CLAVATA3 Signaling Buffers Arabidopsis Shoot Apical Meristem Activity in Response to Photoperiod
Abstract
:1. Introduction
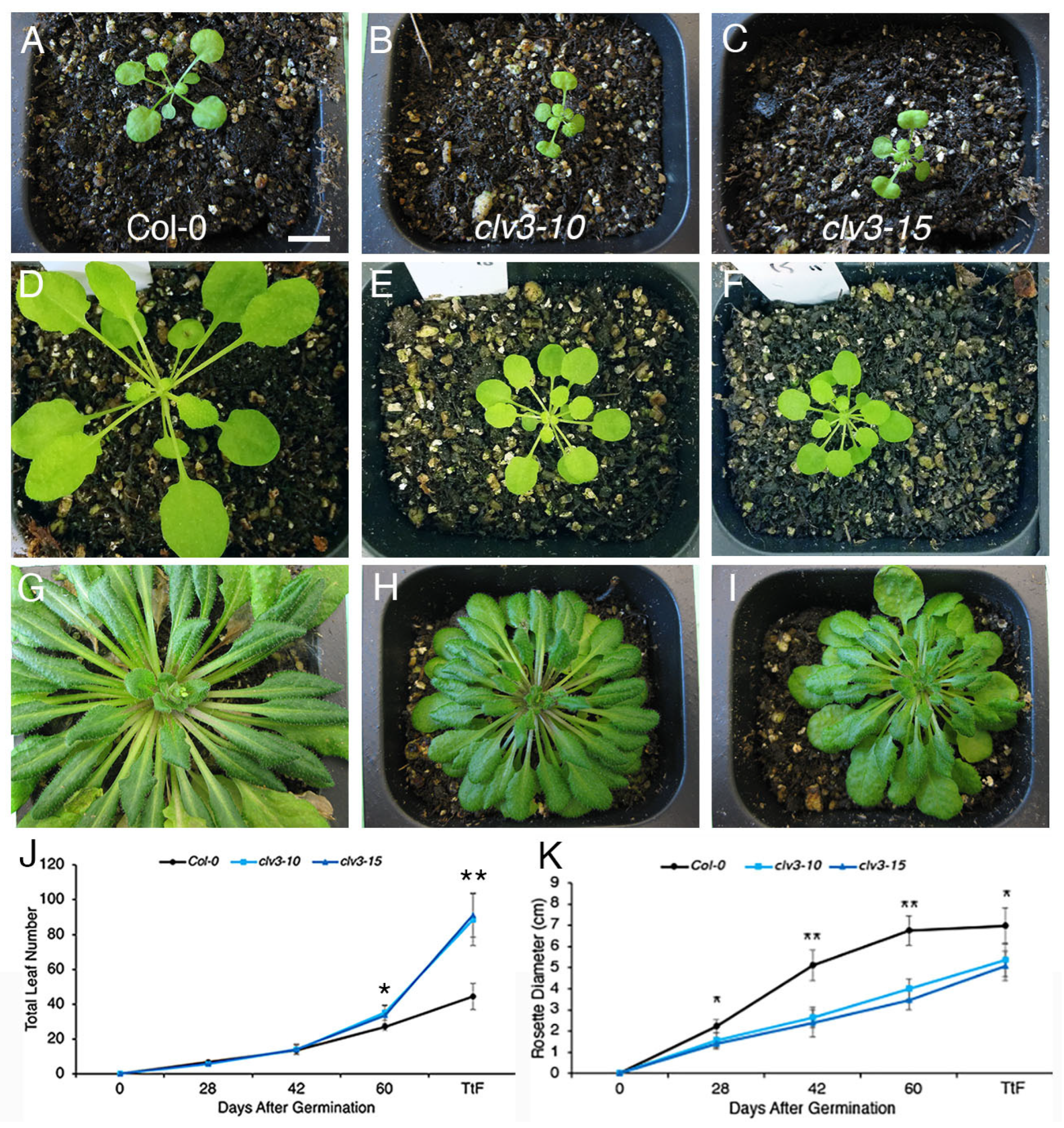
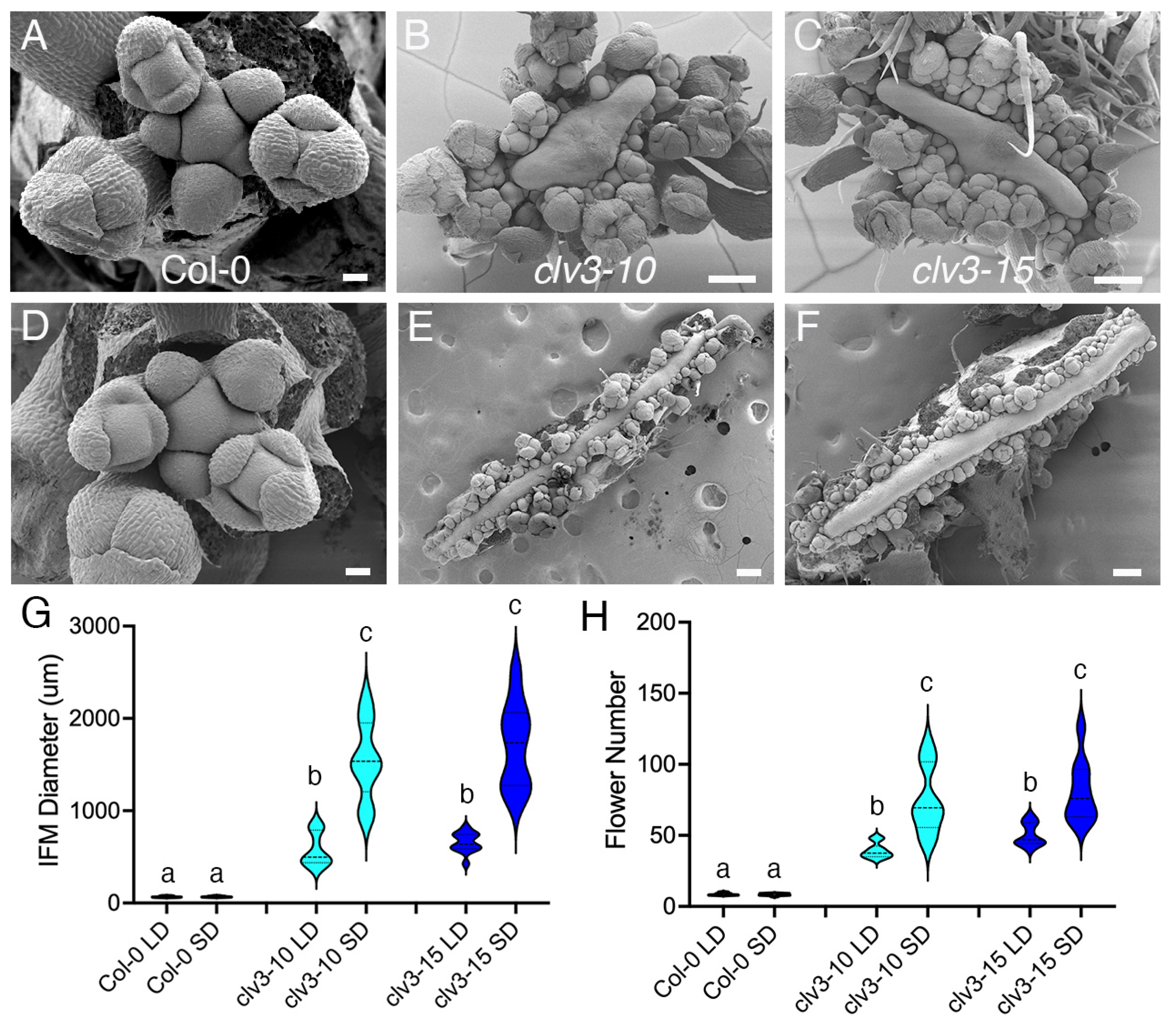
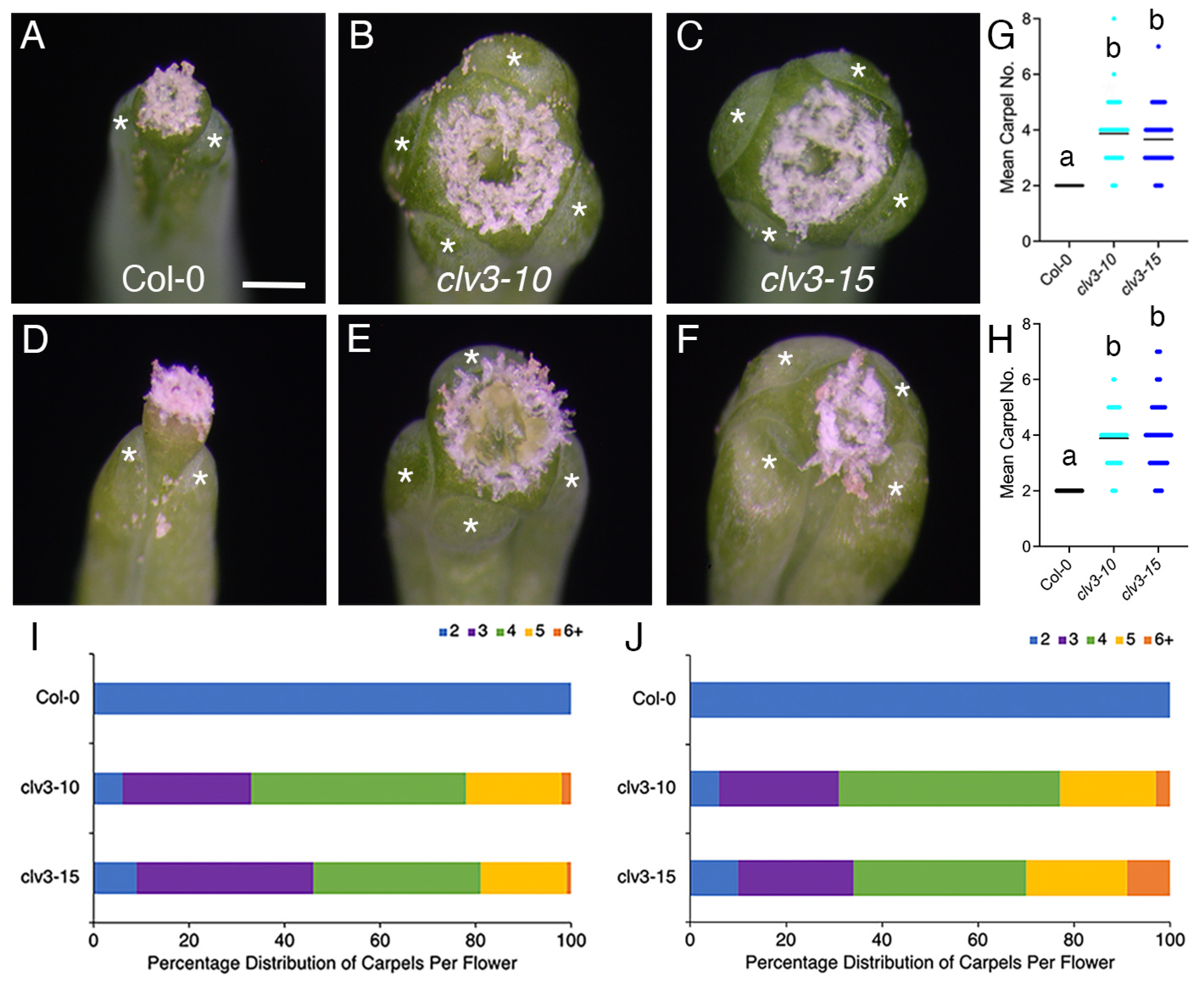
2. Results
3. Discussion
4. Materials and Methods
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barton, M.K. Twenty years on: The inner workings of the shoot apical meristem, a developmental dynamo. Dev. Biol. 2010, 341, 95–113. [Google Scholar] [CrossRef]
- Hong, L.; Fletcher, J.C. Stem cells: Engines of plant growth and development. Int. J. Mol. Sci. 2023, 24, 14889. [Google Scholar] [CrossRef]
- Brand, U.; Fletcher, J.C.; Hobe, M.; Meyerowitz, E.M.; Simon, R. Dependence of stem cell fate in Arabidopsis on a feedback loop regulated by CLV3 activity. Science 2000, 289, 617–619. [Google Scholar] [CrossRef]
- Schoof, H.; Lenhard, M.; Haecker, A.; Mayer, K.F.X.; Jurgens, G.; Laux, T. The stem cell population of Arabidopsis shoot meristems is maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 2000, 100, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Geng, X.; Zhao, Z.; Zhou, W. Tipping the balance: The dynamics of stem cell maintenance and stress responses in plant meristems. Curr. Opin. Plant Biol. 2024, 78, 102510. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, A.; Richter, R. Genetic and molecular basis of floral induction in Arabidopsis thaliana. J. Exp. Bot. 2020, 71, 2490–2504. [Google Scholar] [CrossRef]
- Jaeger, K.E.; Wigge, P.A. FT protein acts as a long-range signal in Arabidopsis. Curr. Biol. 2007, 17, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Corbesier, L.; Vincent, C.; Jang, S.; Fornara, F.; Fan, Q.; Searle, I.; Giakountis, A.; Farrona, S.; Gissot, L.; Turnbull, C.; et al. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 2007, 316, 1030–1033. [Google Scholar] [CrossRef]
- Mathieu, J.; Warthmann, N.; Kuttner, F.; Schmid, M. Export of FT protein from phloem companion cells is sufficient for floral induction in Arabidopsis. Curr. Biol. 2007, 17, 1055–1060. [Google Scholar] [CrossRef]
- Abe, M.; Kosaka, S.; Shibuta, M.; Nagata, K.; Uemura, T.; Nakano, A.; Kaya, H. Transient activity of the florigan complex during the floral transition in Arabidopsis thaliana. Development 2019, 146, dev171504. [Google Scholar] [CrossRef]
- Taoka, K.; Ohki, I.; Tsuji, H.; Furuita, K.; Hayashi, K.; Yanase, T.; Yamaguchi, M.; Nakashima, C.; Purwestri, Y.A.; Tamaki, S.; et al. 14-3-3 proteins act as intercellular receptors for rice Hd3a florigen. Nature 2011, 476, 332–335. [Google Scholar] [CrossRef]
- Collani, S.; Neumann, M.; Yant, L.; Schmid, M. FT modulates genome-wide DNA-binding of the bZIP transcription factor FD. Plant Physiol. 2019, 180, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, U.; Hohmann, S.; Nettesheim, K.; Wisman, E.; Saedler, H.; Huijser, P. Molecular cloning of SVP: A negative regulator of the floral transition in Arabidopsis. Plant J. 2000, 21, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, C.; Shen, L.; Wu, Y.; Chen, H.; Robertson, M.; Helliwell, C.A.; Ito, T.; Meyerowitz, E.M.; Yu, H. A repressor complex governs the integration of flowering signals in Arabidopsis. Dev. Cell 2008, 15, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Torti, S.; Coupland, G. Genetic and spatial interactions between FT, TSF and SVP during the early stages of floral induction in Arabidopsis. Plant J. 2009, 60, 614–625. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoo, S.J.; Park, S.H.; Hwang, I.; Lee, J.S.; Ahn, J.H. Role of SVP in the control of flowering time by ambient temperature in Arabidopsis. Genes. Dev. 2007, 21, 397–402. [Google Scholar] [CrossRef]
- Kwiatkowska, D. Flowering and apical meristem growth dynamics. J. Exp. Bot. 2008, 59, 187–201. [Google Scholar] [CrossRef]
- Jacqmard, A.; Gadisseur, I.; Bernier, G. Cell division and morphological changes in the shoot apex of Arabidopsis thaliana during floral transition. Ann. Bot. 2003, 91, 571–576. [Google Scholar] [CrossRef]
- Kinoshita, A.; Vayssieres, A.; Richter, R.; Sang, Q.; Roggen, A.; van Driel, A.D.; Smith, R.S.; Coupland, G. Regulation of shoot meristem shape by photoperiodic signaling and phytohormones during floral induction of Arabidopsis. eLife 2020, 9, e60661. [Google Scholar] [CrossRef]
- Tal, L.; Friedlander, G.; Segal Gilboa, N.; Unger, T.; Gilad, S.; Eshed, Y. Coordination of meristem doming and the floral transition by Late Termination, a Kelch Repeat protein. Plant Cell 2017, 29, 681–696. [Google Scholar] [CrossRef]
- Jeong, S.; Clark, S.E. Photoperiod regulates flower meristem development in Arabidopsis thaliana. Genetics 2005, 169, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Kayes, J.M.; Clark, S.E. CLAVATA2, a regulator of meristem and organ development in Arabidopsis. Development 1998, 125, 3843–3851. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.E.; Running, M.P.; Meyerowitz, E.M. CLAVATA3 is a specific regulator of shoot and floral meristem development affecting the same processes as CLAVATA1. Development 1995, 121, 2057–2067. [Google Scholar] [CrossRef]
- Forner, J.; Pfeiffer, A.; Langenecker, T.; Manavella, P.; Lohmann, J.U. Germline-tranmitted genome editing in Arabidopsis thaliana using TAL-Effector-Nucleases. PLoS ONE 2015, 10, e0121056. [Google Scholar]
- Dievart, A.; Dalal, M.; Tax, F.E.; Lacey, A.D.; Huttly, A.; Li, J.; Clark, S.E. CLAVATA1 dominant-negative alleles reveal functional overlap between multiple receptor kinases that regulate meristem and organ development. Plant Cell 2003, 15, 1198–1211. [Google Scholar] [CrossRef] [PubMed]
- Asai, K.; Satoh, N.; Sasaki, H.; Satoh, H.; Nagato, Y. A rice heterochronic mutant, mori1, is defective in the juvenile-adult phase change. Development 2002, 129, 265–273. [Google Scholar] [CrossRef]
- Chenu, K.; Franck, N.; Dauzat, J.; Barczi, J.-F.; Rey, H.; Lecoeur, J. Integrated responses of rosette organogenesis, morphogenesis and architecture to reduced incident light in Arabidopsis thaliana results in higher efficiency of light interception. Funct. Plant Biol. 2005, 32, 1123–1134. [Google Scholar] [CrossRef]
- Wang, G.; Ellendorff, U.; Kemp, B.; Mansfield, J.W.; Forsyth, A.; Mitchell, K.; Bastas, K.; Liu, C.-M.; Woods-Tor, A.; Zipfel, C.; et al. A genome-wide functional investigation into the roles of receptor-like proteins in Arabidopsis. Plant Physiol. 2008, 147, 503–517. [Google Scholar] [CrossRef]
- Lee, B.; Yu, S.; Jackson, D. Control of plant architecture: The role of phyllotaxy and plastochron. J. Plant Biol. 2009, 52, 277–282. [Google Scholar] [CrossRef]
- Landrein, B.; Refahi, Y.; Besnard, F.; Hervieux, N.; Mirabet, V.; Boudaoud, A.; Vernoux, T.; Hamant, O. Meristem size contributes to the robustness of phyllotaxis in Arabidopsis. J. Exp. Bot. 2015, 66, 1317–1324. [Google Scholar] [CrossRef]
- Fletcher, J.C.; Brand, U.; Running, M.P.; Simon, R.; Meyerowitz, E.M. Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science 1999, 283, 1911–1914. [Google Scholar] [CrossRef] [PubMed]
- Ponteau, S.; Ferret, V.; Gaudin, V.; Lefebvre, D.; Sabar, M.; Zhao, G.; Prunus, F. Extensive phenotypic variation in early flowering mutants of Arabidopsis. Plant Physiol. 2004, 135, 201–211. [Google Scholar] [CrossRef]
- Chaudhury, A.M.; Letham, L.; Howell, S.H. amp1—A mutant with high cytokinin levels and altered embryonic pattern, faster vegetative growth, constitutive photomorphogenesis and precocious flowering. Plant J. 1993, 4, 906–916. [Google Scholar] [CrossRef]
- Lee, B. Ecotype-dependent genetic regulation of bolting time in the Arabidopsis mutants with increased number of leaves. J. Microbiol. Biotechnol. 2009, 19, 542–546. [Google Scholar]
- Schlegel, J.; Denay, G.; Wink, R.; Pinto, K.G.; Stahl, Y.; Schmid, J.; Blumke, P.; Simon, R.G.W. Control of Arabidopsis shoot stem cell homeostasis by two antagonistic CLE peptide signalling pathways. eLife 2021, 10, e70934. [Google Scholar] [CrossRef] [PubMed]
- Rojo, E.; Sharma, V.K.; Kovaleva, V.; Raikhel, N.V.; Fletcher, J.C. CLV3 is localized to the extracellular space, where it activates the Arabidopsis CLAVATA stem cell signaling pathway. Plant Cell 2002, 14, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Sawa, S.; Kinoshita, A.; Mizuno, S.; Kakimoto, T.; Fukuda, H.; Sakagami, Y. A plant peptide encoded by CLV3 identified by in situ MALDI-TOF MS analysis. Science 2006, 313, 845–848. [Google Scholar] [CrossRef]
- Plong, A.; Rodriguez, K.; Alber, M.; Chen, W.; Reddy, G.V. CLAVATA3 mediated simultaneous control of transcriptional and post-translational processes provides robustness to the WUSCHEL gradient. Nat. Comm. 2021, 12, 6361. [Google Scholar] [CrossRef]
- Dao, T.Q.; Weksler, N.; Liu, H.M.-H.; Leiboff, S.; Fletcher, J.C. Interactive CLV3, CLE16 and CLE17 signaling mediates stem cell homeostasis in the Arabidopsis shoot apical meristem. Development 2022, 149, dev200787. [Google Scholar] [CrossRef]
- Pfieffer, A.; Janocha, D.; Dong, Y.-H.; Medzihradszky, A.; Schone, S.; Daum, G.; Suzaki, T.; Forner, J.; Langenecker, T.; Rempel, E.; et al. Integration of light and metabolic signals for stem cell activation at the shoot apical meristem. eLife 2016, 5, e17023. [Google Scholar] [CrossRef]
- Peoples, M.B.; Dalling, M.J. The interplay between proteolysis and amino acid metabolism during senescence and nitrogen reallocation. In Senescence and Aging in Plants; Noodén, L.D., Leopold, A.C., Eds.; Academic Press: San Diego, CA, USA, 1988; pp. 181–217. [Google Scholar]
- Ahmad, N.; Malagoli, M.; Wirtz, M.; Hell, R. Drought stress in maize causes differential acclimation responses of glutathione and sulfur metabolism in leaves and roots. BMC Plant Biol. 2016, 16, 247. [Google Scholar] [CrossRef]
- Li, J.; Li, Q.; Guo, N.; Xian, Q.; Lan, B.; Nangia, V.; Mo, F.; Liu, Y. Polyamines mediate the inhibitory effect of drought stress on nitrogen reallocation and utilization to regulate grain number in wheat. J. Exp. Bot. 2024, 75, 1016–1035. [Google Scholar] [CrossRef]
- He, Z.; Webster, S.; He, S.Y. Growth-defense trade-offs in plants. Curr. Biol. 2022, 32, R634–R639. [Google Scholar] [CrossRef] [PubMed]
- Karasov, T.L.; Chae, E.; Herman, J.J.; Bergelson, J. Mechanisms to mitigate the trade-off between growth and defense. Plant Cell 2017, 29, 666–680. [Google Scholar] [CrossRef] [PubMed]
- Landrein, B.; Formosa-Jordan, P.; Malivert, A.; Schuster, C.; Melnyk, C.W.; Yang, W.; Turnbull, C.; Meyerowitz, E.M.; Locke, J.C.W.; Jonsson, H. Nitrate modulates stem cell dynamics in Arabidopsis shoot meristems through cytokinins. Proc. Natl. Acad. Sci. USA 2018, 115, 1383–1387. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhong, Z.; Ma, L.; Xiang, C.; Huang, X.-Y.; Xu, P.; Xiong, Y. Sulfate-TOR signalling controls transcriptional reprogramming for shoot apex activation. New Phytol. 2022, 236, 1326–1338. [Google Scholar] [CrossRef]
- Julkowska, M.M.; Klei, K.; Fokkens, L.; Haring, M.A.; Schranz, M.E.; Testerink, C. Natural variation in rosette size under salt stress conditions corresponds to developmental differences between Arabidopsis accessions and allelic variation in the LRR-KISS gene. J. Exp. Bot. 2016, 67, 2127–2138. [Google Scholar] [CrossRef]
- Xue, S.; Zou, J.; Liu, Y.; Wang, M.; Zhang, C.; Le, J. Involvement of BIG5 and BIG3 in BRI1 Trafficking Reveals Diverse Functions of BIG-subfamily ARF-GEFs in Plant Growth and Gravitropism. Int. J. Mol. Sci. 2019, 20, 2339. [Google Scholar] [CrossRef]
- Hall, A.; Bleecker, A.B. Analysis of combinatorial loss-of-function mutants in the Arabidopsis ethylene receptors reveals that the ers1 etr1 double mutant has severe developmental defects that are EIN2 dependent. Plant Cell 2003, 15, 2032–2041. [Google Scholar] [CrossRef]
- Riefler, M.; Novak, O.; Strnad, M.; Schmulling, T. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell 2005, 18, 40–54. [Google Scholar] [CrossRef]
- Beltramino, M.; Ercoli, M.F.; Debernardi, J.M.; Goldy, C.; Rojas, A.M.L.; Nota, F.; Alvarez, M.E.; Vercruyssen, L.; Inze, D.; Palatnik, J.F.; et al. Robust increase of leaf size by Arabidopsis thaliana GRF3-like transcripton factors under different growth conditions. Sci. Rep. 2018, 8, 13447. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, D.; Kende, H. The AtGRF family of putative transcription factors is involved in leaf and cotyledon growth and development. Plant J. 2003, 36, 94–104. [Google Scholar] [CrossRef]
- Wu, Y.; Shi, L.; Li, L.; Fu, L.; Liu, Y.; Xiong, Y.; Sheen, J. Integration of nutrient, energy, light and hormone signalling via TOR in plant. J. Exp. Bot. 2019, 70, 2227–2238. [Google Scholar] [CrossRef]
- Fu, L.; Wang, P.; Xiong, Y. Target of Rapamycin signaling in plant stress response. Plant Physiol. 2020, 182, 1613–1623. [Google Scholar] [CrossRef]
- Burkart, G.M.; Brandizzi, F. A tour of TOR complex signaling in plants. Trends Biochem. Sci. 2021, 46, 417–428. [Google Scholar] [CrossRef]
- Deprost, D.; Yao, L.; Sormani, R.; Moreau, M.; Leterreux, G.; Nicolai, M.; Bedu, M.; Robaglia, C.; Meyer, C. The Arabidopsis TOR kinase links plant growth, yield, stress resistance and mRNA translation. EMBO Rep. 2007, 8, 864–870. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; Liu, Y.; Li, H.; Fu, L.; Liu, Z.; Xu, L.; Liu, H.; Xu, T.; Xiong, Y. Differential TOR activation and cell proliferation in Arabidopsis root and shoot apexes. Proc. Natl. Acad. Sci. USA 2017, 114, 2765–2770. [Google Scholar] [CrossRef]
- Ye, R.; Wang, M.; Du, H.; Chhajed, S.; Koh, J.; Liu, K.; Shin, J.; Wu, Y.; Shi, L.; Chen, S.; et al. Glucose-driven TOR-FIE-PRC2 signalling controls plant development. Nature 2022, 609, 986–993. [Google Scholar] [CrossRef]
- Smyth, D.R.; Bowman, J.L.; Meyerowitz, E.M. Early flower development in Arabidopsis. Plant Cell 1990, 2, 755–767. [Google Scholar]
- Wang, H.; Deng, X.W. Arabidopsis FHY3 defines a key phytochrome A signaling component directly interacting with its homologous partner FAR1. EMBO J. 2002, 21, 1339–1349. [Google Scholar] [CrossRef]
- Seaton, D.D.; Toledo-Ortiz, G.; Ganpudi, A.; Kubota, A.; Imaizumi, T.; Halliday, K.J. Dawn and photoperiod sensing by phytochrome A. Proc. Natl. Acad. Sci. USA 2018, 115, 10523–10528. [Google Scholar] [CrossRef]
- Li, D.; Fu, X.; Guo, L.; Huang, Z.; Li, Y.; Liu, Y.; He, Z.; Cao, X.; Ma, X.; Zhao, M.; et al. FAR-RED ELONGATED HYPOCOTYL3 activates SEPALLATA2 but inhibits CLAVATA3 to regulate meristem determinacy and maintenance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2016, 113, 9375–9380. [Google Scholar] [CrossRef]
- Muller, R.; Bleckmann, A.; Simon, R. The receptor kinase CORYNE of Arabidopsis transmits the stem cell-limiting signal CLAVATA3 independently of CLAVATA1. Plant Cell 2008, 20, 934–946. [Google Scholar] [CrossRef]
- Mandel, T.; Moreau, F.; Kutsher, Y.; Fletcher, J.C.; Carles, C.C.; Eshed Williams, L. The ERECTA receptor kinase regulates Arabidopsis shoot apical meristem size, phyllotaxy and floral meristem identity. Development 2014, 141, 830–841. [Google Scholar] [CrossRef]
- Mandel, T.; Candela, H.; Landau, U.; Asis, L.; Zelinger, E.; Carles, C.C.; Williams, L.E. Differential regulation of meristem size, morphology and organization by the ERECTA, CLAVATA and class III HD-ZIP pathways. Development 2016, 143, 1612–1622. [Google Scholar] [CrossRef]
- Zhang, L.; DeGennaro, D.; Lin, G.; Chai, J.; Shpak, E.D. ERECTA family signaling constrains CLAVATA3 and WUSCHEL to the center of the shoot apical meristem. Development 2021, 148, dev189753. [Google Scholar] [CrossRef]
- Kimura, Y.; Tasaka, M.; Torii, K.U.; Uchida, N. ERECTA-family genes coordinate stem cell functions between the epidermal and internal layers of the shoot apical meristem. Development 2018, 145, dev156380. [Google Scholar] [CrossRef]
- Monfared, M.M.; Fletcher, J.C. Genetic and phenotypic analysis of shoot apical and floral meristem development. Methods Mol. Biol. 2014, 1110, 157–189. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fletcher, J.C. CLAVATA3 Signaling Buffers Arabidopsis Shoot Apical Meristem Activity in Response to Photoperiod. Int. J. Mol. Sci. 2024, 25, 9357. https://doi.org/10.3390/ijms25179357
Fletcher JC. CLAVATA3 Signaling Buffers Arabidopsis Shoot Apical Meristem Activity in Response to Photoperiod. International Journal of Molecular Sciences. 2024; 25(17):9357. https://doi.org/10.3390/ijms25179357
Chicago/Turabian StyleFletcher, Jennifer C. 2024. "CLAVATA3 Signaling Buffers Arabidopsis Shoot Apical Meristem Activity in Response to Photoperiod" International Journal of Molecular Sciences 25, no. 17: 9357. https://doi.org/10.3390/ijms25179357




