Syndecan-1 Plays a Role in the Pathogenesis of Sjögren’s Disease by Inducing B-Cell Chemotaxis through CXCL13–Heparan Sulfate Interaction
Abstract
1. Introduction
2. Results
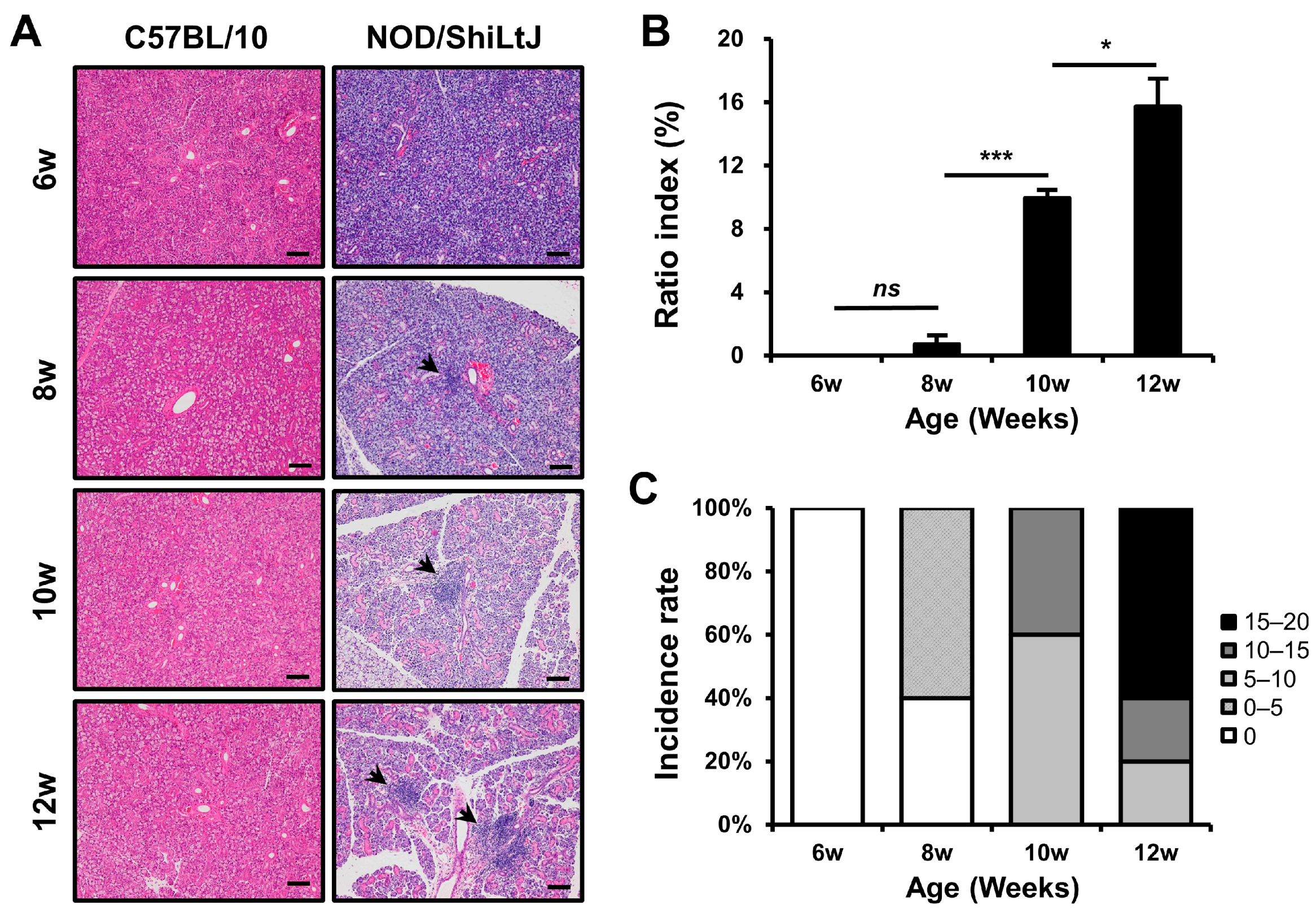
2.1. Histopathological Changes in the Salivary Glands of NOD/ShiLtJ Mice
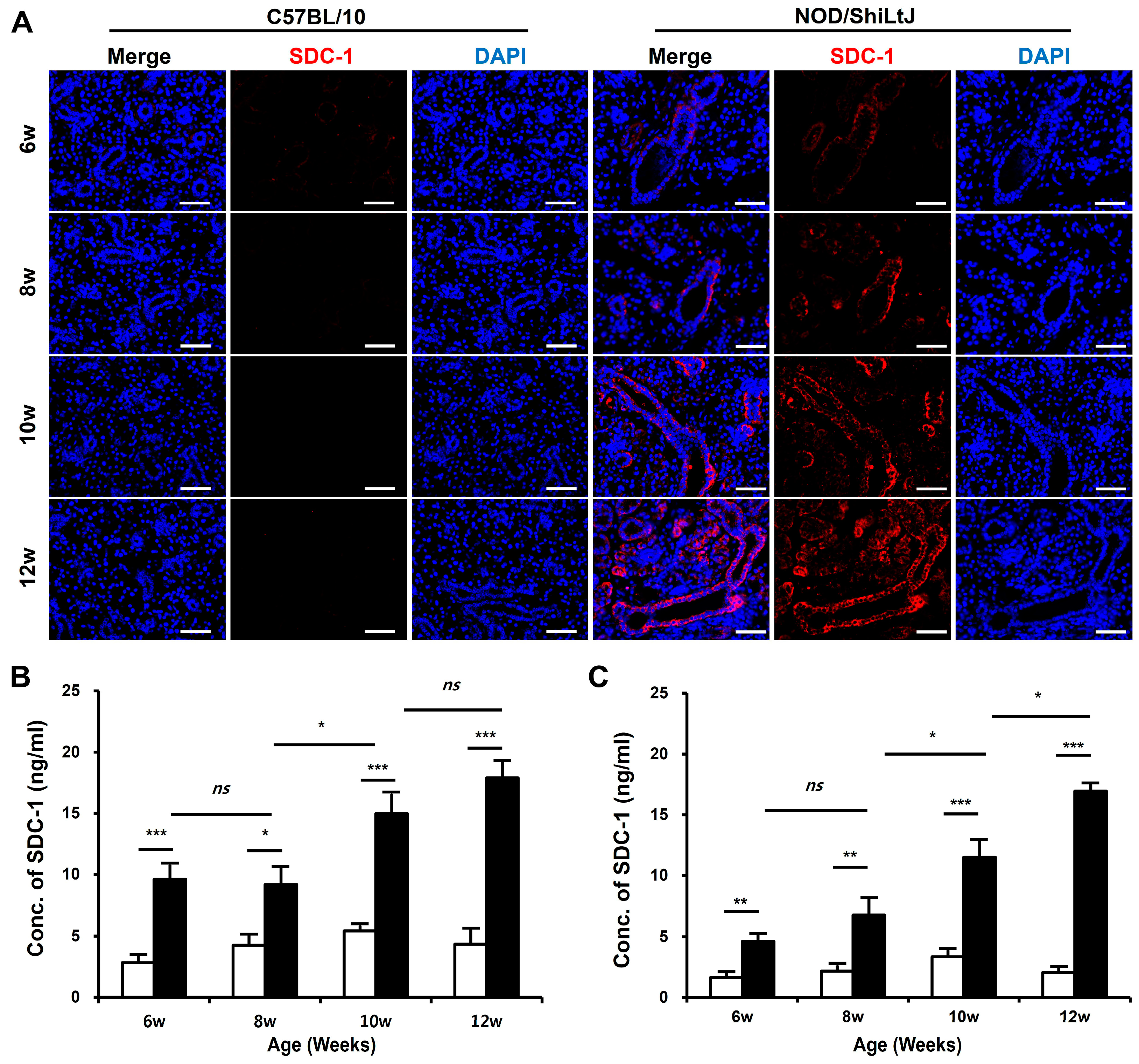
2.2. Increased SDC-1 Expression in Blood and SMGs of NOD/ShiLtJ Mice
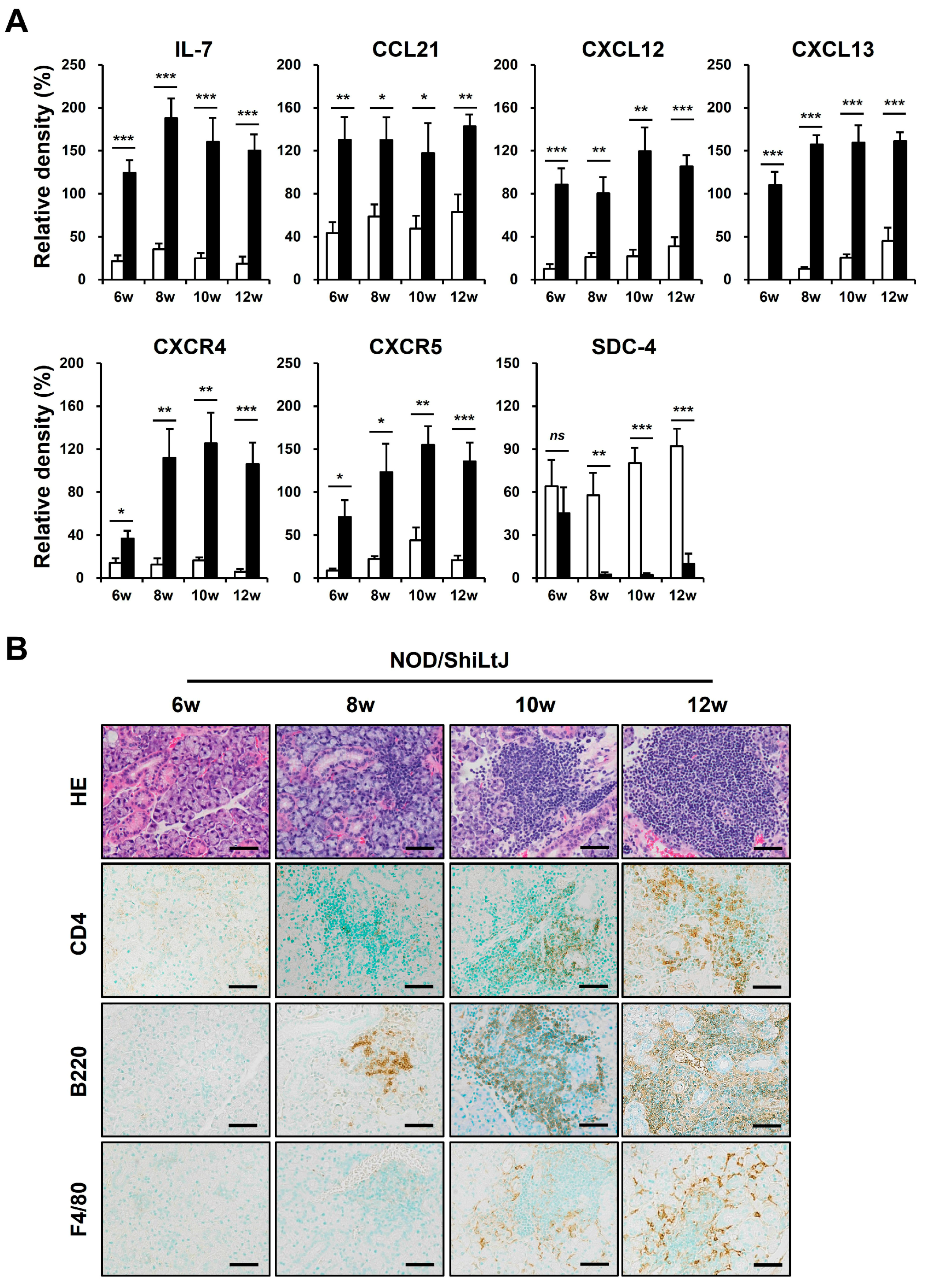
2.3. Expression of B-Cell Chemokines and Their Receptors in SMG Tissues of NOD/ShiLtJ Mice
2.4. Identification of CXCL13 Binding to SDC-1
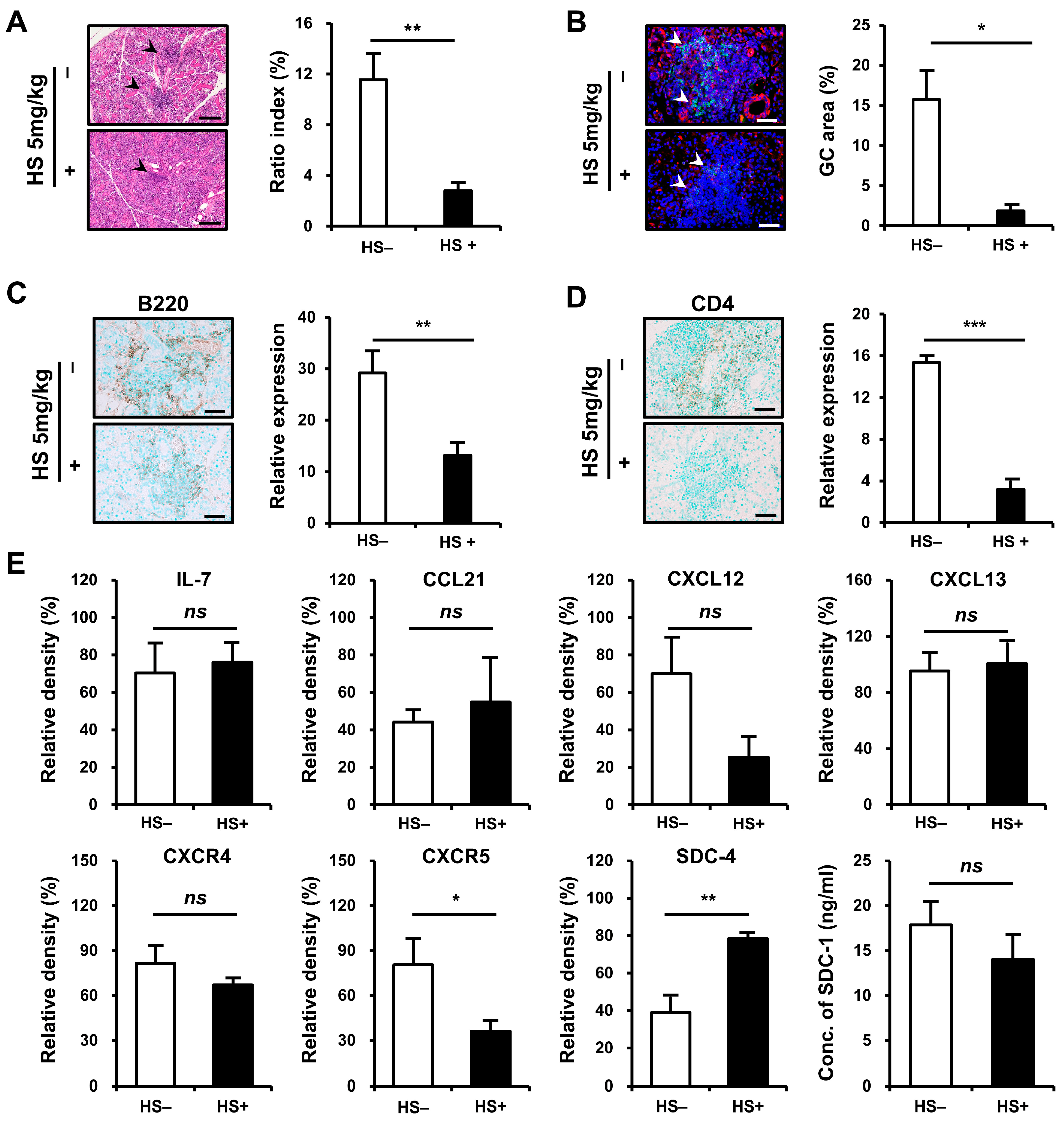
2.5. Improvement of Salivary Gland Inflammation in an HS-Dependent Manner
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Animal Experiments
4.3. Cell Culture
4.4. Histology and Immunohistochemistry (IHC) Staining
4.5. IF Staining
4.6. Western Blotting Analysis
4.7. Dot-Blotting Analysis
4.8. IP Assay
4.9. Semi-Quantitative Reverse Transcription PCR (RT-PCR)
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goules, A.V.; Kapsogeorgou, E.K.; Tzioufas, A.G. Insight into pathogenesis of Sjogren’s syndrome: Dissection on autoimmune infiltrates and epithelial cells. Clin. Immunol. 2017, 182, 30–40. [Google Scholar] [CrossRef]
- Verstappen, G.M.; Pringle, S.; Bootsma, H.; Kroese, F.G.M. Epithelial-immune cell interplay in primary Sjogren syndrome salivary gland pathogenesis. Nat. Rev. Rheumatol. 2021, 17, 333–348. [Google Scholar] [CrossRef]
- Tzioufas, A.G.; Kapsogeorgou, E.K.; Moutsopoulos, H.M. Pathogenesis of Sjogren’s syndrome: What we know and what we should learn. J. Autoimmun. 2012, 39, 4–8. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, Y.; Wang, X.; Che, N.; Tian, J.; Man, K.; Rui, K.; Peng, N.; Lu, L. The role of epithelial cells in the immunopathogenesis of Sjogren’s syndrome. J. Leukoc. Biol. 2024, 115, 57–67. [Google Scholar] [CrossRef]
- Pan, Z.; Zhu, T.; Liu, Y.; Zhang, N. Role of the CXCL13/CXCR5 Axis in Autoimmune Diseases. Front. Immunol. 2022, 13, 850998. [Google Scholar] [CrossRef] [PubMed]
- Bombardieri, M.; Lewis, M.; Pitzalis, C. Ectopic lymphoid neogenesis in rheumatic autoimmune diseases. Nat. Rev. Rheumatol. 2017, 13, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Cho, M.L.; Kim, H.R.; Ju, J.H.; Park, M.K.; Oh, H.J.; Kim, J.S.; Park, S.H.; Lee, S.H.; Kim, H.Y. Up-regulation of stromal cell-derived factor 1 (CXCL12) production in rheumatoid synovial fibroblasts through interactions with T lymphocytes: Role of interleukin-17 and CD40L-CD40 interaction. Arthritis Rheum. 2007, 56, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Bernfield, M.; Gotte, M.; Park, P.W.; Reizes, O.; Fitzgerald, M.L.; Lincecum, J.; Zako, M. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 1999, 68, 729–777. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.H.; Aquino, R.S.; Park, P.W. Molecular functions of syndecan-1 in disease. Matrix Biol. 2012, 31, 3–16. [Google Scholar] [CrossRef]
- Bartlett, A.H.; Hayashida, K.; Park, P.W. Molecular and cellular mechanisms of syndecans in tissue injury and inflammation. Mol. Cells 2007, 24, 153–166. [Google Scholar] [CrossRef]
- Gotte, M. Syndecans in inflammation. FASEB J. 2003, 17, 575–591. [Google Scholar] [CrossRef]
- Middleton, J.; Neil, S.; Wintle, J.; Clark-Lewis, I.; Moore, H.; Lam, C.; Auer, M.; Hub, E.; Rot, A. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell 1997, 91, 385–395. [Google Scholar] [CrossRef]
- Kuschert, G.S.; Coulin, F.; Power, C.A.; Proudfoot, A.E.; Hubbard, R.E.; Hoogewerf, A.J.; Wells, T.N. Glycosaminoglycans interact selectively with chemokines and modulate receptor binding and cellular responses. Biochemistry 1999, 38, 12959–12968. [Google Scholar] [CrossRef]
- Lee, N.Y.; Kim, N.R.; Kang, J.W.; Kim, G.; Han, M.S.; Jang, J.A.; Ahn, D.; Jeong, J.H.; Han, M.H.; Nam, E.J. Increased salivary syndecan-1 level is associated with salivary gland function and inflammation in patients with Sjogren’s syndrome. Scand. J. Rheumatol. 2022, 51, 220–229. [Google Scholar] [CrossRef]
- Monneau, Y.R.; Luo, L.; Sankaranarayanan, N.V.; Nagarajan, B.; Vives, R.R.; Baleux, F.; Desai, U.R.; Arenzana-Seidedos, F.; Lortat-Jacob, H. Solution structure of CXCL13 and heparan sulfate binding show that GAG binding site and cellular signalling rely on distinct domains. Open Biol. 2017, 7, 170133. [Google Scholar] [CrossRef]
- Witas, R.; Gupta, S.; Nguyen, C.Q. Contributions of Major Cell Populations to Sjogren’s Syndrome. J. Clin. Med. 2020, 9, 3057. [Google Scholar] [CrossRef]
- Edwards, J.C.; Cambridge, G. B-cell targeting in rheumatoid arthritis and other autoimmune diseases. Nat. Rev. Immunol. 2006, 6, 394–403. [Google Scholar] [CrossRef]
- Klein, U.; Rajewsky, K.; Kuppers, R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J. Exp. Med. 1998, 188, 1679–1689. [Google Scholar] [CrossRef]
- Agematsu, K.; Nagumo, H.; Yang, F.C.; Nakazawa, T.; Fukushima, K.; Ito, S.; Sugita, K.; Mori, T.; Kobata, T.; Morimoto, C.; et al. B cell subpopulations separated by CD27 and crucial collaboration of CD27+ B cells and helper T cells in immunoglobulin production. Eur. J. Immunol. 1997, 27, 2073–2079. [Google Scholar] [CrossRef]
- Klein, U.; Casola, S.; Cattoretti, G.; Shen, Q.; Lia, M.; Mo, T.; Ludwig, T.; Rajewsky, K.; Dalla-Favera, R. Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nat. Immunol. 2006, 7, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, E.; Kamada, A. Expression of the cell-surface heparan sulfate proteoglycan mRNA in monkey submandibular gland. J. Osaka Dent. Univ. 1999, 33, 83–87. [Google Scholar]
- Chen, W.; Lin, J.; Cao, H.; Xu, D.; Xu, B.; Xu, L.; Yue, L.; Sun, C.; Wu, G.; Qian, W. Local and Systemic IKKepsilon and NF-kappaB Signaling Associated with Sjogren’s Syndrome Immunopathogenesis. J. Immunol. Res. 2015, 2015, 534648. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Mabuchi, T.; Satoh, E.; Furuya, K.; Zhang, L.; Maeda, S.; Naganuma, H. Expression of syndecans, a heparan sulfate proteoglycan, in malignant gliomas: Participation of nuclear factor-kappaB in upregulation of syndecan-1 expression. J. Neurooncol. 2006, 77, 25–32. [Google Scholar] [CrossRef]
- Mason, G.I.; Hamburger, J.; Bowman, S.; Matthews, J.B. Salivary gland expression of transforming growth factor beta isoforms in Sjogren’s syndrome and benign lymphoepithelial lesions. Mol. Pathol. 2003, 56, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, K.; Johnston, D.R.; Goldberger, O.; Park, P.W. Syndecan-1 expression in epithelial cells is induced by transforming growth factor beta through a PKA-dependent pathway. J. Biol. Chem. 2006, 281, 24365–24374. [Google Scholar] [CrossRef]
- Fears, C.Y.; Woods, A. The role of syndecans in disease and wound healing. Matrix Biol. 2006, 25, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Manon-Jensen, T.; Multhaupt, H.A.; Couchman, J.R. Mapping of matrix metalloproteinase cleavage sites on syndecan-1 and syndecan-4 ectodomains. FEBS J. 2013, 280, 2320–2331. [Google Scholar] [CrossRef]
- Brule, S.; Charnaux, N.; Sutton, A.; Ledoux, D.; Chaigneau, T.; Saffar, L.; Gattegno, L. The shedding of syndecan-4 and syndecan-1 from HeLa cells and human primary macrophages is accelerated by SDF-1/CXCL12 and mediated by the matrix metalloproteinase-9. Glycobiology 2006, 16, 488–501. [Google Scholar] [CrossRef]
- Endo, K.; Takino, T.; Miyamori, H.; Kinsen, H.; Yoshizaki, T.; Furukawa, M.; Sato, H. Cleavage of syndecan-1 by membrane type matrix metalloproteinase-1 stimulates cell migration. J. Biol. Chem. 2003, 278, 40764–40770. [Google Scholar] [CrossRef]
- Pruessmeyer, J.; Martin, C.; Hess, F.M.; Schwarz, N.; Schmidt, S.; Kogel, T.; Hoettecke, N.; Schmidt, B.; Sechi, A.; Uhlig, S.; et al. A disintegrin and metalloproteinase 17 (ADAM17) mediates inflammation-induced shedding of syndecan-1 and -4 by lung epithelial cells. J. Biol. Chem. 2010, 285, 555–564. [Google Scholar] [CrossRef]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef] [PubMed]
- Pérez, P.; Goicovich, E.; Alliende, C.; Aguilera, S.; Leyton, C.; Molina, C.; Pinto, R.; Romo, R.; Martinez, B.; Gonzalez, M.J. Differential expression of matrix metalloproteinases in labial salivary glands of patients with primary Sjogren’s syndrome. Arthritis Rheum. 2000, 43, 2807–2817. [Google Scholar] [CrossRef]
- Chen, W.S.; Lin, K.C.; Chen, C.H.; Liao, H.T.; Wang, H.P.; Li, W.Y.; Lee, H.T.; Tsai, C.Y.; Chou, C.T. Autoantibody and biopsy grading are associated with expression of ICAM-1, MMP-3, and TRAIL in salivary gland mononuclear cells of Chinese patients with Sjogren’s syndrome. J. Rheumatol. 2009, 36, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Konttinen, Y.T.; Halinen, S.; Hanemaaijer, R.; Sorsa, T.; Hietanen, J.; Ceponis, A.; Xu, J.W.; Manthorpe, R.; Whittington, J.; Larsson, A.; et al. Matrix metalloproteinase (MMP)-9 type IV collagenase/gelatinase implicated in the pathogenesis of Sjogren’s syndrome. Matrix Biol. 1998, 17, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Ram, M.; Sherer, Y.; Shoenfeld, Y. Matrix metalloproteinase-9 and autoimmune diseases. J. Clin. Immunol. 2006, 26, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Lisi, S.; Sisto, M.; Lofrumento, D.D.; D’Amore, M.; De Lucro, R.; Ribatti, D. GRO-alpha/CXCR2 system and ADAM17 correlated expression in Sjogren’s syndrome. Inflammation 2013, 36, 759–766. [Google Scholar] [CrossRef]
- Azuma, M.; Aota, K.; Tamatani, T.; Motegi, K.; Yamashita, T.; Ashida, Y.; Hayashi, Y.; Sato, M. Suppression of tumor necrosis factor alpha-induced matrix metalloproteinase 9 production in human salivary gland acinar cells by cepharanthine occurs via down-regulation of nuclear factor kappaB: A possible therapeutic agent for preventing the destruction of the acinar structure in the salivary glands of Sjogren’s syndrome patients. Arthritis Rheum. 2002, 46, 1585–1594. [Google Scholar] [CrossRef]
- Okada, Y.; Tsuchiya, H.; Shimizu, H.; Tomita, K.; Nakanishi, I.; Sato, H.; Seiki, M.; Yamashita, K.; Hayakawa, T. Induction and stimulation of 92-kDa gelatinase/type IV collagenase production in osteosarcoma and fibrosarcoma cell lines by tumor necrosis factor alpha. Biochem. Biophys. Res. Commun. 1990, 171, 610–617. [Google Scholar] [CrossRef]
- Lisi, S.; D’Amore, M.; Sisto, M. ADAM17 at the interface between inflammation and autoimmunity. Immunol. Lett. 2014, 162, 159–169. [Google Scholar] [CrossRef]
- Sisto, M.; Lisi, S.; Lofrumento, D.D.; D’Amore, M.; Frassanito, M.A.; Ribatti, D. Sjogren’s syndrome pathological neovascularization is regulated by VEGF-A-stimulated TACE-dependent crosstalk between VEGFR2 and NF-kappaB. Genes Immun. 2012, 13, 411–420. [Google Scholar] [CrossRef]
- Lisi, S.; Sisto, M.; Lofrumento, D.D.; D’Amore, M. Sjogren’s syndrome autoantibodies provoke changes in gene expression profiles of inflammatory cytokines triggering a pathway involving TACE/NF-kappaB. Lab. Investig. 2012, 92, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Rivière, E.; Pascaud, J.; Virone, A.; Dupre, A.; Ly, B.; Paoletti, A.; Seror, R.; Tchitchek, N.; Mingueneau, M.; Smith, N.; et al. Interleukin-7/Interferon Axis Drives T Cell and Salivary Gland Epithelial Cell Interactions in Sjogren’s Syndrome. Arthritis Rheumatol. 2021, 73, 631–640. [Google Scholar] [CrossRef]
- Ogawa, N.; Ping, L.; Zhenjun, L.; Takada, Y.; Sugai, S. Involvement of the interferon-gamma-induced T cell-attracting chemokines, interferon-gamma-inducible 10-kd protein (CXCL10) and monokine induced by interferon-gamma (CXCL9), in the salivary gland lesions of patients with Sjogren’s syndrome. Arthritis Rheum. 2002, 46, 2730–2741. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.; Lipsky, P.E.; Dorner, T. B cells in Sjogren’s syndrome: Indications for disturbed selection and differentiation in ectopic lymphoid tissue. Arthritis Res. Ther. 2007, 9, 218. [Google Scholar] [CrossRef]
- Gatto, D.; Brink, R. The germinal center reaction. J. Allergy Clin. Immunol. 2010, 126, 898–907; quiz 908–899. [Google Scholar] [CrossRef] [PubMed]
- Aloisi, F.; Pujol-Borrell, R. Lymphoid neogenesis in chronic inflammatory diseases. Nat. Rev. Immunol. 2006, 6, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Luther, S.A.; Bidgol, A.; Hargreaves, D.C.; Schmidt, A.; Xu, Y.; Paniyadi, J.; Matloubian, M.; Cyster, J.G. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J. Immunol. 2002, 169, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Barata, J.T.; Durum, S.K.; Seddon, B. Flip the coin: IL-7 and IL-7R in health and disease. Nat. Immunol. 2019, 20, 1584–1593. [Google Scholar] [CrossRef]
- Borghesi, L.A.; Yamashita, Y.; Kincade, P.W. Heparan sulfate proteoglycans mediate interleukin-7-dependent B lymphopoiesis. Blood 1999, 93, 140–148. [Google Scholar] [CrossRef]
- Proudfoot, A.E.; Handel, T.M.; Johnson, Z.; Lau, E.K.; LiWang, P.; Clark-Lewis, I.; Borlat, F.; Wells, T.N.; Kosco-Vilbois, M.H. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc. Natl. Acad. Sci. USA 2003, 100, 1885–1890. [Google Scholar] [CrossRef]
- Hamon, M.; Mbemba, E.; Charnaux, N.; Slimani, H.; Brule, S.; Saffar, L.; Vassy, R.; Prost, C.; Lievre, N.; Starzec, A.; et al. A syndecan-4/CXCR4 complex expressed on human primary lymphocytes and macrophages and HeLa cell line binds the CXC chemokine stromal cell-derived factor-1 (SDF-1). Glycobiology 2004, 14, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Tanino, Y.; Wang, X.; Nikaido, T.; Misa, K.; Sato, Y.; Togawa, R.; Kawamata, T.; Kikuchi, M.; Frevert, C.W.; Tanino, M.; et al. Syndecan-4 Inhibits the Development of Pulmonary Fibrosis by Attenuating TGF-beta Signaling. Int. J. Mol. Sci. 2019, 20, 4989. [Google Scholar] [CrossRef] [PubMed]
- Polte, T.; Petzold, S.; Bertrand, J.; Schutze, N.; Hinz, D.; Simon, J.C.; Lehmann, I.; Echtermeyer, F.; Pap, T.; Averbeck, M. Critical role for syndecan-4 in dendritic cell migration during development of allergic airway inflammation. Nat. Commun. 2015, 6, 7554. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Litwack, E.D.; Stanley, M.J.; Langford, J.K.; Lander, A.D.; Sanderson, R.D. Heparan sulfate proteoglycans as adhesive and anti-invasive molecules. Syndecans and glypican have distinct functions. J. Biol. Chem. 1998, 273, 22825–22832. [Google Scholar] [CrossRef]
- Langford, J.K.; Yang, Y.; Kieber-Emmons, T.; Sanderson, R.D. Identification of an invasion regulatory domain within the core protein of syndecan-1. J. Biol. Chem. 2005, 280, 3467–3473. [Google Scholar] [CrossRef]
- Bacsa, S.; Karasneh, G.; Dosa, S.; Liu, J.; Valyi-Nagy, T.; Shukla, D. Syndecan-1 and syndecan-2 play key roles in herpes simplex virus type-1 infection. J. Gen. Virol. 2011, 92, 733–743. [Google Scholar] [CrossRef]
- McQuade, K.J.; Beauvais, D.M.; Burbach, B.J.; Rapraeger, A.C. Syndecan-1 regulates alphavbeta5 integrin activity in B82L fibroblasts. J. Cell Sci. 2006, 119, 2445–2456. [Google Scholar] [CrossRef]
- Iida, J.; Dorchak, J.; Clancy, R.; Slavik, J.; Ellsworth, R.; Katagiri, Y.; Pugacheva, E.N.; van Kuppevelt, T.H.; Mural, R.J.; Cutler, M.L.; et al. Role for chondroitin sulfate glycosaminoglycan in NEDD9-mediated breast cancer cell growth. Exp. Cell Res. 2015, 330, 358–370. [Google Scholar] [CrossRef]
- Martin, R.; Brady, J.; Lew, A. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J. Immunol. Methods 1998, 212, 187–192. [Google Scholar] [CrossRef]
- Kiripolsky, J.; Shen, L.; Liang, Y.; Li, A.; Suresh, L.; Lian, Y.; Li, Q.Z.; Gaile, D.P.; Kramer, J.M. Systemic manifestations of primary Sjögren’s syndrome in the NOD.B10Sn-H2b/J mouse model. Clin. Immunol. 2017, 183, 225–232. [Google Scholar] [CrossRef]
- Kato, M.; Wang, H.; Bernfield, M.; Gallagher, J.T.; Turnbull, J.E. Cell surface syndecan-1 on distinct cell types differs in fine structure and ligand binding of its heparan sulfate chains. J. Biol. Chem. 1994, 269, 18881–18890. [Google Scholar] [CrossRef] [PubMed]
- Allushi, B.; Bagavant, H.; Papinska, J.; Deshmukh, U.S. Hyperglycemia and Salivary Gland Dysfunction in the Non-obese Diabetic Mouse: Caveats for Preclinical Studies in Sjögren’s Syndrome. Sci. Rep. 2019, 9, 17969. [Google Scholar] [CrossRef] [PubMed]
- Kurien, B.T.; Scofield, R.H. Western blotting. Methods 2006, 38, 283–293. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, N.Y.; Ture, H.Y.; Lee, E.J.; Jang, J.A.; Kim, G.; Nam, E.J. Syndecan-1 Plays a Role in the Pathogenesis of Sjögren’s Disease by Inducing B-Cell Chemotaxis through CXCL13–Heparan Sulfate Interaction. Int. J. Mol. Sci. 2024, 25, 9375. https://doi.org/10.3390/ijms25179375
Lee NY, Ture HY, Lee EJ, Jang JA, Kim G, Nam EJ. Syndecan-1 Plays a Role in the Pathogenesis of Sjögren’s Disease by Inducing B-Cell Chemotaxis through CXCL13–Heparan Sulfate Interaction. International Journal of Molecular Sciences. 2024; 25(17):9375. https://doi.org/10.3390/ijms25179375
Chicago/Turabian StyleLee, Nan Young, Hirut Yadeta Ture, Eun Ju Lee, Ji Ae Jang, Gunwoo Kim, and Eon Jeong Nam. 2024. "Syndecan-1 Plays a Role in the Pathogenesis of Sjögren’s Disease by Inducing B-Cell Chemotaxis through CXCL13–Heparan Sulfate Interaction" International Journal of Molecular Sciences 25, no. 17: 9375. https://doi.org/10.3390/ijms25179375
APA StyleLee, N. Y., Ture, H. Y., Lee, E. J., Jang, J. A., Kim, G., & Nam, E. J. (2024). Syndecan-1 Plays a Role in the Pathogenesis of Sjögren’s Disease by Inducing B-Cell Chemotaxis through CXCL13–Heparan Sulfate Interaction. International Journal of Molecular Sciences, 25(17), 9375. https://doi.org/10.3390/ijms25179375




