Insights into Iron Metabolism Parameters in Ischemic Stroke: A Single-Center Prospective Cohort Study
Abstract
1. Introduction
2. Results
2.1. Patient-Specific Data
2.2. Iron Metabolism Parameters in Relation to Time Course and Treatment
2.3. Analysis of Iron Metabolism Parameters Depending on Selected Clinimetric Scales
2.3.1. Stroke Severity (NIHSS)
2.3.2. Stroke Etiology (TOAST)
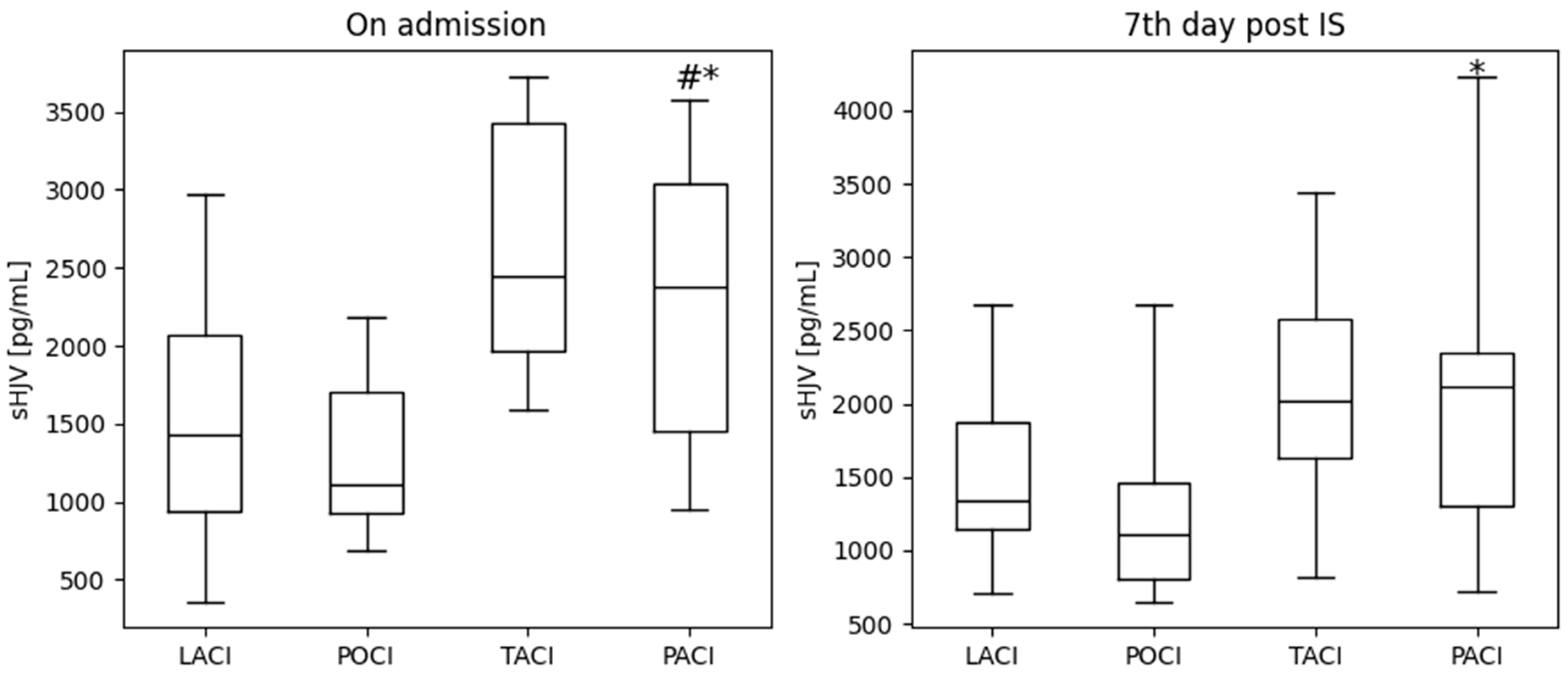
2.3.3. Stroke Location (OCSP)
3. Discussion
4. Material and Methods
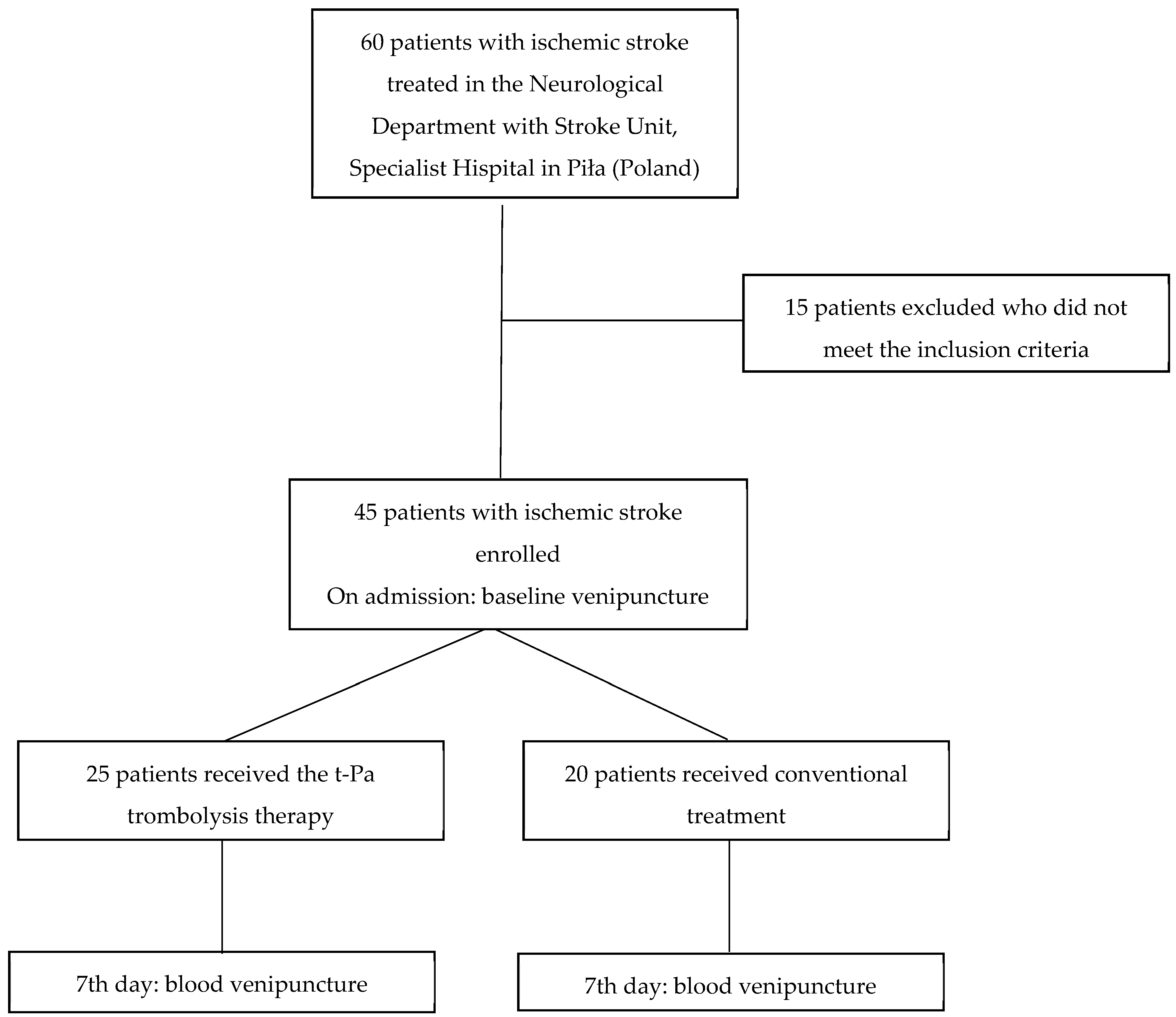
4.1. Study Population
4.2. Blood Samples and Laboratory Tests
4.3. Iron Metabolism Parameters
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saini, V.; Guada, L.; Yavagal, D.R. Global Epidemiology of Stroke and Access to Acute Ischemic Stroke Interventions. Neurology 2020, 97, S6–S16. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Feske, S. Ischemic Stroke. Am. J. Med. 2021, 134, 1457–1464. [Google Scholar] [CrossRef]
- Zi, W.; Qiu, Z.; Li, F.; Sang, H.; Wu, D.; Luo, W.; Liu, S.; Yuan, J.; Song, J.; Shi, Z.; et al. Effect of Endovascular Treatment Alone vs. Intravenous Alteplase Plus Endovascular Treatment on Functional Independence in Patients With Acute Ischemic Stroke: The DEVT Randomized Clinical Trial. JAMA 2021, 325, 234–243. [Google Scholar] [CrossRef] [PubMed]
- García-Yébenes, I.; García-Culebras, A.; Peña-Martínez, C.; Fernández-López, D.; Díaz-Guzmán, J.; Negredo, P.; Avendaño, C.; Castellanos, M.; Gasull, T.; Dávalos, A.; et al. Iron Overload Exacerbates the Risk of Hemorrhagic Transformation After tPA (Tissue-Type Plasminogen Activator) Administration in Thromboembolic Stroke Mice. Stroke 2018, 49, 2163–2172. [Google Scholar] [CrossRef]
- Wan, J.; Ren, H.; Wang, J. Iron toxicity, lipid peroxidation and ferroptosis after intracerebral haemorrhage. Stroke Vasc. Neurol. 2019, 4, 93–95. [Google Scholar] [CrossRef]
- Wei, Y.; Song, X.; Gao, Y.; Gao, Y.; Li, Y.; Gu, L. Iron toxicity in intracerebral hemorrhage: Physiopathological and therapeutic implications. Brain Res. Bull. 2022, 178, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Pang, X.; Yeo, A.J.; Xie, S.; Xiang, M.; Shi, B.; Yu, G.; Li, C. The Molecular Mechanisms of Ferroptosis and Its Role in Blood-Brain Barrier Dysfunction. Front. Cell Neurosci. 2022, 16, 889765. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lu, X.; Tai, B.; Li, W.; Li, T. eFerroptosis and Its Multifaceted Roles in Cerebral Stroke. Front. Cell Neurosci. 2021, 15, 615372. [Google Scholar] [CrossRef]
- Wang, P.; Ren, Q.; Shi, M.; Bai, H.; Liu, Y.; Chang, Y.-Z. Overexpression of Mitochondrial Ferritin Enhances Blood-Brain Barrier Integrity Following Ischemic Stroke in Mice by Maintaining Iron Homeostasis in Endothelial Cells. Antioxidants 2022, 11, 1257. [Google Scholar] [CrossRef]
- Vela, D. Hepcidin, an emerging and important player in brain iron homeostasis. J. Transl. Med. 2018, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- DeGregorio-Rocasolano, N.; Martí-Sistac, O.; Gasull, T. Deciphering the Iron Side of Stroke: Neurodegeneration at the Crossroads Between Iron Dyshomeostasis, Excitotoxicity, and Ferroptosis. Front. Neurosci. 2019, 13, 85. [Google Scholar] [CrossRef]
- Guo, J.; Tuo, Q.-Z.; Lei, P. Iron, ferroptosis, and ischemic stroke. J. Neurochem. 2023, 165, 487–520. [Google Scholar] [CrossRef]
- Lattanzi, S.; Norata, D.; Broggi, S.; Meletti, S.; Świtońsk, M.; Słomka, A.; Silvestrini, M. Neutrophil-to-Lymphocyte Ratio Predicts Early Neurological Deterioration after Endovascular Treatment in Patients with Ischemic Stroke. Life 2022, 12, 1415. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Guo, L.; Gao, W.; Tang, T.L.; Yan, M. Interaction between macrophages and ferroptosis. Cell Death Dis. 2022, 13, 355. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, R.C.; Kosman, D.J. Glial cell ceruloplasmin and hepcidin differentially regulate iron efflux from brain microvascular endothelial cells. PLoS ONE 2014, 9, e89003. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Yu, P.P.; Dong, T.; Guo, W.; Chang, S.; Zheng, B.; Ci, Y.; Wang, F.; Yu, P.; Gao, G.; et al. Astrocyte-derived hepcidin controls iron traffic at the blood-brain-barrier via regulating ferroportin 1 of microvascular endothelial cells. Cell Death Dis. 2022, 13, 667. [Google Scholar] [CrossRef]
- Muckenthaler, M.U.; Rivella, S.; Hentze, M.W.; Galy, B. A Red Carpet for Iron Metabolism. Cell 2017, 168, 344–361. [Google Scholar] [CrossRef]
- Zhang, A.-S. Control of systemic iron homeostasis by the hemojuvelin-hepcidin axis. Adv. Nutr. 2010, 1, 38–45. [Google Scholar] [CrossRef]
- Zhang, A.S.; Gao, J.; Koeberl, D.D.; Enns, C.A. The role of hepatocyte hemojuvelin in the regulation of bone morphogenic protein-6 and hepcidin expression in vivo. J. Biol. Chem. 2010, 285, 16416–16423. [Google Scholar] [CrossRef]
- Young, G.-H.; Tang, S.-C.; Wu, V.-C.; Wang, K.-C.; Nong, J.-Y.; Huang, P.-Y.; Hu, C.-J.; Chiou, H.-Y.; Jeng, J.-S.; Hsu, C.Y. The functional role of hemojuvelin in acute ischemic stroke. J. Cereb. Blood Flow. Metab. 2020, 40, 1316–1327. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Derdeyn, C.P.; Biller, J.; Coffey, C.S.; Hoh, B.L.; Jauch, E.C.; Johnston, K.C.; Johnston, S.C.; Khalessi, A.A.; Kidwell, C.S.; et al. American Heart Association Stroke Council. 2015 American Heart Association/American Stroke Association Focused Update of the 2013 Guidelines for the Early Management of Patients With Acute Ischemic Stroke Regarding Endovascular Treatment. Stroke 2015, 46, 3020–3035. [Google Scholar] [CrossRef] [PubMed]
- Gill, D.; Monori, G.; Tzoulaki, I.; Dehghan, A. Iron Status and Risk of Stroke. Stroke 2018, 49, 2815–2821. [Google Scholar] [CrossRef] [PubMed]
- Reggiani, M.; Società Inter-Regionale Piemonte e Valle d’Aosta per le Cerebrovasculopatie Group. Five-year survival after first-ever ischaemic stroke is worse in total anterior circulation infarcts: The SINPAC cohort. Cerebrovasc. Dis. 2009, 27, 29–36. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, A.; Zhao, X.; Liu, L.; Zheng, H.; Wang, Y.; Cao, Y.; Wang, Y. The Oxfordshire Community Stroke Project classification system predicts clinical outcomes following intravenous thrombolysis: A prospective cohort study. Ther. Clin. Risk Manag. 2016, 12, 1049–1056. [Google Scholar] [CrossRef][Green Version]
- Fredriksson, L.; Lawrence, D.A.; Medcalf, R.L. tPA Modulation of the Blood-Brain Barrier: A Unifying Explanation for the Pleiotropic Effects of tPA in the CNS. Semin. Thromb. Hemost. 2017, 43, 154–168. [Google Scholar]
- Krause, A.; Neitz, S.; Mägert, H.J.; Schulz, A.; Forssmann, W.G.; Schulz-Knappe, P.; Adermann, K. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000, 480, 147–150. [Google Scholar] [CrossRef]
- Zechel, S.; Huber-Wittmer, K.; von Bohlen und Halbach, O. Distribution of the iron-regulating protein hepcidin in the murine central nervous system. J. Neurosci. Res. 2006, 84, 790–800. [Google Scholar] [CrossRef]
- Raha-Chowdhury, R.; Raha, A.A.; Forostyak, S.; Zhao, J.-W.; Stott, S.R.W.; Bomford, A. Expression and cellular localization of hepcidin mRNA and protein in normal rat brain. BMC Neurosci. 2015, 16, 24. [Google Scholar] [CrossRef]
- Słomka, A.; Świtońska, M.; Żekanowska, E. Hepcidin levels are increased in patients with acute ischemic stroke: Preliminary report. J. Stroke Cerebrovasc. Dis. 2015, 24, 1570–1576. [Google Scholar] [CrossRef]
- Poli, M.; Asperti, M.; Ruzzenenti, P.; Mandelli, L.; Campostrini, N.; Martini, G.; Di Somma, M.; Maccarinelli, F.; Girelli, D.; Naggi, A.; et al. Oversulfated heparins with low anticoagulant activity are strong and fast inhibitors of hepcidin expression in vitro and in vivo. Biochem. Pharmacol. 2014, 92, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Poli, M.; Asperti, M.; Ruzzenenti, P.; Regoni, M.; Arosio, P. Hepcidin antagonists for potential treatments of disorders with hepcidin excess. Front. Pharmacol. 2014, 5, 86. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Wu, S.; Wong, T.M.; Chung, S.K.; Chung, S.S.M. Polyol pathway mediates iron—Induced oxidative injury in ischemic—Reperfused rat heart. Free Radic. Biol. Med. 2008, 45, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Seham, F.A.; Nagwa, E.A.; Mohamed, A.A. Serum hepcidin levels in childhood—Onset ischemic stroke: A case—Control study. Medicine 2016, 95, e2921. [Google Scholar]
| Variable | Total (n = 45) | Type of Treatment | p Value | ||
|---|---|---|---|---|---|
| Thrombolysis (n = 25) | Conventional (n = 20) | ||||
| Age, y | 69 (63–75) | 71 (62–79) | 68 (63–72) | 0.37 | |
| Male-sex, n (%) | 24 (53) | 12 (48) | 12 (60) | 0.42 | |
| BMI, kg/m2 | 27 (25–29) | 28 (25–30) | 27 (24–29) | 0.36 | |
| WHR | 1.09 (0.99–1.19) | 1.11 (1.01–1.19) | 1.08 (0.99–1.16) | 0.24 | |
| SBP, mmHg | 148 (130–160) | 151 (140–160) | 143 (130–160) | 0.34 | |
| DBP, mmHg | 80 (79–90) | 80 (80–90) | 83 (75–90) | 0.55 | |
| Clinical history n/total (%) | |||||
| CAD | 8/45 (18) | 5/25 (20) | 3/20 (15) | 0.66 | |
| AMI | 10/45 (22) | 7/25 (28) | 3/20 (15) | 0.42 | |
| AF | 12/45 (27) | 8/25 (32) | 4/20 (20) | 0.37 | |
| Hypertension | 32/45 (71) | 19/25 (76) | 13/20 (65) | 0.30 | |
| Diabetes mellitus | 12/45 (27) | 6/25 (24) | 6/20 (30) | 0.65 | |
| Dyslipidemia | 37/45 (82) | 22/25 (88) | 15/20 (75) | 0.26 | |
| Smokers | 12/45 (27) | 6/25 (24) | 6/20 (30) | 0.65 | |
| TOAST | CE | 13/45 (29) | 9/25 (36) | 4/20 (20) | 0.20 |
| LAA | 8/45 (18) | 3/25 (12) | 5/20 (25) | ||
| SVO | 7/45 (15) | 2/25 (8) | 5/20 (25) | ||
| SUE | 17/45 (38) | 11/25 (44) | 6/20 (30) | ||
| OCSP | LACI | 17/45 (38) | 9/25 (36) | 8/20 (40) | 0.17 |
| POCI | 8/45 (18) | 2/25 (8) | 6/20 (30) | ||
| TACI | 5/45 (11) | 4/25 (16) | 1/20 (5) | ||
| PACI | 15/45 (33) | 10 (40) | 5/20 (25) | ||
| Stroke scales | |||||
| NIHSS | 6 (4–11) | 7 (3–11) | 5.5 (4–11) | 0.44 | |
| mRS | 3 (2–4) | 3 (2–4) | 3 (2–4) | 0.50 | |
| BI | 80 (42.5–90) | 80 (40–85) | 80 (55–90) | 0.22 | |
| On Admission | 7th Day | p Value | ||
|---|---|---|---|---|
| Group | Median IQT | Median IQT | ||
| Ferritin, ng/mL | Whole study group | 171.40 101.50; 243.60 | 208.80 151.80; 282.10 | 0.03 |
| Thrombolysis | 173.70 99.16; 228.60 | 194.10 118,80; 264,10 | 0.02 | |
| Conventional treatment | 146.15 106.65; 251.20 | 215.95 170.00; 285.80 | 0.47 | |
| p value * | 0.98 | 0.86 | ||
| sTfR, µg/mL | Whole study group | 1.31 0.93; 1.75 | 1.27 1.04; 1.70 | 0.42 |
| Thrombolysis | 1.44 1.12; 1.88 | 1.43 1.11; 1.84 | 0.30 | |
| Conventional treatment | 1.21 0.70; 1.60 | 1.17 0.91; 1.48 | 0.91 | |
| p value * | 0.14 | 0.10 | ||
| Hepcidin, ng/mL | Whole study group | 47.16 29.33; 84.81 | 37.88 19.96; 72.09 | 0.04 |
| Thrombolysis | 58.43 29.66; 118.40 | 48.82 27.03; 66.12 | 0.08 | |
| Conventional treatment | 44.12 22.96; 64.88 | 30.76 8.33; 76.00 | 0.24 | |
| p value * | 0.24 | 0.32 | ||
| sHJV, pg/mL | Whole study group | 1862.00 1088.00; 2539.00 | 1609.00 1140.00; 2136.00 | 0.21 |
| Thrombolysis | 2468.00 1962.00; 2972.00 | 2067.00 1609.00; 2342.00 | 0.10 | |
| Conventional treatment | 1115.50 916.45; 1463.50 | 1175.50 794.30; 1383.50 | 0.97 | |
| p value * | <0.0001 | 0.0002 | ||
| NIHSS < 8 n = 27 | NIHSS 8–15 n = 13 | NIHSS ≥ 16 n = 5 | p Value | |
|---|---|---|---|---|
| Median IQT | Median IQT | Median IQT | ||
| Ferritin, ng/mL on admission | 195.40 110.60; 310.50 | 141.80 95.76; 191.70 | 204.10 120.00; 211.30 | 0.32 |
| Ferritin, ng/mL 7th day | 234.30 158.70; 345.30 | 169.60 132.00; 193.20 | 250.00 170.30; 414.90 | 0.07 |
| sTfR, µg/mL on admission | 1.24 0.82; 1.72 | 1.31 0.84; 1.92 | 1.50 1.44; 1.51 | 0.34 |
| sTfR, µg/mL 7th day | 1.27 1.11; 1.69 | 1.04 0.76; 1.61 | 1.84 1.58; 1.88 | 0.19 |
| Hepcidin, ng/mL on admission | 50.57 27.75; 79.25 | 42,86 29.66; 84.81 | 82.72 47.13; 141.30 | 0.75 |
| Hepcidin, ng/mL 7th day | 38.77 16.05; 89.13 | 30.63 25.51; 51.34 | 51.34 33.81; 67.45 | 0.68 |
| sHJV, pg/mL on admission | 1682.00 970.30; 2539.00 | 1861.00 1455.00; 2468.00 | 2238.00 1287.00; 2968.00 | 0.64 |
| sHJV, pg/mL 7th day | 1426.00 1059.00; 2067.00 | 1900.00 1140.00; 2327.00 | 1759.00 1314.00; 2122.00 | 0.86 |
| Stroke Subtype | |||||
|---|---|---|---|---|---|
| Undetermined Etiology n = 17 | Cardioembolism n = 13 | Small-Vessel Occlusion n = 7 | Large-Vessel Occlusion n = 8 | p Value | |
| Median IQT | Median IQT | Median IQT | Median IQT | ||
| Ferritin, ng/mL on admission | 171.40 101.50; 204.10 | 141.80 95.69; 228.60 | 211.20 95.76; 310.50 | 205.20 133.25; 258.95 | 0.82 |
| Ferritin, ng/mL 7th day | 208.80 169.60; 234.30 | 151.80 104.10; 345.30 | 192.30 132.00; 282.10 | 299.10 179.60; 382.50 | 0.54 |
| sTfR, µg/ml on admission | 1.51 1.15; 1.82 | 1.38 1.22; 2.02 | 0.93 0.72; 1.70 | 0.86 0.66; 1.38 | 0.02 * |
| sTfR, µg/mL 7th day | 1.32 0.98; 1.70 | 1.25 1.11; 2.13 | 1.27 0.76; 1.52 | 1.28 0.79; 1.63 | 0.69 |
| Hepcidin, ng/mL on admission | 50.57 29.37; 96.37 | 34.36 10.33; 67.35 | 47.16 32.12; 79.25 | 65.99 30.58; 129.85 | 0.54 |
| Hepcidin, ng/mL 7th day | 38.77 27.03; 67,45 | 30.63 19.96; 66.12 | 31.85 2.98; 50.74 | 70.33 29.44; 107.70 | 0.40 |
| sHJV, pg/mL on admission | 1962.00 1424.0; 2468.0 | 2449.00 1523.0; 2972.0 | 1398.00 883.1; 1682.1 | 1115.50 973.8; 2423.3 | 0.11 |
| sHJV, pg/mL 7th day | 1609.00 1258.0; 2277.0 | 1900.00 1303.0; 2375.0 | 1059.00 745.4; 1303.0 | 1532.92 1257.0; 1891.0 | 0.13 |
| LACI n = 17 | POCI n = 8 | TACI n = 5 | PACI n = 15 | p Value | |
|---|---|---|---|---|---|
| Median IQT | Median IQT | Median IQT | Median IQT | ||
| Ferritin, ng/mL on admission | 147.20 110.60; 282.60 | 192.45 153.00; 284.65 | 141.80 92.19; 206,30 | 171.40 69.43; 208.10 | 0.33 |
| Ferritin, ng/mL 7th day | 194.10 170.30; 368.70 | 259.95 198,60; 327,00 | 169.60 151.80; 350.10 | 193.20 107.00; 234.30 | 0.24 |
| sTfR, µg/mL on admission | 1.37 0.82; 1.70 | 0.98 0.68; 1.34 | 1.88 1.26; 3.03 | 1.40 1.15; 1.92 | 0.12 |
| sTfR, µg/mL 7th day | 1.26 0.89; 1.69 | 1.17 0.86; 1.32 | 1.71 1.62; 3.22 | 1.32 0.98; 1.74 | 0.09 |
| Hepcidin, ng/mL on admission | 45.25 27.75; 111.00 | 58.80 45.08; 66.00 | 13.32 10.33; 118.40 | 50.57 29.66; 84.81 | 0.91 |
| Hepcidin, ng/mL 7th day | 33.81 16.05; 61.54 | 63.04 22.49; 81.33 | 25.51 23.59; 48.82 | 37.88 27.03; 67.45 | 0.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boinska, J.; Słomka, A.; Sury, M.; Wiszniewska, M.; Pisarek, E.; Żekanowska, E. Insights into Iron Metabolism Parameters in Ischemic Stroke: A Single-Center Prospective Cohort Study. Int. J. Mol. Sci. 2024, 25, 9352. https://doi.org/10.3390/ijms25179352
Boinska J, Słomka A, Sury M, Wiszniewska M, Pisarek E, Żekanowska E. Insights into Iron Metabolism Parameters in Ischemic Stroke: A Single-Center Prospective Cohort Study. International Journal of Molecular Sciences. 2024; 25(17):9352. https://doi.org/10.3390/ijms25179352
Chicago/Turabian StyleBoinska, Joanna, Artur Słomka, Magdalena Sury, Małgorzata Wiszniewska, Ewa Pisarek, and Ewa Żekanowska. 2024. "Insights into Iron Metabolism Parameters in Ischemic Stroke: A Single-Center Prospective Cohort Study" International Journal of Molecular Sciences 25, no. 17: 9352. https://doi.org/10.3390/ijms25179352
APA StyleBoinska, J., Słomka, A., Sury, M., Wiszniewska, M., Pisarek, E., & Żekanowska, E. (2024). Insights into Iron Metabolism Parameters in Ischemic Stroke: A Single-Center Prospective Cohort Study. International Journal of Molecular Sciences, 25(17), 9352. https://doi.org/10.3390/ijms25179352






