Mining Candidate Genes for Leaf Angle in Brassica napus L. by Combining QTL Mapping and RNA Sequencing Analysis
Abstract
1. Introduction
2. Results
2.1. Phenotypic Performance of RIL Population for LA Trait
2.2. QTLs Mapping for LA Trait
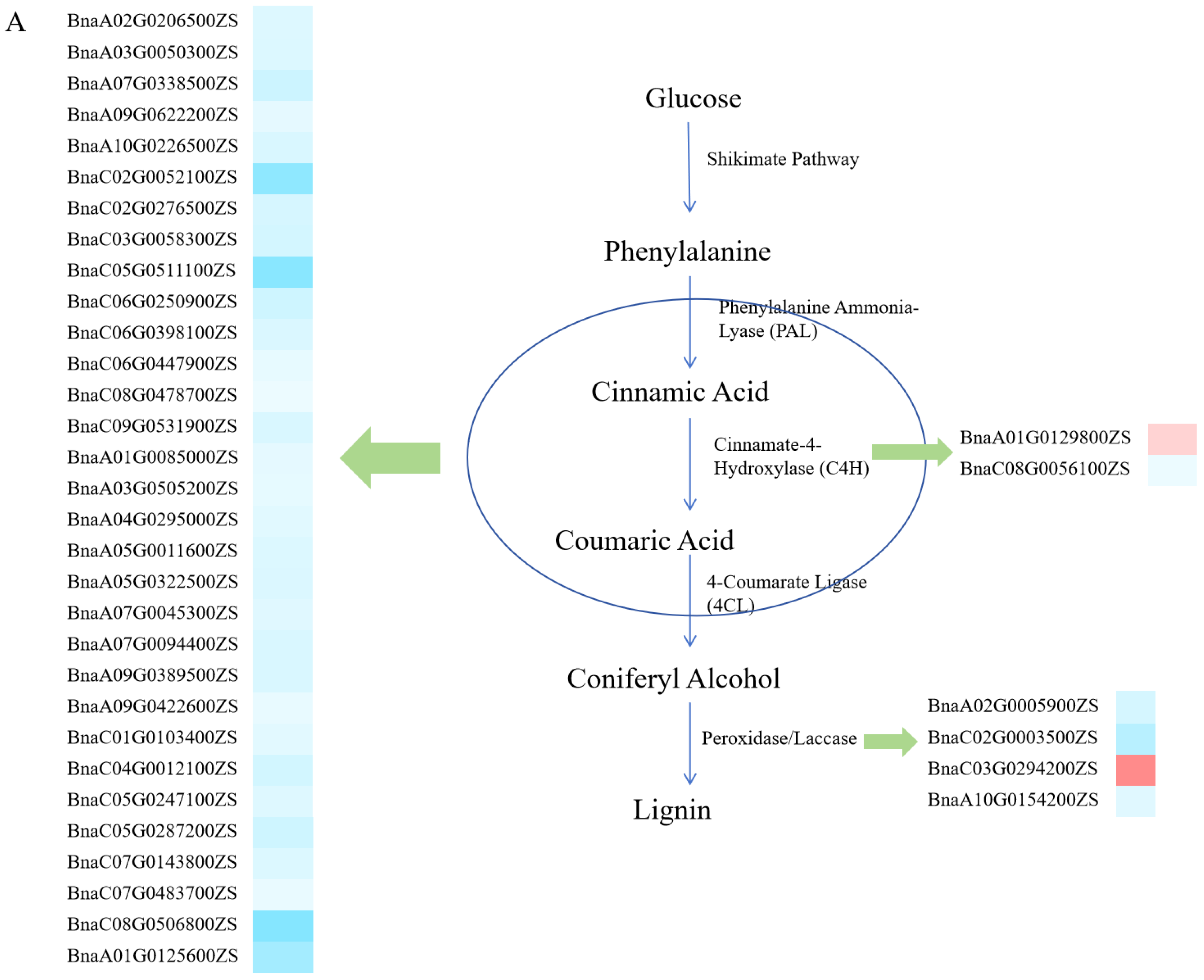
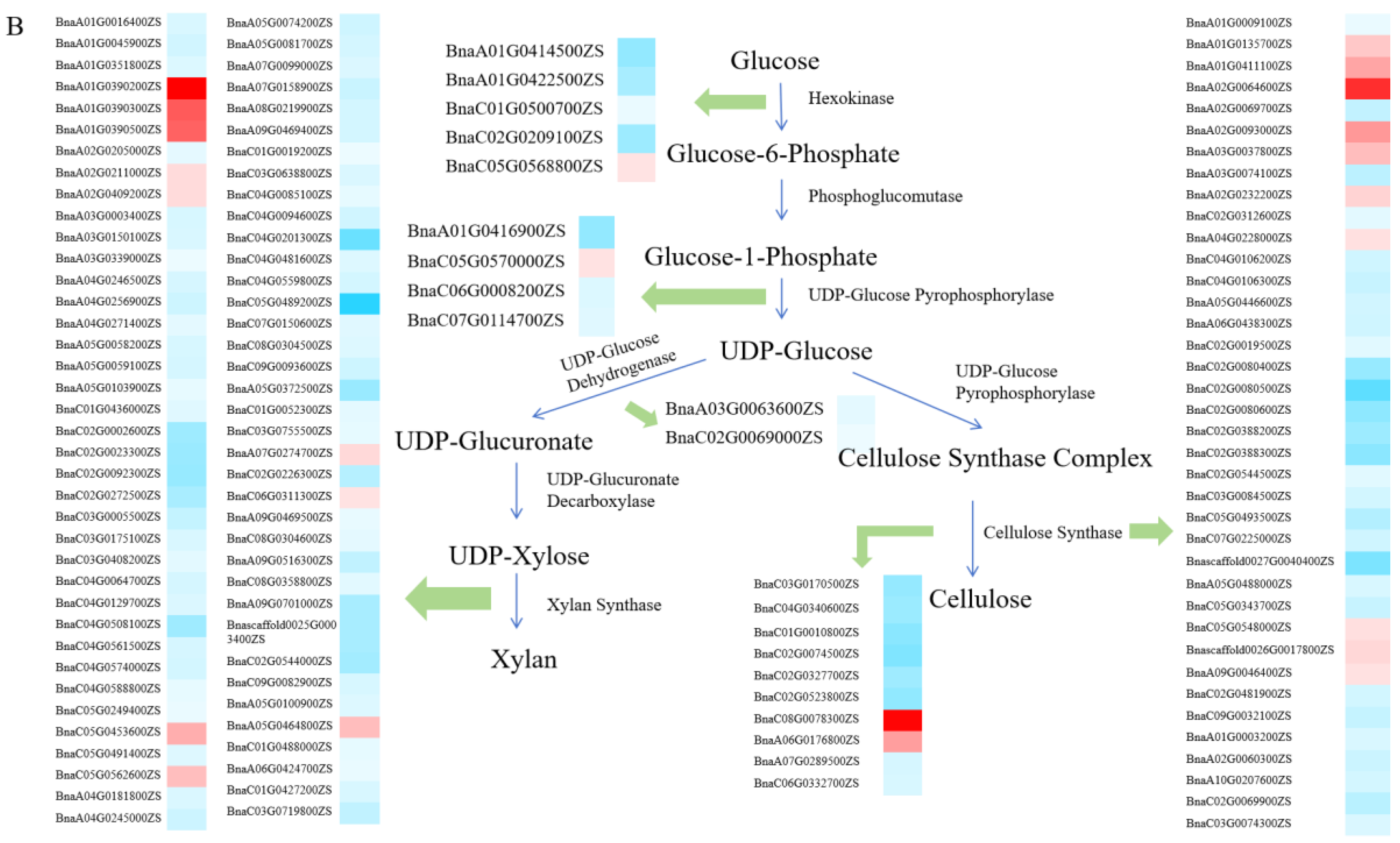
2.3. Transcriptome Analysis of LPA
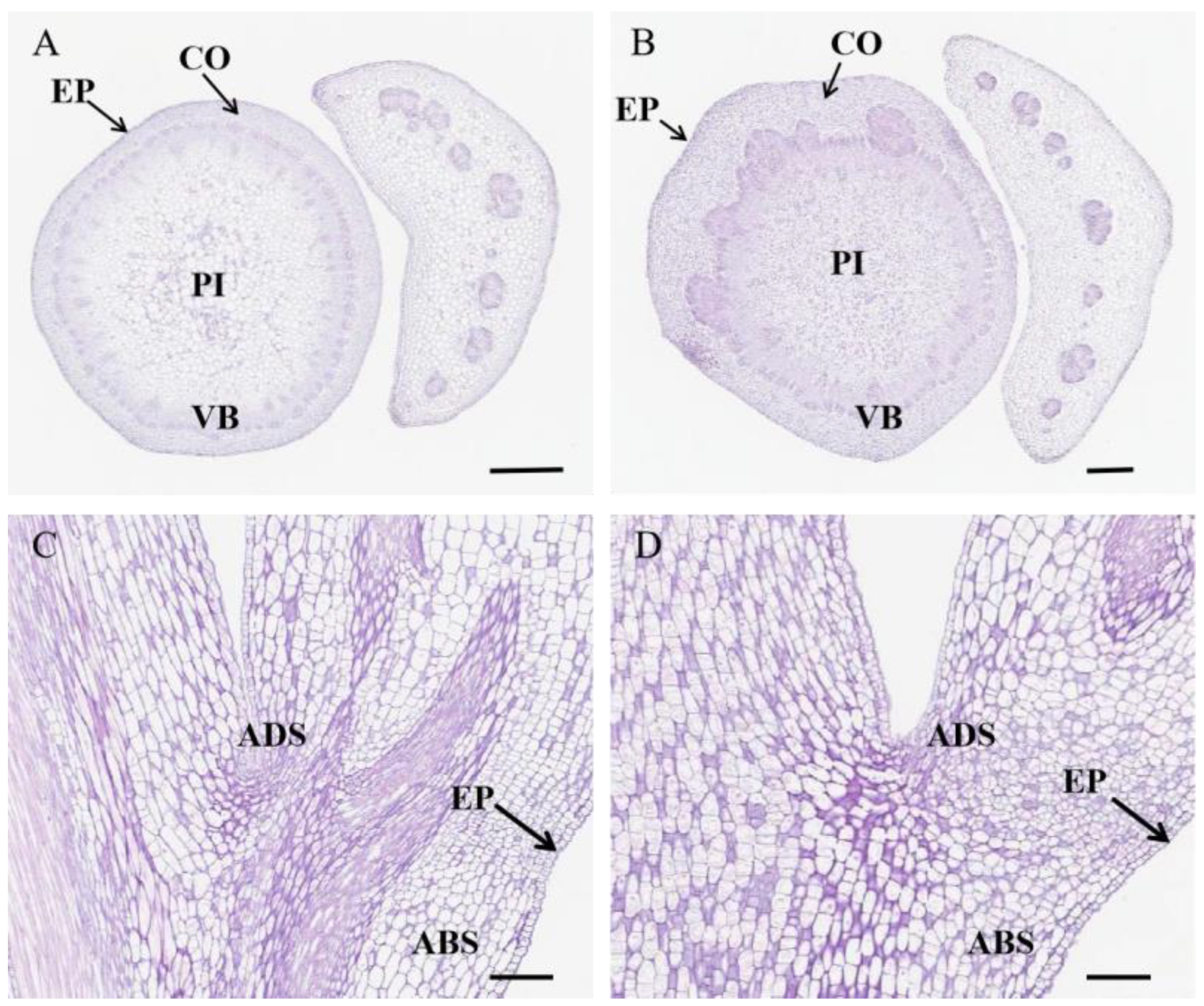
2.4. The Anatomical Structure of Stem
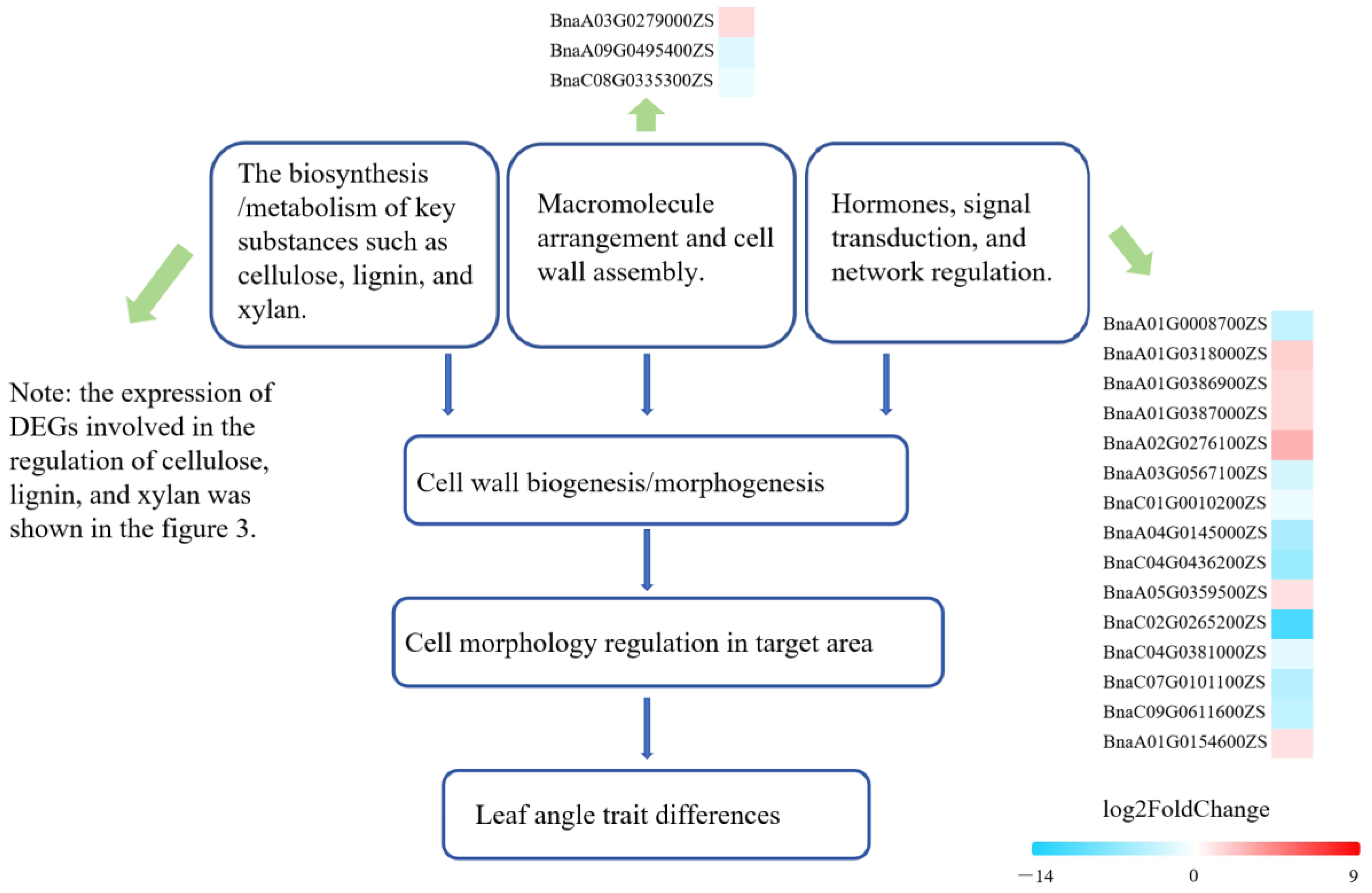
2.5. Combined Analysis of QTL and DEGs
3. Discussion
3.1. Novel Loci Identified for LA in Rapeseed
3.2. Characteristics of DEGs and Cytological Analysis
3.3. Potential Regulatory Model for LPA and Identification of Candidate Genes in Brassica napus L.
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. LPA and LIA Evaluation
4.3. QTL Mapping
4.4. Transcriptome Analysis
4.5. Anatomical Structure of Stem-Petiole Junction
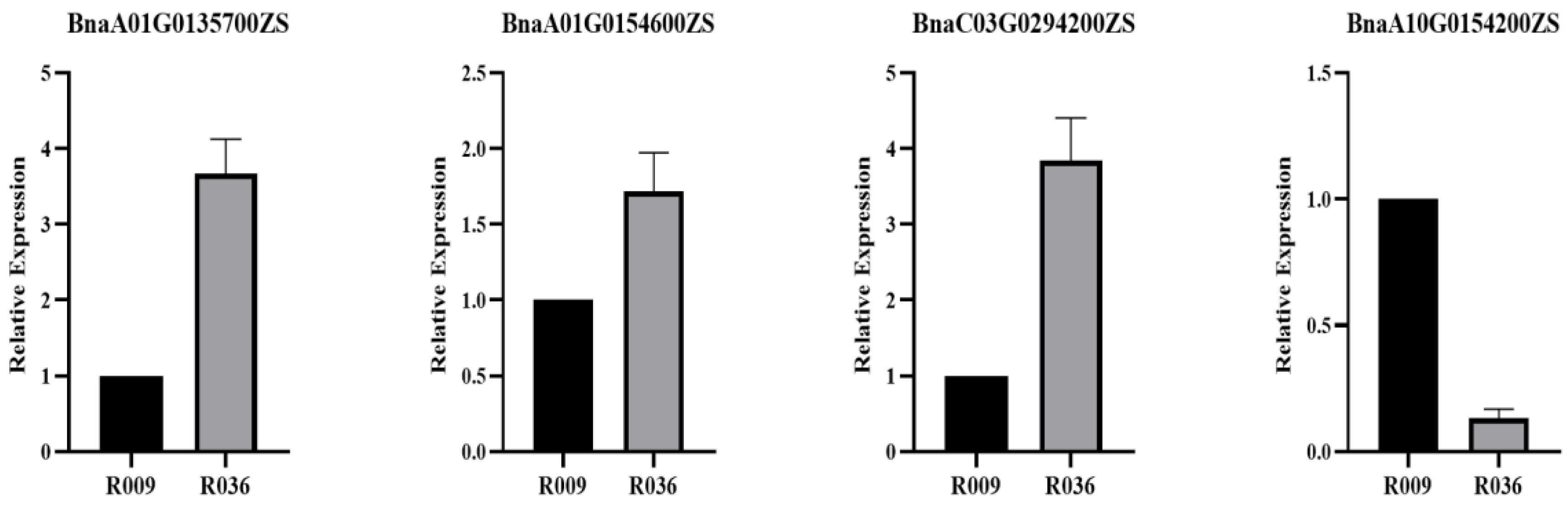
4.6. qPCR Analyses of the Candidate Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, X.; Zhang, J.; Zhang, Z.; Deng, A.; Zhang, W. Dense Planting with Less Basal Nitrogen Fertilization Might Benefit Rice Cropping for High Yield with Less Environmental Impacts. Eur. J. Agron. 2016, 75, 50–59. [Google Scholar] [CrossRef]
- Khan, S.; Anwar, S.; Kuai, J.; Ullah, S.; Fahad, S.; Zhou, G. Optimization of Nitrogen Rate and Planting Density for Improving Yield, Nitrogen Use Efficiency, and Lodging Resistance in Oilseed Rape. Front. Plant Sci. 2017, 8, 532. [Google Scholar] [CrossRef]
- Tian, J.; Wang, C.; Xia, J.; Wu, L.; Xu, G.; Wu, W.; Li, D.; Qin, W.; Han, X.; Chen, Q.; et al. Teosinte Ligule Allele Narrows Plant Architecture and Enhances High-Density Maize Yields. Science 2019, 365, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Li, Z.; Yao, X. Changes in Photosynthetic Traits and Their Responses to Increasing Fertilization Rates in Soybean (Glycine max (L.) Merr.) during Decades of Genetic Improvement. J. Sci. Food Agric. 2021, 101, 4715–4723. [Google Scholar] [CrossRef]
- Hou, S.; Ren, H.; Fan, F.; Zhao, M.; Zhou, W.; Zhou, B.; Li, C. The Effects of Plant Density and Nitrogen Fertilization on Maize Yield and Soil Microbial Communities in the Black Soil Region of Northeast China. Geoderma 2023, 430, 116325. [Google Scholar] [CrossRef]
- Jie, K.; Wang, J.; Zuo, Q.; Chen, H.; Gao, J.; WANG, B.; Zhou, J.; Fu, T. Effects and Mechanism of Higher Plant Density on Directly-Sown Rapeseed in the Yangtze River Basin of China. Sci. Agric. Sin. 2018, 51, 4625–4632. [Google Scholar] [CrossRef]
- Bai, G.; Liu, K.; Tan, Y.; Yi, Y.; Yu, H.; Wang, H. Effect of Agronomic Traits on Seed Yield in High-Yielding Rapeseed Populations. Crops 2015, 6, 33–38. [Google Scholar] [CrossRef]
- Cao, Y.; Zhong, Z.; Wang, H.; Shen, R. Leaf Angle: A Target of Genetic Improvement in Cereal Crops Tailored for High-Density Planting. Plant Biotechnol. J. 2022, 20, 426–436. [Google Scholar] [CrossRef]
- Li, X.; Wu, P.; Lu, Y.; Guo, S.; Zhong, Z.; Shen, R.; Xie, Q. Synergistic Interaction of Phytohormones in Determining Leaf Angle in Crops. Int. J. Mol. Sci. 2020, 21, 5052. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, L.; Ke, M.; Gao, Z.; Tu, T.; Huang, L.; Chen, J.; Guan, Y.; Huang, X.; Chen, X. GmPIN1-Mediated Auxin Asymmetry Regulates Leaf Petiole Angle and Plant Architecture in Soybean. J. Integr. Plant Biol. 2022, 64, 1325–1338. [Google Scholar] [CrossRef]
- ANTEN, N.P.R. Optimal Photosynthetic Characteristics of Individual Plants in Vegetation Stands and Implications for Species Coexistence. Ann. Bot. 2004, 95, 495–506. [Google Scholar] [CrossRef]
- Cao, Y.; Dou, D.; Zhang, D.; Zheng, Y.; Ren, Z.; Su, H.; Sun, C.; Hu, X.; Bao, M.; Zhu, B.; et al. ZmDWF1 Regulates Leaf Angle in Maize. Plant Sci. 2022, 325, 111459. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, X.; Zhang, Q.; Zhang, X.; Liu, X.; Jiang, J. Major Quantitative Trait Locus qLA3.1 Is Related to Tomato Leaf Angle by Regulating Cell Length at the Petiole Base. TAG Theor. Appl. Genet. 2024, 137, 145. [Google Scholar] [CrossRef]
- Song, Y.; Niu, R.; Yu, H.; Guo, J.; Du, C.; Zhang, Z.; Wei, Y.; Li, J.; Zhang, S. OsSLA1 Functions in Leaf Angle Regulation by Enhancing the Interaction between OsBRI1 and OsBAK1 in Rice. Plant J. 2022, 110, 1111–1127. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.M.; Park, S.J.; Huang, J.; Lee, E.J.; Xuan, Y.H.; Je, B.I.; Kumar, V.; Priatama, R.A.; Raj K, V.; Kim, S.H.; et al. Loose Plant Architecture1 (LPA1) Determines Lamina Joint Bending by Suppressing Auxin Signalling That Interacts with C-22-Hydroxylated and 6-Deoxo Brassinosteroids in Rice. J. Exp. Bot. 2016, 67, 1883–1895. [Google Scholar] [CrossRef]
- Tanaka, A.; Nakagawa, H.; Tomita, C.; Shimatani, Z.; Ohtake, M.; Nomura, T.; Jiang, C.-J.; Dubouzet, J.G.; Kikuchi, S.; Sekimoto, H.; et al. BRASSINOSTEROID UPREGULATED1, Encoding a Helix-Loop-Helix Protein, Is a Novel Gene Involved in Brassinosteroid Signaling and Controls Bending of the Lamina Joint in Rice. Plant Physiol. 2009, 151, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-Y.; Bai, M.-Y.; Wu, J.; Zhu, J.-Y.; Wang, H.; Zhang, Z.; Wang, W.; Sun, Y.; Zhao, J.; Sun, X.; et al. Antagonistic HLH/bHLH Transcription Factors Mediate Brassinosteroid Regulation of Cell Elongation and Plant Development in Rice and Arabidopsis. Plant Cell 2009, 21, 3767–3780. [Google Scholar] [CrossRef]
- Jang, S.; An, G.; Li, H.-Y. Rice Leaf Angle and Grain Size Are Affected by the OsBUL1 Transcriptional Activator Complex. Plant Physiol. 2017, 173, 688–702. [Google Scholar] [CrossRef]
- Huang, P.; Zhao, J.; Hong, J.; Zhu, B.; Xia, S.; Zhu, E.; Han, P.; Zhang, K. Cytokinins Regulate Rice Lamina Joint Development and Leaf Angle. Plant Physiol. 2023, 191, 56–69. [Google Scholar] [CrossRef]
- Gao, J.; Yang, S.; Cheng, W.; Fu, Y.; Leng, J.; Yuan, X.; Jiang, N.; Ma, J.; Feng, X. GmILPA1, Encoding an APC8-like Protein, Controls Leaf Petiole Angle in Soybean. Plant Physiol. 2017, 174, 1167–1176. [Google Scholar] [CrossRef]
- Jin, Y.; Li, J.; Zhu, Q.; Du, X.; Liu, F.; Li, Y.; Ahmar, S.; Zhang, X.; Sun, J.; Xue, F. GhAPC8 Regulates Leaf Blade Angle by Modulating Multiple Hormones in Cotton (Gossypium hirsutum L.). Int. J. Biol. Macromol. 2022, 195, 217–228. [Google Scholar] [CrossRef] [PubMed]
- An, G.; Qi, Y.; Zhang, W.; Gao, H.; Qian, J.; Larkin, R.M.; Chen, J.; Kuang, H. LsNRL4 Enhances Photosynthesis and Decreases Leaf Angles in Lettuce. Plant Biotechnol. J. 2022, 20, 1956–1967. [Google Scholar] [CrossRef]
- Tian, J.; Wang, C.; Chen, F.; Qin, W.; Yang, H.; Zhao, S.; Xia, J.; Du, X.; Zhu, Y.; Wu, L.; et al. Maize Smart-Canopy Architecture Enhances Yield at High Densities. Nature 2024, 632, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhu, Y.; Varshney, R.K.; Zhan, J.; Zheng, X.; Shi, J.; Wang, X.; Liu, G.; Wang, H. A Systematic Dissection of the Mechanisms Underlying the Natural Variation of Silique Number in Rapeseed (Brassica napus L.) Germplasm. Plant Biotechnol. J. 2020, 18, 568–580. [Google Scholar] [CrossRef]
- Hu, J.; Chen, B.; Zhao, J.; Zhang, F.; Xie, T.; Xu, K.; Gao, G.; Yan, G.; Li, H.; Li, L.; et al. Genomic Selection and Genetic Architecture of Agronomic Traits during Modern Rapeseed Breeding. Nat. Genet. 2022, 54, 694–704. [Google Scholar] [CrossRef]
- Li, N.; Song, D.; Peng, W.; Zhan, J.; Shi, J.; Wang, X.; Liu, G.; Wang, H. Maternal Control of Seed Weight in Rapeseed (Brassica napus L.): The Causal Link between the Size of Pod (Mother, Source) and Seed (Offspring, Sink). Plant Biotechnol. J. 2019, 17, 736–749. [Google Scholar] [CrossRef]
- Xu, P.; Wu, X.; Muñoz-Amatriaín, M.; Wang, B.; Wu, X.; Hu, Y.; Huynh, B.-L.; Close, T.J.; Roberts, P.A.; Zhou, W.; et al. Genomic Regions, Cellular Components and Gene Regulatory Basis Underlying Pod Length Variations in Cowpea (V. unguiculata L. Walp). Plant Biotechnol. J. 2017, 15, 547–557. [Google Scholar] [CrossRef]
- Carpita, N.; Tierney, M.; Campbell, M. Molecular Biology of the Plant Cell Wall: Searching for the Genes That Define Structure, Architecture and Dynamics. In Plant Cell Walls; Springer: Dordrecht, The Netherlands, 2001; Volume 47, pp. 1–5. [Google Scholar]
- Nakano, Y.; Yamaguchi, M.; Endo, H.; Rejab, N.A.; Ohtani, M. NAC-MYB-Based Transcriptional Regulation of Secondary Cell Wall Biosynthesis in Land Plants. Front. Plant Sci. 2015, 6, 288. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Yoon, J.; Kim, H.; Lee, S.-J.; Kim, T.; Kang, K.; Paek, N.-C. OsMYB7 Determines Leaf Angle at the Late Developmental Stage of Lamina Joints in Rice. Front. Plant Sci. 2023, 14, 1167202. [Google Scholar] [CrossRef]
- Yuan, Y.; Teng, Q.; Zhong, R.; Ye, Z.-H. TBL3 and TBL31, Two Arabidopsis DUF231 Domain Proteins, Are Required for 3-O-Monoacetylation of Xylan. Plant Cell Physiol. 2016, 57, 35–45. [Google Scholar] [CrossRef]
- Zeng, W.; Lampugnani, E.R.; Picard, K.L.; Song, L.; Wu, A.-M.; Farion, I.M.; Zhao, J.; Ford, K.; Doblin, M.S.; Bacic, A. Asparagus IRX9, IRX10, and IRX14A Are Components of an Active Xylan Backbone Synthase Complex That Forms in the Golgi Apparatus. Plant Physiol. 2016, 171, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhang, R.; Tao, Z.; Li, X.; Li, Y.; Huang, J.; Li, X.; Han, X.; Feng, S.; Zhang, G.; et al. Cellulose Synthase Mutants Distinctively Affect Cell Growth and Cell Wall Integrity for Plant Biomass Production in Arabidopsis. Plant Cell Physiol. 2018, 59, 1144–1157. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, A.; Zhu, C.; Chen, W.; Dixit, R. FRA1 Kinesin Modulates the Lateral Stability of Cortical Microtubules through Cellulose Synthase-Microtubule Uncoupling Proteins. Plant Cell 2020, 32, 2508–2524. [Google Scholar] [CrossRef] [PubMed]
- Delmer, D.; Dixon, R.A.; Keegstra, K.; Mohnen, D. The Plant Cell Wall-Dynamic, Strong, and Adaptable-Is a Natural Shapeshifter. Plant Cell 2024, 36, 1257–1311. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Structure and Growth of Plant Cell Walls. Nat. Rev. Mol. Cell Biol. 2024, 25, 340–358. [Google Scholar] [CrossRef]
- Sun, S.; Chen, D.; Li, X.; Qiao, S.; Shi, C.; Li, C.; Shen, H.; Wang, X. Brassinosteroid Signaling Regulates Leaf Erectness in Oryza Sativa via the Control of a Specific U-Type Cyclin and Cell Proliferation. Dev. Cell 2015, 34, 220–228. [Google Scholar] [CrossRef]
- Pedreira, J.M.V.; Herrera, M.T.; Zarra, I.; Revilla, G. The Overexpression of AtPrx37, an Apoplastic Peroxidase, Reduces Growth in Arabidopsis. Physiol. Plant. 2011, 141, 177–187. [Google Scholar] [CrossRef]
- Berthet, S.; Demont-Caulet, N.; Pollet, B.; Bidzinski, P.; Cézard, L.; Le Bris, P.; Borrega, N.; Hervé, J.; Blondet, E.; Balzergue, S.; et al. Disruption of LACCASE4 and 17 Results in Tissue-Specific Alterations to Lignification of Arabidopsis thaliana Stems. Plant Cell 2011, 23, 1124–1137. [Google Scholar] [CrossRef]
- Zhao, Q.; Nakashima, J.; Chen, F.; Yin, Y.; Fu, C.; Yun, J.; Shao, H.; Wang, X.; Wang, Z.-Y.; Dixon, R.A. LACCASE Is Necessary and Nonredundant with PEROXIDASE for Lignin Polymerization during Vascular Development in Arabidopsis. Plant Cell 2013, 25, 3976–3987. [Google Scholar] [CrossRef]
- Geng, P.; Zhang, S.; Liu, J.; Zhao, C.; Wu, J.; Cao, Y.; Fu, C.; Han, X.; He, H.; Zhao, Q. MYB20, MYB42, MYB43, and MYB85 Regulate Phenylalanine and Lignin Biosynthesis during Secondary Cell Wall Formation. Plant Physiol. 2020, 182, 1272–1283. [Google Scholar] [CrossRef]
- Xiao, R.; Zhang, C.; Guo, X.; Li, H.; Lu, H. MYB Transcription Factors and Its Regulation in Secondary Cell Wall Formation and Lignin Biosynthesis during Xylem Development. Int. J. Mol. Sci. 2021, 22, 3560. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Zhang, W.; Xu, W.; Li, S.; Chen, X.; Chen, H. Genome-Wide Bioinformatics Analysis of Cellulose Synthase Gene Family in Common Bean (Phaseolus vulgaris L.) and the Expression in the Pod Development. BMC Genom. Data 2022, 23, 9. [Google Scholar] [CrossRef]
- Cho, M.; Cho, H.-T. The Function of ABCB Transporters in Auxin Transport. Plant Signal. Behav. 2013, 8, e22990. [Google Scholar] [CrossRef] [PubMed]
- Majda, M.; Robert, S. The Role of Auxin in Cell Wall Expansion. Int. J. Mol. Sci. 2018, 19, 951. [Google Scholar] [CrossRef] [PubMed]
- Jenness, M.K.; Tayengwa, R.; Murphy, A.S. An ATP-Binding Cassette Transporter, ABCB19, Regulates Leaf Position and Morphology during Phototropin1-Mediated Blue Light Responses. Plant Physiol. 2020, 184, 1601–1612. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Song, M.; Zhang, M.; Zha, G.; Yin, J.; Cheng, C.; Chen, J.; Lou, Q. A Mutation in CsABCB19 Encoding an ATP-Binding Cassette Auxin Transporter Leads to Erect and Compact Leaf Architecture in Cucumber (Cucumis sativus L.). Plant Sci. 2023, 329, 111625. [Google Scholar] [CrossRef]
- Chen, J.; Wang, B.; Zhang, Y.; Yue, X.; Li, Z.; Liu, K. High-Density ddRAD Linkage and Yield-Related QTL Mapping Delimits a Chromosomal Region Responsible for Oil Content in Rapeseed (Brassica napus L.). Breed. Sci. 2017, 67, 296–306. [Google Scholar] [CrossRef]
- Chen, X.; Tong, C.; Zhang, X.; Song, A.; Hu, M.; Dong, W.; Chen, F.; Wang, Y.; Tu, J.; Liu, S.; et al. A High-quality Brassica napus Genome Reveals Expansion of Transposable Elements, Subgenome Evolution and Disease Resistance. Plant Biotechnol. J. 2020, 19, 615–630. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A Fast Spliced Aligner with Low Memory Requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
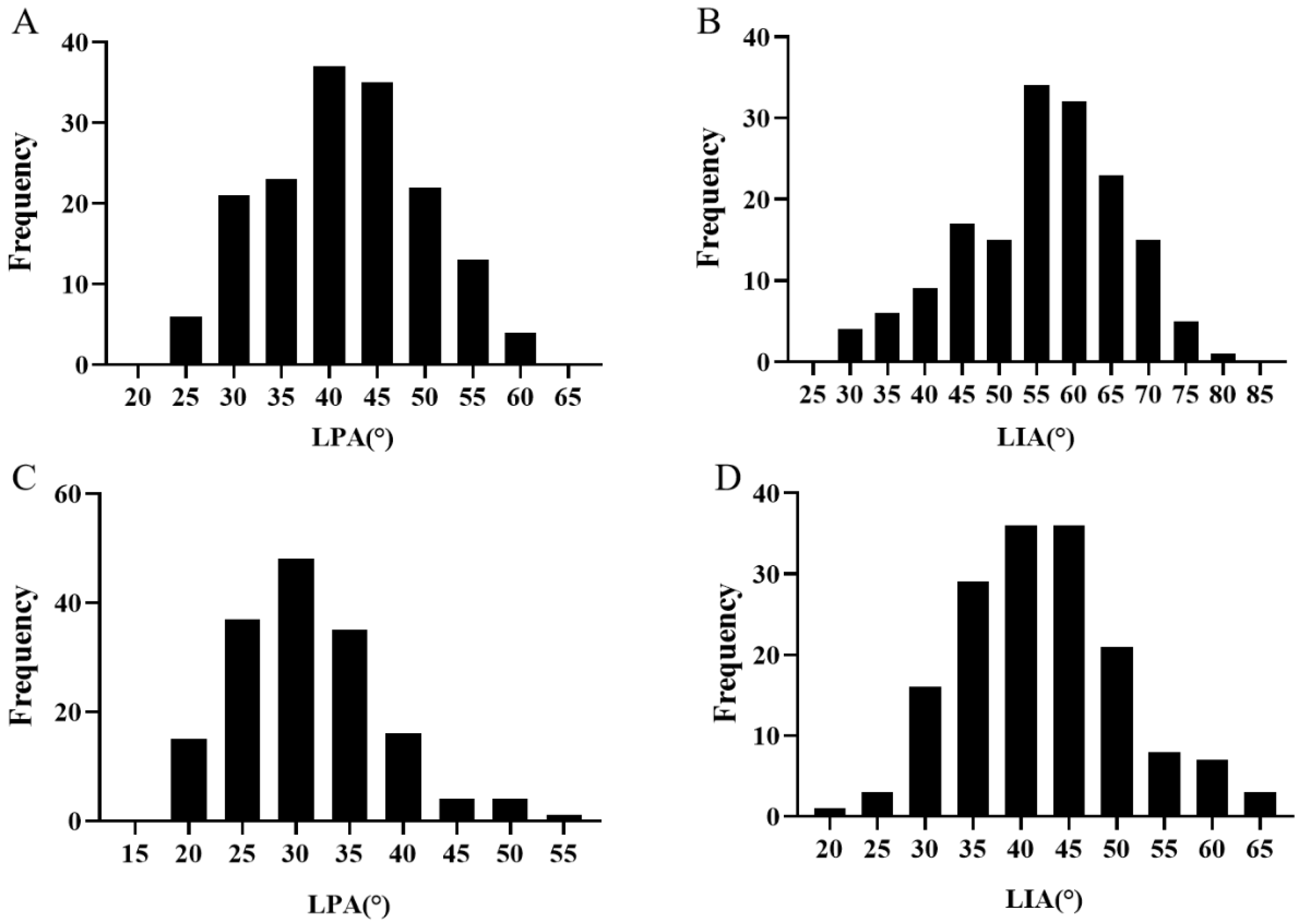

| Environment | Materials | LPA | LIA | |
|---|---|---|---|---|
| Gansu | Parents | M201 | 29.75° ± 0.095 | 50.30° ± 1.20 |
| M352 | 54.89° ± 1.36 | 68.07° ± 0.90 | ||
| Pt-test | 0.016 * | 0.018 * | ||
| RIL | Min | 22.73° | 29.68° | |
| Max | 61.69° | 81.04° | ||
| Mean ± SD | 41.61° ± 8.26 | 55.79° ± 10.43 | ||
| CV | 19.85% | 18.70% | ||
| Nanchang | Parents | M201 | 25.75° ± 0.49 | 34.70° ± 0.22 |
| M352 | 34.76° ± 0.52 | 45.39° ± 1.20 | ||
| Pt-test | 0.036 * | 0.020 * | ||
| RIL | Min | 18.44° | 21.31° | |
| Max | 52.82° | 64.07° | ||
| Mean ± SD | 31.16° ± 6.98 | 42.31° ± 8.52 | ||
| CV | 22.40% | 20.14% |
| Trait | QTL | Chromosome | Confidence Interval | LOD | PVE (%) | Add (%) | Environment | Physical Location |
|---|---|---|---|---|---|---|---|---|
| LPA | qLPAA01 | A01 | 52.5–66.5 | 2.5872 | 7.7860 | −2.5216 | Gansu | 6,239,139–9,453,887 |
| qLPAA10.a | A10 | 39.5–40.5 | 2.8796 | 6.7048 | 2.3380 | Gansu | 18,971,020–19,039,409 | |
| qLPAA10.b | A10 | 28.5–33.5 | 2.6689 | 0.4409 | −1.8276 | Nanchang | 18,091,839–18,522,230 | |
| qLPAC03 | C03 | 107.5–108.5 | 37.4487 | 11.2860 | 9.2192 | Nanchang | 18,838,385–19,430,815 | |
| LIA | qLIAA06 | A06 | 17.5–19.5 | 3.1420 | 0.9141 | 2.3155 | Nanchang | 3,105,210–3,152,990 |
| qLIAC03.a | C03 | 107.5–108.5 | 34.1515 | 17.4869 | 10.0803 | Nanchang | 18,838,385–19,430,815 | |
| qLIAC03.b | C03 | 144.5–150.5 | 2.8696 | 0.8706 | −2.2593 | Nanchang | 7,166,121–8,185,264 |
| No. | Cell Diameter (μm) | Cell Surface Area (μm2) |
|---|---|---|
| R009 | 19.13 ± 6.33 | 200.82 ± 114.40 |
| R036 | 11.79 ± 3.14 | 74.20 ± 38.68 |
| p-value | 4.01 × 10−5 | 3.49 × 10−5 |
| QTL | Rapeseed Gene | Arabidopsis Gene | Gene Function |
|---|---|---|---|
| qLPAA01 | BnaA01G0125600ZS | AT4G22680 | Encodes a putative transcription factor |
| BnaA01G0135700ZS | AT4G24000 | Cellulose synthase-like protein G2 | |
| BnaA01G0154600ZS | AT4G25960 | P-glycoprotein 2 | |
| qLPAA10.a | BnaA10G0154200ZS | AT5G60020 | Laccase-17 |
| qLPAC03 | BnaC03G0294200ZS | AT4G08770 | peroxidase Prx37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, A.; Li, S.; Wang, Y.; Cheng, F.; Chen, J.; Zheng, X.; Xiong, J.; Ding, G.; Zhang, B.; Zhai, W.; et al. Mining Candidate Genes for Leaf Angle in Brassica napus L. by Combining QTL Mapping and RNA Sequencing Analysis. Int. J. Mol. Sci. 2024, 25, 9325. https://doi.org/10.3390/ijms25179325
Peng A, Li S, Wang Y, Cheng F, Chen J, Zheng X, Xiong J, Ding G, Zhang B, Zhai W, et al. Mining Candidate Genes for Leaf Angle in Brassica napus L. by Combining QTL Mapping and RNA Sequencing Analysis. International Journal of Molecular Sciences. 2024; 25(17):9325. https://doi.org/10.3390/ijms25179325
Chicago/Turabian StylePeng, Aoyi, Shuyu Li, Yuwen Wang, Fengjie Cheng, Jun Chen, Xiaoxiao Zheng, Jie Xiong, Ge Ding, Bingchao Zhang, Wen Zhai, and et al. 2024. "Mining Candidate Genes for Leaf Angle in Brassica napus L. by Combining QTL Mapping and RNA Sequencing Analysis" International Journal of Molecular Sciences 25, no. 17: 9325. https://doi.org/10.3390/ijms25179325
APA StylePeng, A., Li, S., Wang, Y., Cheng, F., Chen, J., Zheng, X., Xiong, J., Ding, G., Zhang, B., Zhai, W., Song, L., Wei, W., & Chen, L. (2024). Mining Candidate Genes for Leaf Angle in Brassica napus L. by Combining QTL Mapping and RNA Sequencing Analysis. International Journal of Molecular Sciences, 25(17), 9325. https://doi.org/10.3390/ijms25179325





