Olfactory Projections to Locomotor Control Centers in the Sea Lamprey
Abstract
:1. Introduction
2. Results
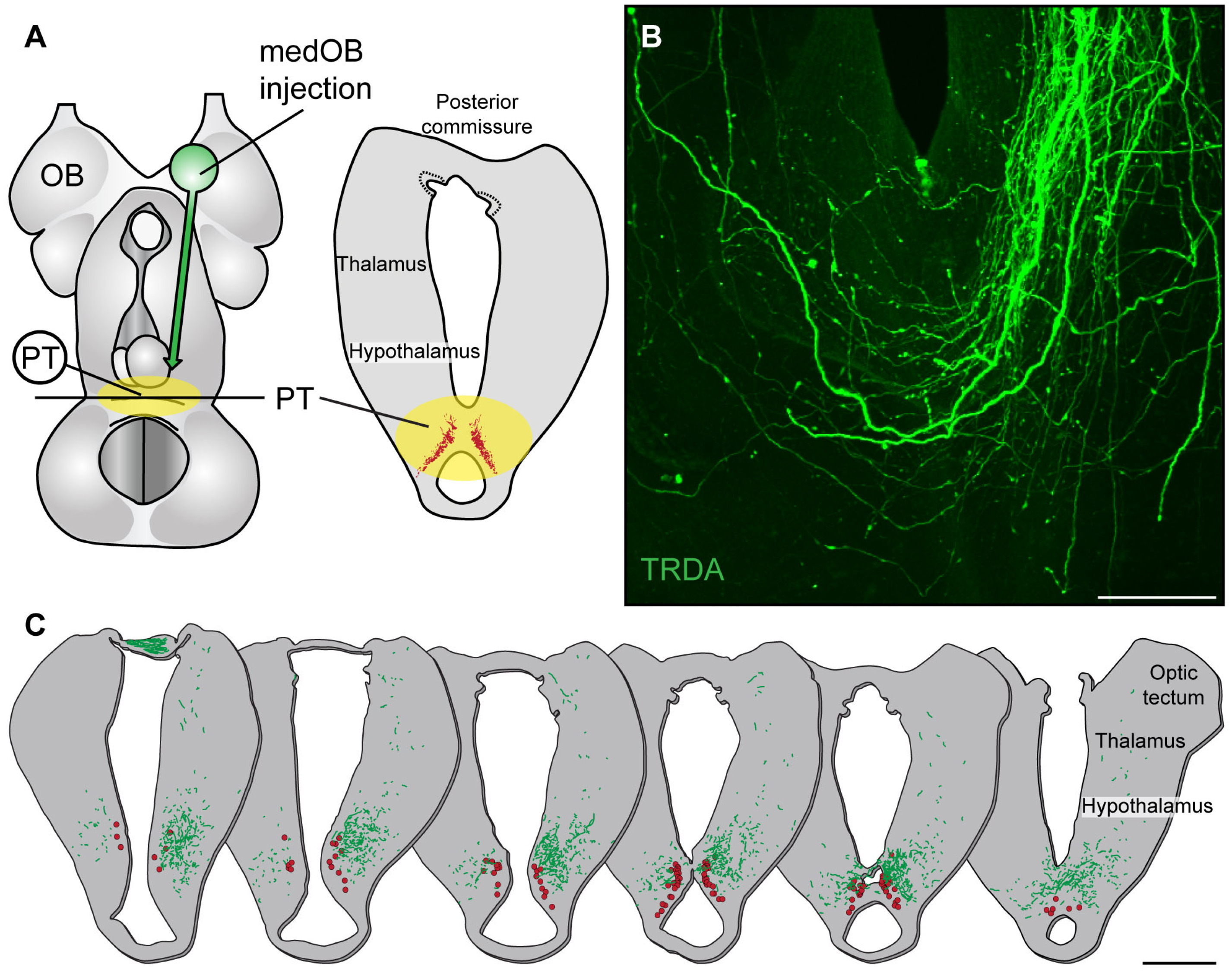
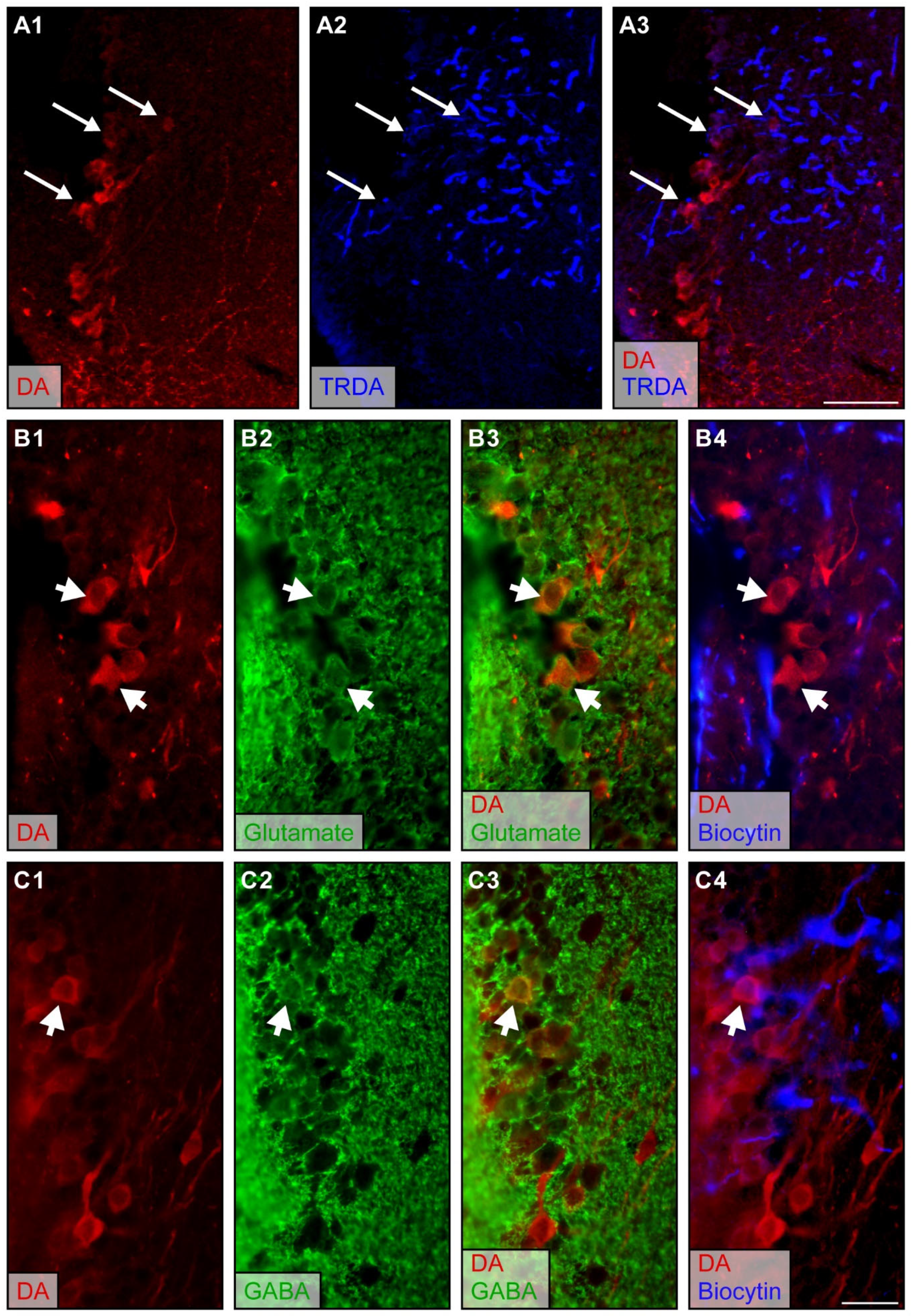
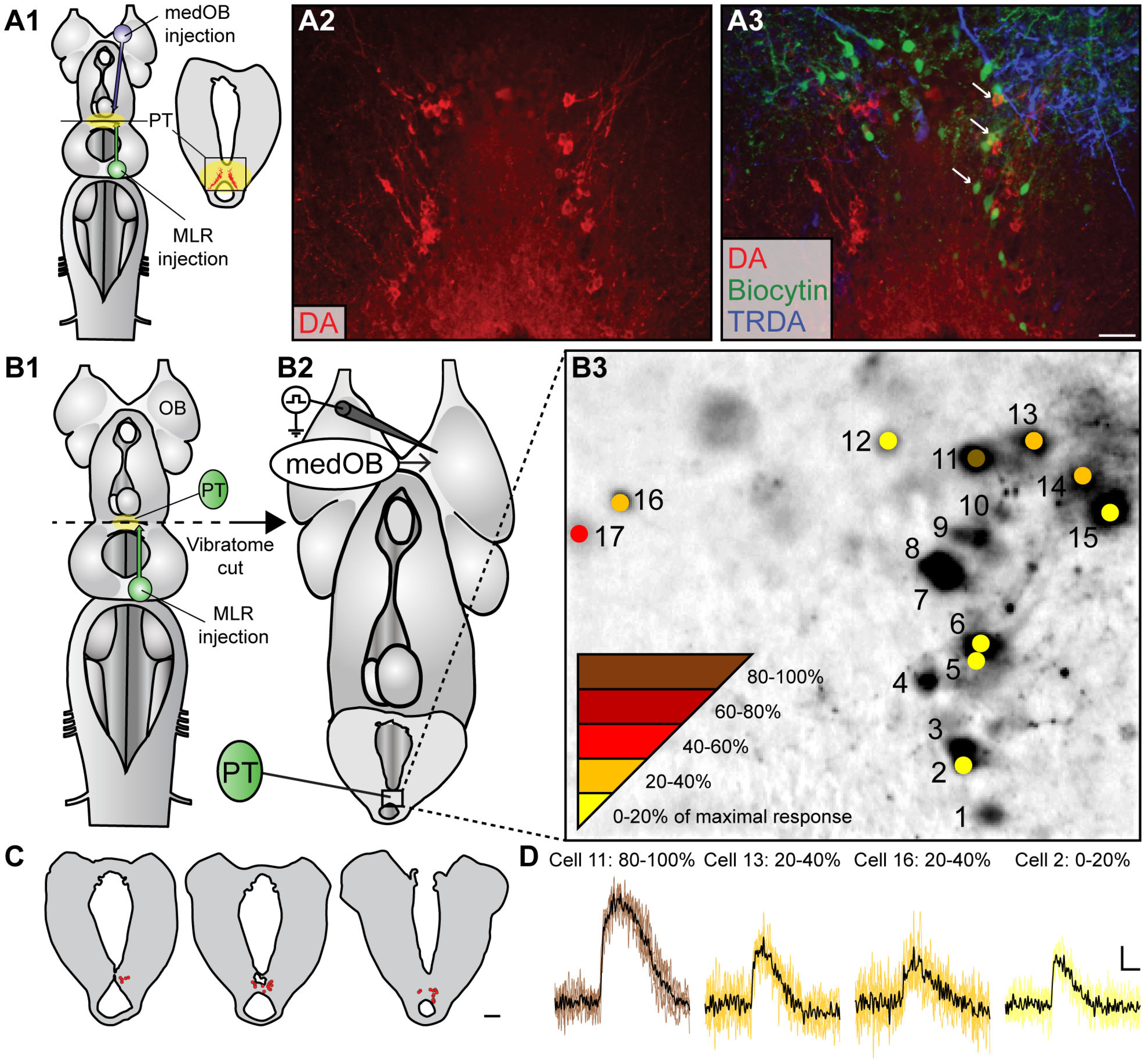
2.1. The medOB Projects to DA Neurons of the PT
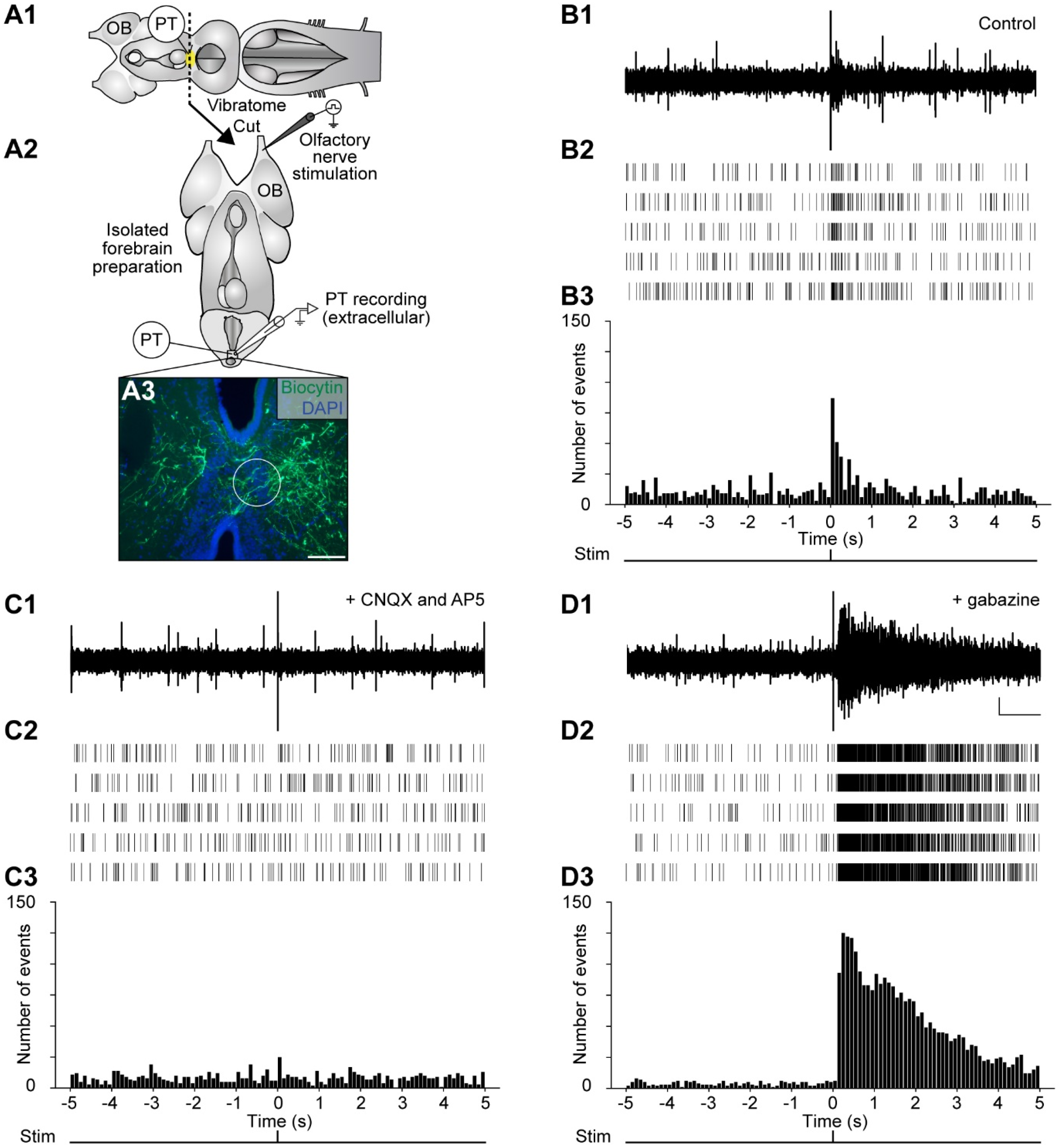
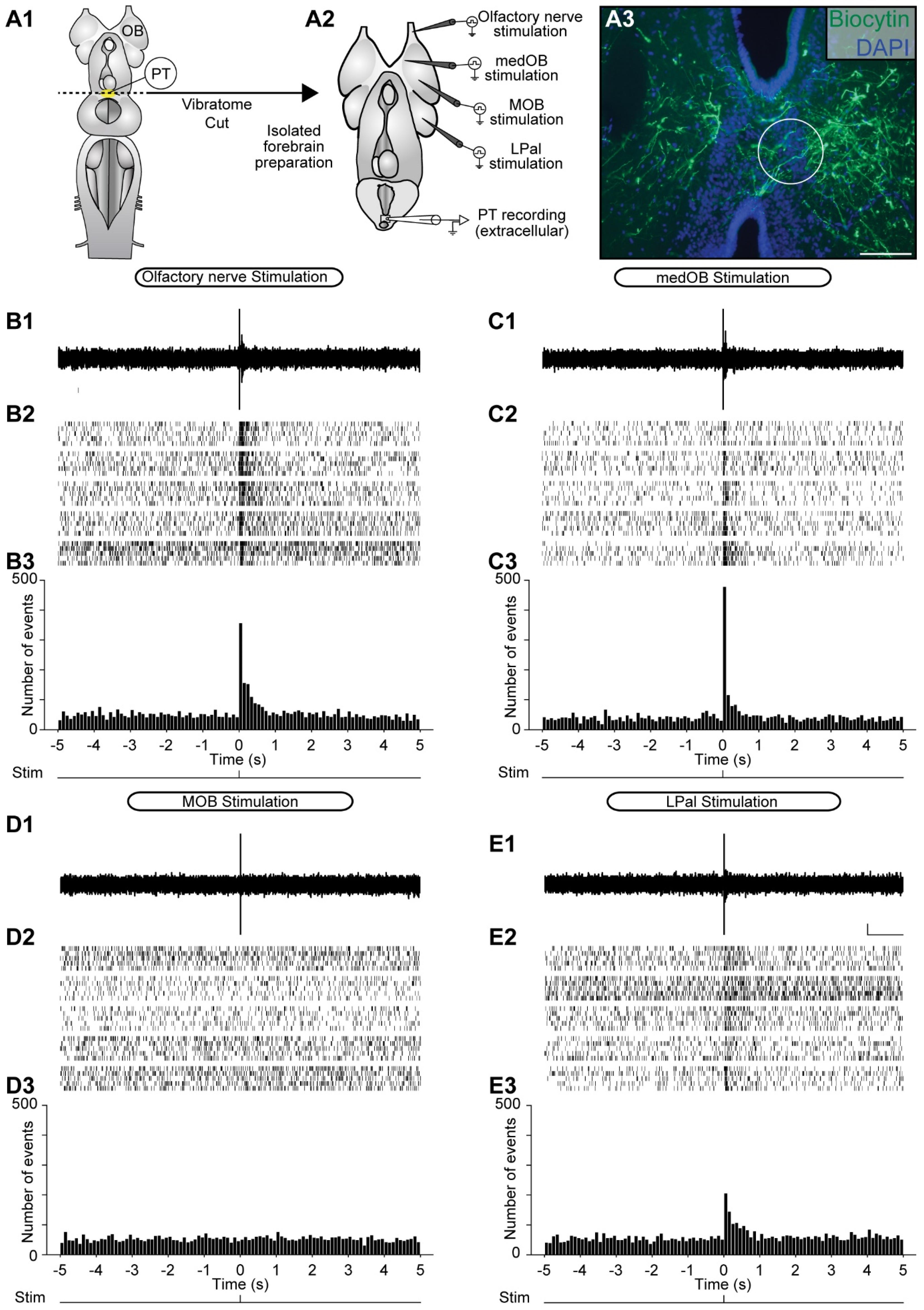
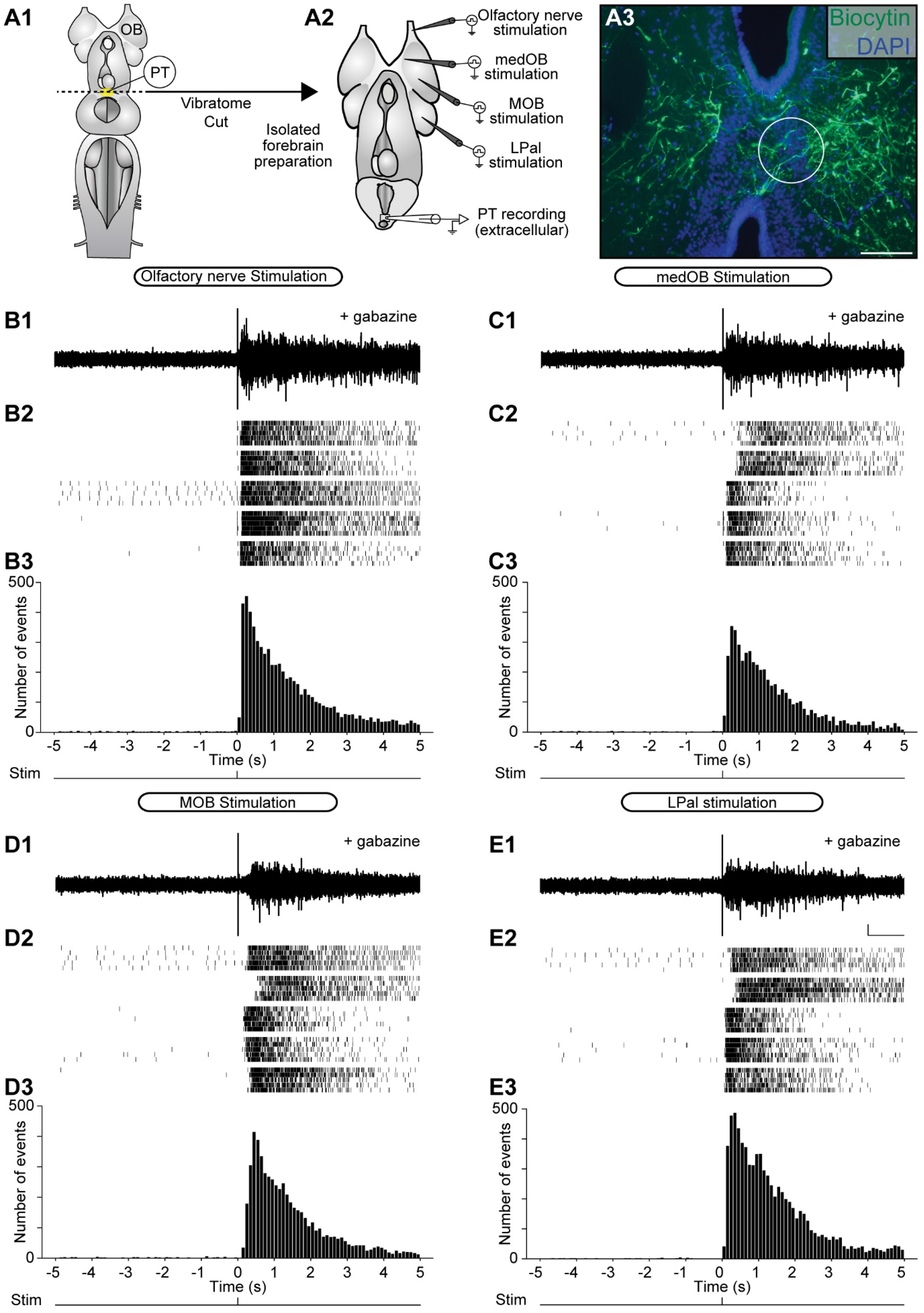
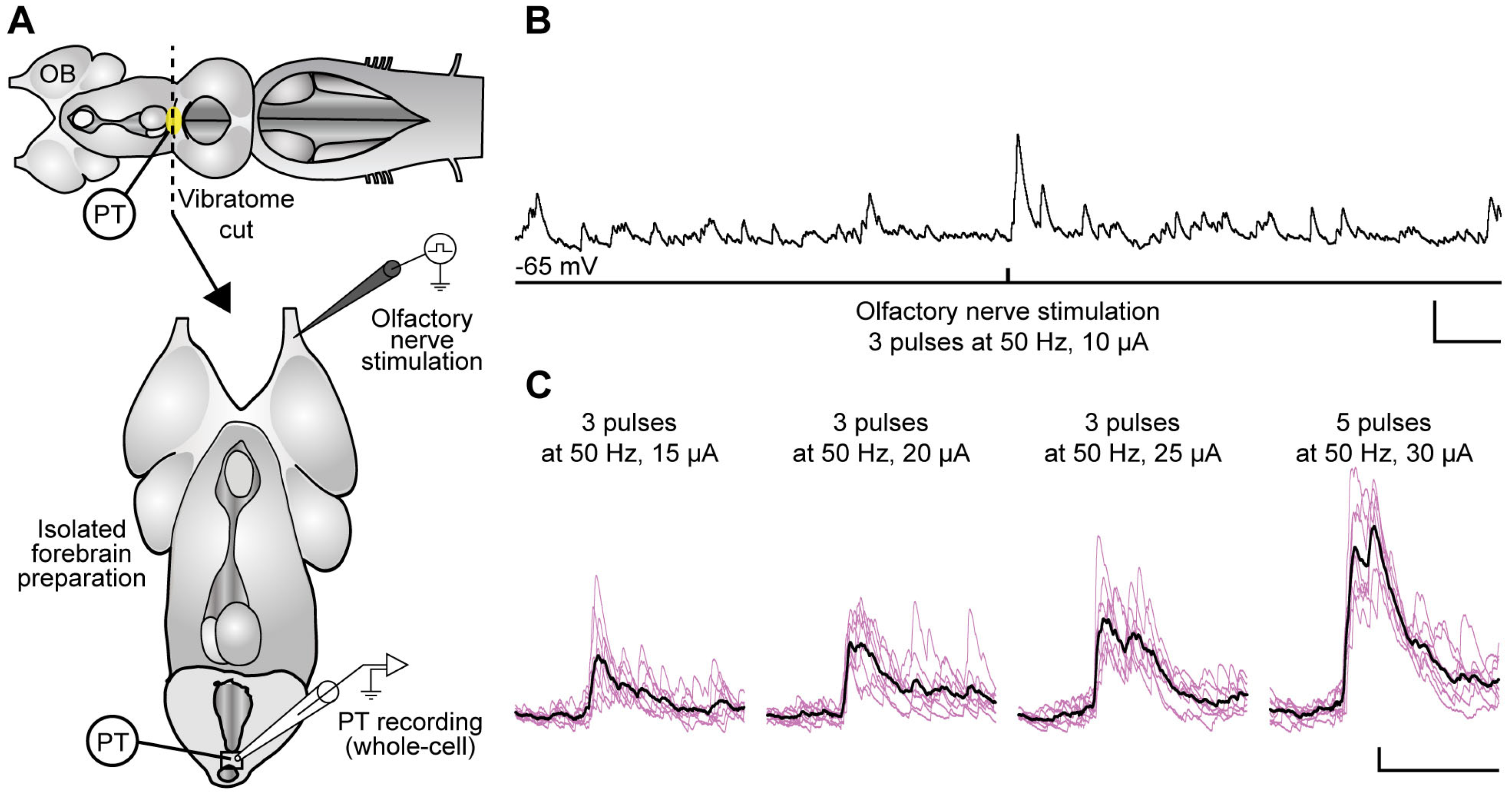
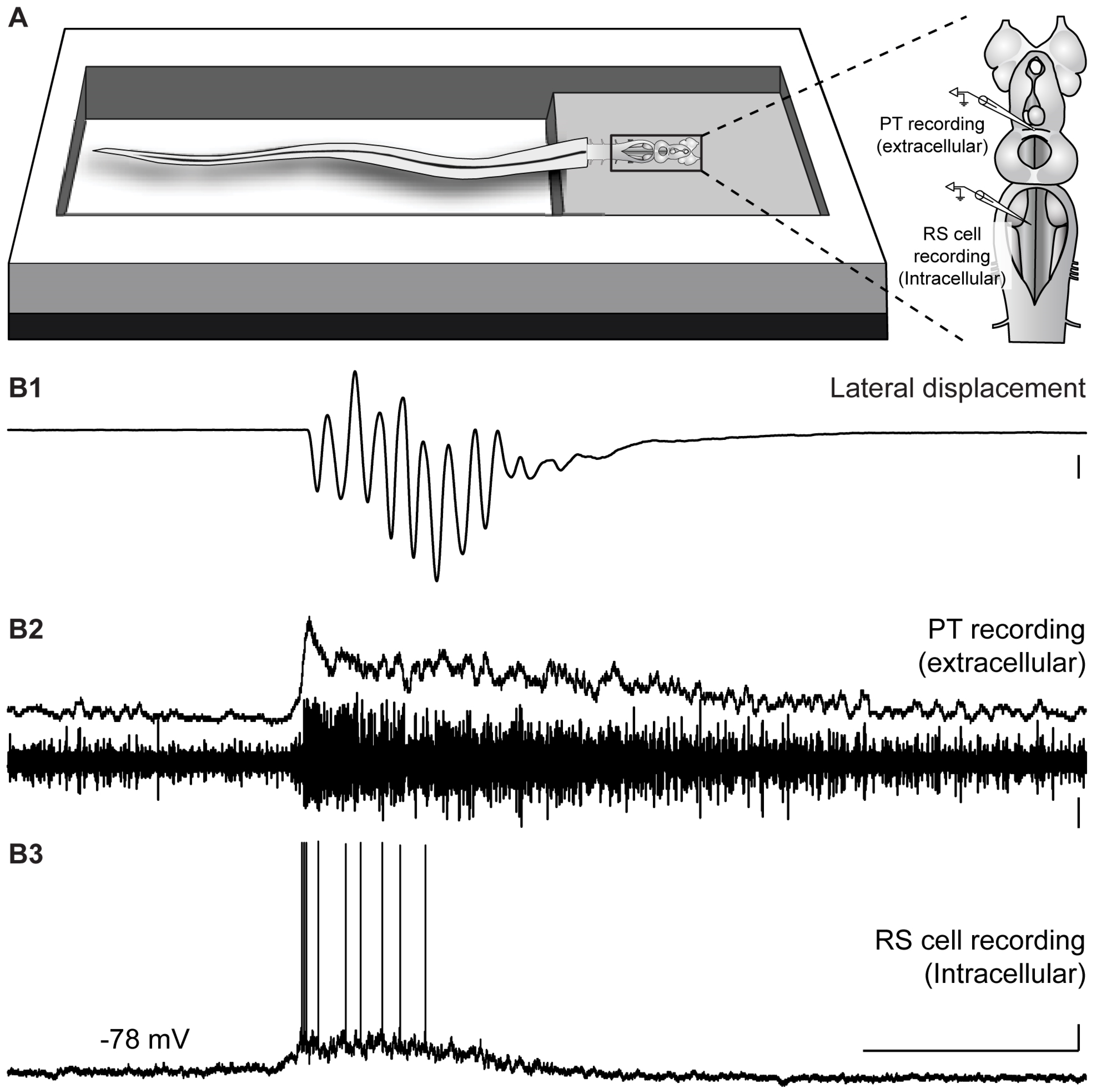
2.2. Stimulation of the Olfactomotor Circuitry Induces Neuronal Responses in the PT
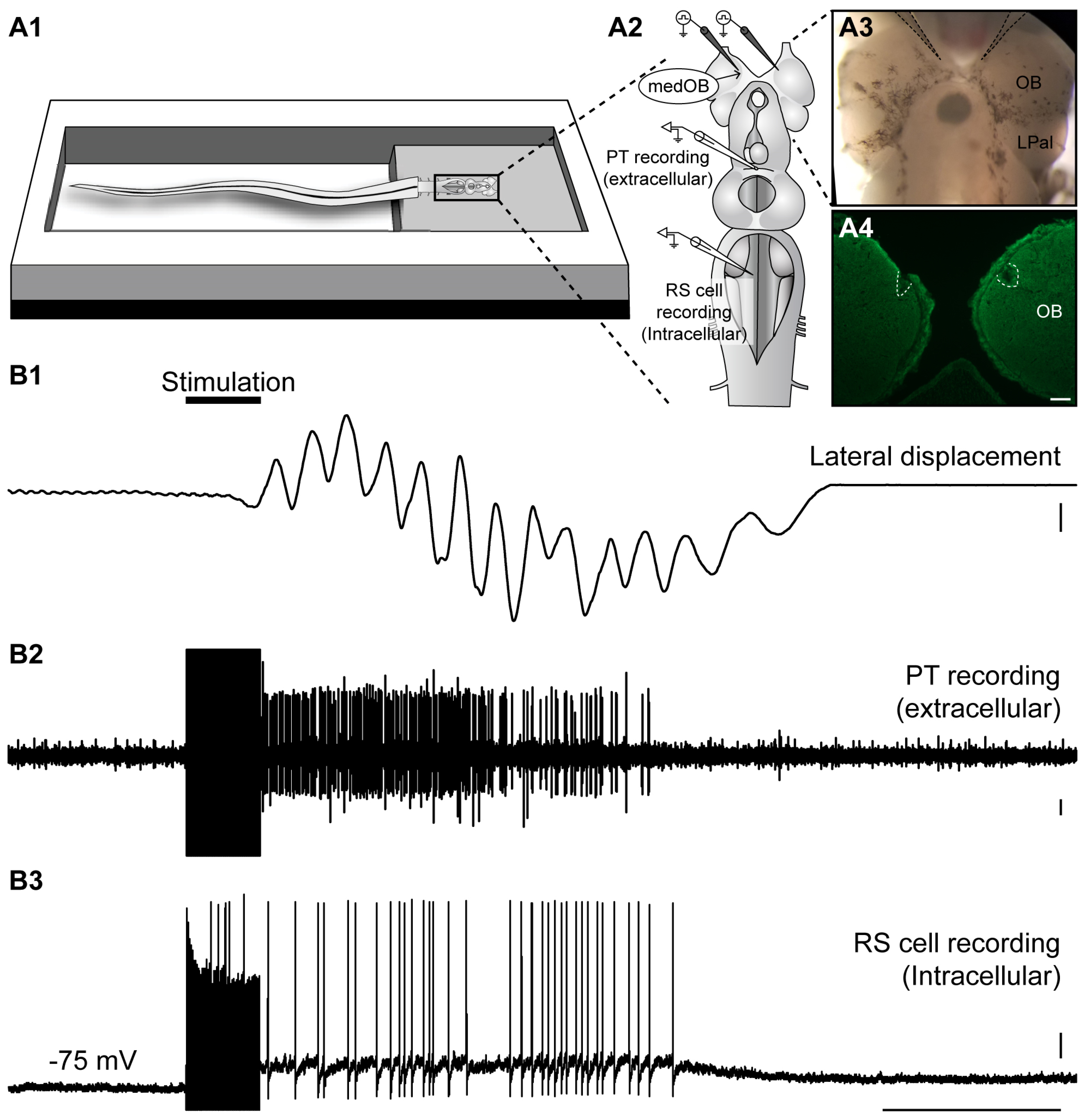
2.3. Neural Activity in the PT during Locomotion Induced by the Olfactomotor Circuitry
2.4. Neural Activity in the PT during Spontaneous Locomotion
3. Discussion
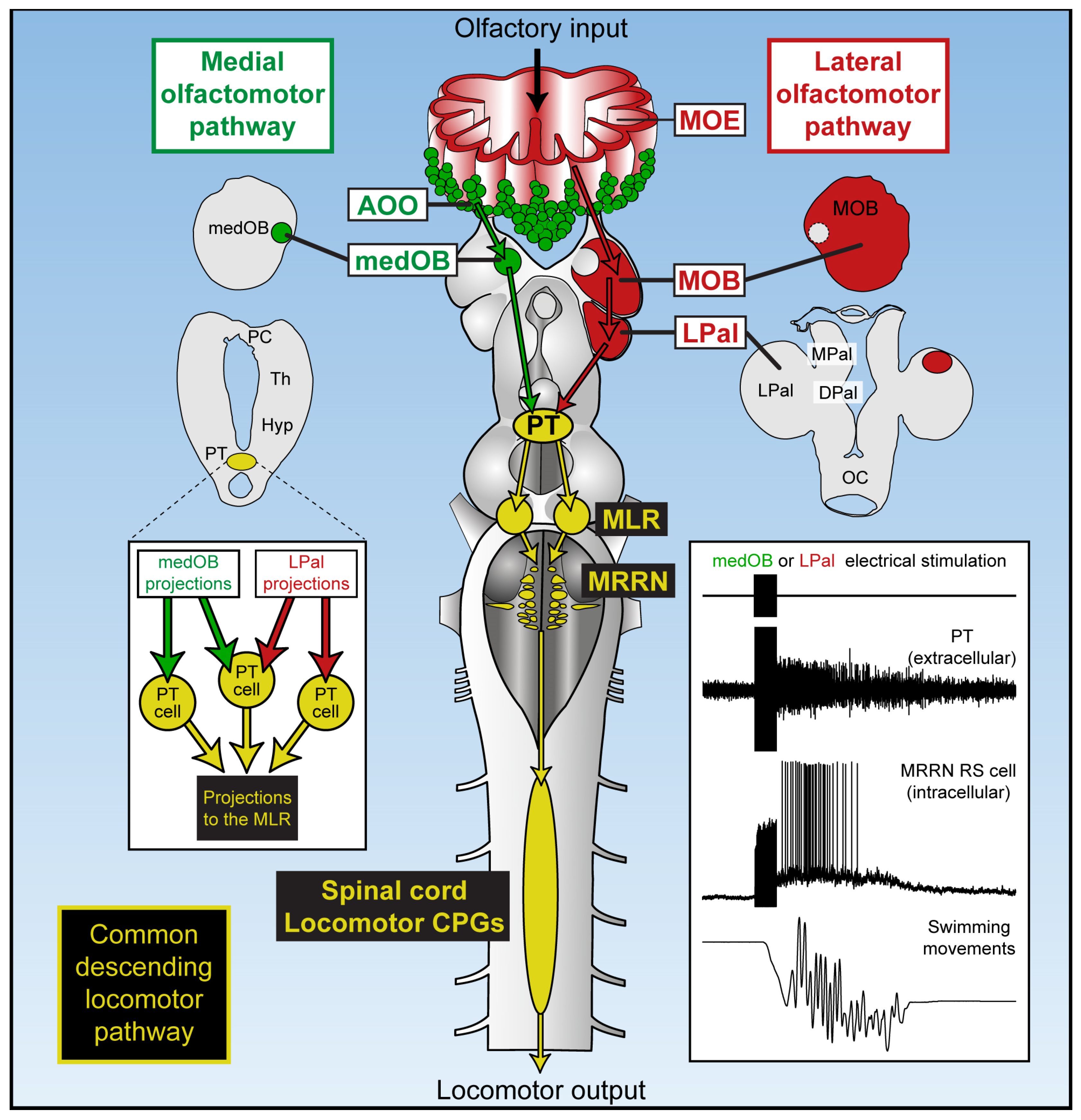
3.1. medOB Projections to DA Neurons of the PT
3.2. MOB and LPal Projections to the PT
3.3. Convergence of the Medial and Lateral Olfactomotor Pathways in the PT
3.4. The Role of the PT in Olfactory-Induced Locomotion
3.5. The Role of the PT in Locomotion
4. Materials and Methods
4.1. Animals
4.2. Anatomical Experiments
4.2.1. Isolated Whole Brain Preparation
4.2.2. Tracer Injection
4.2.3. Dopamine, Glutamate, and GABA Immunofluorescence
4.2.4. Fluorescence and Confocal Microscopy
4.2.5. Cleared Brain Whole Mount
4.3. Physiological Experiments
4.3.1. Isolated Forebrain Preparation
4.3.2. Electrical Stimulation
4.3.3. Drug Application
4.3.4. Extracellular Recordings
4.3.5. Whole-Cell Patch Clamp
4.3.6. Calcium Imaging
4.3.7. Intracellular Recordings (Sharp Glass Microelectrodes)
4.3.8. Semi-Intact Preparation
4.3.9. Kinematic Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baumgarten, H.G. Biogenic monoamines in the cyclostome and lower vertebrate brain. Prog. Histochem. Cytochem. 1972, 4, 1–90. [Google Scholar] [CrossRef] [PubMed]
- Pombal, M.A.; El Manira, A.; Grillner, S. Afferents of the lamprey striatum with special reference to the dopaminergic system: A combined tracing and immunohistochemical study. J. Comp. Neurol. 1997, 386, 71–91. [Google Scholar] [CrossRef]
- Pierre, J.; Mahouche, M.; Suderevskaya, E.I.; Repérant, J.; Ward, R. Immunocytochemical localization of dopamine and its synthetic enzymes in the central nervous system of the lamprey Lampetra fluviatilis. J. Comp. Neurol. 1997, 380, 119–135. [Google Scholar] [CrossRef]
- Pierre-Simons, J.; Repérant, J.; Mahouche, M.; Ward, R. Development of tyrosine hydroxylase-immunoreactive systems in the brain of the larval lamprey Lampetra fluviatilis. J. Comp. Neurol. 2002, 447, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Stephenson-Jones, M.; Ericsson, J.; Robertson, B.; Grillner, S. Evolution of the basal ganglia: Dual-output pathways conserved throughout vertebrate phylogeny. J. Comp. Neurol. 2012, 520, 2957–2973. [Google Scholar] [CrossRef] [PubMed]
- Stephenson-Jones, M.; Kardamakis, A.A.; Robertson, B.; Grillner, S. Independent circuits in the basal ganglia for the evaluation and selection of actions. Proc. Natl. Acad. Sci. USA 2013, 110, E3670–E3679. [Google Scholar] [CrossRef]
- Pérez-Fernández, J.; Stephenson-Jones, M.; Suryanarayana, S.M.; Robertson, B.; Grillner, S. Evolutionarily conserved organization of the dopaminergic system in lamprey: SNc/VTA afferent and efferent connectivity and D2 receptor expression. J. Comp. Neurol. 2014, 522, 3775–3794. [Google Scholar] [CrossRef]
- Pérez-Fernández, J.; Kardamakis, A.A.; Suzuki, D.G.; Robertson, B.; Grillner, S. Direct dopaminergic projections from the SNc modulate visuomotor transformation in the lamprey tectum. Neuron 2017, 96, 910–924.e5. [Google Scholar] [CrossRef]
- von Twickel, A.; Kowatschew, D.; Saltürk, M.; Schauer, M.; Robertson, B.; Korsching, S.; Walkowiak, W.; Grillner, S.; Pérez-Fernández, J. Individual dopaminergic neurons of lamprey SNc/VTA project to both the striatum and optic tectum but restrict co-release of glutamate to striatum only. Curr. Biol. 2019, 29, 677–685.e6. [Google Scholar] [CrossRef]
- Suryanarayana, S.M.; Pérez-Fernández, J.; Robertson, B.; Grillner, S. The lamprey forebrain—Evolutionary implications. Brain Behav. Evol. 2022, 96, 318–333. [Google Scholar] [CrossRef]
- Robertson, B.; Huerta-Ocampo, I.; Ericsson, J.; Stephenson-Jones, M.; Pérez-Fernández, J.; Bolam, J.P.; Diaz-Heijtz, R.; Grillner, S. The dopamine D2 receptor gene in lamprey, its expression in the striatum and cellular effects of D2 receptor activation. PLoS ONE 2012, 7, e35642. [Google Scholar] [CrossRef]
- Ericsson, J.; Stephenson-Jones, M.; Pérez-Fernández, J.; Robertson, B.; Silberberg, G.; Grillner, S. Dopamine differentially modulates the excitability of striatal neurons of the direct and indirect pathways in lamprey. J. Neurosci. 2013, 33, 8045–8054. [Google Scholar] [CrossRef] [PubMed]
- Ehringer, H.; Hornykiewicz, O. Distribution of noradrenaline and dopamine (3-hydroxytyramine) in the human brain and their behavior in diseases of the extrapyramidal system. Klin. Wochenschr. 1960, 38, 1236–1239. [Google Scholar] [CrossRef] [PubMed]
- Hornykiewicz, O. The tropical localization and content of noradrenalin and dopamine (3-hydroxytyramine) in the substantia nigra of normal persons and patients with Parkinson’s disease. Wien. Klin. Wochenschr. 1963, 75, 309–312. [Google Scholar]
- Poirier, L.J.; Sourkes, T.L. Influence of locus niger on the concentration of catecholamines in the striatum. J. Physiol. 1964, 56, 426–427. [Google Scholar]
- Dahlström, A.; Fuxe, K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol. Scand. 1964, 62 (Suppl. S232), 231–255. [Google Scholar]
- Bernheimer, H.; Birkmayer, W.; Hornykiewicz, O.; Jellinger, K.; Seitelberger, F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J. Neurol. Sci. 1973, 20, 415–455. [Google Scholar] [CrossRef]
- Olanow, C.W.; Tatton, W.G. Etiology and pathogenesis of Parkinson’s disease. Annu. Rev. Neurosci. 1999, 22, 123–144. [Google Scholar] [CrossRef]
- Hornykiewicz, O. Basic research on dopamine in Parkinson’s disease and the discovery of the nigrostriatal dopamine pathway: The view of an eyewitness. Neurodegener. Dis. 2008, 5, 114–117. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef]
- Ryczko, D.; Grätsch, S.; Auclair, F.; Dubé, C.; Bergeron, S.; Alpert, M.H.; Cone, J.J.; Roitman, M.F.; Alford, S.; Dubuc, R. Forebrain dopamine neurons project down to a brainstem region controlling locomotion. Proc. Natl. Acad. Sci. USA 2013, 110, E3235–E3242. [Google Scholar] [CrossRef]
- Ryczko, D.; Grätsch, S.; Schläger, L.; Keuyalian, A.; Boukhatem, Z.; Garcia, C.; Auclair, F.; Büschges, A.; Dubuc, R. Nigral glutamatergic neurons control the speed of locomotion. J. Neurosci. 2017, 37, 9759–9770. [Google Scholar] [CrossRef] [PubMed]
- Ryczko, D.; Grätsch, S.; Alpert, M.H.; Cone, J.J.; Kasemir, J.; Ruthe, A.; Beauséjour, P.A.; Auclair, F.; Roitman, M.F.; Alford, S.; et al. Descending dopaminergic inputs to reticulospinal neurons promote locomotor movements. J. Neurosci. 2020, 40, 8478–8490. [Google Scholar] [CrossRef] [PubMed]
- Derjean, D.; Moussaddy, A.; Atallah, E.; St-Pierre, M.; Auclair, F.; Chang, S.; Ren, X.; Zielinski, B.S.; Dubuc, R. A novel neural substrate for the transformation of olfactory inputs into motor output. PLoS Biol. 2010, 8, e1000567. [Google Scholar] [CrossRef] [PubMed]
- Heier, P. Fundamental principles in the structure of the brain. A study of the brain of Petromyzon fluviatilis. Acta Anat. 1948, 8, 3–213. [Google Scholar]
- Nieuwenhuys, R. The brain of the lamprey in a comparative perspective. Ann. N. Y. Acad. Sci. 1977, 299, 97–145. [Google Scholar] [CrossRef]
- Northcutt, R.G.; Puzdrowski, R.L. Projections of the olfactory bulb and nervus terminalis in the silver lamprey. Brain Behav. Evol. 1988, 32, 96–107. [Google Scholar] [CrossRef]
- Polenova, O.A.; Vesselkin, N.P. Olfactory and nonolfactory projections in the river lamprey (Lampetra fluviatilis) telencephalon. J. Hirnforsch. 1993, 34, 261–279. [Google Scholar]
- Green, W.W.; Basilious, A.; Dubuc, R.; Zielinski, B.S. The neuroanatomical organization of projection neurons associated with different olfactory bulb pathways in the sea lamprey, Petromyzon marinus. PLoS ONE 2013, 8, e69525. [Google Scholar] [CrossRef]
- Daghfous, G.; Auclair, F.; Clotten, F.; Létourneau, J.L.; Atallah, E.; Millette, J.P.; Derjean, D.; Robitaille, R.; Zielinski, B.S.; Dubuc, R. GABAergic modulation of olfactomotor transmission in lampreys. PLoS Biol. 2018, 16, e2005512. [Google Scholar] [CrossRef]
- Beauséjour, P.A.; Auclair, F.; Daghfous, G.; Ngovandan, C.; Veilleux, D.; Zielinski, B.S.; Dubuc, R. Dopaminergic modulation of olfactory-evoked motor output in sea lampreys (Petromyzon marinus L.). J. Comp. Neurol. 2020, 528, 114–134. [Google Scholar] [CrossRef] [PubMed]
- Suryanarayana, S.M.; Pérez-Fernández, J.; Robertson, B.; Grillner, S. Olfaction in lamprey pallium revisited—Dual projections of mitral and tufted cells. Cell Rep. 2021, 34, 108596. [Google Scholar] [CrossRef] [PubMed]
- Kleerekoper, H.; Mogensen, J. Role of olfaction in the orientation of Petromyzon marinus. I. Response to a single amine in prey’s body odor. Physiol Zool. 1963, 36, 347–360. [Google Scholar] [CrossRef]
- Bjerselius, R.; Li, W.; Teeter, J.H.; Seelye, J.G.; Johnsen, P.B.; Maniak, P.J.; Grant, G.C.; Polkinghorne, C.N.; Sorensen, P.W. Direct behavioral evidence that unique bile acids released by larval sea lamprey (Petromyzon marinus) function as a migratory pheromone. Can. J. Fish. Aquat. Sci. 2000, 57, 557–569. [Google Scholar] [CrossRef]
- Wickelgren, W.O. Physiological and anatomical characteristics of reticulospinal neurones in lamprey. J. Physiol. 1977, 270, 89–114. [Google Scholar] [CrossRef] [PubMed]
- Wickelgren, W.O. Post-tetanic potentiation, habituation and facilitation of synaptic potentials in reticulospinal neurones of lamprey. J. Physiol. 1977, 270, 115–131. [Google Scholar] [CrossRef]
- Rovainen, C.M. Synaptic interactions of reticulospinal neurons and nerve cells in the spinal cord of the sea lamprey. J. Comp. Neurol. 1974, 154, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, J.T.; Cohen, A.H. Activities of identified interneurons, motoneurons, and muscle fibers during fictive swimming in the lamprey and effects of reticulospinal and dorsal cell stimulation. J. Neurophysiol. 1982, 47, 948–960. [Google Scholar] [CrossRef]
- Buchanan, J.T.; Brodin, L.; Dale, N.; Grillner, S. Reticulospinal neurones activate excitatory amino acid receptors. Brain Res. 1987, 408, 321–325. [Google Scholar] [CrossRef]
- McClellan, A.D. Brainstem command systems for locomotion in the lamprey: Localization of descending pathways in the spinal cord. Brain Res. 1988, 457, 338–349. [Google Scholar] [CrossRef]
- Ohta, Y.; Grillner, S. Monosynaptic excitatory amino acid transmission from the posterior rhombencephalic reticular nucleus to spinal neurons involved in the control of locomotion in lamprey. J. Neurophysiol. 1989, 62, 1079–1089. [Google Scholar] [CrossRef]
- Viana Di Prisco, G.; Pearlstein, E.; Robitaille, R.; Dubuc, R. Role of sensory-evoked NMDA plateau potentials in the initiation of locomotion. Science 1997, 278, 1122–1125. [Google Scholar] [CrossRef]
- Beauséjour, P.A.; Zielinski, B.S.; Dubuc, R. Olfactory-induced locomotion in lampreys. Cell Tissue Res. 2022, 387, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Ocaña, F.M.; Suryanarayana, S.M.; Saitoh, K.; Kardamakis, A.A.; Capantini, L.; Robertson, B.; Grillner, S. The lamprey pallium provides a blueprint of the mammalian motor projections from cortex. Curr. Biol. 2015, 25, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Sirota, M.G.; Viana Di Prisco, G.; Dubuc, R. Stimulation of the mesencephalic locomotor region elicits controlled swimming in semi-intact lampreys. Eur. J. Neurosci. 2000, 12, 4081–4092. [Google Scholar] [CrossRef] [PubMed]
- Grätsch, S.; Auclair, F.; Demers, O.; Auguste, E.; Hanna, A.; Büschges, A.; Dubuc, R. A brainstem neural substrate for stopping locomotion. J. Neurosci. 2019, 39, 1044–1057. [Google Scholar] [CrossRef]
- Dubuc, R.; Brocard, F.; Antri, M.; Fénelon, K.; Gariépy, J.F.; Smetana, R.; Ménard, A.; Le Ray, D.; Viana Di Prisco, G.; Pearlstein, E.; et al. Initiation of locomotion in lampreys. Brain Res. Rev. 2008, 57, 172–182. [Google Scholar] [CrossRef]
- Ryczko, D.; Dubuc, R. The multifunctional mesencephalic locomotor region. Curr. Pharm. Des. 2013, 19, 4448–4470. [Google Scholar] [CrossRef]
- Grätsch, S.; Büschges, A.; Dubuc, R. Descending control of locomotor circuits. Curr. Opin. Physiol. 2019, 8, 94–98. [Google Scholar] [CrossRef]
- Le Ray, D.; Juvin, L.; Ryczko, D.; Dubuc, R. Supraspinal control of locomotion: The mesencephalic locomotor region. Prog. Brain Res. 2011, 188, 51–70. [Google Scholar] [CrossRef]
- Sulzer, D.; Joyce, M.P.; Lin, L.; Geldwert, D.; Haber, S.N.; Hattori, T.; Rayport, S. Dopamine neurons make glutamatergic synapses in vitro. J. Neurosci. 1998, 18, 4588–4602. [Google Scholar] [CrossRef]
- Morales, M.; Margolis, E.B. Ventral tegmental area: Cellular heterogeneity, connectivity and behaviour. Nat. Rev. Neurosci. 2017, 18, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Chang, S.; Laframboise, A.J.; Green, W.W.; Dubuc, R.; Zielinski, B.S. Projections from the accessory olfactory organ into the medial region of the olfactory bulb in the sea lamprey (Petromyzon marinus): A novel vertebrate sensory structure? J. Comp. Neurol. 2009, 516, 105–116. [Google Scholar] [CrossRef]
- Green, W.W.; Boyes, K.; McFadden, C.; Daghfous, G.; Auclair, F.; Zhang, H.; Li, W.; Dubuc, R.; Zielinski, B.S. Odorant organization in the olfactory bulb of the sea lamprey. J. Exp. Biol. 2017, 220, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Yáñez, J.; Folgueira, M.; Köhler, E.; Martínez, C.; Anadón, R. Connections of the terminal nerve and the olfactory system in two galeomorph sharks: An experimental study using a carbocyanine dye. J. Comp. Neurol. 2011, 519, 3202–3217. [Google Scholar] [CrossRef] [PubMed]
- Rooney, D.; Døving, K.B.; Ravaille-Veron, M.; Szabo, T. The central connections of the olfactory bulbs in cod, Gadus morhua L. J. Hirnforsch. 1992, 33, 63–75. [Google Scholar] [PubMed]
- Matz, S.P. Connections of the olfactory bulb in the chinook salmon (Oncorhynchus tshawytscha). Brain Behav. Evol. 1995, 46, 108–120. [Google Scholar] [CrossRef] [PubMed]
- von Bartheld, C.S.; Meyer, D.L.; Fiebig, E.; Ebbesson, S.O.E. Central connections of the olfactory bulb in the goldfish, Carassius auratus. Cell Tissue Res. 1984, 238, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Northcutt, R.G. Olfactory projections in the white sturgeon, Acipenser transmontanus: An experimental study. J. Comp. Neurol. 2011, 519, 1999–2022. [Google Scholar] [CrossRef]
- Northcutt, R.G.; Rink, E. Olfactory projections in the lepidosirenid lungfishes. Brain Behav. Evol. 2012, 79, 4–25. [Google Scholar] [CrossRef]
- Miyasaka, N.; Arganda-Carreras, I.; Wakisaka, N.; Masuda, M.; Sümbül, U.; Seung, H.S.; Yoshihara, Y. Olfactory projectome in the zebrafish forebrain revealed by genetic single-neuron labelling. Nat. Commun. 2014, 5, 3639. [Google Scholar] [CrossRef] [PubMed]
- Filippi, A.; Mueller, T.; Driever, W. vglut2 and gad expression reveal distinct patterns of dual GABAergic versus glutamatergic cotransmitter phenotypes of dopaminergic and noradrenergic neurons in the zebrafish brain. J. Comp. Neurol. 2014, 522, 2019–2037. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Wang, H.L.; Li, X.; Ng, T.H.; Morales, M. Mesocorticolimbic glutamatergic pathway. J. Neurosci. 2011, 31, 8476–8490. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Wang, H.L.; Morales, M. Glutamate neurons in the substantia nigra compacta and retrorubral field. Eur. J. Neurosci. 2013, 38, 3602–3610. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Qi, J.; Wang, H.L.; Zhang, S.; Morales, M. Glutamatergic and dopaminergic neurons in the mouse ventral tegmental area. Eur. J. Neurosci. 2015, 41, 760–772. [Google Scholar] [CrossRef]
- Root, D.H.; Wang, H.L.; Liu, B.; Barker, D.J.; Mód, L.; Szocsics, P.; Silva, A.C.; Maglóczky, Z.; Morales, M. Glutamate neurons are intermixed with midbrain dopamine neurons in nonhuman primates and humans. Sci. Rep. 2016, 6, 30615. [Google Scholar] [CrossRef]
- Fernández-López, B.; Sobrido-Cameán, D.; Anadón, R.; Rodicio, M.C.; Barreiro-Iglesias, A. Restricted co-localization of glutamate and dopamine in neurons of the adult sea lamprey brain. J. Anat. 2017, 231, 776–784. [Google Scholar] [CrossRef]
- Barreiro-Iglesias, A.; Villar-Cerviño, V.; Anadón, R.; Rodicio, M.C. Dopamine and gamma-aminobutyric acid are colocalized in restricted groups of neurons in the sea lamprey brain: Insights into the early evolution of neurotransmitter colocalization in vertebrates. J. Anat. 2009, 215, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.B. The brain of Petromyzon. J. Comp. Neurol. 1902, 12, 1–86. [Google Scholar] [CrossRef]
- Edinger, L. Die deutung des vorderhirns bei Petromyzon. Anat. Anz. 1905, 26, 633–635. [Google Scholar]
- Ariëns Kappers, C.U.; Theunissen, W.F. Die phylogenese des rhinencephalons des corpus striatum und der vorderhirnkommissuren. Folia Neurobiol. 1908, 1, 173–288. [Google Scholar]
- Herrick, C.J.; Obenchain, J.B. Notes on the anatomy of a cyclostome brain: Ichthyomyzon concolor. J. Comp. Neurol. 1913, 23, 635–675. [Google Scholar]
- Nieuwenhuys, R. Comparative anatomy of olfactory centres and tracts. Prog. Brain Res. 1967, 23, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Northcutt, R.G.; Wicht, H. Afferent and efferent connections of the lateral and medial pallia of the silver lamprey. Brain Behav. Evol. 1997, 49, 1–19. [Google Scholar] [CrossRef]
- Suryanarayana, S.M.; Robertson, B.; Wallén, P.; Grillner, S. The lamprey pallium provides a blueprint of the mammalian layered cortex. Curr. Biol. 2017, 27, 3264–3277.e5. [Google Scholar] [CrossRef] [PubMed]
- Suryanarayana, S.M.; Pérez-Fernández, J.; Robertson, B.; Grillner, S. The evolutionary origin of visual and somatosensory representation in the vertebrate pallium. Nat. Ecol. Evol. 2020, 4, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Villar-Cerviño, V.; Barreiro-Iglesias, A.; Mazan, S.; Rodicio, M.C.; Anadón, R. Glutamatergic neuronal populations in the forebrain of the sea lamprey, Petromyzon marinus: An in situ hybridization and immunocytochemical study. J. Comp. Neurol. 2011, 519, 1712–1735. [Google Scholar] [CrossRef]
- Ménard, A.; Auclair, F.; Bourcier-Lucas, C.; Grillner, S.; Dubuc, R. Descending GABAergic projections to the mesencephalic locomotor region in the lamprey Petromyzon marinus. J. Comp. Neurol. 2007, 501, 260–273. [Google Scholar] [CrossRef]
- Ericsson, J.; Silberberg, G.; Robertson, B.; Wikström, M.A.; Grillner, S. Striatal cellular properties conserved from lampreys to mammals. J. Physiol. 2011, 589, 2979–2992. [Google Scholar] [CrossRef]
- Stephenson-Jones, M.; Samuelsson, E.; Ericsson, J.; Robertson, B.; Grillner, S. Evolutionary conservation of the basal ganglia as a common vertebrate mechanism for action selection. Curr. Biol. 2011, 21, 1081–1091. [Google Scholar] [CrossRef]
- Ericsson, J.; Stephenson-Jones, M.; Kardamakis, A.A.; Robertson, B.; Silberberg, G.; Grillner, S. Evolutionarily conserved differences in pallial and thalamic short-term synaptic plasticity in striatum. J. Physiol. 2013, 591, 859–874. [Google Scholar] [CrossRef] [PubMed]
- Grillner, S.; Robertson, B.; Stephenson-Jones, M. The evolutionary origin of the vertebrate basal ganglia and its role in action selection. J. Physiol. 2013, 591, 5425–5431. [Google Scholar] [CrossRef] [PubMed]
- Grillner, S.; Robertson, B. The basal ganglia over 500 million years. Curr. Biol. 2016, 26, R1088–R1100. [Google Scholar] [CrossRef]
- Kravitz, A.V.; Freeze, B.S.; Parker, P.R.; Kay, K.; Thwin, M.T.; Deisseroth, K.; Kreitzer, A.C. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 2010, 466, 622–626. [Google Scholar] [CrossRef]
- Roseberry, T.K.; Lee, A.M.; Lalive, A.L.; Wilbrecht, L.; Bonci, A.; Kreitzer, A.C. Cell-type-specific control of brainstem locomotor circuits by basal ganglia. Cell 2016, 164, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Ryczko, D.; Cone, J.J.; Alpert, M.H.; Goetz, L.; Auclair, F.; Dubé, C.; Parent, M.; Roitman, M.F.; Alford, S.; Dubuc, R. A descending dopamine pathway conserved from basal vertebrates to mammals. Proc. Natl. Acad. Sci. USA 2016, 113, E2440–E2449. [Google Scholar] [CrossRef]
- Juárez Tello, A.; van der Zouwen, C.I.; Dejas, L.; Duque-Yate, J.; Boutin, J.; Medina-Ortiz, K.; Suresh, J.S.; Swiegers, J.; Sarret, P.; Ryczko, D. Dopamine-sensitive neurons in the mesencephalic locomotor region control locomotion initiation, stop, and turns. Cell Rep. 2024, 43, 114187. [Google Scholar] [CrossRef]
- Howe, M.W.; Dombeck, D.A. Rapid signalling in distinct dopaminergic axons during locomotion and reward. Nature 2016, 535, 505–510. [Google Scholar] [CrossRef]
- Ryczko, D.; Dubuc, R. Dopamine and the brainstem locomotor networks: From lamprey to human. Front. Neurosci. 2017, 11, 295. [Google Scholar] [CrossRef]
- Ryczko, D.; Dubuc, R. Dopamine control of downstream motor centers. Curr. Opin. Neurobiol. 2023, 83, 102785. [Google Scholar] [CrossRef]
- da Silva, J.A.; Tecuapetla, F.; Paixão, V.; Costa, R.M. Dopamine neuron activity before action initiation gates and invigorates future movements. Nature 2018, 554, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Poirier, L.J. Experimental and histological study of midbrain dyskinesias. J. Neurophysiol. 1960, 23, 534–551. [Google Scholar] [CrossRef]
- Poirier, L.J.; Sourkes, T.L. Influence of the substantia nigra on the catecholamine content of the striatum. Brain 1965, 88, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, P.; Lozano, A.; Stanzione, P.; Galati, S.; Scarnati, E.; Peppe, A.; Stefani, A. Implantation of human pedunculopontine nucleus: A safe and clinically relevant target in Parkinson’s disease. NeuroReport 2005, 16, 1877–1881. [Google Scholar] [CrossRef] [PubMed]
- Plaha, P.; Gill, S.S. Bilateral deep brain stimulation of the pedunculopontine nucleus for Parkinson’s disease. NeuroReport 2005, 16, 1883–1887. [Google Scholar] [CrossRef]
- Thevathasan, W.; Pogosyan, A.; Hyam, J.A.; Jenkinson, N.; Foltynie, T.; Limousin, P.; Bogdanovic, M.; Zrinzo, L.; Green, A.L.; Aziz, T.Z.; et al. Alpha oscillations in the pedunculopontine nucleus correlate with gait performance in parkinsonism. Brain 2012, 135, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Shine, J.M.; Matar, E.; Ward, P.B.; Bolitho, S.J.; Gilat, M.; Pearson, M.; Naismith, S.L.; Lewis, S.J. Exploring the cortical and subcortical functional magnetic resonance imaging changes associated with freezing in Parkinson’s disease. Brain 2013, 136, 1204–1215. [Google Scholar] [CrossRef]
- Ryczko, D. The mesencephalic locomotor region: Multiple cell types, multiple behavioral roles, and multiple implications for disease. Neuroscientist 2024, 30, 347–366. [Google Scholar] [CrossRef]
- Mellone, S.; Mancini, M.; King, L.A.; Horak, F.B.; Chiari, L. The quality of turning in Parkinson’s disease: A compensatory strategy to prevent postural instability? J. Neuroeng. Rehabil. 2016, 13, 39. [Google Scholar] [CrossRef]
- Gao, C.; Liu, J.; Tan, Y.; Chen, S. Freezing of gait in Parkinson’s disease: Pathophysiology, risk factors and treatments. Transl. Neurodegener. 2020, 9, 12. [Google Scholar] [CrossRef]
- Rahimpour, S.; Gaztanaga, W.; Yadav, A.P.; Chang, S.J.; Krucoff, M.O.; Cajigas, I.; Turner, D.A.; Wang, D.D. Freezing of gait in Parkinson’s disease: Invasive and noninvasive neuromodulation. Neuromodulation 2021, 24, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Rolland, A.S.; Tandé, D.; Herrero, M.T.; Luquin, M.R.; Vazquez-Claverie, M.; Karachi, C.; Hirsch, E.C.; Francois, C. Evidence for a dopaminergic innervation of the pedunculopontine nucleus in monkeys, and its drastic reduction after MPTP intoxication. J. Neurochem. 2009, 110, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, M.F. Dopamine signaling in substantia nigra and its impact on locomotor function—Not a new concept, but neglected reality. Int. J. Mol. Sci. 2024, 25, 1131. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.H.; Ménard, A.; Pombal, M.A.; Grillner, S. Forebrain dopamine depletion impairs motor behavior in lamprey. Eur. J. Neurosci. 2008, 27, 1452–1460. [Google Scholar] [CrossRef]
- Wullimann, M.F.; Rink, E. Detailed immunohistology of Pax6 protein and tyrosine hydroxylase in the early zebrafish brain suggests role of Pax6 gene in development of dopaminergic diencephalic neurons. Dev. Brain Res. 2001, 131, 173–191. [Google Scholar] [CrossRef] [PubMed]
- Tay, T.L.; Ronneberger, O.; Ryu, S.; Nitschke, R.; Driever, W. Comprehensive catecholaminergic projectome analysis reveals single-neuron integration of zebrafish ascending and descending dopaminergic systems. Nat. Commun. 2011, 2, 171. [Google Scholar] [CrossRef]
- Rink, E.; Wullimann, M.F. The teleostean (zebrafish) dopaminergic system ascending to the subpallium (striatum) is located in the basal diencephalon (posterior tuberculum). Brain Res. 2001, 889, 316–330. [Google Scholar] [CrossRef]
- Kastenhuber, E.; Kratochwil, C.F.; Ryu, S.; Schweitzer, J.; Driever, W. Genetic dissection of dopaminergic and noradrenergic contributions to catecholaminergic tracts in early larval zebrafish. J. Comp. Neurol. 2010, 518, 439–458. [Google Scholar] [CrossRef]
- Jay, M.; De Faveri, F.; McDearmid, J.R. Firing dynamics and modulatory actions of supraspinal dopaminergic neurons during zebrafish locomotor behavior. Curr. Biol. 2015, 25, 435–444. [Google Scholar] [CrossRef]
- Reinig, S.; Driever, W.; Arrenberg, A.B. The descending diencephalic dopamine system is tuned to sensory stimuli. Curr. Biol. 2017, 27, 318–333. [Google Scholar] [CrossRef]
- Barrios, J.P.; Wang, W.C.; England, R.; Reifenberg, E.; Douglass, A.D. Hypothalamic dopamine neurons control sensorimotor behavior by modulating brainstem premotor nuclei in zebrafish. Curr. Biol. 2020, 30, 4606–4618.e4. [Google Scholar] [CrossRef]
- Mu, Y.; Li, X.Q.; Zhang, B.; Du, J.L. Visual input modulates audiomotor function via hypothalamic dopaminergic neurons through a cooperative mechanism. Neuron 2012, 75, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Jha, U.; Thirumalai, V. Neuromodulatory selection of motor neuron recruitment patterns in a visuomotor behavior increases speed. Curr. Biol. 2020, 30, 788–801.e3. [Google Scholar] [CrossRef]
- McPherson, A.D.; Barrios, J.P.; Luks-Morgan, S.J.; Manfredi, J.P.; Bonkowsky, J.L.; Douglass, A.D.; Dorsky, R.I. Motor behavior mediated by continuously generated dopaminergic neurons in the zebrafish hypothalamus recovers after cell ablation. Curr. Biol. 2016, 26, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Ericsson, J.; Robertson, B.; Wikström, M.A. A lamprey striatal brain slice preparation for patch-clamp recordings. J. Neurosci. Methods 2007, 165, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Li, K. The Image Stabilizer Plugin for ImageJ. 2008. Available online: http://www.cs.cmu.edu/~kangli/code/Image_Stabilizer.html (accessed on 30 June 2021).
- Gray, J. Studies in animal locomotion: II. The relationship between waves of muscular contraction and the propulsive mechanism of the eel. J. Exp. Biol. 1933, 10, 386–390. [Google Scholar] [CrossRef]
- Scott, W.B. Notes on the development of Petromyzon. J. Morphol. 1896, 1, 253–310. [Google Scholar] [CrossRef]
- Hagelin, L.O.; Johnels, A.G. On the structure and function of the accessory olfactory organ in lampreys. Acta Zool. 1955, 36, 113–125. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beauséjour, P.-A.; Veilleux, J.-C.; Condamine, S.; Zielinski, B.S.; Dubuc, R. Olfactory Projections to Locomotor Control Centers in the Sea Lamprey. Int. J. Mol. Sci. 2024, 25, 9370. https://doi.org/10.3390/ijms25179370
Beauséjour P-A, Veilleux J-C, Condamine S, Zielinski BS, Dubuc R. Olfactory Projections to Locomotor Control Centers in the Sea Lamprey. International Journal of Molecular Sciences. 2024; 25(17):9370. https://doi.org/10.3390/ijms25179370
Chicago/Turabian StyleBeauséjour, Philippe-Antoine, Jean-Christophe Veilleux, Steven Condamine, Barbara S. Zielinski, and Réjean Dubuc. 2024. "Olfactory Projections to Locomotor Control Centers in the Sea Lamprey" International Journal of Molecular Sciences 25, no. 17: 9370. https://doi.org/10.3390/ijms25179370
APA StyleBeauséjour, P. -A., Veilleux, J. -C., Condamine, S., Zielinski, B. S., & Dubuc, R. (2024). Olfactory Projections to Locomotor Control Centers in the Sea Lamprey. International Journal of Molecular Sciences, 25(17), 9370. https://doi.org/10.3390/ijms25179370




