CLAVATA3 Signaling Buffers Arabidopsis Shoot Apical Meristem Activity in Response to Photoperiod
Abstract
1. Introduction
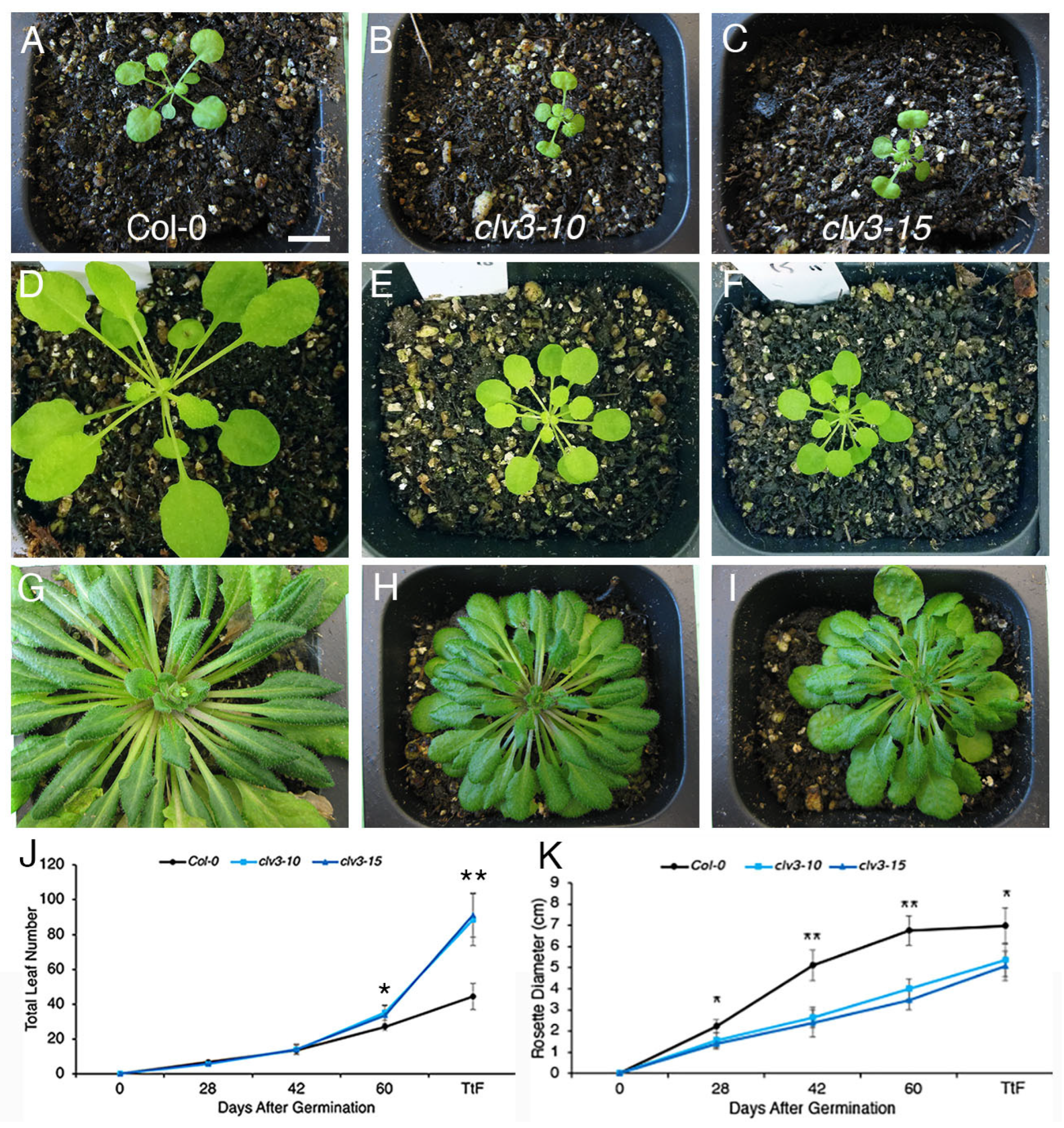
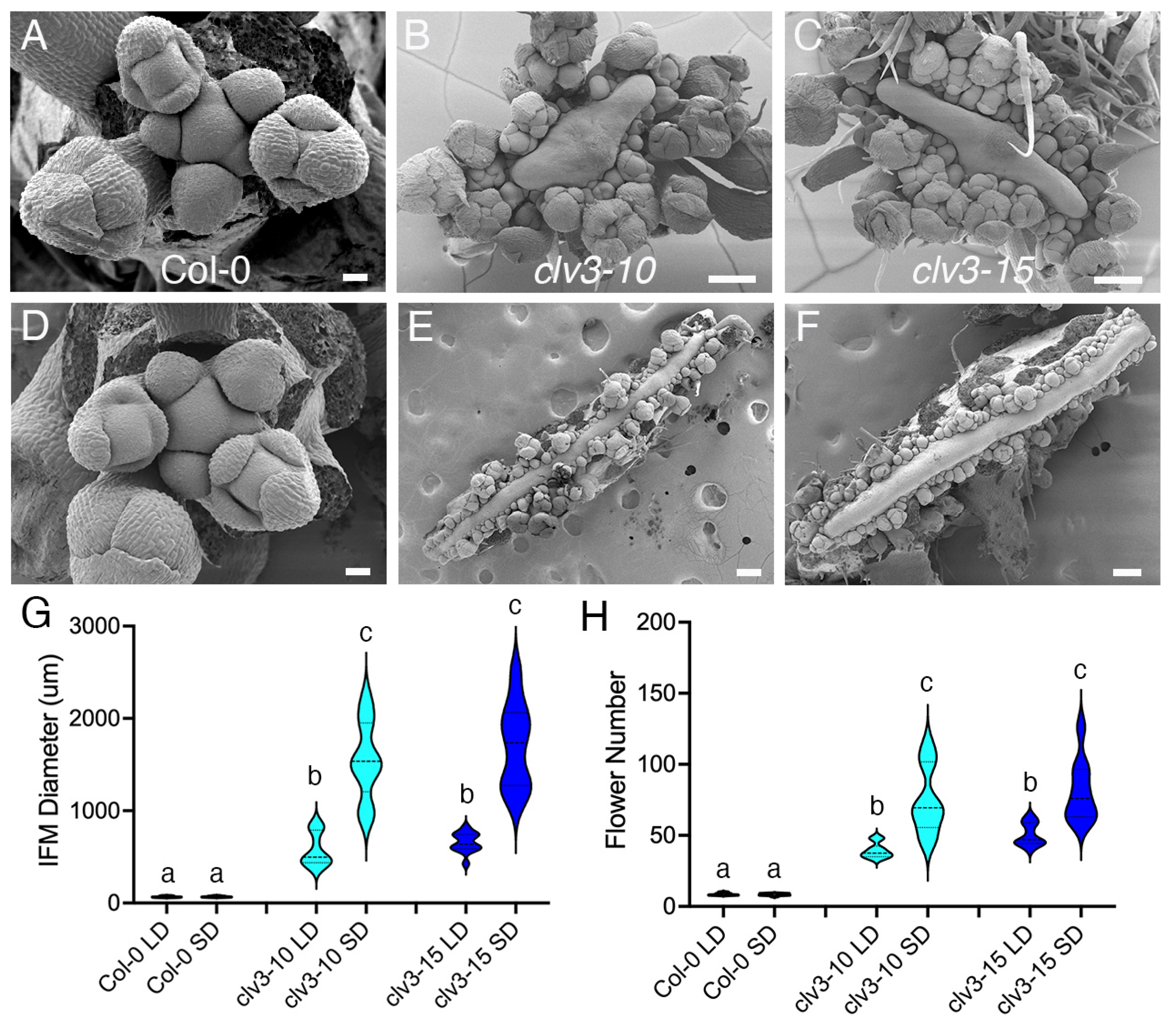
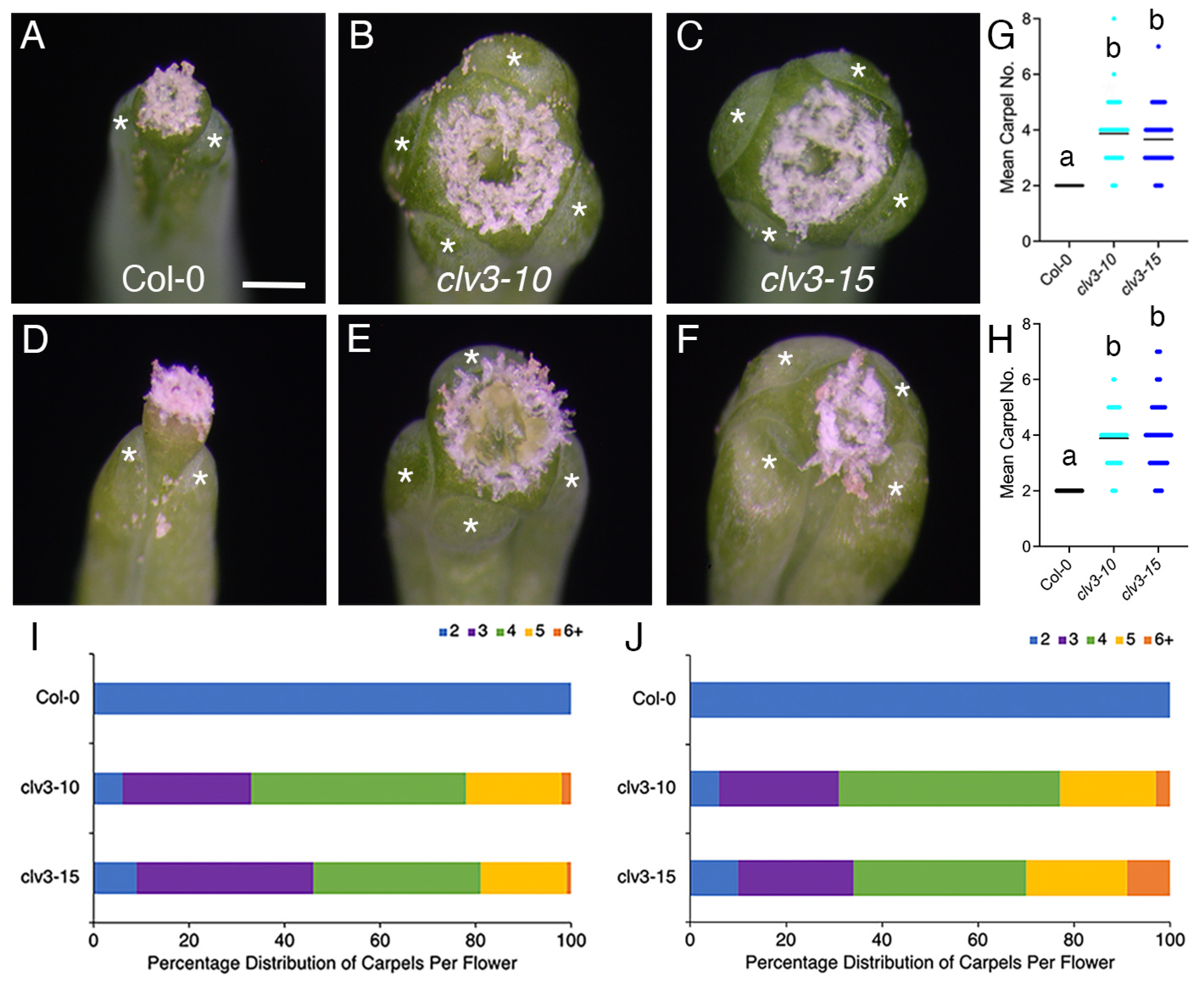
2. Results
3. Discussion
4. Materials and Methods
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barton, M.K. Twenty years on: The inner workings of the shoot apical meristem, a developmental dynamo. Dev. Biol. 2010, 341, 95–113. [Google Scholar] [CrossRef]
- Hong, L.; Fletcher, J.C. Stem cells: Engines of plant growth and development. Int. J. Mol. Sci. 2023, 24, 14889. [Google Scholar] [CrossRef]
- Brand, U.; Fletcher, J.C.; Hobe, M.; Meyerowitz, E.M.; Simon, R. Dependence of stem cell fate in Arabidopsis on a feedback loop regulated by CLV3 activity. Science 2000, 289, 617–619. [Google Scholar] [CrossRef]
- Schoof, H.; Lenhard, M.; Haecker, A.; Mayer, K.F.X.; Jurgens, G.; Laux, T. The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 2000, 100, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Geng, X.; Zhao, Z.; Zhou, W. Tipping the balance: The dynamics of stem cell maintenance and stress responses in plant meristems. Curr. Opin. Plant Biol. 2024, 78, 102510. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, A.; Richter, R. Genetic and molecular basis of floral induction in Arabidopsis thaliana. J. Exp. Bot. 2020, 71, 2490–2504. [Google Scholar] [CrossRef]
- Jaeger, K.E.; Wigge, P.A. FT protein acts as a long-range signal in Arabidopsis. Curr. Biol. 2007, 17, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Corbesier, L.; Vincent, C.; Jang, S.; Fornara, F.; Fan, Q.; Searle, I.; Giakountis, A.; Farrona, S.; Gissot, L.; Turnbull, C.; et al. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 2007, 316, 1030–1033. [Google Scholar] [CrossRef]
- Mathieu, J.; Warthmann, N.; Kuttner, F.; Schmid, M. Export of FT protein from phloem companion cells is sufficient for floral induction in Arabidopsis. Curr. Biol. 2007, 17, 1055–1060. [Google Scholar] [CrossRef]
- Abe, M.; Kosaka, S.; Shibuta, M.; Nagata, K.; Uemura, T.; Nakano, A.; Kaya, H. Transient activity of the florigan complex during the floral transition in Arabidopsis thaliana. Development 2019, 146, dev171504. [Google Scholar] [CrossRef]
- Taoka, K.; Ohki, I.; Tsuji, H.; Furuita, K.; Hayashi, K.; Yanase, T.; Yamaguchi, M.; Nakashima, C.; Purwestri, Y.A.; Tamaki, S.; et al. 14-3-3 proteins act as intercellular receptors for rice Hd3a florigen. Nature 2011, 476, 332–335. [Google Scholar] [CrossRef]
- Collani, S.; Neumann, M.; Yant, L.; Schmid, M. FT modulates genome-wide DNA-binding of the bZIP transcription factor FD. Plant Physiol. 2019, 180, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, U.; Hohmann, S.; Nettesheim, K.; Wisman, E.; Saedler, H.; Huijser, P. Molecular cloning of SVP: A negative regulator of the floral transition in Arabidopsis. Plant J. 2000, 21, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, C.; Shen, L.; Wu, Y.; Chen, H.; Robertson, M.; Helliwell, C.A.; Ito, T.; Meyerowitz, E.M.; Yu, H. A repressor complex governs the integration of flowering signals in Arabidopsis. Dev. Cell 2008, 15, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Torti, S.; Coupland, G. Genetic and spatial interactions between FT, TSF and SVP during the early stages of floral induction in Arabidopsis. Plant J. 2009, 60, 614–625. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoo, S.J.; Park, S.H.; Hwang, I.; Lee, J.S.; Ahn, J.H. Role of SVP in the control of flowering time by ambient temperature in Arabidopsis. Genes. Dev. 2007, 21, 397–402. [Google Scholar] [CrossRef]
- Kwiatkowska, D. Flowering and apical meristem growth dynamics. J. Exp. Bot. 2008, 59, 187–201. [Google Scholar] [CrossRef]
- Jacqmard, A.; Gadisseur, I.; Bernier, G. Cell division and morphological changes in the shoot apex of Arabidopsis thaliana during floral transition. Ann. Bot. 2003, 91, 571–576. [Google Scholar] [CrossRef]
- Kinoshita, A.; Vayssieres, A.; Richter, R.; Sang, Q.; Roggen, A.; van Driel, A.D.; Smith, R.S.; Coupland, G. Regulation of shoot meristem shape by photoperiodic signaling and phytohormones during floral induction of Arabidopsis. eLife 2020, 9, e60661. [Google Scholar] [CrossRef]
- Tal, L.; Friedlander, G.; Segal Gilboa, N.; Unger, T.; Gilad, S.; Eshed, Y. Coordination of meristem doming and the floral transition by Late Termination, a Kelch Repeat protein. Plant Cell 2017, 29, 681–696. [Google Scholar] [CrossRef]
- Jeong, S.; Clark, S.E. Photoperiod regulates flower meristem development in Arabidopsis thaliana. Genetics 2005, 169, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Kayes, J.M.; Clark, S.E. CLAVATA2, a regulator of meristem and organ development in Arabidopsis. Development 1998, 125, 3843–3851. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.E.; Running, M.P.; Meyerowitz, E.M. CLAVATA3 is a specific regulator of shoot and floral meristem development affecting the same processes as CLAVATA1. Development 1995, 121, 2057–2067. [Google Scholar] [CrossRef]
- Forner, J.; Pfeiffer, A.; Langenecker, T.; Manavella, P.; Lohmann, J.U. Germline-tranmitted genome editing in Arabidopsis thaliana using TAL-Effector-Nucleases. PLoS ONE 2015, 10, e0121056. [Google Scholar]
- Dievart, A.; Dalal, M.; Tax, F.E.; Lacey, A.D.; Huttly, A.; Li, J.; Clark, S.E. CLAVATA1 dominant-negative alleles reveal functional overlap between multiple receptor kinases that regulate meristem and organ development. Plant Cell 2003, 15, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- Asai, K.; Satoh, N.; Sasaki, H.; Satoh, H.; Nagato, Y. A rice heterochronic mutant, mori1, is defective in the juvenile-adult phase change. Development 2002, 129, 265–273. [Google Scholar] [CrossRef]
- Chenu, K.; Franck, N.; Dauzat, J.; Barczi, J.-F.; Rey, H.; Lecoeur, J. Integrated responses of rosette organogenesis, morphogenesis and architecture to reduced incident light in Arabidopsis thaliana results in higher efficiency of light interception. Funct. Plant Biol. 2005, 32, 1123–1134. [Google Scholar] [CrossRef]
- Wang, G.; Ellendorff, U.; Kemp, B.; Mansfield, J.W.; Forsyth, A.; Mitchell, K.; Bastas, K.; Liu, C.-M.; Woods-Tor, A.; Zipfel, C.; et al. A genome-wide functional investigation into the roles of receptor-like proteins in Arabidopsis. Plant Physiol. 2008, 147, 503–517. [Google Scholar] [CrossRef]
- Lee, B.; Yu, S.; Jackson, D. Control of plant architecture: The role of phyllotaxy and plastochron. J. Plant Biol. 2009, 52, 277–282. [Google Scholar] [CrossRef]
- Landrein, B.; Refahi, Y.; Besnard, F.; Hervieux, N.; Mirabet, V.; Boudaoud, A.; Vernoux, T.; Hamant, O. Meristem size contributes to the robustness of phyllotaxis in Arabidopsis. J. Exp. Bot. 2015, 66, 1317–1324. [Google Scholar] [CrossRef]
- Fletcher, J.C.; Brand, U.; Running, M.P.; Simon, R.; Meyerowitz, E.M. Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science 1999, 283, 1911–1914. [Google Scholar] [CrossRef] [PubMed]
- Ponteau, S.; Ferret, V.; Gaudin, V.; Lefebvre, D.; Sabar, M.; Zhao, G.; Prunus, F. Extensive phenotypic variation in early flowering mutants of Arabidopsis. Plant Physiol. 2004, 135, 201–211. [Google Scholar] [CrossRef]
- Chaudhury, A.M.; Letham, L.; Howell, S.H. amp1—A mutant with high cytokinin levels and altered embryonic pattern, faster vegetative growth, constitutive photomorphogenesis and precocious flowering. Plant J. 1993, 4, 906–916. [Google Scholar] [CrossRef]
- Lee, B. Ecotype-dependent genetic regulation of bolting time in the Arabidopsis mutants with increased number of leaves. J. Microbiol. Biotechnol. 2009, 19, 542–546. [Google Scholar]
- Schlegel, J.; Denay, G.; Wink, R.; Pinto, K.G.; Stahl, Y.; Schmid, J.; Blumke, P.; Simon, R.G.W. Control of Arabidopsis shoot stem cell homeostasis by two antagonistic CLE peptide signalling pathways. eLife 2021, 10, e70934. [Google Scholar] [CrossRef] [PubMed]
- Rojo, E.; Sharma, V.K.; Kovaleva, V.; Raikhel, N.V.; Fletcher, J.C. CLV3 is localized to the extracellular space, where it activates the Arabidopsis CLAVATA stem cell signaling pathway. Plant Cell 2002, 14, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Sawa, S.; Kinoshita, A.; Mizuno, S.; Kakimoto, T.; Fukuda, H.; Sakagami, Y. A plant peptide encoded by CLV3 identified by in situ MALDI-TOF MS analysis. Science 2006, 313, 845–848. [Google Scholar] [CrossRef]
- Plong, A.; Rodriguez, K.; Alber, M.; Chen, W.; Reddy, G.V. CLAVATA3 mediated simultaneous control of transcriptional and post-translational processes provides robustness to the WUSCHEL gradient. Nat. Comm. 2021, 12, 6361. [Google Scholar] [CrossRef]
- Dao, T.Q.; Weksler, N.; Liu, H.M.-H.; Leiboff, S.; Fletcher, J.C. Interactive CLV3, CLE16 and CLE17 signaling mediates stem cell homeostasis in the Arabidopsis shoot apical meristem. Development 2022, 149, dev200787. [Google Scholar] [CrossRef]
- Pfieffer, A.; Janocha, D.; Dong, Y.-H.; Medzihradszky, A.; Schone, S.; Daum, G.; Suzaki, T.; Forner, J.; Langenecker, T.; Rempel, E.; et al. Integration of light and metabolic signals for stem cell activation at the shoot apical meristem. eLife 2016, 5, e17023. [Google Scholar] [CrossRef]
- Peoples, M.B.; Dalling, M.J. The interplay between proteolysis and amino acid metabolism during senescence and nitrogen reallocation. In Senescence and Aging in Plants; Noodén, L.D., Leopold, A.C., Eds.; Academic Press: San Diego, CA, USA, 1988; pp. 181–217. [Google Scholar]
- Ahmad, N.; Malagoli, M.; Wirtz, M.; Hell, R. Drought stress in maize causes differential acclimation responses of glutathione and sulfur metabolism in leaves and roots. BMC Plant Biol. 2016, 16, 247. [Google Scholar] [CrossRef]
- Li, J.; Li, Q.; Guo, N.; Xian, Q.; Lan, B.; Nangia, V.; Mo, F.; Liu, Y. Polyamines mediate the inhibitory effect of drought stress on nitrogen reallocation and utilization to regulate grain number in wheat. J. Exp. Bot. 2024, 75, 1016–1035. [Google Scholar] [CrossRef]
- He, Z.; Webster, S.; He, S.Y. Growth-defense trade-offs in plants. Curr. Biol. 2022, 32, R634–R639. [Google Scholar] [CrossRef] [PubMed]
- Karasov, T.L.; Chae, E.; Herman, J.J.; Bergelson, J. Mechanisms to mitigate the trade-off between growth and defense. Plant Cell 2017, 29, 666–680. [Google Scholar] [CrossRef] [PubMed]
- Landrein, B.; Formosa-Jordan, P.; Malivert, A.; Schuster, C.; Melnyk, C.W.; Yang, W.; Turnbull, C.; Meyerowitz, E.M.; Locke, J.C.W.; Jonsson, H. Nitrate modulates stem cell dynamics in Arabidopsis shoot meristems through cytokinins. Proc. Natl. Acad. Sci. USA 2018, 115, 1383–1387. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhong, Z.; Ma, L.; Xiang, C.; Huang, X.-Y.; Xu, P.; Xiong, Y. Sulfate-TOR signalling controls transcriptional reprogramming for shoot apex activation. New Phytol. 2022, 236, 1326–1338. [Google Scholar] [CrossRef]
- Julkowska, M.M.; Klei, K.; Fokkens, L.; Haring, M.A.; Schranz, M.E.; Testerink, C. Natural variation in rosette size under salt stress conditions corresponds to developmental differences between Arabidopsis accessions and allelic variation in the LRR-KISS gene. J. Exp. Bot. 2016, 67, 2127–2138. [Google Scholar] [CrossRef]
- Xue, S.; Zou, J.; Liu, Y.; Wang, M.; Zhang, C.; Le, J. Involvement of BIG5 and BIG3 in BRI1 Trafficking Reveals Diverse Functions of BIG-subfamily ARF-GEFs in Plant Growth and Gravitropism. Int. J. Mol. Sci. 2019, 20, 2339. [Google Scholar] [CrossRef]
- Hall, A.; Bleecker, A.B. Analysis of combinatorial loss-of-function mutants in the Arabidopsis ethylene receptors reveals that the ers1 etr1 double mutant has severe developmental defects that are EIN2 dependent. Plant Cell 2003, 15, 2032–2041. [Google Scholar] [CrossRef]
- Riefler, M.; Novak, O.; Strnad, M.; Schmulling, T. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell 2005, 18, 40–54. [Google Scholar] [CrossRef]
- Beltramino, M.; Ercoli, M.F.; Debernardi, J.M.; Goldy, C.; Rojas, A.M.L.; Nota, F.; Alvarez, M.E.; Vercruyssen, L.; Inze, D.; Palatnik, J.F.; et al. Robust increase of leaf size by Arabidopsis thaliana GRF3-like transcripton factors under different growth conditions. Sci. Rep. 2018, 8, 13447. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, D.; Kende, H. The AtGRF family of putative transcription factors is involved in leaf and cotyledon growth and development. Plant J. 2003, 36, 94–104. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, L.; Li, L.; Fu, L.; Liu, Y.; Xiong, Y.; Sheen, J. Integration of nutrient, energy, light and hormone signalling via TOR in plant. J. Exp. Bot. 2019, 70, 2227–2238. [Google Scholar] [CrossRef]
- Fu, L.; Wang, P.; Xiong, Y. Target of Rapamycin signaling in plant stress response. Plant Physiol. 2020, 182, 1613–1623. [Google Scholar] [CrossRef]
- Burkart, G.M.; Brandizzi, F. A tour of TOR complex signaling in plants. Trends Biochem. Sci. 2021, 46, 417–428. [Google Scholar] [CrossRef]
- Deprost, D.; Yao, L.; Sormani, R.; Moreau, M.; Leterreux, G.; Nicolai, M.; Bedu, M.; Robaglia, C.; Meyer, C. The Arabidopsis TOR kinase links plant growth, yield, stress resistance and mRNA translation. EMBO Rep. 2007, 8, 864–870. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; Liu, Y.; Li, H.; Fu, L.; Liu, Z.; Xu, L.; Liu, H.; Xu, T.; Xiong, Y. Differential TOR activation and cell proliferation in Arabidopsis root and shoot apexes. Proc. Natl. Acad. Sci. USA 2017, 114, 2765–2770. [Google Scholar] [CrossRef]
- Ye, R.; Wang, M.; Du, H.; Chhajed, S.; Koh, J.; Liu, K.; Shin, J.; Wu, Y.; Shi, L.; Chen, S.; et al. Glucose-driven TOR-FIE-PRC2 signalling controls plant development. Nature 2022, 609, 986–993. [Google Scholar] [CrossRef]
- Smyth, D.R.; Bowman, J.L.; Meyerowitz, E.M. Early flower development in Arabidopsis. Plant Cell 1990, 2, 755–767. [Google Scholar]
- Wang, H.; Deng, X.W. Arabidopsis FHY3 defines a key phytochrome A signaling component directly interacting with its homologous partner FAR1. EMBO J. 2002, 21, 1339–1349. [Google Scholar] [CrossRef]
- Seaton, D.D.; Toledo-Ortiz, G.; Ganpudi, A.; Kubota, A.; Imaizumi, T.; Halliday, K.J. Dawn and photoperiod sensing by phytochrome A. Proc. Natl. Acad. Sci. USA 2018, 115, 10523–10528. [Google Scholar] [CrossRef]
- Li, D.; Fu, X.; Guo, L.; Huang, Z.; Li, Y.; Liu, Y.; He, Z.; Cao, X.; Ma, X.; Zhao, M.; et al. FAR-RED ELONGATED HYPOCOTYL3 activates SEPALLATA2 but inhibits CLAVATA3 to regulate meristem determinacy and maintenance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2016, 113, 9375–9380. [Google Scholar] [CrossRef]
- Muller, R.; Bleckmann, A.; Simon, R. The receptor kinase CORYNE of Arabidopsis transmits the stem cell-limiting signal CLAVATA3 independently of CLAVATA1. Plant Cell 2008, 20, 934–946. [Google Scholar] [CrossRef]
- Mandel, T.; Moreau, F.; Kutsher, Y.; Fletcher, J.C.; Carles, C.C.; Eshed Williams, L. The ERECTA receptor kinase regulates Arabidopsis shoot apical meristem size, phyllotaxy and floral meristem identity. Development 2014, 141, 830–841. [Google Scholar] [CrossRef]
- Mandel, T.; Candela, H.; Landau, U.; Asis, L.; Zelinger, E.; Carles, C.C.; Williams, L.E. Differential regulation of meristem size, morphology and organization by the ERECTA, CLAVATA and class III HD-ZIP pathways. Development 2016, 143, 1612–1622. [Google Scholar] [CrossRef]
- Zhang, L.; DeGennaro, D.; Lin, G.; Chai, J.; Shpak, E.D. ERECTA family signaling constrains CLAVATA3 and WUSCHEL to the center of the shoot apical meristem. Development 2021, 148, dev189753. [Google Scholar] [CrossRef]
- Kimura, Y.; Tasaka, M.; Torii, K.U.; Uchida, N. ERECTA-family genes coordinate stem cell functions between the epidermal and internal layers of the shoot apical meristem. Development 2018, 145, dev156380. [Google Scholar] [CrossRef]
- Monfared, M.M.; Fletcher, J.C. Genetic and phenotypic analysis of shoot apical and floral meristem development. Methods Mol. Biol. 2014, 1110, 157–189. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fletcher, J.C. CLAVATA3 Signaling Buffers Arabidopsis Shoot Apical Meristem Activity in Response to Photoperiod. Int. J. Mol. Sci. 2024, 25, 9357. https://doi.org/10.3390/ijms25179357
Fletcher JC. CLAVATA3 Signaling Buffers Arabidopsis Shoot Apical Meristem Activity in Response to Photoperiod. International Journal of Molecular Sciences. 2024; 25(17):9357. https://doi.org/10.3390/ijms25179357
Chicago/Turabian StyleFletcher, Jennifer C. 2024. "CLAVATA3 Signaling Buffers Arabidopsis Shoot Apical Meristem Activity in Response to Photoperiod" International Journal of Molecular Sciences 25, no. 17: 9357. https://doi.org/10.3390/ijms25179357
APA StyleFletcher, J. C. (2024). CLAVATA3 Signaling Buffers Arabidopsis Shoot Apical Meristem Activity in Response to Photoperiod. International Journal of Molecular Sciences, 25(17), 9357. https://doi.org/10.3390/ijms25179357





