Selenium-Binding Protein 1 (SBP1): A New Putative Player of Stress Sensing in Plants
Abstract
:1. The Discovery and Importance of Selenium in Plants
2. Discovery of SBP
3. New Insights into the Role of SBPs
4. Exploring the Role of SBPs in Plant Physiology
5. Structure
6. Exploring Gene Expression and Selenium Tolerance in Plants
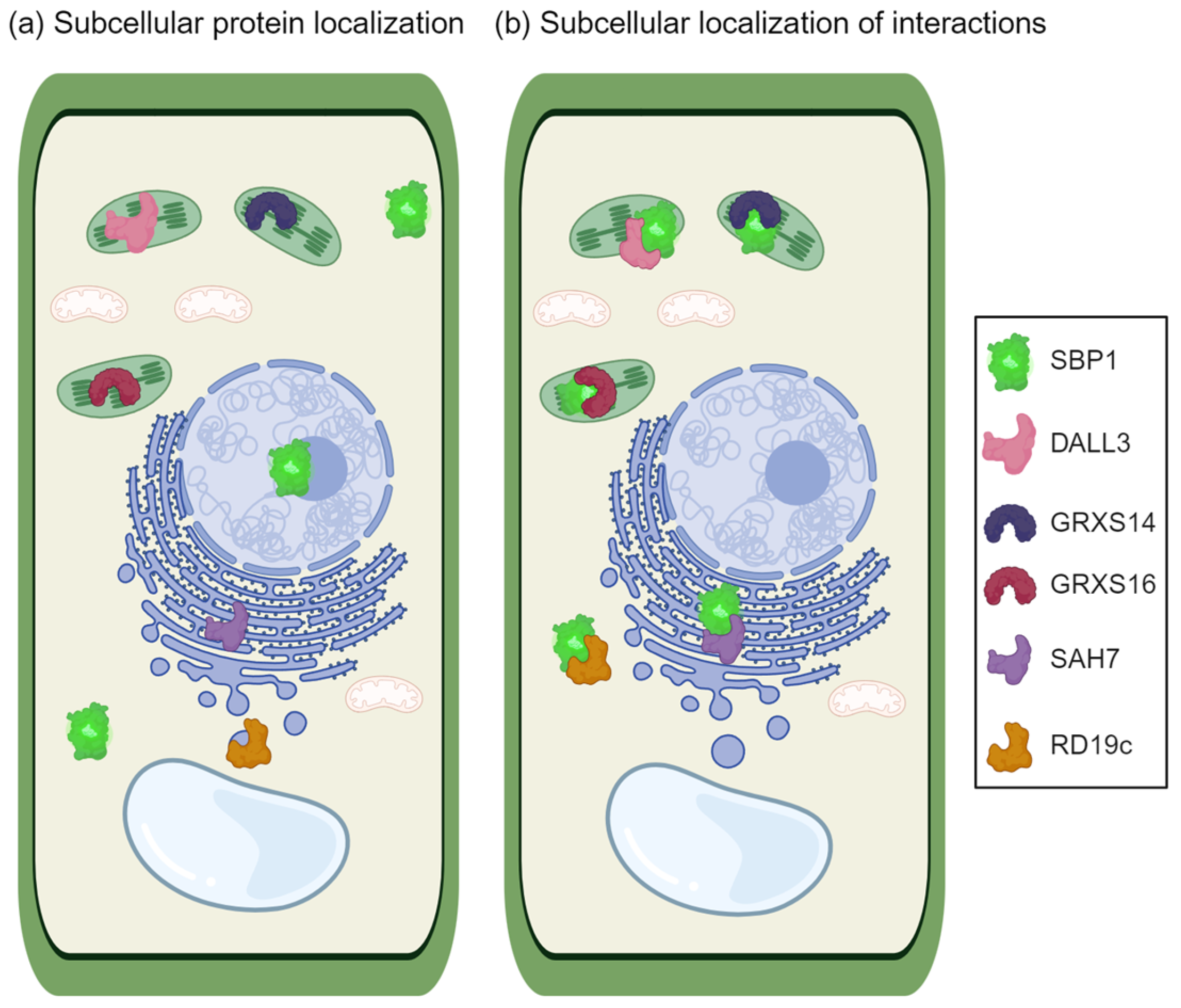
7. Unveiling the Intricate Web of Protein Interactions in Plant Selenium Metabolism
8. Proposed Biochemical/Molecular Action of SBP
9. Conclusions
Funding
Conflicts of Interest
References
- Schwarz, K.; Foltz, C.M. Selenium as an Integral Part of Factor 3 against Dietary Necrotic Liver Degeneration. J. Am. Chem. Soc. 1957, 79, 3293. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Raza, A.; Hawrylak-Nowak, B.; Matraszek-Gawron, R.; Mahmud, J.A.; Nahar, K.; Fujita, M. Selenium in Plants: Boon or Bane? Environ. Exp. Bot. 2020, 178, 104170. [Google Scholar] [CrossRef]
- Floor, G.H.; Román-Ross, G. Selenium in Volcanic Environments: A Review. Appl. Geochem. 2012, 27, 517–531. [Google Scholar] [CrossRef]
- Boyd, R. Selenium Stories. Nat. Chem. 2011, 3, 570. [Google Scholar] [CrossRef] [PubMed]
- Reich, H.J.; Hondal, R.J. Why Nature Chose Selenium. ACS Chem. Biol. 2016, 11, 821–841. [Google Scholar] [CrossRef]
- Lu, J.; Holmgren, A. Selenoproteins. J. Biol. Chem. 2009, 284, 723–727. [Google Scholar] [CrossRef]
- Bodnar, M.; Konieczka, P.; Namiesnik, J. The Properties, Functions, and Use of Selenium Compounds in Living Organisms. J. Environ. Sci. Health Part C 2012, 30, 225–252. [Google Scholar] [CrossRef]
- Agalou, A.; Spaink, H.P.; Roussis, A. Novel Interaction of Selenium-Binding Protein with Glyceraldehyde-3-Phosphate Dehydrogenase and Fructose-Bisphosphate Aldolase of Arabidopsis Thaliana. Funct. Plant Biol. 2006, 33, 847–856. [Google Scholar] [CrossRef]
- Dervisi, I.; Valassakis, C.; Koletti, A.; Kouvelis, V.N.; Flemetakis, E.; Ouzounis, C.A.; Roussis, A. Evolutionary Aspects of Selenium Binding Protein (SBP). J. Mol. Evol. 2023, 91, 471–481. [Google Scholar] [CrossRef]
- Bansal, M.P.; Mukhopadhyay, T.; Scott, J.; Cook, R.G.; Mukhopadhyay, R.; Medina, D. DNA Sequencing of a Mouse Liver Protein That Binds Selenium: Implications for Selenium’s Mechanism of Action in Cancer Prevention. Carcinogenesis 1990, 11, 2071–2073. [Google Scholar] [CrossRef]
- Bansal, M.P.; Cook, R.G.; Danielson, K.G.; Medina, D. A 14-Kilodalton Selenium-Binding Protein in Mouse Liver Is Fatty Acid-Binding Protein. J. Biol. Chem. 1989, 264, 13780–13784. [Google Scholar] [CrossRef] [PubMed]
- Giometti, C.S.; Liang, X.; Tollaksen, S.L.; Wall, D.B.; Lubman, D.M.; Subbarao, V.; Sambasiva Rao, M. Mouse Liver Selenium-Binding Protein Decreased in Abundance by Peroxisome Proliferators. Electrophoresis 2000, 21, 2162–2169. [Google Scholar] [CrossRef]
- Chang, P.W.G.; Tsui, S.K.W.; Liew, C.; Lee, C.; Waye, M.M.Y.; Fung, K. Isolation, Characterization, and Chromosomal Mapping of a Novel cDNA Clone Encoding Human Selenium Binding Protein. J. Cell. Biochem. 1997, 64, 217–224. [Google Scholar] [CrossRef]
- Torrealba, J.R.; Colburn, M.; Golner, S.; Chang, Z.; Scheunemann, T.; Fechner, J.H.; Roenneburg, D.; Hu, H.; Alam, T.; Kim, H.T.; et al. Selenium-Binding Protein-1 in Smooth Muscle Cells Is Downregulated in a Rhesus Monkey Model of Chronic Allograft Nephropathy. Am. J. Transplant. 2005, 5, 58–67. [Google Scholar] [CrossRef]
- Chen, G.; Wang, H.; Miller, C.T.; Thomas, D.G.; Gharib, T.G.; Misek, D.E.; Giordano, T.J.; Orringer, M.B.; Hanash, S.M.; Beer, D.G. Reduced Selenium-binding Protein 1 Expression Is Associated with Poor Outcome in Lung Adenocarcinomas. J. Pathol. 2004, 202, 321–329. [Google Scholar] [CrossRef]
- Brown, L.M.; Helmke, S.M.; Hunsucker, S.W.; Netea-Maier, R.T.; Chiang, S.A.; Heinz, D.E.; Shroyer, K.R.; Duncan, M.W.; Haugen, B.R. Quantitative and Qualitative Differences in Protein Expression between Papillary Thyroid Carcinoma and Normal Thyroid Tissue. Mol. Carcinog. 2006, 45, 613–626. [Google Scholar] [CrossRef]
- Zhu, Y.; Pu, Q.; Zhang, Q.; Liu, Y.; Ma, Y.; Yuan, Y.; Liu, L.; Zhu, W. Selenium-Binding Protein 1 Inhibits Malignant Progression and Induces Apoptosis via Distinct Mechanisms in Non-Small Cell Lung Cancer. Cancer Med. 2023, 12, 17149–17170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, W.; Pan, W.; Wang, N.; Li, G.; Fan, X.; Xu, X.; Shen, S.; Das, U.N. Selenium-Binding Protein 1 May Decrease Gastric Cellular Proliferation and Migration. Int. J. Oncol. 2013, 42, 1620–1629. [Google Scholar] [CrossRef]
- Xia, Y.-J.; Ma, Y.-Y.; He, X.-J.; Wang, H.-J.; Ye, Z.-Y.; Tao, H.-Q. Suppression of Selenium-Binding Protein 1 in Gastric Cancer Is Associated with Poor Survival. Hum. Pathol. 2011, 42, 1620–1628. [Google Scholar] [CrossRef]
- Gao, P.-T.; Ding, G.-Y.; Yang, X.; Dong, R.-Z.; Hu, B.; Zhu, X.-D.; Cai, J.-B.; Ji, Y.; Shi, G.-M.; Shen, Y.-H.; et al. Invasive Potential of Hepatocellular Carcinoma Is Enhanced by Loss of Selenium-Binding Protein 1 and Subsequent Upregulation of CXCR4. Am. J. Cancer Res. 2018, 8, 1040–1049. [Google Scholar]
- Huang, C.; Ding, G.; Gu, C.; Zhou, J.; Kuang, M.; Ji, Y.; He, Y.; Kondo, T.; Fan, J. Decreased Selenium-Binding Protein 1 Enhances Glutathione Peroxidase 1 Activity and Downregulates HIF-1α to Promote Hepatocellular Carcinoma Invasiveness. Clin. Cancer Res. 2012, 18, 3042–3053. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.-S.; Lee, G.T.; Kim, Y.-H.; Kwon, S.Y.; Choi, S.H.; Kim, T.-H.; Kwon, T.G.; Yun, S.J.; Kim, I.Y.; Kim, W.-J. Decreased Selenium-Binding Protein 1 mRNA Expression Is Associated with Poor Prognosis in Renal Cell Carcinoma. World J. Surg. Oncol. 2014, 12, 288. [Google Scholar] [CrossRef]
- Huang, K.; Park, D.C.; Ng, S.; Lee, J.Y.; Ni, X.; Ng, W.; Bandera, C.A.; Welch, W.R.; Berkowitz, R.S.; Mok, S.C.; et al. Selenium Binding Protein 1 in Ovarian Cancer. Int. J. Cancer 2006, 118, 2433–2440. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.E.; Zhang, P.; Liu, F.; Sung, C.J.; Steinhoff, M.M.; Quddus, M.R.; Lawrence, W.D. Progressive Loss of Selenium-Binding Protein 1 Expression Correlates with Increasing Epithelial Proliferation and Papillary Complexity in Ovarian Serous Borderline Tumor and Low-Grade Serous Carcinoma. Hum. Pathol. 2010, 41, 255–261. [Google Scholar] [CrossRef]
- Yu-Rice, Y.; Edassery, S.L.; Urban, N.; Hellstrom, I.; Hellstrom, K.E.; Deng, Y.; Li, Y.; Luborsky, J.L. Selenium-Binding Protein 1 (SBP1) Autoantibodies in Ovarian Disorders and Ovarian Cancer. Reproduction 2017, 153, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, F.; Younes, M.; Liu, H.; Chen, C.; Yao, Q. Reduced Selenium-Binding Protein 1 in Breast Cancer Correlates with Poor Survival and Resistance to the Anti-Proliferative Effects of Selenium. PLoS ONE 2013, 8, e63702. [Google Scholar] [CrossRef]
- Ansong, E.; Ying, Q.; Ekoue, D.N.; Deaton, R.; Hall, A.R.; Kajdacsy-Balla, A.; Yang, W.; Gann, P.H.; Diamond, A.M. Evidence That Selenium Binding Protein 1 Is a Tumor Suppressor in Prostate Cancer. PLoS ONE 2015, 10, e0127295. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-Y.; Zhou, J.-R.; Gao, C.; Feldman, L.; Sytkowski, A.J. Human Selenium Binding Protein-1 (hSP56) Is a Negative Regulator of HIF-1α and Suppresses the Malignant Characteristics of Prostate Cancer Cells. BMB Rep. 2014, 47, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Elhodaky, M.; Hong, L.K.; Kadkol, S.; Diamond, A.M. Selenium-Binding Protein 1 Alters Energy Metabolism in Prostate Cancer Cells. Prostate 2020, 80, 962–976. [Google Scholar] [CrossRef]
- Wang, N.; Chen, Y.; Yang, X.; Jiang, Y. Selenium-Binding Protein 1 Is Associated with the Degree of Colorectal Cancer Differentiation and Is Regulated by Histone Modification. Oncol. Rep. 2014, 31, 2506–2514. [Google Scholar] [CrossRef]
- Zhang, X.; Hong, R.; Bei, L.; Yang, J.; Zhao, X.; Hu, Z.; Chen, L.; Meng, H.; Zhang, Q.; Niu, G.; et al. Selenium Binding Protein 1 Inhibits Tumor Angiogenesis in Colorectal Cancers by Blocking the Delta-like Ligand 4/Notch1 Signaling Pathway. Transl. Oncol. 2022, 18, 101365. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chen, C.; Qu, Y.; Xiang, H.; Ai, Q.; Yang, F.; Tan, X.; Zhou, Y.; Jiang, G.; Zhang, Z. Selenium-Binding Protein 1 in Head and Neck Cancer Is Low-Expression and Associates with the Prognosis of Nasopharyngeal Carcinoma. Medicine 2016, 95, e4592. [Google Scholar] [CrossRef] [PubMed]
- Schott, M.; de Jel, M.M.; Engelmann, J.C.; Renner, P.; Geissler, E.K.; Bosserhoff, A.K.; Kuphal, S. Selenium-Binding Protein 1 Is down-Regulated in Malignant Melanoma. Oncotarget 2018, 9, 10445–10456. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, X.; Wang, Y.; Liu, Y.; Dai, J.; Zhang, L.; Wu, X.; Zhang, J.; Xiang, H.; Yang, Y.; et al. Knocking out Selenium Binding Protein 1 Induces Depressive-Like Behavior in Mice. Biol. Trace Elem. Res. 2024, 202, 3149–3162. [Google Scholar] [CrossRef]
- Glatt, S.J.; Everall, I.P.; Kremen, W.S.; Corbeil, J.; Šášik, R.; Khanlou, N.; Han, M.; Liew, C.-C.; Tsuang, M.T. Comparative Gene Expression Analysis of Blood and Brain Provides Concurrent Validation of SELENBP1 up-Regulation in Schizophrenia. Proc. Natl. Acad. Sci. USA 2005, 102, 15533–15538. [Google Scholar] [CrossRef]
- Kanazawa, T.; Chana, G.; Glatt, S.J.; Mizuno, H.; Masliah, E.; Yoneda, H.; Tsuang, M.T.; Everall, I.P. The Utility of SELENBP1 Gene Expression as a Biomarker for Major Psychotic Disorders: Replication in Schizophrenia and Extension to Bipolar Disorder with Psychosis. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2008, 147B, 686–689. [Google Scholar] [CrossRef]
- Chau, E.J.; Mostaid, M.S.; Cropley, V.; McGorry, P.; Pantelis, C.; Bousman, C.A.; Everall, I.P. Downregulation of Plasma SELENBP1 Protein in Patients with Recent-Onset Schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 85, 1–6. [Google Scholar] [CrossRef]
- Eyice, Ö.; Myronova, N.; Pol, A.; Carrión, O.; Todd, J.D.; Smith, T.J.; Gurman, S.J.; Cuthbertson, A.; Mazard, S.; Mennink-Kersten, M.A.; et al. Bacterial SBP56 Identified as a Cu-Dependent Methanethiol Oxidase Widely Distributed in the Biosphere. ISME J. 2018, 12, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Pol, A.; Renkema, G.H.; Tangerman, A.; Winkel, E.G.; Engelke, U.F.; De Brouwer, A.P.M.; Lloyd, K.C.; Araiza, R.S.; Van Den Heuvel, L.; Omran, H.; et al. Mutations in SELENBP1, Encoding a Novel Human Methanethiol Oxidase, Cause Extraoral Halitosis. Nat. Genet. 2018, 50, 120–129. [Google Scholar] [CrossRef]
- Blom, H.J.; Tangerman, A. Methanethiol Metabolism in Whole Blood. J. Lab. Clin. Med. 1988, 111, 606–610. [Google Scholar]
- Fang, W.; Goldberg, M.L.; Pohl, N.M.; Bi, X.; Tong, C.; Xiong, B.; Koh, T.J.; Diamond, A.M.; Yang, W. Functional and Physical Interaction between the Selenium-Binding Protein 1 (SBP1) and the Glutathione Peroxidase 1 Selenoprotein. Carcinogenesis 2010, 31, 1360–1366. [Google Scholar] [CrossRef]
- Philipp, T.M.; Gernoth, L.; Will, A.; Schwarz, M.; Ohse, V.A.; Kipp, A.P.; Steinbrenner, H.; Klotz, L.-O. Selenium-Binding Protein 1 (SELENBP1) Is a Copper-Dependent Thiol Oxidase. Redox Biol. 2023, 65, 102807. [Google Scholar] [CrossRef]
- Machuka, J.; Bashiardes, S.; Ruben, E.; Spooner, K.; Cuming, A.; Knight, C.; Cove, D. Sequence Analysis of Expressed Sequence Tags from an ABA-Treated cDNA Library Identifies Stress Response Genes in the Moss Physcomitrella Patens. Plant Cell Physiol. 1999, 40, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Iwata, M. Isolation of Blast Fungal Cerebroside Elicitor-Responsive Genes in Rice Plants. J. Gen. Plant Pathol. 2002, 68, 128–133. [Google Scholar] [CrossRef]
- Sawada, K.; Tokuda, L.; Shinmyo, A. Characterization of the Rice Blast Fungal Elicitor Responsive Gene OSSBP Encoding a Homolog to the Mammalian Selenium—Binding Protelns. Plant Biotechnol. 2003, 20, 177–181. [Google Scholar] [CrossRef]
- Sawada, K.; Hasegawa, M.; Tokuda, L.; Kameyama, J.; Kodama, O.; Kohchi, T.; Yoshida, K.; Shinmyo, A. Enhanced Resistance to Blast Fungus and Bacterial Blight in Transgenic Rice Constitutively Expressing OsSBP, a Rice Homologue of Mammalian Selenium-Binding Proteins. Biosci. Biotechnol. Biochem. 2004, 68, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Gloudemans, T.; Bisseling, T. Plant Gene Expression in Early Stages of Rhizobium-Legume Symbiosis. Plant Sci. 1989, 65, 1–14. [Google Scholar] [CrossRef]
- Flemetakis, E.; Agalou, A.; Kavroulakis, N.; Dimou, M.; Martsikovskaya, A.; Slater, A.; Spaink, H.P.; Roussis, A.; Katinakis, P. Lotus japonicus Gene Ljsbp Is Highly Conserved Among Plants and Animals and Encodes a Homologue to the Mammalian Selenium-Binding Proteins. Mol. Plant-Microbe Interact. 2002, 15, 313–322. [Google Scholar] [CrossRef]
- Oehrle, N.W.; Sarma, A.D.; Waters, J.K.; Emerich, D.W. Proteomic Analysis of Soybean Nodule Cytosol. Phytochemistry 2008, 69, 2426–2438. [Google Scholar] [CrossRef]
- Agalou, A.; Roussis, A.; Spaink, H.P. The Arabidopsis Selenium-Binding Protein Confers Tolerance to Toxic Levels of Selenium. Funct. Plant Biol. 2005, 32, 881–890. [Google Scholar] [CrossRef]
- Valassakis, C.; Dervisi, I.; Agalou, A.; Papandreou, N.; Kapetsis, G.; Podia, V.; Haralampidis, K.; Iconomidou, V.A.; Spaink, H.P.; Roussis, A. Novel Interactions of Selenium Binding Protein Family with the PICOT Containing Proteins AtGRXS14 and AtGRXS16 in Arabidopsis Thaliana. Plant Sci. 2019, 281, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Dervisi, I.; Valassakis, C.; Agalou, A.; Papandreou, N.; Podia, V.; Haralampidis, K.; Iconomidou, V.A.; Kouvelis, V.N.; Spaink, H.P.; Roussis, A. Investigation of the Interaction of DAD1-LIKE LIPASE 3 (DALL3) with Selenium Binding Protein 1 (SBP1) in Arabidopsis Thaliana. Plant Sci. 2020, 291, 110357. [Google Scholar] [CrossRef] [PubMed]
- Dervisi, I.; Haralampidis, K.; Roussis, A. Investigation of the Interaction of a Papain-like Cysteine Protease (RD19c) with Selenium-Binding Protein 1 (SBP1) in Arabidopsis Thaliana. Plant Sci. 2022, 315, 111157. [Google Scholar] [CrossRef]
- Dervisi, I.; Petropoulos, O.; Agalou, A.; Podia, V.; Papandreou, N.; Iconomidou, V.A.; Haralampidis, K.; Roussis, A. The SAH7 Homologue of the Allergen Ole e 1 Interacts with the Putative Stress Sensor SBP1 (Selenium-Binding Protein 1) in Arabidopsis Thaliana. Int. J. Mol. Sci. 2023, 24, 3580. [Google Scholar] [CrossRef]
- Luo, F.; Zhu, D.; Sun, H.; Zou, R.; Duan, W.; Liu, J.; Yan, Y. Wheat Selenium-Binding Protein TaSBP-A Enhances Cadmium Tolerance by Decreasing Free Cd2+ and Alleviating the Oxidative Damage and Photosynthesis Impairment. Front. Plant Sci. 2023, 14, 1103241. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Qiang, J.; Sun, H.; Luo, F.; Li, X.; Yan, Y. Overexpression of Wheat Selenium-Binding Protein Gene TaSBP-A Enhances Plant Growth and Grain Selenium Accumulation under Spraying Sodium Selenite. Int. J. Mol. Sci. 2024, 25, 7007. [Google Scholar] [CrossRef]
- Martins Alves, A.M.; Pereira Menezes, S.; Matos Lima, E.; Peres Gramacho, K.; Silva Andrade, B.; Macêdo Ferreira, M.; Pirovani, C.P.; Micheli, F. The Selenium-Binding Protein of Theobroma Cacao: A Thermostable Protein Involved in the Witches’ Broom Disease Resistance. Plant Physiol. Biochem. 2019, 142, 472–481. [Google Scholar] [CrossRef]
- Koletti, A.; Dervisi, I.; Kalloniati, C.; Zografaki, M.-E.; Rennenberg, H.; Roussis, A.; Flemetakis, E. Selenium-Binding Protein 1 (SBD1): A Stress Response Regulator in Chlamydomonas reinhardtii. Plant Physiol. 2022, 189, 2368–2381. [Google Scholar] [CrossRef]
- Schild, F.; Kieffer-Jaquinod, S.; Palencia, A.; Cobessi, D.; Sarret, G.; Zubieta, C.; Jourdain, A.; Dumas, R.; Forge, V.; Testemale, D.; et al. Biochemical and Biophysical Characterization of the Selenium-Binding and Reducing Site in Arabidopsis Thaliana Homologue to Mammals Selenium-Binding Protein 1. J. Biol. Chem. 2014, 289, 31765–31776. [Google Scholar] [CrossRef]
- Stornaiuolo, M.; Lotti, L.V.; Borgese, N.; Torrisi, M.-R.; Mottola, G.; Martire, G.; Bonatti, S. KDEL and KKXX Retrieval Signals Appended to the Same Reporter Protein Determine Different Trafficking between Endoplasmic Reticulum, Intermediate Compartment, and Golgi Complex. Mol. Biol. Cell 2003, 14, 889–902. [Google Scholar] [CrossRef]
- Hillier, J.; Allcott, G.J.; Guest, L.A.; Heaselgrave, W.; Tonks, A.; Conway, M.E.; Cherry, A.L.; Coles, S.J. The BCAT1 CXXC Motif Provides Protection against ROS in Acute Myeloid Leukaemia Cells. Antioxidants 2022, 11, 683. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.A.; Koc, A.; Cerny, R.L.; Gladyshev, V.N. Reaction Mechanism, Evolutionary Analysis, and Role of Zinc in Drosophila Methionine-R-Sulfoxide Reductase. J. Biol. Chem. 2002, 277, 37527–37535. [Google Scholar] [CrossRef] [PubMed]
- Anelli, T. ERp44, a Novel Endoplasmic Reticulum Folding Assistant of the Thioredoxin Family. EMBO J. 2002, 21, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Fomenko, D.E.; Gladyshev, V.N. CxxS: Fold-independent Redox Motif Revealed by Genome-wide Searches for Thiol/Disulfide Oxidoreductase Function. Protein Sci. 2002, 11, 2285–2296. [Google Scholar] [CrossRef]
- Fomenko, D.E.; Gladyshev, V.N. Identity and Functions of CxxC-Derived Motifs. Biochemistry 2003, 42, 11214–11225. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.-C.; Hou, L.; Cao, P.-R.; Wu, L.; Zhang, Q.-S.; Yang, H.-Y.; Zang, Y.; Ding, J.-P.; Li, J. Functional Role of Histidine in the Conserved His-x-Asp Motif in the Catalytic Core of Protein Kinases. Sci. Rep. 2015, 5, 10115. [Google Scholar] [CrossRef]
- Yadav, U.; Rai, T.K.; Sethi, S.C.; Chandraker, A.; Khan, M.A.; Komath, S.S. Characterising N-Acetylglucosaminylphosphatidylinositol de-N-Acetylase (CaGpi12), the Enzyme That Catalyses the Second Step of GPI Biosynthesis in Candida Albicans. FEMS Yeast Res. 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, L.; Hou, Z.; Lin, H.; Gao, H.; Zhang, L. Structural Basis for the Substrate Recognition Mechanism of ATP-Sulfurylase Domain of Human PAPS Synthase 2. Biochem. Biophys. Res. Commun. 2022, 586, 1–7. [Google Scholar] [CrossRef]
- Valassakis, C.; Livanos, P.; Minopetrou, M.; Haralampidis, K.; Roussis, A. Promoter Analysis and Functional Implications of the Selenium Binding Protein (SBP) Gene Family in Arabidopsis Thaliana. J. Plant Physiol. 2018, 224–225, 19–29. [Google Scholar] [CrossRef]
- Dutilleul, C.; Jourdain, A.; Bourguignon, J.; Hugouvieux, V. The Arabidopsis Putative Selenium-Binding Protein Family: Expression Study and Characterization of SBP1 as a Potential New Player in Cadmium Detoxification Processes. Plant Physiol. 2008, 147, 239–251. [Google Scholar] [CrossRef]
- Hugouvieux, V.; Dutilleul, C.; Jourdain, A.; Reynaud, F.; Lopez, V.; Bourguignon, J. Arabidopsis Putative Selenium-Binding Protein1 Expression Is Tightly Linked to Cellular Sulfur Demand and Can Reduce Sensitivity to Stresses Requiring Glutathione for Tolerance. Plant Physiol. 2009, 151, 768–781. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.P.; Fladvad, M.; Berndt, C.; Andrésen, C.; Lillig, C.H.; Neubauer, P.; Sunnerhagen, M.; Holmgren, A.; Vlamis-Gardikas, A. A Novel Monothiol Glutaredoxin (Grx4) from Escherichia Coli Can Serve as a Substrate for Thioredoxin Reductase. J. Biol. Chem. 2005, 280, 24544–24552. [Google Scholar] [CrossRef] [PubMed]
- Meyer, Y.; Buchanan, B.B.; Vignols, F.; Reichheld, J.-P. Thioredoxins and Glutaredoxins: Unifying Elements in Redox Biology. Annu. Rev. Genet. 2009, 43, 335–367. [Google Scholar] [CrossRef]
- Rouhier, N.; Gelhaye, E.; Jacquot, J.-P. Plant Glutaredoxins: Still Mysterious Reducing Systems. Cell. Mol. Life Sci. CMLS 2004, 61, 1266–1277. [Google Scholar] [CrossRef]
- Rouhier, N.; Lemaire, S.D.; Jacquot, J.-P. The Role of Glutathione in Photosynthetic Organisms: Emerging Functions for Glutaredoxins and Glutathionylation. Annu. Rev. Plant Biol. 2008, 59, 143–166. [Google Scholar] [CrossRef] [PubMed]
- Rey, P.; Becuwe, N.; Tourrette, S.; Rouhier, N. Involvement of Arabidopsis Glutaredoxin S14 in the Maintenance of Chlorophyll Content. Plant Cell Environ. 2017, 40, 2319–2332. [Google Scholar] [CrossRef]
- Cheng, N.-H.; Hirschi, K.D. Cloning and Characterization of CXIP1, a Novel PICOT Domain-Containing Arabidopsis Protein That Associates with CAX1. J. Biol. Chem. 2003, 278, 6503–6509. [Google Scholar] [CrossRef] [PubMed]
- Upchurch, R.G. Fatty Acid Unsaturation, Mobilization, and Regulation in the Response of Plants to Stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef]
- Yang, L.; Ji, J.; Harris-Shultz, K.R.; Wang, H.; Wang, H.; Abd-Allah, E.F.; Luo, Y.; Hu, X. The Dynamic Changes of the Plasma Membrane Proteins and the Protective Roles of Nitric Oxide in Rice Subjected to Heavy Metal Cadmium Stress. Front. Plant Sci. 2016, 7, 190. [Google Scholar] [CrossRef]
- Chmielowska-Bąk, J.; Gzyl, J.; Rucińska-Sobkowiak, R.; Arasimowicz-Jelonek, M.; Deckert, J. The New Insights into Cadmium Sensing. Front. Plant Sci. 2014, 5, 245. [Google Scholar] [CrossRef]
- Maksymiec, W.; Wianowska, D.; Dawidowicz, A.L.; Radkiewicz, S.; Mardarowicz, M.; Krupa, Z. The Level of Jasmonic Acid in Arabidopsis Thaliana and Phaseolus Coccineus Plants under Heavy Metal Stress. J. Plant Physiol. 2005, 162, 1338–1346. [Google Scholar] [CrossRef]
- Maksymiec, W. Signaling Responses in Plants to Heavy Metal Stress. Acta Physiol. Plant. 2007, 29, 177–187. [Google Scholar] [CrossRef]
- Koeduka, T.; Matsui, K.; Hasegawa, M.; Akakabe, Y.; Kajiwara, T. Rice Fatty Acid α-Dioxygenase Is Induced by Pathogen Attack and Heavy Metal Stress: Activation through Jasmonate Signaling. J. Plant Physiol. 2005, 162, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Mithöfer, A.; Schulze, B.; Boland, W. Biotic and Heavy Metal Stress Response in Plants: Evidence for Common Signals. FEBS Lett. 2004, 566, 1–5. [Google Scholar] [CrossRef]
- Maksymiec, W. Effects of Jasmonate and Some Other Signalling Factors on Bean and Onion Growth during the Initial Phase of Cadmium Action. Biol. Plant. 2011, 55, 112–118. [Google Scholar] [CrossRef]
- Swindell, W.R. The Association Among Gene Expression Responses to Nine Abiotic Stress Treatments in Arabidopsis Thaliana. Genetics 2006, 174, 1811–1824. [Google Scholar] [CrossRef] [PubMed]
- Bernoux, M.; Timmers, T.; Jauneau, A.; Brière, C.; De Wit, P.J.G.M.; Marco, Y.; Deslandes, L. RD19, an Arabidopsis Cysteine Protease Required for RRS1-R–Mediated Resistance, Is Relocalized to the Nucleus by the Ralstonia solanacearum PopP2 Effector. Plant Cell 2008, 20, 2252–2264. [Google Scholar] [CrossRef]
- Cheng, Z.; Guo, X.; Zhang, J.; Liu, Y.; Wang, B.; Li, H.; Lu, H. βVPE Is Involved in Tapetal Degradation and Pollen Development by Activating Proprotease Maturation in Arabidopsis Thaliana. J. Exp. Bot. 2020, 71, 1943–1955. [Google Scholar] [CrossRef] [PubMed]
- Köhnlein, K.; Urban, N.; Guerrero-Gómez, D.; Steinbrenner, H.; Urbánek, P.; Priebs, J.; Koch, P.; Kaether, C.; Miranda-Vizuete, A.; Klotz, L.-O. A Caenorhabditis Elegans Ortholog of Human Selenium-Binding Protein 1 Is a pro-Aging Factor Protecting against Selenite Toxicity. Redox Biol. 2020, 28, 101323. [Google Scholar] [CrossRef]
| Conserved Motif | Position (aa) | Function |
|---|---|---|
| CC | 21–22 | Se binding |
| KDEL | 86–89 | Endoplasmic reticulum signal |
| CSSC | 97–100 | Redox activity |
| HxD | 101–103, 347–349 | Metal binding |
| HXXHC | 154–158 | Substrate binding |
| Clathrin-binding boxes (pLφpφp) | 331–336, 460–465 | Membrane trafficking |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dervisi, I.; Koletti, A.; Agalou, A.; Haralampidis, K.; Flemetakis, E.; Roussis, A. Selenium-Binding Protein 1 (SBP1): A New Putative Player of Stress Sensing in Plants. Int. J. Mol. Sci. 2024, 25, 9372. https://doi.org/10.3390/ijms25179372
Dervisi I, Koletti A, Agalou A, Haralampidis K, Flemetakis E, Roussis A. Selenium-Binding Protein 1 (SBP1): A New Putative Player of Stress Sensing in Plants. International Journal of Molecular Sciences. 2024; 25(17):9372. https://doi.org/10.3390/ijms25179372
Chicago/Turabian StyleDervisi, Irene, Aikaterini Koletti, Adamantia Agalou, Kosmas Haralampidis, Emmanouil Flemetakis, and Andreas Roussis. 2024. "Selenium-Binding Protein 1 (SBP1): A New Putative Player of Stress Sensing in Plants" International Journal of Molecular Sciences 25, no. 17: 9372. https://doi.org/10.3390/ijms25179372
APA StyleDervisi, I., Koletti, A., Agalou, A., Haralampidis, K., Flemetakis, E., & Roussis, A. (2024). Selenium-Binding Protein 1 (SBP1): A New Putative Player of Stress Sensing in Plants. International Journal of Molecular Sciences, 25(17), 9372. https://doi.org/10.3390/ijms25179372







