Preterm Piglets Born by Cesarean Section as a Suitable Animal Model for the Study of Iron Metabolism in Premature Infants
Abstract
:1. Introduction
2. Results
2.1. Substantial Decrease in Body Weight in Premature Piglets
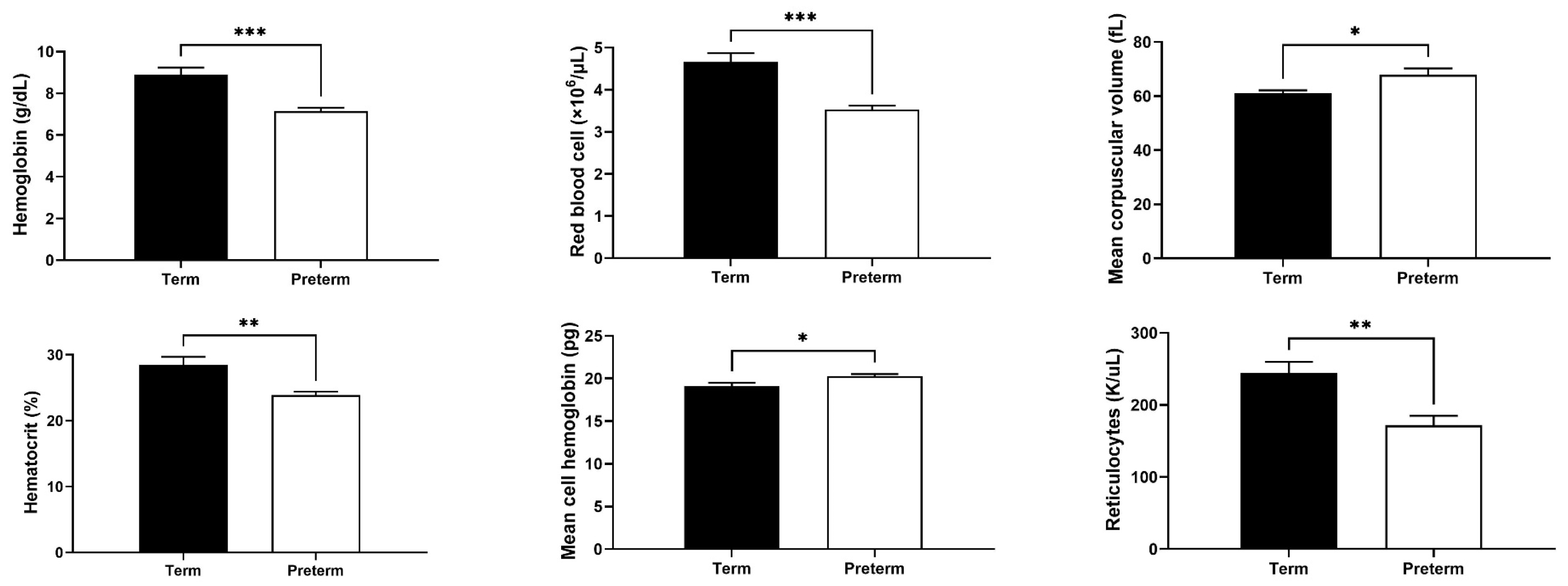
2.2. Changes in Red Blood Cell (RBC) and Reticulocyte Indices in Premature Piglets
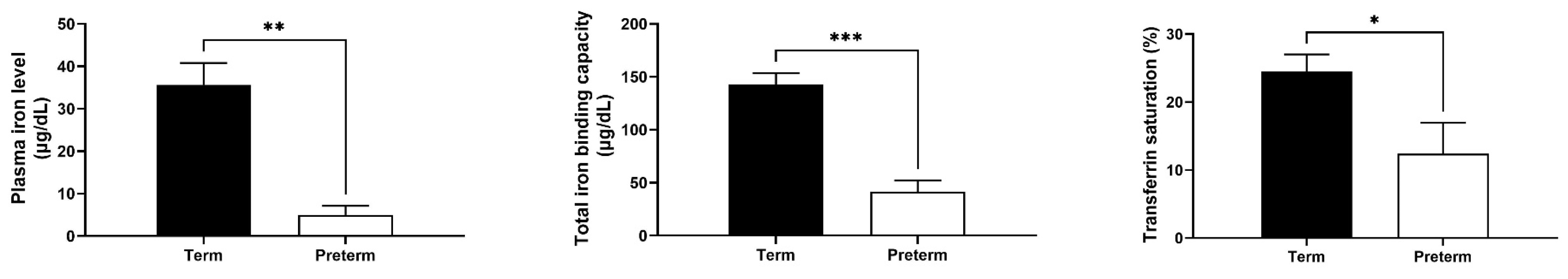
2.3. Strong Decrease in Biochemical Plasma Iron Parameters in Premature Piglets
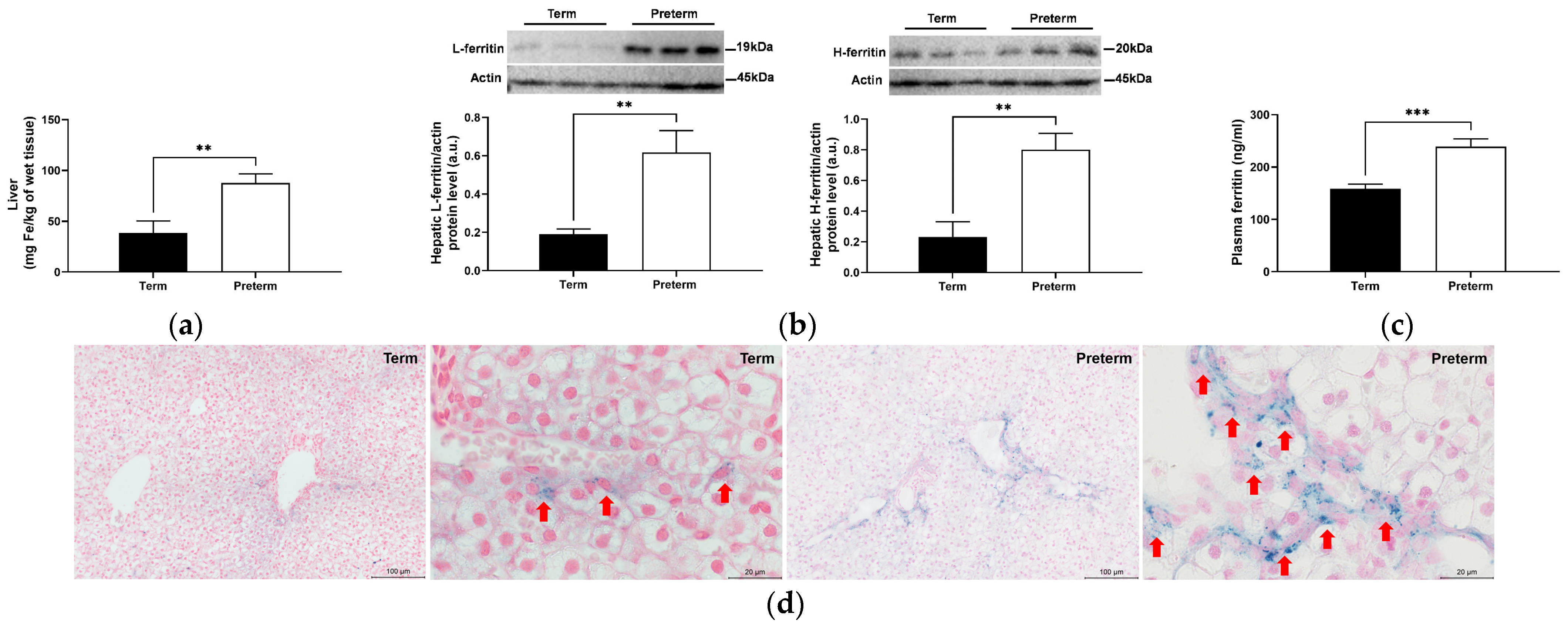
2.4. Increased Hepatic Iron Status in Premature Piglets
2.5. Splenic and Bone Marrow Iron Status in Preterm and Term Piglets
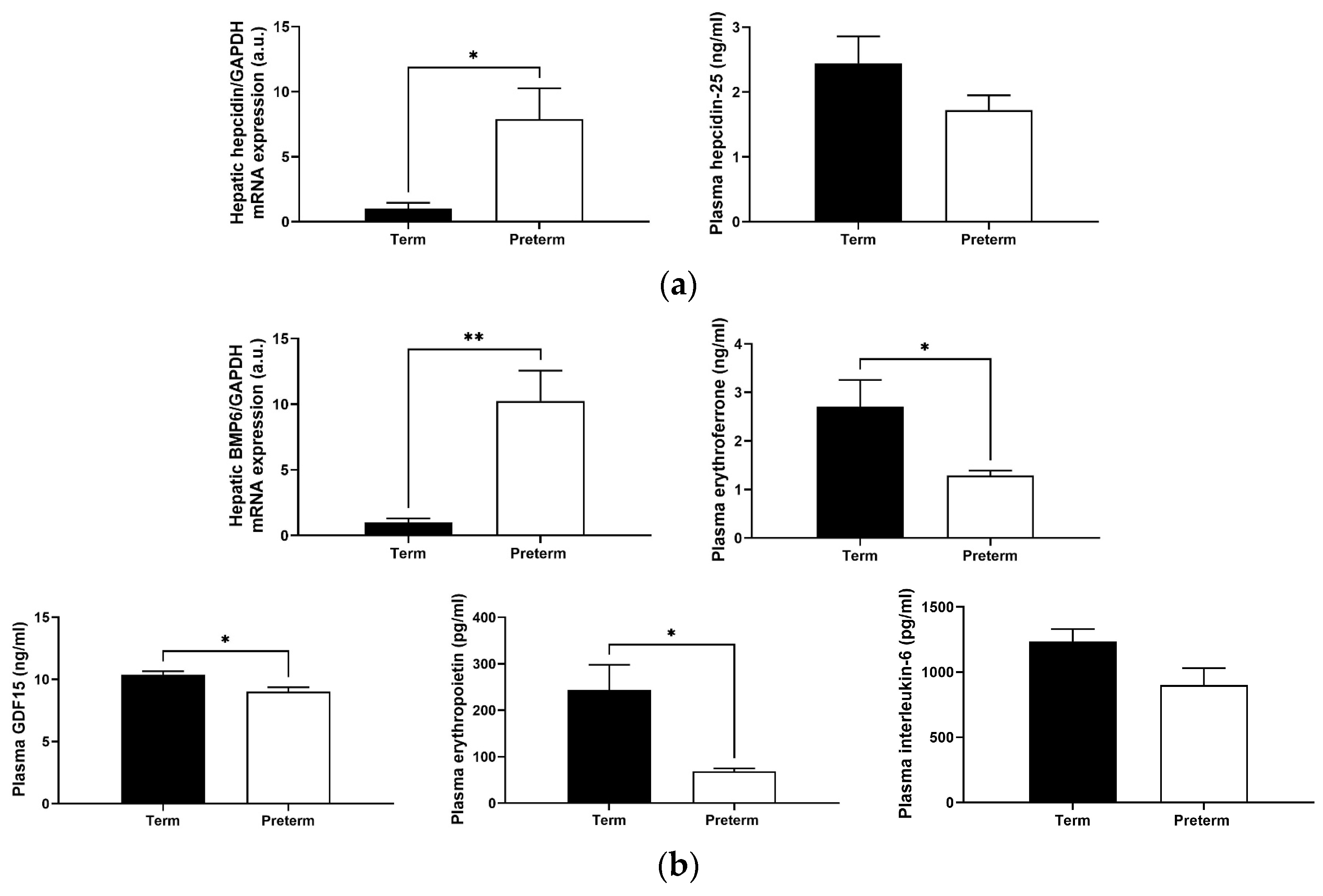
2.6. Changes in Levels of Hepcidin and Its Regulators in Premature Piglets
2.7. Similar Hepatic and Splenic Ferroportin Levels in Term and Preterm Piglets
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Biological Sample Collection
4.3. Measurement of RBC Indices and Blood Plasma Iron Parameters
4.4. Measurement of Iron Content in Tissues
4.5. Perls’ Staining
4.6. Measurement of Plasma Hepcidin, Erythropoietin, Erythroferrone, and GDF15 Level
4.7. RNA Isolation and Real-Time Quantitative RT-PCR Analysis
4.8. Western Blot Analysis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Collard, K.J. Iron homeostasis in the neonate. Pediatrics 2009, 123, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Petry, C.D.; Eaton, M.A.; Wobken, J.D.; Mills, M.M.; Johnson, D.E.; Georgieff, M.K. Iron deficiency of liver, heart, and brain in newborn infants of diabetic mothers. J. Pediatr. 1992, 121, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Sandri, B.J.; Lubach, G.R.; Lock, E.F.; Kling, P.J.; Georgieff, M.K.; Coe, C.L.; Rao, R.B. Correcting iron deficiency anemia with iron dextran alters the serum metabolomic profile of the infant rhesus monkey. Am. J. Clin. Nutr. 2021, 113, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Schoennagel, B.P.; Remus, C.C.; Wedegaertner, U.; Salzmann, I.; Grabhorn, E.; Adam, G.; Fischer, R.; Harmatz, P.; Kooijman, H.; Yamamura, J. Quantification of prenatal liver and spleen iron in a sheep model and assessment of iron stores in a human neonate with neonatal hemochromatosis using R2* mapping. Magn. Reson. Med. Sci. 2014, 13, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Berndt, M.; Buttenberg, M.; Graw, J.A. Large animal models for simulating physiology of transfusion of red cell concentrates-a scoping review of the literature. Medicina 2022, 58, 1735. [Google Scholar] [CrossRef]
- Eiby, Y.A.; Wright, L.L.; Kalanjati, V.P.; Miller, S.M.; Bjorkman, S.T.; Keates, H.L.; Lumbers, E.R.; Colditz, P.B.; Lingwood, B.E. A pig model of the preterm neonate: Anthropometric and physiological characteristics. PLoS ONE 2013, 8, e68763. [Google Scholar] [CrossRef]
- Kopeć, Z.; Starzyński, R.R.; Lenartowicz, M.; Grzesiak, M.; Opiela, J.; Smorąg, Z.; Gajda, B.; Nicpoń, J.; Ogłuszka, M.; Wang, X.; et al. Comparison of molecular potential for iron transfer across the placenta in domestic pigs with varied litter sizes and wild boars. Int. J. Mol. Sci. 2024, 25, 9638. [Google Scholar] [CrossRef]
- Rincker, M.J.; Clarke, S.L.; Eisenstein, R.S.; Link, J.E.; Hill, G.M. Effects of iron supplementation on binding activity of iron regulatory proteins and the subsequent effect on growth performance and indices of hematological and mineral status of young pigs. J. Anim. Sci. 2005, 83, 2137–2145. [Google Scholar] [CrossRef]
- Peters, J.C.; Mahan, D.C. Effects of neonatal iron status, iron injections at birth, and weaning in young pigs from sows fed either organic or inorganic trace minerals. J. Anim. Sci. 2008, 86, 2261–2269. [Google Scholar] [CrossRef]
- Kruszewski, M.; Iwaneńko, T.; Bartłomiejczyk, T.; Woliński, J.; Starzyński, R.R.; Gralak, M.A.; Zabielski, R.; Lipiński, P. Hepatic iron content corresponds with the susceptibility of lymphocytes to oxidative stress in neonatal pigs. Mutat. Res. Genetic Toxicol. Environ. Mutagen. 2008, 657, 146–149. [Google Scholar] [CrossRef]
- Lipiński, P.; Starzyński, R.R.; Canonne-Hergaux, F.; Tudek, B.; Oliński, R.; Kowalczyk, P.; Dziaman, T.; Thibaudeau, O.; Gralak, M.A.; Smuda, E.; et al. Benefits and risks of iron supplementation in anemic neonatal pigs. Am. J. Pathol. 2010, 177, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Staroń, R.; Lipiński, P.; Lenartowicz, M.; Bednarz, A.; Gajowiak, A.; Smuda, E.; Krzeptowski, W.; Pieszka, M.; Korolonek, T.; Hamza, I.; et al. Dietary hemoglobin rescues young piglets from severe iron deficiency anemia: Duodenal expression profile of genes involved in heme iron absorption. PLoS ONE 2017, 12, e0181117. [Google Scholar] [CrossRef] [PubMed]
- Szudzik, M.; Lipiński, P.; Jończy, A.; Mazgaj, R.; Pieszka, M.; Kamyczek, M.; Smuda, E.; Starzyński, R.R. Long-term effect of split iron dextran/hemoglobin supplementation on erythrocyte and iron status, growth performance, carcass parameters, and meat quality of Polish Large White and 990 Line pigs. Biol. Trace Elem. Res. 2020, 196, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Arosio, P.; Elia, L.; Poli, M. Ferritin, cellular iron storage and regulation. IUBMB Life 2017, 69, 414–422. [Google Scholar] [CrossRef]
- Viatte, L.; Vaulont, S. Hepcidin, the iron watcher. Biochimie 2009, 91, 1223–1228. [Google Scholar] [CrossRef]
- Parrow, N.L.; Fleming, R.E. Bone morphogenetic proteins as regulators of iron metabolism. Annu. Rev. Nutr. 2014, 34, 77–94. [Google Scholar] [CrossRef]
- Cross, J.H.; Prentice, A.M.; Cerami, C. Hepcidin, serum iron, and transferrin saturation in full-term and premature infants during the first month of life: A state-of-the-art review of existing evidence in humans. Curr. Dev. Nutr. 2020, 4, nzaa104. [Google Scholar] [CrossRef]
- Hentze, M.W.; Muckenthaler, M.U.; Galy, B.; Camaschella, C. Two to tango: Regulation of mammalian iron metabolism. Cell 2010, 142, 24–38. [Google Scholar] [CrossRef]
- Arad, I.; Konijn, A.M.; Linder, N.; Goldstein, M.; Kaufmann, N.A. Serum ferritin levels in preterm infants after multiple blood transfusions. Am. J. Perinatol. 1988, 5, 40–43. [Google Scholar] [CrossRef]
- Alur, P.; Devapatla, S.S.; Super, D.M.; Danish, E.; Stern, T.; Inagandla, R.; Moore, J.J. Impact of race and gestational age on red blood cell indices in very low birth weight infants. Pediatrics 2000, 106, 306–310. [Google Scholar] [CrossRef]
- Holter, P.H.; Framstad, T.; Aulie, A.; Refsum, H.E.; Sjaastad, O.V. Effect of iron treatment on erythrocyte parameters in postnatal anemia of the pig. Pediatr. Hematol. Oncol. 1991, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Olivares, M.; Llaguno, S.; Marin, V.; Hertrampf, E.; Mena, P.; Milad, M. Iron status in low-birth-weight infants, small and appropriate for gestational age. A follow-up study. Acta Paediatr. 1992, 81, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Lurie, S.; Mamet, Y. Red blood cell survival and kinetics during pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 93, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Rolim, A.C.B.; Lambert, M.A.; Borges, J.P.G.; Abbas, S.A.; Bordin, J.O.; Langhi Junior, D.M.; Chiba, A.K.; Santos, A.M.N.D. Blood cells profile in umbilical cord of late preterm and term newborns. Rev. Paul. Pediatr. 2019, 37, 264–274. [Google Scholar] [CrossRef]
- Yamada, R.T.; Leone, C.R. Hematological and iron content evolution in exclusively breastfed late-preterm newborns. Clinics 2014, 69, 792–798. [Google Scholar] [CrossRef]
- Sweet, D.G.; Savage, G.A.; Tubman, R.; Lappin, T.R.; Halliday, H.L. Cord blood transferrin receptors to assess fetal iron status. Arch. Dis. Child. Fetal Neonatal Ed. 2001, 85, F46–F48. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Li, T.; Wang, X.; Zhu, C. Iron metabolism and brain development in premature infants. Front. Physiol. 2019, 10, 463. [Google Scholar] [CrossRef]
- Raffaeli, G.; Manzoni, F.; Cortesi, V.; Cavallaro, G.; Mosca, F.; Ghirardello, S. Iron homeostasis disruption and oxidative stress in preterm newborns. Nutrients 2020, 12, 1554. [Google Scholar] [CrossRef]
- Franz, A.R.; Mihatsch, W.A.; Sander, S.; Kron, M.; Pohlandt, F. Prospective randomized trial of early versus late enteral iron supplementation in infants with a birth weight of less than 1301 grams. Pediatrics 2000, 106, 700–706. [Google Scholar] [CrossRef]
- Georgieff, M.K.; Mills, M.M.; Gordon, K.; Wobken, J.D. Reduced neonatal liver iron concentrations after uteroplacental insufficiency. J. Pediatr. 1995, 127, 304–308. [Google Scholar] [CrossRef]
- Siddappa, A.M.; Rao, R.; Long, J.D.; Widness, J.A.; Georgieff, M.K. The assessment of newborn iron stores at birth: A review of the literature and standards for ferritin concentrations. Neonatology 2007, 92, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, S.; Seip, M. Erythrocyte production and iron stores in premature infants during the first months of life; the anemia of prematurity-etiology, pathogenesis, iron requirement. Acta Paediatr. 1956, 45, 600–617. [Google Scholar]
- Palis, J. Primitive and definitive erythropoiesis in mammals. Front. Physiol. 2014, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Hom, J.; Dulmovits, B.M.; Mohandas, N.; Blanc, L. The erythroblastic island as an emerging paradigm in the anemia of inflammation. Immunol. Res. 2015, 63, 75–89. [Google Scholar] [CrossRef]
- Punia, J.N.; Elghetany, M.T. Hematopoiesis. In Rudolph’s Pediatrics; Kline, M.W., Ed.; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Mazgaj, R.; Lipiński, P.; Edison, E.S.; Bednarz, A.; Staroń, R.; Haberkiewicz, O.; Lenartowicz, M.; Smuda, E.; Jończy, A.; Starzyński, R.R. Marginally reduced maternal hepatic and splenic ferroportin under severe nutritional iron deficiency in pregnancy maintains systemic iron supply. Am. J. Hematol. 2021, 96, 659–670. [Google Scholar] [CrossRef]
- Wang, C.Y.; Babitt, J.L. Liver iron sensing and body iron homeostasis. Blood 2019, 133, 18–29. [Google Scholar] [CrossRef]
- Kautz, L.; Nemeth, E. Molecular liaisons between erythropoiesis and iron metabolism. Blood 2014, 124, 479–482. [Google Scholar] [CrossRef]
- Tanno, T.; Bhanu, N.V.; Oneal, P.A.; Goh, S.H.; Staker, P.; Lee, Y.T.; Moroney, J.W.; Reed, C.H.; Luban, N.L.C.; Wang, R.H.; et al. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat. Med. 2007, 13, 1096–1101. [Google Scholar] [CrossRef]
- Kling, P.J. Anemia of prematurity and indications for erythropoietin therapy. In Neonatal Hematology; de Alcaron, P., Werner, E., Eds.; Cambridge University Press: Cambridge, UK, 2005; pp. 59–67. [Google Scholar]
- Aher, S.M.; Ohlsson, A. Early versus late erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst. Rev. 2012, 10, CD004865. [Google Scholar] [CrossRef]
- Liu, W.; Wu, Y.; Zhang, N.; Liu, S.; Zhou, L. Disordered maternal and fetal iron metabolism occurs in preterm births in human. Dis. Markers 2022, 2022, 1664474. [Google Scholar] [CrossRef]
- Ruan, S.; Yang, S.; Li, J.; Xiong, F.; Qie, D.; Lu, Y.; Tang, Z.; Yang, F.; Ruan, S.; Yang, S.; et al. Hepcidin and iron metabolism in preterm infants. AIMS Mol. Sci. 2023, 10, 99–108. [Google Scholar] [CrossRef]
- Mazgaj, R.; Lipiński, P.; Szudzik, M.; Jończy, A.; Kopeć, Z.; Stankiewicz, A.M.; Kamyczek, M.; Swinkels, D.; Żelazowska, B.; Starzyński, R.R. Comparative evaluation of Sucrosomial Iron and iron oxide nanoparticles as oral supplements in iron deficiency anemia in piglets. Int. J. Mol. Sci. 2021, 22, 9930. [Google Scholar] [CrossRef] [PubMed]
- Hunter, H.N.; Fulton, D.B.; Ganz, T.; Vogel, H.J. The solution structure of human hepcidin, a peptide hormone with antimicrobial activity that is involved in iron uptake and hereditary hemochromatosis. J. Biol. Chem. 2002, 277, 37597–37603. [Google Scholar] [CrossRef] [PubMed]
- Valore, E.V.; Ganz, T. Posttranslational processing of hepcidin in human hepatocytes is mediated by the prohormone convertase furin. Blood Cells Mol. Dis. 2008, 40, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Tabbah, S.M.; Buhimschi, C.S.; Rodewald-Millen, K.; Pierson, C.R.; Bhandari, V.; Samuels, P.; Buhimschi, I.A. Hepcidin, an iron regulatory hormone of innate immunity, is differentially expressed in premature fetuses with early-onset neonatal sepsis. Am. J. Perinatol. 2018, 35, 865–872. [Google Scholar] [CrossRef]
- Torrance, J.D.; Bothwell, T.H. Tissue iron stores. In Methods in Haematology; Cook, J.D., Ed.; Churchill Livingstone: New York, NY, USA, 1980; pp. 90–115. [Google Scholar]
- Kopeć, Z.; Mazgaj, R.; Starzyński, R.R.; Wang, X.; Opiela, J.; Smorąg, Z.; Gajda, B.; Nicpoń, J.; Lenartowicz, M.; Ogłuszka, M.; et al. Impact of litter size on the hematological and iron status of gilts, sows and newborn piglets: A comparative study of domestic pigs and wild boars. BMC Vet. Res. 2024, 20, 64. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Starzyński, R.R.; Canonne-Hergaux, F.; Lenartowicz, M.; Krzeptowski, W.; Willemetz, A.; Styś, A.; Bierła, J.; Pietrzak, P.; Dziaman, T.; Lipiński, P. Ferroportin expression in haem oxygenase 1-deficient mice. Biochem. J. 2013, 449, 69–78. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Lenartowicz, M.; Mazgaj, R.; Ogłuszka, M.; Szkopek, D.; Zaworski, K.; Kopeć, Z.; Żelazowska, B.; Lipiński, P.; Woliński, J.; et al. Preterm Piglets Born by Cesarean Section as a Suitable Animal Model for the Study of Iron Metabolism in Premature Infants. Int. J. Mol. Sci. 2024, 25, 11215. https://doi.org/10.3390/ijms252011215
Wang X, Lenartowicz M, Mazgaj R, Ogłuszka M, Szkopek D, Zaworski K, Kopeć Z, Żelazowska B, Lipiński P, Woliński J, et al. Preterm Piglets Born by Cesarean Section as a Suitable Animal Model for the Study of Iron Metabolism in Premature Infants. International Journal of Molecular Sciences. 2024; 25(20):11215. https://doi.org/10.3390/ijms252011215
Chicago/Turabian StyleWang, Xiuying, Małgorzata Lenartowicz, Rafał Mazgaj, Magdalena Ogłuszka, Dominika Szkopek, Kamil Zaworski, Zuzanna Kopeć, Beata Żelazowska, Paweł Lipiński, Jarosław Woliński, and et al. 2024. "Preterm Piglets Born by Cesarean Section as a Suitable Animal Model for the Study of Iron Metabolism in Premature Infants" International Journal of Molecular Sciences 25, no. 20: 11215. https://doi.org/10.3390/ijms252011215
APA StyleWang, X., Lenartowicz, M., Mazgaj, R., Ogłuszka, M., Szkopek, D., Zaworski, K., Kopeć, Z., Żelazowska, B., Lipiński, P., Woliński, J., & Starzyński, R. R. (2024). Preterm Piglets Born by Cesarean Section as a Suitable Animal Model for the Study of Iron Metabolism in Premature Infants. International Journal of Molecular Sciences, 25(20), 11215. https://doi.org/10.3390/ijms252011215




