost, D.; Bauch, J.; Coffinier, C.; Majumdar, S.; Bergo, M.O.; Young, S.G.; Fong, L.G.A. Farnesyltransferase Inhibitor Improves Disease Phenotypes in Mice with a Hutchinson-Gilford Progeria Syndrome Mutation. J. Clin. Investig. 2006, 116, 2115–2121. [Google Scholar] [CrossRef]
- Gordon, L.B.; Shappell, H.; Massaro, J.; D’Agostino, R.B.; Brazier, J.; Campbell, S.E.; Kleinman, M.E.; Kieran, M.W. Association of Lonafarnib Treatment vs No Treatment With Mortality Rate in Patients With Hutchinson-Gilford Progeria Syndrome. JAMA 2018, 319, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Gordon, L.B.; Massaro, J.; D’Agostino, R.B.; Campbell, S.E.; Brazier, J.; Brown, W.T.; Kleinman, M.E.; Kieran, M.W.; Progeria Clinical Trials Collaborative. Impact of Farnesylation Inhibitors on Survival in Hutchinson-Gilford Progeria Syndrome. Circulation 2014, 130, 27–34. [Google Scholar] [CrossRef]
- Erdos, M.R.; Cabral, W.A.; Tavarez, U.L.; Cao, K.; Gvozdenovic-Jeremic, J.; Narisu, N.; Zerfas, P.M.; Crumley, S.; Boku, Y.; Hanson, G.; et al. A Targeted Antisense Therapeutic Approach for Hutchinson-Gilford Progeria Syndrome. Nat. Med. 2021, 27, 536–545. [Google Scholar] [CrossRef]
- Santiago-Fernández, O.; Osorio, F.G.; Quesada, V.; Rodríguez, F.; Basso, S.; Maeso, D.; Rolas, L.; Barkaway, A.; Nourshargh, S.; Folgueras, A.R.; et al. Development of a CRISPR/Cas9-Based Therapy for Hutchinson-Gilford Progeria Syndrome. Nat. Med. 2019, 25, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Doubaj, Y.; De Sandre-Giovannoli, A.; Vera, E.-V.; Navarro, C.L.; Elalaoui, S.C.; Tajir, M.; Lévy, N.; Sefiani, A. An Inherited LMNA Gene Mutation in Atypical Progeria Syndrome. Am. J. Med. Genet. A 2012, 158, 2881–2887. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Subramanyam, L.; Agarwal, A.K.; Simha, V.; Levine, B.; D’Apice, M.R.; Novelli, G.; Crow, Y. Atypical Progeroid Syndrome Due to Heterozygous Missense LMNA Mutations. J. Clin. Endocrinol. Metab. 2009, 94, 4971–4983. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Patni, N.; Ueda, M.; Sorkina, E.; Valerio, C.M.; Cochran, E.; Brown, R.J.; Peeden, J.; Tikhonovich, Y.; Tiulpakov, A.; et al. A Novel Generalized Lipodystrophy-Associated Progeroid Syndrome Due to Recurrent Heterozygous LMNA p.T10I Mutation. J. Clin. Endocrinol. Metab. 2018, 103, 1005–1014. [Google Scholar] [CrossRef]
- Hussain, I.; Jin, R.R.; Baum, H.B.A.; Greenfield, J.R.; Devery, S.; Xing, C.; Hegele, R.A.; Carranza-Leon, B.G.; Linton, M.F.; Vuitch, F.; et al. Multisystem Progeroid Syndrome With Lipodystrophy, Cardiomyopathy, and Nephropathy Due to an LMNA p.R349W Variant. J. Endocr. Soc. 2020, 4, bvaa104. [Google Scholar] [CrossRef]
- Magno, S.; Ceccarini, G.; Pelosini, C.; Ferrari, F.; Prodam, F.; Gilio, D.; Maffei, M.; Sessa, M.R.; Barison, A.; Ciccarone, A.; et al. Atypical Progeroid Syndrome and Partial Lipodystrophy Due to LMNA Gene p.R349W Mutation. J. Endocr. Soc. 2020, 4, bvaa108. [Google Scholar] [CrossRef]
- Motegi, S.; Yokoyama, Y.; Uchiyama, A.; Ogino, S.; Takeuchi, Y.; Yamada, K.; Hattori, T.; Hashizume, H.; Ishikawa, Y.; Goto, M.; et al. First Japanese Case of Atypical Progeroid Syndrome/Atypical Werner Syndrome with Heterozygous LMNA Mutation. J. Dermatol. 2014, 41, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Csoka, A.B.; Cao, H.; Sammak, P.J.; Constantinescu, D.; Schatten, G.P.; Hegele, R.A. Novel Lamin A/C Gene (LMNA) Mutations in Atypical Progeroid Syndromes. J. Med. Genet. 2004, 41, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Mory, P.B.; Crispim, F.; Kasamatsu, T.; Gabbay, M.A.L.; Dib, S.A.; Moisés, R.S. Atypical Generalized Lipoatrophy and Severe Insulin Resistance Due to a Heterozygous LMNA p.T10I Mutation. Arq. Bras. Endocrinol. Metabol. 2008, 52, 1252–1256. [Google Scholar] [CrossRef] [PubMed]
- Saadi, A.; Navarro, C.; Ozalp, O.; Lourenco, C.M.; Fayek, R.; Da Silva, N.; Chaouch, A.; Benahmed, M.; Kubisch, C.; Munnich, A.; et al. A Recurrent Homozygous LMNA Missense Variant p.Thr528Met Causes Atypical Progeroid Syndrome Characterized by Mandibuloacral Dysostosis, Severe Muscular Dystrophy, and Skeletal Deformities. Am. J. Med. Genet. A 2023, 191, 2274–2289. [Google Scholar] [CrossRef]
- Araujo-Vilar, D.; Sánchez-Iglesias, S.; Guillín-Amarelle, C.; Castro, A.; Lage, M.; Pazos, M.; Rial, J.M.; Blasco, J.; Guillén-Navarro, E.; Domingo-Jiménez, R.; et al. Recombinant Human Leptin Treatment in Genetic Lipodystrophic Syndromes: The Long-Term Spanish Experience. Endocrine 2015, 49, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Soyaltin, U.E.; Simsir, I.Y.; Akinci, B.; Altay, C.; Adiyaman, S.C.; Lee, K.; Onay, H.; Oral, E.A. Homozygous LMNA p.R582H Pathogenic Variant Reveals Increasing Effect on the Severity of Fat Loss in Lipodystrophy. Clin. Diabetes. Endocrinol. 2020, 6, 13. [Google Scholar] [CrossRef]
- Ng, K.K.; Kaye, G. A Case of Lamin C Gene-Mutation with Preserved Systolic Function and Ventricular Dysrrhythmia. Australas. Med. J. 2013, 6, 75–78. [Google Scholar] [CrossRef]
- Benedetti, S.; Bertini, E.; Iannaccone, S.; Angelini, C.; Trisciani, M.; Toniolo, D.; Sferrazza, B.; Carrera, P.; Comi, G.; Ferrari, M.; et al. Dominant LMNA Mutations Can Cause Combined Muscular Dystrophy and Peripheral Neuropathy. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1019–1021. [Google Scholar] [CrossRef]
- Patni, N.; Xing, C.; Agarwal, A.K.; Garg, A. Juvenile-Onset Generalized Lipodystrophy Due to a Novel Heterozygous Missense LMNA Mutation Affecting Lamin C. Am. J. Med. Genet. A 2017, 173, 2517–2521. [Google Scholar] [CrossRef]
- Kane, M.S.; Lindsay, M.E.; Judge, D.P.; Barrowman, J.; Ap Rhys, C.; Simonson, L.; Dietz, H.C.; Michaelis, S. LMNA-Associated Cardiocutaneous Progeria: An Inherited Autosomal Dominant Premature Aging Syndrome with Late Onset. Am. J. Med. Genet. A 2013, 161, 1599–1611. [Google Scholar] [CrossRef]
- Cenni, V.; D’Apice, M.R.; Garagnani, P.; Columbaro, M.; Novelli, G.; Franceschi, C.; Lattanzi, G. Mandibuloacral Dysplasia: A Premature Ageing Disease with Aspects of Physiological Ageing. Ageing Res. Rev. 2018, 42, 1–13. [Google Scholar] [CrossRef]
- Novelli, G.; Muchir, A.; Sangiuolo, F.; Helbling-Leclerc, A.; D’Apice, M.R.; Massart, C.; Capon, F.; Sbraccia, P.; Federici, M.; Lauro, R.; et al. Mandibuloacral Dysplasia Is Caused by a Mutation in LMNA-Encoding Lamin A/C. Am. J. Hum. Genet. 2002, 71, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, F.; Gullotta, F.; Columbaro, M.; Filareto, A.; D’Adamo, M.; Vielle, A.; Guglielmi, V.; Nardone, A.M.; Azzolini, V.; Grosso, E.; et al. Compound Heterozygosity for Mutations in LMNA in a Patient with a Myopathic and Lipodystrophic Mandibuloacral Dysplasia Type A Phenotype. J. Clin. Endocrinol. Metab. 2007, 92, 4467–4471. [Google Scholar] [CrossRef]
- Simha, V.; Agarwal, A.K.; Oral, E.A.; Fryns, J.-P.; Garg, A. Genetic and Phenotypic Heterogeneity in Patients with Mandibuloacral Dysplasia-Associated Lipodystrophy. J. Clin. Endocrinol. Metab. 2003, 88, 2821–2824. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Agarwal, A.K.; Fryns, J.-P.; Auchus, R.J.; Garg, A. Zinc Metalloproteinase, ZMPSTE24, Is Mutated in Mandibuloacral Dysplasia. Hum. Mol. Genet. 2003, 12, 1995–2001. [Google Scholar] [CrossRef]
- Miyoshi, Y.; Akagi, M.; Agarwal, A.K.; Namba, N.; Kato-Nishimura, K.; Mohri, I.; Yamagata, M.; Nakajima, S.; Mushiake, S.; Shima, M.; et al. Severe Mandibuloacral Dysplasia Caused by Novel Compound Heterozygous ZMPSTE24 Mutations in Two Japanese Siblings. Clin. Genet. 2008, 73, 535–544. [Google Scholar] [CrossRef]
- Young, L.W.; Radebaugh, J.F.; Rubin, P.; Sensenbrenner, J.A.; Fiorelli, G.; McKusick, V.A. New Syndrome Manifested by Mandibular Hypoplasia, Acroosteolysis, Stiff Joints and Cutaneous Atrophy (Mandibuloacral Dysplasia) in Two Unrelated Boys. Birth Defects Orig. Artic. Ser. 1971, 7, 291–297. [Google Scholar]
- Cunningham, V.J.; D’Apice, M.R.; Licata, N.; Novelli, G.; Cundy, T. Skeletal Phenotype of Mandibuloacral Dysplasia Associated with Mutations in ZMPSTE24. Bone 2010, 47, 591–597. [Google Scholar] [CrossRef]
- Camozzi, D.; D’Apice, M.R.; Schena, E.; Cenni, V.; Columbaro, M.; Capanni, C.; Maraldi, N.M.; Squarzoni, S.; Ortolani, M.; Novelli, G.; et al. Altered Chromatin Organization and SUN2 Localization in Mandibuloacral Dysplasia Are Rescued by Drug Treatment. Histochem. Cell. Biol. 2012, 138, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, F.; Fasciglione, G.F.; D’Apice, M.R.; Vielle, A.; D’Adamo, M.; Sbraccia, P.; Marini, S.; Borgiani, P.; Coletta, M.; Novelli, G. Increased Release and Activity of Matrix Metalloproteinase-9 in Patients with Mandibuloacral Dysplasia Type A, a Rare Premature Ageing Syndrome. Clin. Genet. 2008, 74, 374–383. [Google Scholar] [CrossRef]
- Jéru, I.; Nabil, A.; El-Makkawy, G.; Lascols, O.; Vigouroux, C.; Abdalla, E. Two Decades after Mandibuloacral Dysplasia Discovery: Additional Cases and Comprehensive View of Disease Characteristics. Genes 2021, 12, 1508. [Google Scholar] [CrossRef] [PubMed]
- Ozer, L.; Unsal, E.; Aktuna, S.; Baltaci, V.; Celikkol, P.; Akyigit, F.; Sen, A.; Ayvaz, O.; Balci, S. Mandibuloacral Dysplasia and LMNA A529V Mutation in Turkish Patients with Severe Skeletal Changes and Absent Breast Development. Clin. Dysmorphol. 2016, 25, 91–97. [Google Scholar] [CrossRef]
- Avnet, S.; Pallotta, R.; Perut, F.; Baldini, N.; Pittis, M.G.; Saponari, A.; Lucarelli, E.; Dozza, B.; Greggi, T.; Maraldi, N.M.; et al. Osteoblasts from a Mandibuloacral Dysplasia Patient Induce Human Blood Precursors to Differentiate into Active Osteoclasts. Biochim. Biophys. Acta 2011, 1812, 711–718. [Google Scholar] [CrossRef]
- Garavelli, L.; D’Apice, M.R.; Rivieri, F.; Bertoli, M.; Wischmeijer, A.; Gelmini, C.; De Nigris, V.; Albertini, E.; Rosato, S.; Virdis, R.; et al. Mandibuloacral Dysplasia Type A in Childhood. Am. J. Med. Genet. A 2009, 149, 2258–2264. [Google Scholar] [CrossRef] [PubMed]
- Camozzi, D.; Capanni, C.; Cenni, V.; Mattioli, E.; Columbaro, M.; Squarzoni, S.; Lattanzi, G. Diverse Lamin-Dependent Mechanisms Interact to Control Chromatin Dynamics. Focus on Laminopathies. Nucleus 2014, 5, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Evangelisti, C.; Bernasconi, P.; Cavalcante, P.; Cappelletti, C.; D’Apice, M.R.; Sbraccia, P.; Novelli, G.; Prencipe, S.; Lemma, S.; Baldini, N.; et al. Modulation of TGFbeta 2 Levels by Lamin A in U2-OS Osteoblast-like Cells: Understanding the Osteolytic Process Triggered by Altered Lamins. Oncotarget 2015, 6, 7424–7437. [Google Scholar] [CrossRef]
- Fong, L.G.; Ng, J.K.; Meta, M.; Coté, N.; Yang, S.H.; Stewart, C.L.; Sullivan, T.; Burghardt, A.; Majumdar, S.; Reue, K.; et al. Heterozygosity for Lmna Deficiency Eliminates the Progeria-like Phenotypes in Zmpste24-Deficient Mice. Proc. Natl. Acad. Sci. USA 2004, 101, 18111–18116. [Google Scholar] [CrossRef]
- Navarro, C.L.; De Sandre-Giovannoli, A.; Bernard, R.; Boccaccio, I.; Boyer, A.; Geneviève, D.; Hadj-Rabia, S.; Gaudy-Marqueste, C.; Smitt, H.S.; Vabres, P.; et al. Lamin A and ZMPSTE24 (FACE-1) Defects Cause Nuclear Disorganization and Identify Restrictive Dermopathy as a Lethal Neonatal Laminopathy. Hum. Mol. Genet. 2004, 13, 2493–2503. [Google Scholar] [CrossRef]
- Simha, V.; Garg, A. Body Fat Distribution and Metabolic Derangements in Patients with Familial Partial Lipodystrophy Associated with Mandibuloacral Dysplasia. J. Clin. Endocrinol. Metab. 2002, 87, 776–785. [Google Scholar] [CrossRef]
- Hitzert, M.M.; van der Crabben, S.N.; Baldewsingh, G.; van Amstel, H.K.P.; van den Wijngaard, A.; van Ravenswaaij-Arts, C.M.A.; Zijlmans, C.W.R. Mandibuloacral Dysplasia Type B (MADB): A Cohort of Eight Patients from Suriname with a Homozygous Founder Mutation in ZMPSTE24 (FACE1), Clinical Diagnostic Criteria and Management Guidelines. Orphanet J. Rare Dis. 2019, 14, 294. [Google Scholar] [CrossRef] [PubMed]
- Haye, D.; Dridi, H.; Levy, J.; Lambert, V.; Lambert, M.; Agha, M.; Adjimi, F.; Kohlhase, J.; Lipsker, D.; Verloes, A. Failure of Ossification of the Occipital Bone in Mandibuloacral Dysplasia Type B. Am. J. Med. Genet. A 2016, 170, 2750–2755. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Zackai, E.; Medne, L.; Garg, A. Early Onset Mandibuloacral Dysplasia Due to Compound Heterozygous Mutations in ZMPSTE24. Am. J. Med. Genet. A 2010, 152, 2703–2710. [Google Scholar] [CrossRef]
- Varela, I.; Pereira, S.; Ugalde, A.P.; Navarro, C.L.; Suárez, M.F.; Cau, P.; Cadiñanos, J.; Osorio, F.G.; Foray, N.; Cobo, J.; et al. Combined Treatment with Statins and Aminobisphosphonates Extends Longevity in a Mouse Model of Human Premature Aging. Nat. Med. 2008, 14, 767–772. [Google Scholar] [CrossRef]
- Hartinger, R.; Lederer, E.-M.; Schena, E.; Lattanzi, G.; Djabali, K. Impact of Combined Baricitinib and FTI Treatment on Adipogenesis in Hutchinson-Gilford Progeria Syndrome and Other Lipodystrophic Laminopathies. Cells 2023, 12, 1350. [Google Scholar] [CrossRef] [PubMed]
- Akinci, B.; Sankella, S.; Gilpin, C.; Ozono, K.; Garg, A.; Agarwal, A.K. Progeroid Syndrome Patients with ZMPSTE24 Deficiency Could Benefit When Treated with Rapamycin and Dimethylsulfoxide. Cold Spring Harb. Mol. Case Stud. 2017, 3, a001339. [Google Scholar] [CrossRef]
- Odinammadu, K.O.; Shilagardi, K.; Tuminelli, K.; Judge, D.P.; Gordon, L.B.; Michaelis, S. The Farnesyl Transferase Inhibitor (FTI) Lonafarnib Improves Nuclear Morphology in ZMPSTE24-Deficient Fibroblasts from Patients with the Progeroid Disorder MAD-B. Nucleus 2023, 14, 2288476. [Google Scholar] [CrossRef] [PubMed]
- Harhouri, K.; Navarro, C.; Baquerre, C.; Da Silva, N.; Bartoli, C.; Casey, F.; Mawuse, G.K.; Doubaj, Y.; Lévy, N.; De Sandre-Giovannoli, A. Antisense-Based Progerin Downregulation in HGPS-Like Patients’ Cells. Cells 2016, 5, 31. [Google Scholar] [CrossRef]
- Fisher, H.G.; Patni, N.; Scheuerle, A.E. An Additional Case of Néstor-Guillermo Progeria Syndrome Diagnosed in Early Childhood. Am. J. Med. Genet. A 2020, 182, 2399–2402. [Google Scholar] [CrossRef]
- Puente, X.S.; Quesada, V.; Osorio, F.G.; Cabanillas, R.; Cadiñanos, J.; Fraile, J.M.; Ordóñez, G.R.; Puente, D.A.; Gutiérrez-Fernández, A.; Fanjul-Fernández, M.; et al. Exome Sequencing and Functional Analysis Identifies BANF1 Mutation as the Cause of a Hereditary Progeroid Syndrome. Am. J. Hum. Genet. 2011, 88, 650–656. [Google Scholar] [CrossRef]
- Marcelot, A.; Rodriguez-Tirado, F.; Cuniasse, P.; Joiner, M.-L.; Miron, S.; Soshnev, A.A.; Fang, M.; Pufall, M.A.; Mathews, K.D.; Moore, S.A.; et al. A De Novo Sequence Variant in Barrier-to-Autointegration Factor Is Associated with Dominant Motor Neuronopathy. Cells 2023, 12, 847. [Google Scholar] [CrossRef] [PubMed]
- Guillín-Amarelle, C.; Sánchez-Iglesias, S.; Araújo-Vilar, D. Uncommon lipodystrophic syndromes. Med. Clin. 2015, 144, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Hennekam, R.C.M. Pathophysiology of Premature Aging Characteristics in Mendelian Progeroid Disorders. Eur. J. Med. Genet. 2020, 63, 104028. [Google Scholar] [CrossRef] [PubMed]
- Neer, R.M.; Arnaud, C.D.; Zanchetta, J.R.; Prince, R.; Gaich, G.A.; Reginster, J.Y.; Hodsman, A.B.; Eriksen, E.F.; Ish-Shalom, S.; Genant, H.K.; et al. Effect of Parathyroid Hormone (1-34) on Fractures and Bone Mineral Density in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 2001, 344, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Capell, B.C.; Olive, M.; Erdos, M.R.; Cao, K.; Faddah, D.A.; Tavarez, U.L.; Conneely, K.N.; Qu, X.; San, H.; Ganesh, S.K.; et al. A Farnesyltransferase Inhibitor Prevents Both the Onset and Late Progression of Cardiovascular Disease in a Progeria Mouse Model. Proc. Natl. Acad. Sci. USA 2008, 105, 15902–15907. [Google Scholar] [CrossRef]
- Worman, H.J.; Fong, L.G.; Muchir, A.; Young, S.G. Laminopathies and the Long Strange Trip from Basic Cell Biology to Therapy. J. Clin. Investig. 2009, 119, 1825–1836. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Worman, H.J.; Michaelis, S. Prelamin A and ZMPSTE24 in Premature and Physiological Aging. Nucleus 2023, 14, 2270345. [Google Scholar] [CrossRef]
- Akinci, B.; Oral, E.A.; Neidert, A.; Rus, D.; Cheng, W.Y.; Thompson-Leduc, P.; Cheung, H.C.; Bradt, P.; Foss de Freitas, M.C.; Montenegro, R.M.; et al. Comorbidities and Survival in Patients With Lipodystrophy: An International Chart Review Study. J. Clin. Endocrinol. Metab. 2019, 104, 5120–5135. [Google Scholar] [CrossRef]
- Tu, Y.; Sánchez-Iglesias, S.; Araújo-Vilar, D.; Fong, L.G.; Young, S.G. LMNA Missense Mutations Causing Familial Partial Lipodystrophy Do Not Lead to an Accumulation of Prelamin A. Nucleus 2016, 7, 512–521. [Google Scholar] [CrossRef]
- Carboni, N.; Politano, L.; Floris, M.; Mateddu, A.; Solla, E.; Olla, S.; Maggi, L.; Antonietta Maioli, M.; Piras, R.; Cocco, E.; et al. Overlapping Syndromes in Laminopathies: A Meta-Analysis of the Reported Literature. Acta Myol. 2013, 32, 7–17. [Google Scholar] [PubMed]
- Fernández-Pombo, A.; Ossandon-Otero, J.A.; Guillín-Amarelle, C.; Sánchez-Iglesias, S.; Castro, A.I.; González-Méndez, B.; Rodríguez-García, S.; Rodriguez-Cañete, L.; Casanueva, F.F.; Araújo-Vilar, D. Bone Mineral Density in Familial Partial Lipodystrophy. Clin. Endocrinol. 2018, 88, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.N.; Howlett, T.A.; McNally, P.G.; O’Rahilly, S.; Trembath, R.C. Dunnigan-Kobberling Syndrome: An Autosomal Dominant Form of Partial Lipodystrophy. QJM 1997, 90, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Araújo-Vilar, D.; Fernández-Pombo, A.; Cobelo-Gómez, S.; Castro, A.I.; Sánchez-Iglesias, S. Lipodystrophy-Associated Progeroid Syndromes. Hormones 2022, 21, 555–571. [Google Scholar] [CrossRef]
- Kuo, F.-C.; Huang, Y.-C.; Yen, M.-R.; Lee, C.-H.; Hsu, K.-F.; Yang, H.-Y.; Wu, L.-W.; Lu, C.-H.; Hsu, Y.-J.; Chen, P.-Y. Aberrant Overexpression of HOTAIR Inhibits Abdominal Adipogenesis through Remodelling of Genome-Wide DNA Methylation and Transcription. Mol. Metab. 2022, 60, 101473. [Google Scholar] [CrossRef]
- Patni, N.; Garg, A. Congenital Generalized Lipodystrophies--New Insights into Metabolic Dysfunction. Nat. Rev. Endocrinol. 2015, 11, 522–534. [Google Scholar] [CrossRef]
- Gorden, P.; Lupsa, B.C.; Chong, A.Y.; Lungu, A.O. Is There a Human Model for the “metabolic Syndrome” with a Defined Aetiology? Diabetologia 2010, 53, 1534–1536. [Google Scholar] [CrossRef]
- Araújo-Vilar, D.; Santini, F. Diagnosis and Treatment of Lipodystrophy: A Step-by-Step Approach. J. Endocrinol. Investig. 2019, 42, 61–73. [Google Scholar] [CrossRef]
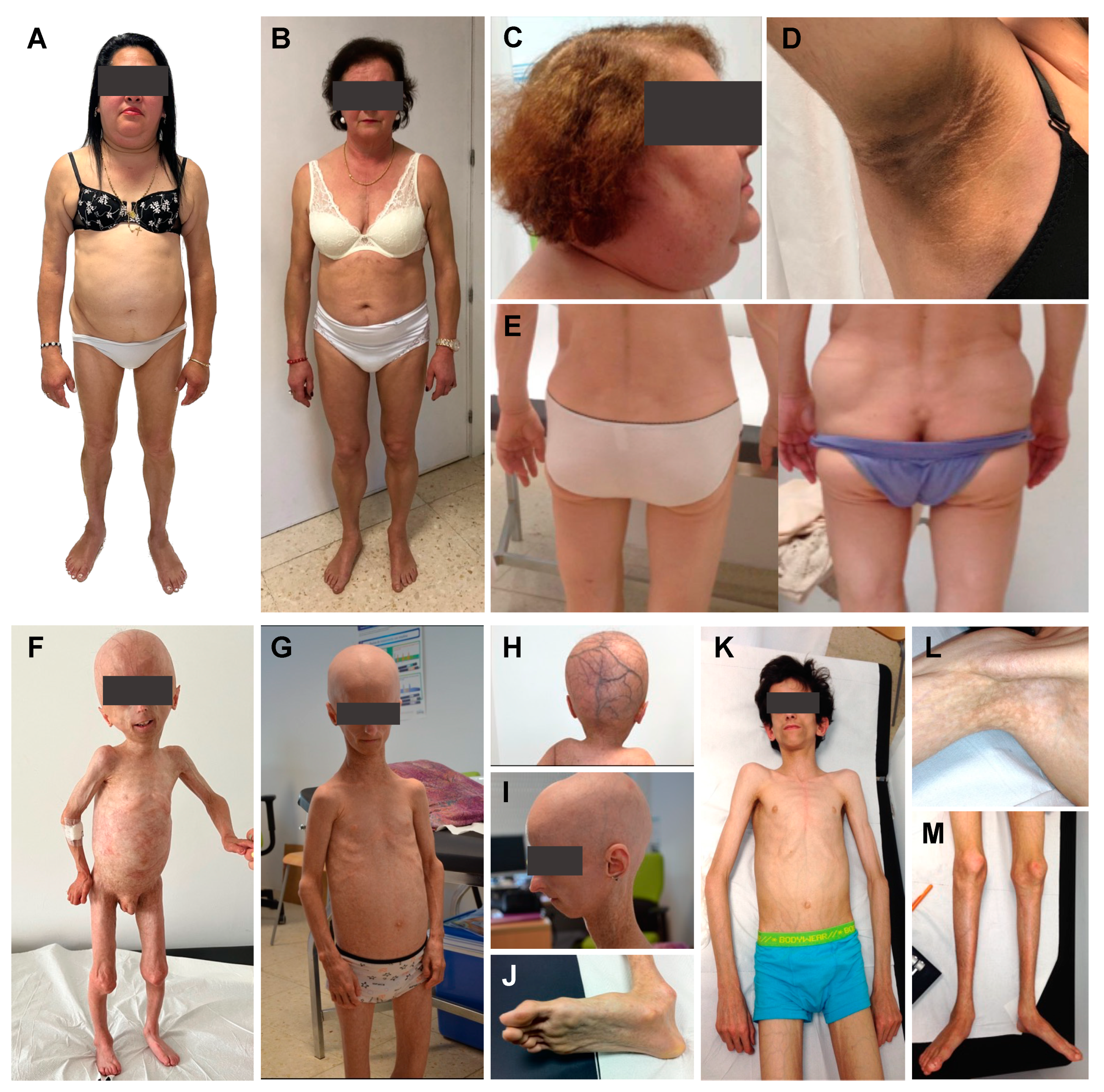

| Dunnigan Disease [30,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87] | Other FPLD Subtypes [88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110] | ||
|---|---|---|---|
| Molecular characteristics | Nuclear lamina alteration Variants in LMNA gene - Classic FPLD2: R482W and R482Q variants in exon 8 - Atypical FPLD2: non-codon 482 variants | - Unknown: FPLD1. - Adipogenesis dysregulation: FPLD3 (PPARG gene), FPLD9 (PLAAT3 gene). - Lipid droplet impairment or lipolysis dysregulation: FPLD4 (PLIN1 gene), FPLD5 (CIDEC gene), FPLD6 (LIPE gene), FPLD8 (ADRA2A gene). - Insulin signal transduction alteration: AKT2-related FPLD. - Caveolar function alteration: FPLD7 (CAV1 gene). - Dysregulation of phosphatidylcholine biosynthesis: PCYT1A-related FPLD. - Mitochondrial dysfunction: MFN2-related FPLD. | |
| Type of inheritance | Autosomal dominant/Semi-dominant inheritance | - Polygenic: FPLD1, FPLD7. - Autosomal dominant: FPLD3, FPLD4, FPLD7, FPLD8 and AKT2-related FPLD. - Autosomal recessive: FPLD5, FPLD6, FPLD9, PCYT1A- and MFN2-related FPLD. | |
| Onset of fat loss | Puberty in women, later in men | - Birth: FPLD7 - Childhood: FPLD1, FPLD4, FPLD5, PCYT1A- and MFN2-related FPLD - Adolescence: FPLD3, FPLD8 and MFN2-related FPLD - Adulthood: FPLD3, FPLD4, FPLD6, AKT2- and MFN2-related FPLD | |
| Abnormal fat pattern | - Loss of fat in the limbs, trunk and gluteal region. - Accumulation of fat in the face, neck, chin, axillae, interscapular area and abdominal viscera. - Hypertrophy of mons pubis fat surrounded by subcutaneous lipoatrophy (“Dunnigan sign”). - Subcutaneous lipomas (20%) | - FPLD3: less severe loss of fat. - FPLD6 and MFN2-related FPLD: multiple lipomatous masses. - FPLD7: loss of fat in the face and upper body. - FPLD9: adipose tissue loss varies from partial to generalised. | |
| Adipokine Disturbance | - Leptin levels ranging from low to normal values. - Lower adiponectin levels in comparison with healthy controls | MFN2-related FPLD: very low leptin concentrations. | |
| Clinical features | - Muscular hypertrophy - Myalgias - Phlebomegaly - Hirsutism in women - Acanthosis nigricans and acrochordons | - FPLD1: KöB index > 3.477 - FPLD3: less prominent musculature, phlebomegaly - FPLD6: muscular dystrophy - PCYT1A-related FPLD: short stature, muscular atrophy. | |
| Organ abnormalities and comorbidities | Metabolic abnormalities - Non-ketotic diabetes - Hypertriglyceridaemia, low HDL cholesterol Heart abnormalities - Cardiac hypertrophy - Atrioventricular conduction defects - Heart failure - Early atherosclerosis - Arrhythmias Liver abnormalities - NAFLD - NASH - Cirrhosis Kidney abnormalities - Proteinuria - Chronic renal failure Reproductive abnormalities - Polycystic ovary syndrome - Fertility problems - Miscarriages and stillbirths Others - Hypertension - Acute pancreatitis | - FPLD3: earlier and more severe metabolic complications - FPLD5: diabetes with ketosis. - FPLD6: auto-fluorescent drusen-like retinal deposits. Increased CK levels. - FPLD7: congenital cataracts. - FPLD9: neurological abnormalities (demyelinating neuropathy, intellectual disability). - MFN2-related FPLD: peripheral axonal neuropathy. | |
| Disease | Gene | Inheritance | Dysfunction | Onset of Phenotype | Fat Loss | Clinical Features | Main Comorbidities |
|---|---|---|---|---|---|---|---|
| FPLD2 or Dunnigan disease [30,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87] #151660 | LMNA | AD | Nuclear lamina alteration | Puberty in women, later in men | Partial | Subutaneous lipomas. Muscular hypertrophy, phlebomegaly. “Dunnigan sign” | Diabetes, hypertriglyceridaemia, hepatic steatosis, fertility problems, PCOS, cardiovascular disease, arrhythmias. May associate cardiomyopathy, muscular dystrophy. |
| HGPS [118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137] #176670 | LMNA | AD | Nuclear lamina alteration | 18–24 months | Generalised | Global alopecia, prominent veins on the scalp, micrognatia, dental crowding. | Bone and joint alterations. Atherosclerosis, cardiovascular disease. Mild metabolic syndrome. |
| APS [138,139,140,141,142,143,144,145,146,147] - | LMNA | AD | Nuclear lamina alteration | Childhood/Early adulthood | Generalised or partial | Phenotypic heterogeneity. Premature greying of hair, alopecia, dental crowding. | Metabolic complications. Valvulopathy, dilated cardiomyopathy. Acroosteolysis and clavicular hypoplasia are absent or mild. |
| MADA [8,14,159,160,161,162,163,164,165,166,167,168] #248370 | LMNA | AR | Nuclear lamina alteration | 2–4 years | Partial | Growth retardation, beaked nose, mottled skin pigmentation, dental crowding | Bone alterations. Metabolic syndrome. Premature adrenal cortical dysfunction in some cases. |
| MADB [131,157,169,170,171,172,173,174,175,176,177,178,179] #608612 | ZMPSTE24 | AR | Nuclear lamina alteration | Perinatal | Generalised | Short stature, delayed closure of fontanels, calcified skin nodules. | Musculoskeletal abnormalities. Metabolic syndrome. |
| NGPS [9,12,180,181,182,183,184,185,186,187] #614008 | BANF1 | AR | Nuclear lamina alteration | 2 years | Generalised | Profound skeletal abnormalities. | Bone alterations. No metabolic complications. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-López, E.J.; Sánchez-Iglesias, S.; Castro, A.I.; Cobelo-Gómez, S.; Prado-Moraña, T.; Araújo-Vilar, D.; Fernandez-Pombo, A. Lipodystrophic Laminopathies: From Dunnigan Disease to Progeroid Syndromes. Int. J. Mol. Sci. 2024, 25, 9324. https://doi.org/10.3390/ijms25179324
Díaz-López EJ, Sánchez-Iglesias S, Castro AI, Cobelo-Gómez S, Prado-Moraña T, Araújo-Vilar D, Fernandez-Pombo A. Lipodystrophic Laminopathies: From Dunnigan Disease to Progeroid Syndromes. International Journal of Molecular Sciences. 2024; 25(17):9324. https://doi.org/10.3390/ijms25179324
Chicago/Turabian StyleDíaz-López, Everardo Josué, Sofía Sánchez-Iglesias, Ana I. Castro, Silvia Cobelo-Gómez, Teresa Prado-Moraña, David Araújo-Vilar, and Antia Fernandez-Pombo. 2024. "Lipodystrophic Laminopathies: From Dunnigan Disease to Progeroid Syndromes" International Journal of Molecular Sciences 25, no. 17: 9324. https://doi.org/10.3390/ijms25179324
APA StyleDíaz-López, E. J., Sánchez-Iglesias, S., Castro, A. I., Cobelo-Gómez, S., Prado-Moraña, T., Araújo-Vilar, D., & Fernandez-Pombo, A. (2024). Lipodystrophic Laminopathies: From Dunnigan Disease to Progeroid Syndromes. International Journal of Molecular Sciences, 25(17), 9324. https://doi.org/10.3390/ijms25179324









