Regulating Leaf Photosynthesis and Soil Microorganisms through Controlled-Release Nitrogen Fertilizer Can Effectively Alleviate the Stress of Elevated Ambient Ozone on Winter Wheat
Abstract
1. Introduction
2. Results
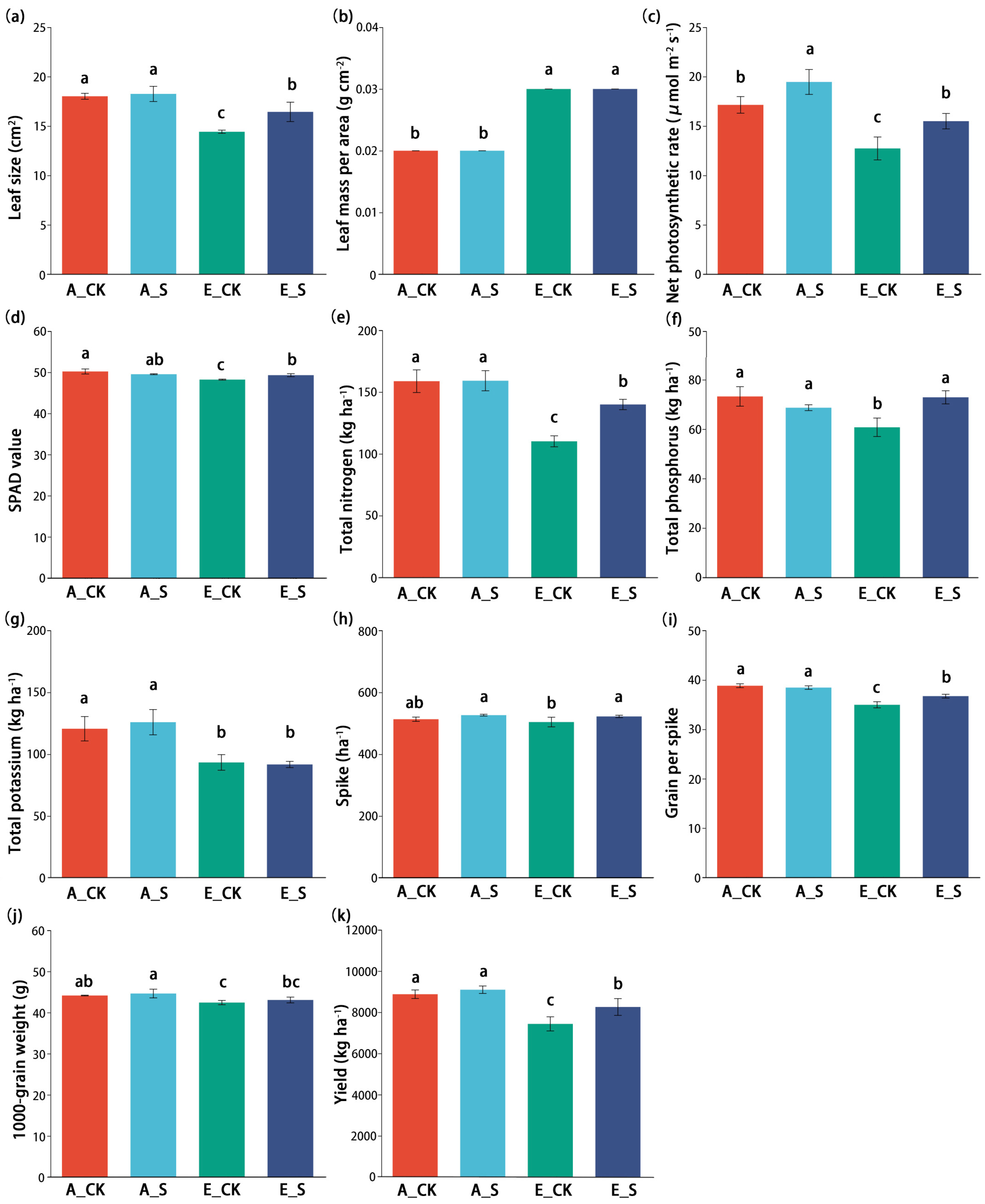
2.1. Effect of O3 and SCNF on Agronomic Parameters
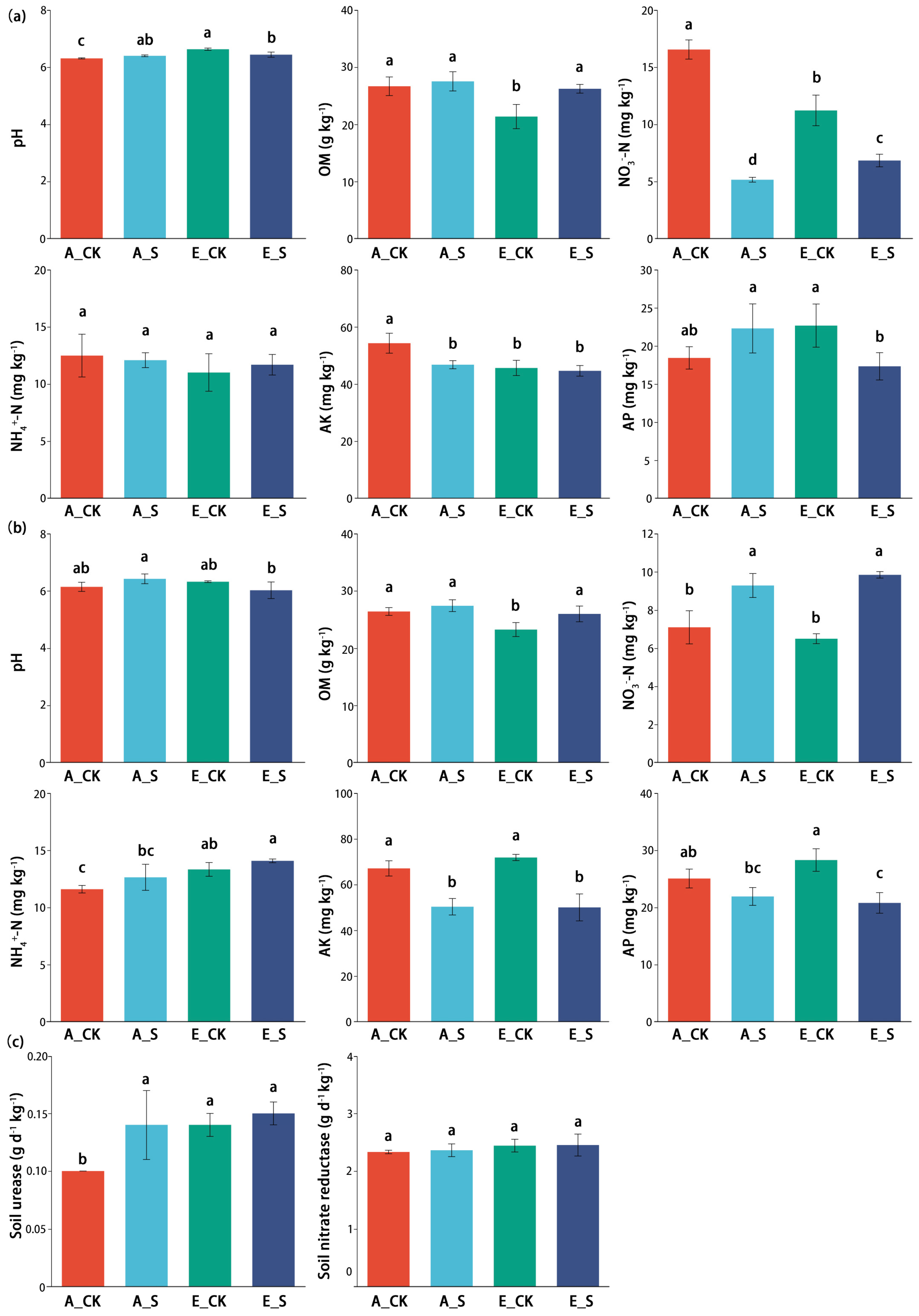
2.2. Effect of O3 and SCNF on Soil Chemical Properties
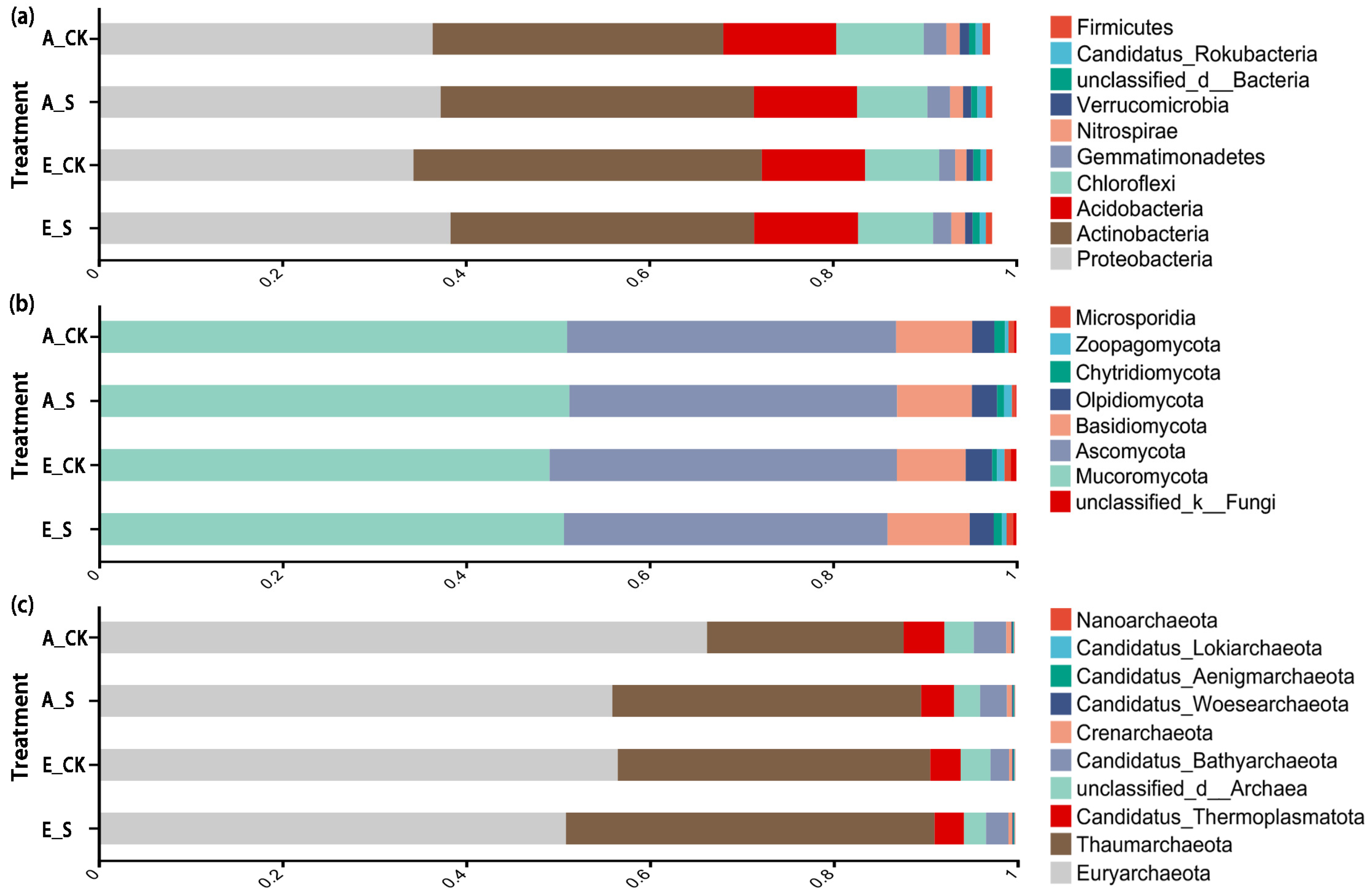
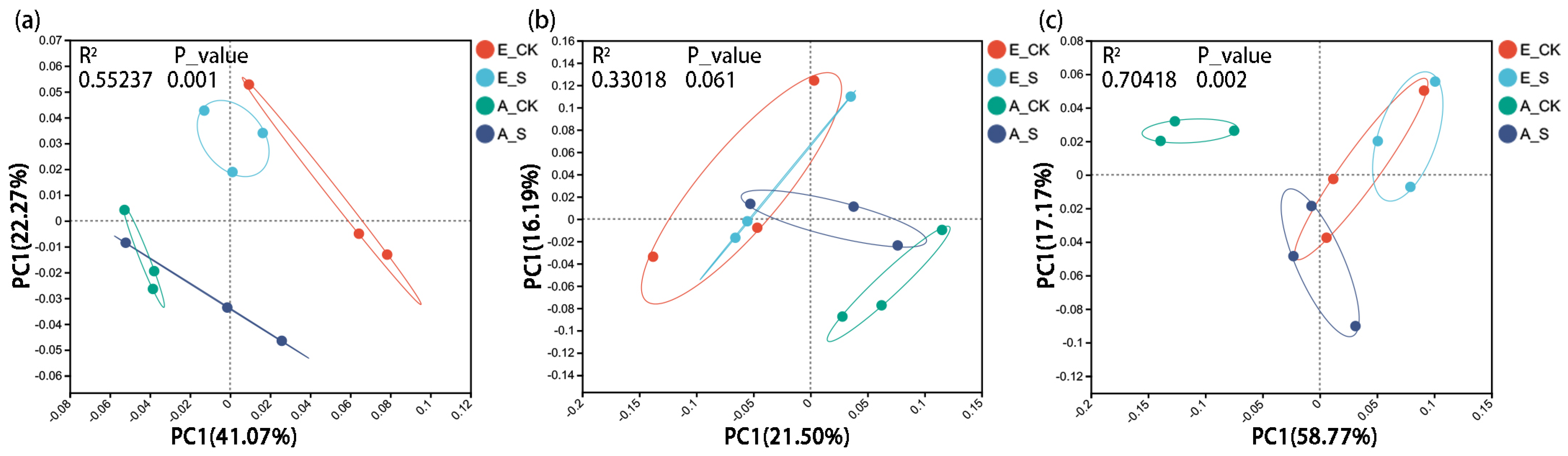
2.3. Effect of O3 and SCNF on Microbial Diversity and Community Structure
2.3.1. Quality Evaluation of Metagenomic Sequences
2.3.2. α Diversity Analysis of Soil Microbial Communities
2.3.3. Structural Compositions and Differences in Soil Microbial Communities
2.3.4. β Diversity Analysis of Soil Microbial Communities
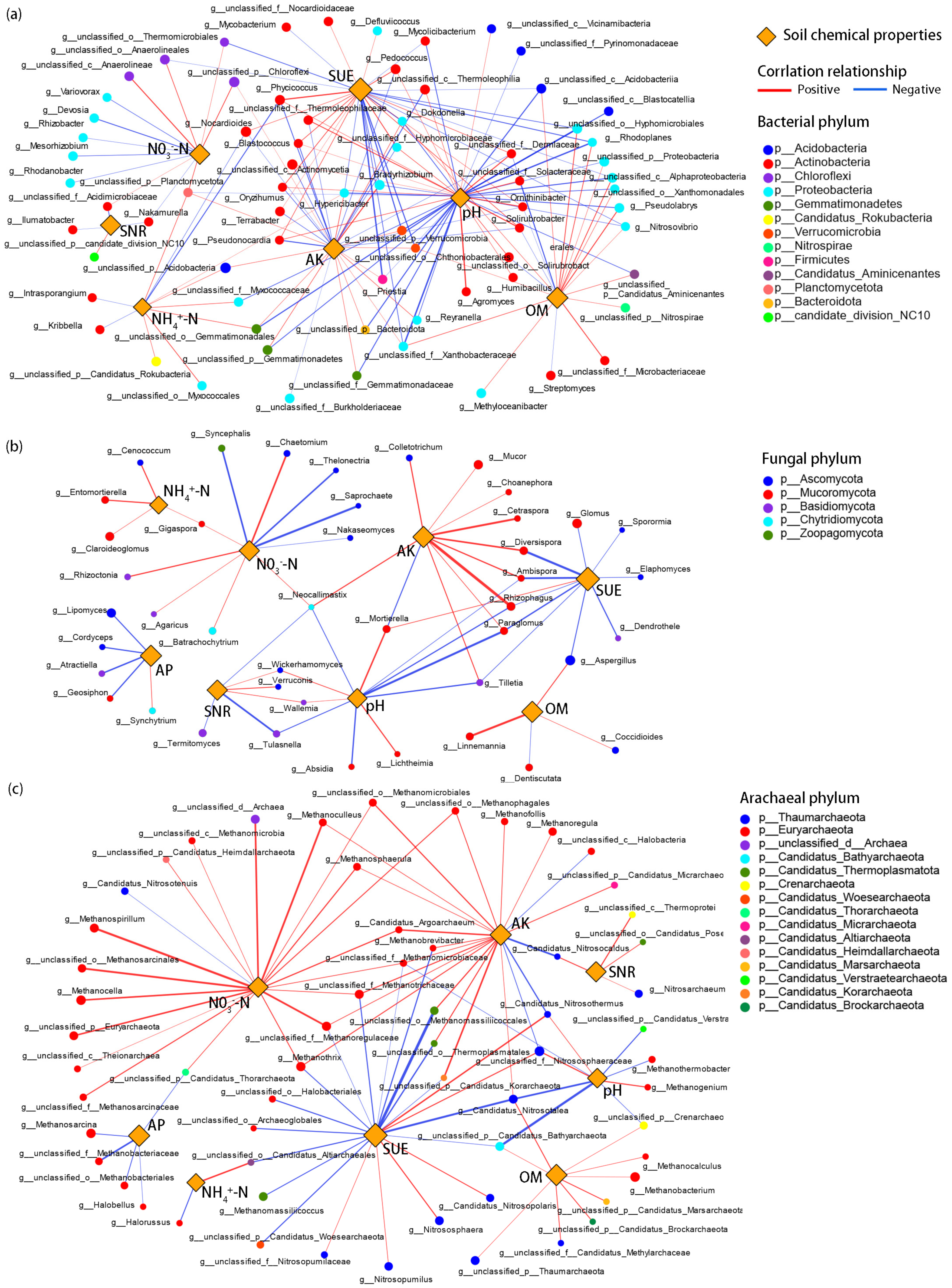
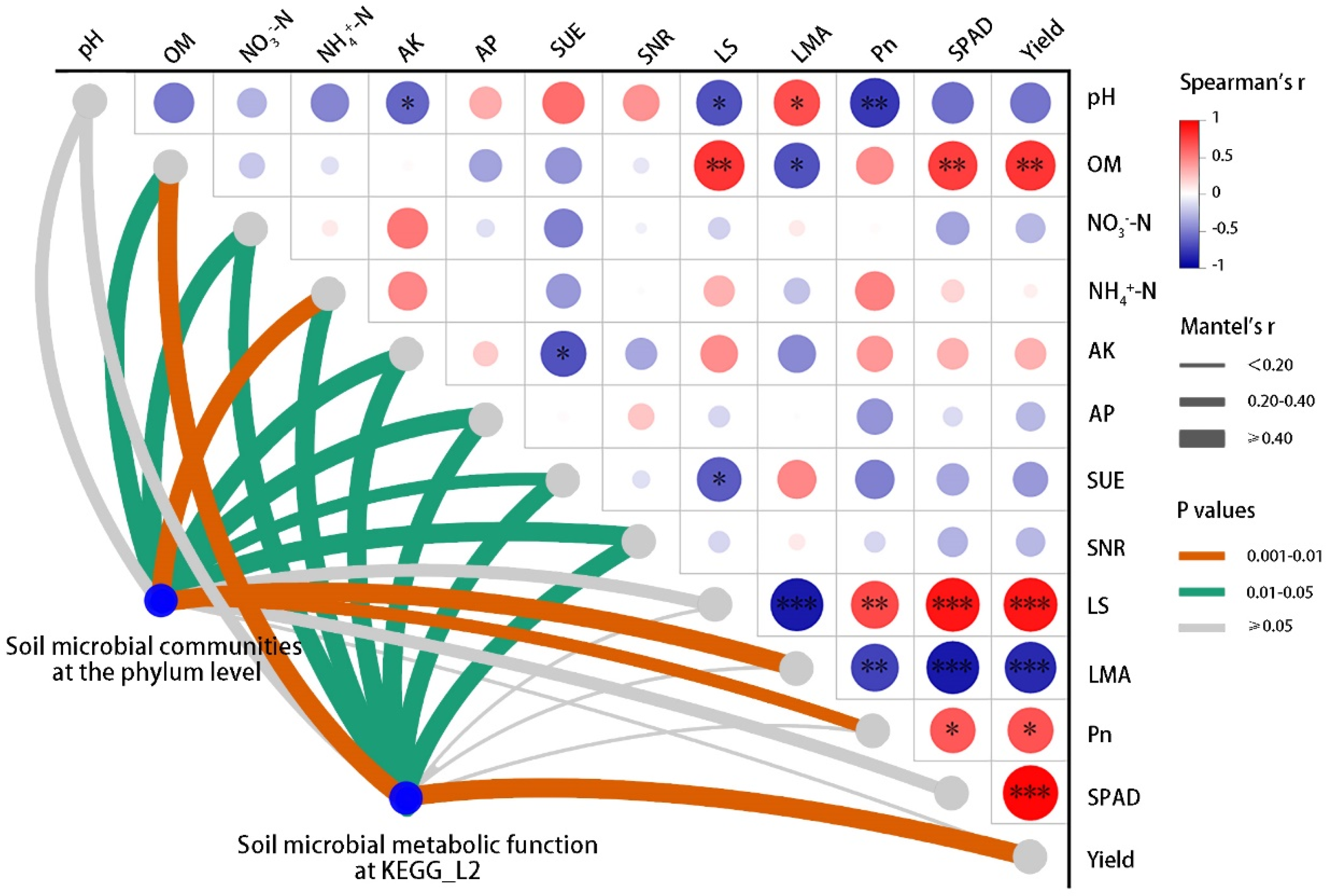
2.4. Interaction among Soil Microbial Communities, Soil Chemical Properties, and Agronomic Parameters
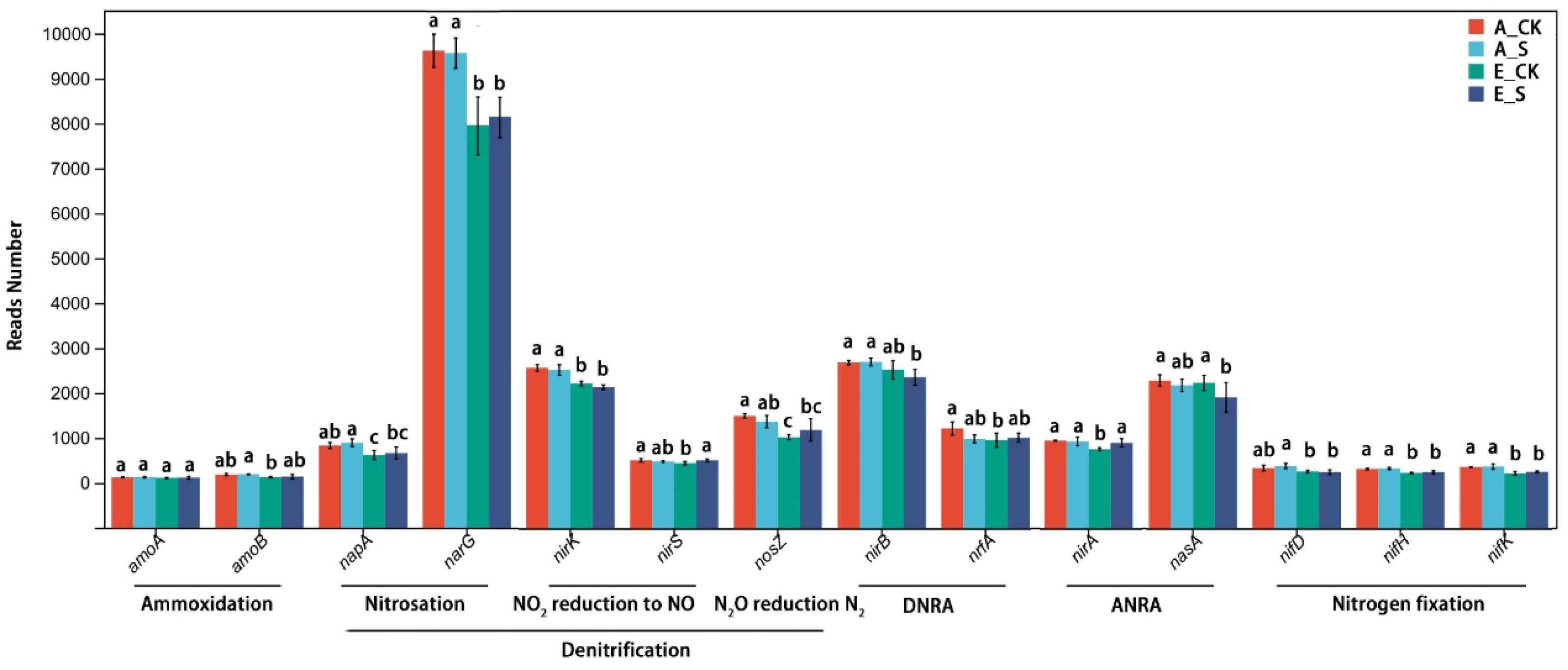
2.5. Comparison of Abundance Differences in N-Cycling Genes
3. Discussion
3.1. Effects of O3 and SCNF on Photosynthetic Parameters of Wheat Flag Leaves
3.2. Regulation of O3 and SCNF on Nutrient Accumulation and Wheat Yield
3.3. Effects of O3 and SCNF on Soil Chemistry Properties
3.4. Responses of the Soil Microbial Community Structure to O3 and SCNF
3.5. Interactions between Environmental Factors and Soil Microorganisms
3.6. Responses of Soil Microbial Nitrogen Metabolism to O3 and SCNF
3.7. Applicability and Limitations
4. Materials and Methods
4.1. Experimental Site
4.2. Materials and Treatments
4.3. Plant Sampling and Analysis
4.4. Soil Sampling and Assessment of Chemical Properties
4.5. Soil Metagenomic DNA Extraction, Sequencing and Data Analysis
4.6. Statistical Analyses and Bioinformatics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ashmore, M.; Emberson, L.; Karlsson, P.E.; Hakan, P. New Directions: A new generation of ozone critical levels for the protection of vegetation in Europe. Atmos. Environ. 2004, 38, 2213–2214. [Google Scholar] [CrossRef]
- Biswas, D.K.; Xu, H.; Li, Y.; Sun, J.; Wang, X.; Han, X.; Jiang, G. Genotypic differences in leaf biochemical, physiological and growth responses to ozone in 20 winter wheat cultivars released over the past 60 years. Global Change Biol. 2008, 14, 46–59. [Google Scholar] [CrossRef]
- Lu, X.; Hong, J.; Zhang, L.; Cooper, O.; Schultz, M.; Xu, X.; Wang, T.; Gao, M.; Zhao, Y. Severe surface ozone pollution in China: A global perspective. Environ. Sci. Technol. 2018, 5, 487–494. [Google Scholar] [CrossRef]
- Wang, N.; Lyu, X.; Deng, X.; Huang, X.; Jiang, F.; Ding, A. Aggravating O3 pollution due to NOX emission control in eastern China. Sci. Total Environ. 2019, 677, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Araminienė, V.; Sicard, P.; Anav, A.; Agathokleous, E.; Stakėnas, V.; DeMarco, A.; Varnagirytė-Kabašinskienė, I.; Paoletti, E.; Girgždienė, R. Trends and interrelationships of ground-level ozone metrics and forest health in Lithuania. Sci. Total Environ. 2019, 658, 1265–1277. [Google Scholar] [CrossRef]
- Aguiar-Silva, C.; Brandão, S.E.; Domingos, M.; Bulbovas, P. Antioxidant responses of Atlantic Forest native tree species as indicators of increasing tolerance to oxidative stress when they are exposed to air pollutants and seasonal tropical climate. Ecol. Indic. 2016, 63, 154–164. [Google Scholar] [CrossRef]
- Chen, Z.; Maltz, M.R.; Cao, J.; Yu, H.; Shang, H.; Aronson, E. Elevated O3 alters soil bacterial and fungal communities and the dynamics of carbon and nitrogen. Sci. Total Environ. 2019, 677, 272–280. [Google Scholar] [CrossRef]
- Zhu, X.; Feng, Z.; Sun, T.; Liu, X.; Tang, H.; Zhu, J.; Guo, W.; Kazuhiko, K. Effects of elevated ozone concentration on yield of four Chinese cultivars of winter wheat under fully open-air field conditions. Global Change Biol. 2011, 17, 2697–2706. [Google Scholar] [CrossRef]
- Mills, G.; Sharps, K.; Simpson, D.; Pleijel, H.; Broberg, M.; Uddling, J.; Jaramillo, F.; Davies, W.J.; Dentener, F.; Van den Berg, M.; et al. Ozone pollution will compromise efforts to increase global wheat production. Global Change Biol. 2018, 24, 3560–3574. [Google Scholar] [CrossRef]
- Ainsworth, E.A. Understanding and improving global crop response to ozone pollution. Plant J. 2017, 90, 886–897. [Google Scholar] [CrossRef]
- Booker, F.; Muntifering, R.; Mcgrath, M.; Burkey, M.; Decoteau, D.; Fiscus, E.; Manning, W.; Krupa, S.; Chappelka, A.; Grantz, D. The ozone component of global change: Potential effects on agricultural and horticultural plant yield, product quality and interactions with invasive species. J. Integr. Plant Biol. 2009, 51, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Mccrady, J.K.; Andersen, C.P. The effect of ozone on below-ground carbon allocation in wheat. Environ. Pollut. 2000, 107, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Deng, Y.; Li, Q.; Lu, C.; Wang, J.; Zhang, H.; Zhu, J.; Zhou, J.; He, Z. Shifts of functional gene representation in wheat rhizosphere microbial communities under elevated ozone. ISME J. 2013, 7, 660–671. [Google Scholar] [CrossRef]
- Miltner, A.; Bombach, P.; Schmidt-Brucken, B.; Kastner, M. SOM genesis: Microbial biomass as a significant source. Biogeochemistry 2012, 111, 41–55. [Google Scholar] [CrossRef]
- Andersen, C.P. Source-sink balance and carbon allocation below ground in plants exposed to ozone. New phytol. 2003, 157, 213–228. [Google Scholar] [CrossRef]
- Liu, P.; Huang, J.J.; Cai, Z.Y.; Chen, H.T.; Huang, X.; Yang, S.N.; Su, Z.X.; Azam, M.; Chen, H.B.; Shen, J.Y. Influence of girdling on growth of litchi (Litchi chinensis) roots during cold-dependent floral induction. Sci. Hortic. 2022, 297, 110928. [Google Scholar] [CrossRef]
- Wang, Z.; Zong, H.; Zheng, H.; Liu, G.; Chen, L.; Xing, B. Reduced nitrification and abundance of ammonia-oxidizing bacteria in acidic soil amended with biochar. Chemosphere 2015, 138, 576–583. [Google Scholar] [CrossRef]
- Baker, B.J.; Anda, V.D.; Seitz, K.W.; Dombrowski, N.; Santoro, A.E.; Lloyd, K.G. Diversity, ecology and evolution of Archaea. Nat. Microbiol. 2020, 5, 887–900. [Google Scholar] [CrossRef]
- Chelsea, C.J.; Dove, N.C.; Beman, J.M.; Stephen, C.H.; Emma, L.A. Meta-analysis reveals ammonia-oxidizing bacteria respond more strongly to nitrogen addition than ammonia-oxidizing archaea. Soil Biol. Biochem. 2016, 99, 158–166. [Google Scholar] [CrossRef]
- Berg, I.A.; Kockelkorn, D.; Buckel, W.; Fuchs, G. A 3-hydroxypropionate/4hydroxybutyrate autotrophic carbon dioxide assimilation pathway in Archaea. Science 2007, 318, 1782–1786. [Google Scholar] [CrossRef]
- Pei, Y.; Yu, Z.; Ji, J.; Khan, A.; Li, X. Microbial community structure and function indicate the severity of chromium contamination of the Yellow River. Front. Microbiol. 2018, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Lin, M.; Xiong, W.; Wang, M.; Zhang, J.; Wang, M.; Sun, Y. Metagenomic insights into the effect of oxytetracycline on microbial structures, functions and functional genes in sediment denitrification. Ecotoxicol. Environ. Saf. 2018, 161, 85–91. [Google Scholar] [CrossRef]
- Wang, M.; Li, G.; Feng, Z.; Liu, Y.; Yuan, X.; Uscola, M. A wider spectrum of avoidance and tolerance mechanisms explained ozone sensitivity of two white poplar ploidy levels. Ann. Bot. 2023, 131, 655–666. [Google Scholar] [CrossRef]
- Tahamolkonan, M.; Ghahsareh, A.M.; Ashtari, M.K.; Honarjoo, N. Tomato (Solanum lycopersicum) growth and fruit quality affected by organic fertilization and ozonated water. Protoplasma 2021, 259, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, D.; Agathokleous, E.; Cheng, C.; Shang, B.; Feng, Z.Z. Soil microbial community involved in nitrogen cycling in rice tields treated with antiozonant under ambient ozone. Appl. Environ. Microb. 2023, 89, 00180. [Google Scholar] [CrossRef]
- Manning, W.J.; Paoletti, E.; Sandermann, H.; Ernst, D. Ethylenediurea (EDU): A research tool for assessment and verification of the effects of ground level ozone on plants under natural conditions. Environ. Pollut. 2011, 159, 3283–3293. [Google Scholar] [CrossRef]
- Peng, J.; Xu, Y.; Shang, B.; Qu, L.; Feng, Z. Impact of ozone pollution on nitrogen fertilization management during maize (Zea mays L.) production. Environ. Pollut. 2020, 266, 115158. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Y.; Blaylock, A.D.; Chen, X. Mixture of controlled release and normal urea to optimize nitrogen management for high-yielding (>15 Mg ha–1) maize. Field Crops Res. 2017, 204, 23–30. [Google Scholar] [CrossRef]
- Li, R.; Gao, Y.; Chen, Q.; Li, Z.; Gao, F.; Meng, Q.; Li, T.; Liu, A.; Wang, Q.; Wu, L.; et al. Blended controlled-release nitrogen fertilizer with straw returning improved soil nitrogen availability, soil microbial community, and root morphology of wheat. Soil Tillage Res. 2021, 212, 105045. [Google Scholar] [CrossRef]
- Ma, Q.; Qian, Y.; Yu, Q.; Cao, Y.; Tao, R.; Zhu, M.; Ding, J.; Li, C.; Guo, W.; Zhu, X. Controlled-release nitrogen fertilizer application mitigated N losses and modified microbial community while improving wheat yield and N use efficiency. Agr. Ecosyst. Environ. 2023, 349, 108445. [Google Scholar] [CrossRef]
- Hu, T.; Liu, S.; Xu, Y.; Feng, Z.; Calatayud, V. Assessment of O3-induced yield and economic losses for wheat in the North China Plain from 2014 to 2017, China. Environ. Pollut. 2020, 258, 113828. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Shang, B.; Feng, Z.; Calatayud, V. Yield and economic losses of winter wheat and rice due to ozone in the Yangtze River Delta during 2014–2019. Sci. Total Environ. 2020, 745, 140847. [Google Scholar] [CrossRef] [PubMed]
- Avnery, S.; Mauzerall, D.L.; Liu, J.; Horowitz, L.W. Global crop yield reductions due to surface ozone exposure: 2. Year 2030 potential crop production losses, economic damage, and implications for world hunger under two scenarios of O3 pollution. Atmos. Environ. 2011, 45, 2297–2309. [Google Scholar] [CrossRef]
- Gelang, J.; Sild, E.; Danielsson, H.; Younis, S.; Sellden, G. Rate and duration of grain filling in relation to flag leaf senescence and grain yield in spring wheat exposed to different concentrations of ozone. Physiol. Plantarum 2000, 110, 366–375. [Google Scholar] [CrossRef]
- Yang, X.; Chen, X.; Ge, Q.; Li, B.; Tong, Y.; Li, Z.; Kuang, T.; Lu, C. Characterization of photosynthesis of flag leaves in a wheat hybrid and its parents grown under field conditions. Plant Physiol. 2007, 164, 318–326. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Giaramida, L.; Reich, P.B.; Khachane, A.N.; Hamonts, K.; Edwards, C.; Lawton, L.A.; Singh, B.K. Lack of functional redundancy in the relationship between microbial diversity and ecosystem functioning. J. Ecol. 2016, 104, 936–946. [Google Scholar] [CrossRef]
- Feng, P.; Wang, B.; Liu, D.; Xing, H.; Ji, F.; Macadam, I.; Ruan, H.; Yu, Q. Impacts of rainfall extremes on wheat yield in semi-arid cropping systems in eastern Australia. Climatic Change 2018, 147, 555–569. [Google Scholar] [CrossRef]
- Agathokleous, E.; Feng, Z.; Oksanen, E.; Sicard, P.; Wang, Q.; Saitanis, C.J.; Araminiene, V.; Blande, J.D.; Hayes, F.; Calatayud, V.; et al. Ozone affects plant, insect, and soil microbial communities: A threat to terrestrial ecosystems and biodiversity. Sci. Adv. 2020, 6, eabc1176. [Google Scholar] [CrossRef]
- Feng, Z.; Büker, P.; Pleijel, H.; Emberson, L.; Karlsson, P.E.; Uddling, J. A unifying explanation for variation in ozone sensitivity among woody plants. Glob. Change Biol. 2018, 24, 78–84. [Google Scholar] [CrossRef]
- Li, X.; Song, X.; Zhao, J.; Lu, H.; Qian, C.; Zhao, X. Shifts and plasticity of plant leaf mass per area and leaf size among slope aspects in a subalpine meadow. Ecol. Evol. 2021, 11, 14042–14055. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, H.; Li, T.; Wu, J.; Nagarajan, R.; Lei, L.; Powers, C.; Kan, C.C.; Hua, W.; Liu, Z.; et al. TaCol-B5 modifies spike architecture and enhances grain yield in wheat. Science 2022, 376, 180. [Google Scholar] [CrossRef] [PubMed]
- Shang, B.; Fu, R.; Agathokleous, E.; Dai, L.; Zhang, G.; Wu, R.; Feng, Z. Ethylenediurea offers moderate protection against ozone-induced rice yield loss under high ozone pollution. Sci. Total Environ. 2022, 806, 151341. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yin, R.; Zhou, H.; Yuan, X.; Feng, Z. Soil pH drives poplar rhizosphere soil microbial community responses to ozone pollution and nitrogen addition. Eur. J. Soil Sci. 2021, 73, 13186. [Google Scholar] [CrossRef]
- Cuhel, J.; Simek, M.; Laughlin, R.J.; Bru, D.; Cheneby, D.; Watson, C.J.; Philippot, L. Insights into the effect of soil pH on N2O and N2 emissions and denitrifier community size and activity. Appl. Environ. Microb. 2010, 76, 1870–1878. [Google Scholar] [CrossRef]
- Wang, H.; Boutton, T.; Xu, W.; Hu, G.; Jiang, P.; Bai, E. Quality of fresh organic matter affects priming of soil organic matter and substrate utilization patterns of microbes. Sci. Rep. 2015, 5, 10102. [Google Scholar] [CrossRef]
- Nacke, H.; Thürmer, A.; Wollherr, A.; Will, C.; Hodac, L.; Herold, N.; Schoning, L.; Schrumpf, M.; Daniel, R. Pyrosequencing-based assessment of bacterial community structure along different management types in German forest and grassland soils. PLoS ONE 2011, 6, e17000. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, J.; Wang, C.; Wang, Q. The 19-years inorganic fertilization increased bacterial diversity and altered bacterial community composition and potential functions in a paddy soil. Appl. Soil Ecol. 2019, 144, 60–67. [Google Scholar] [CrossRef]
- Zhou, J.; Guan, D.; Zhou, B.; Zhao, B.; Ma, M.; Qin, J.; Jiang, X.; Chen, S.; Cao, F.; Shen, D.; et al. Influence of 34-years of fertilization on bacterial communities in an intensively cultivated black soil in northeast China. Soil Biol. Biochem. 2015, 90, 42–51. [Google Scholar] [CrossRef]
- Ai, C.; Zhang, S.; Zhang, X.; Guo, D.; Zhou, W.; Huang, S. Distinct responses of soil bacterial and fungal communities to changes in fertilization regime and crop rotation. Geofis. Int. 2018, 319, 156–166. [Google Scholar] [CrossRef]
- He, Z.; Huang, X.; Fan, Y.; Yang, M.; Zhou, E. Metatranscriptomic analysis reveals rich mycoviral diversity in three major fungal pathogens of rice. Int. J. Mol. Sci. 2022, 23, 91–92. [Google Scholar] [CrossRef]
- Aller, J.Y.; Kemp, P.F. Are Archaea inherently less diverse than Bacteria in the same environments? FEMS Microbiol. Ecol. 2008, 65, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Reji, L.; Francis, C.A. Metagenome-assembled genomes reveal unique metabolic adaptations of a basal marine Thaumarchaeota lineage. ISME J. 2020, 14, 2105–2115. [Google Scholar] [CrossRef]
- Joshi, B.; Chaudhary, A.; Varma, A.; Tripathi, S.; Bhatia, A. Elevated CO2, O3 and their interaction have differential impact on soil microbial diversity and functions in wheat agroecosystems. Rhizosphere 2023, 27, 100777. [Google Scholar] [CrossRef]
- Miao, S.; Qiao, Y.; Jin, J.; Wang, Y.; Tang, C. Greater variation of bacterial community structure in soybean- than maize-grown Mollisol soils in responses to seven-year elevated CO2 and temperature. Sci. Total Environ. 2021, 764, 142836. [Google Scholar] [CrossRef]
- GB/T 17892-1999; High quality wheat—Strong gluten wheat. The State Bureau of Quality and Technical Supervisor: Beijing, China, 1999.
- Cai, F.; Zhang, Y.S.; Mi, N.; Ming, H.Q.; Zhang, S.J.; Zhang, H.; Zhao, X. Maize (Zea mays L.) physiological responses to drought and rewatering, and the associations with water stress degree. Agr. Water Manag. 2020, 241, 106379. [Google Scholar] [CrossRef]
- Liu, J.; Song, M.; Wei, X.; Zhang, H.; Bai, Z.; Zhuang, X. Responses of phyllosphere microbiome to ozone stress: Abundance, community compositions and functions. Microorganisms 2022, 10, 680. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.D. Soil Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Zhou, J.; Deng, Y.; Shen, L.; Wen, C.; Yan, Q.; Ning, D.; Qin, Y.; Xue, K.; Wu, L.; He, Z. Temperature mediates continental-scale diversity of microbes in forest soils. Nat. Commun. 2016, 7, 12083. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, Z.; Zhang, Y.; Shi, Y. Impacts of fertilization optimization on soil nitrogen cycling and wheat nitrogen utilization under water-saving irrigation. Front. Plant Sci. 2022, 13, 878424. [Google Scholar] [CrossRef]
- Zhong, Y.; Yan, W.; Shangguan, Z. Impact of long-term N additions upon coupling between soil microbial community structure and activity, and nutrient-use efficiencies. Soil Biol. Biochem. 2015, 91, 151–159. [Google Scholar] [CrossRef]
- Schmidt, R.; Mitchell, J.; Scow, K. Cover cropping and no-till increase diversity and symbiotroph: Saprotroph ratios of soil fungal communities. Soil Biol. Biochem. 2019, 129, 99–109. [Google Scholar] [CrossRef]
- Mueller, R.; Paula, F.; Mirza, B.; Rodrigues, J.; Nüsslein, K.; Bohannan, B. Links between plant and fungal communities across a deforestation chronosequence in the Amazon rainforest. ISME J. 2014, 8, 1551. [Google Scholar] [CrossRef]
- Buchfink, B.; Reuter, K.; Drost, H. Sensitive protein alignments at tree-of-life scale using DIAMOND. Nat. Methods 2021, 18, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jiang, Y.; Huang, H.; Mou, L.; Ru, J.; Zhao, J.; Xiao, S. Long-term and high-concentration heavy-metal contamination strongly influences the microbiome and functional genes in Yellow River sediments. Sci. Total Environ. 2018, 637, 1400–1412. [Google Scholar] [CrossRef]
- Lawson, C.E.; Wu, S.; Bhattacharjee, A.S.; Hamilton, J.J.; McMahon, K.D.; Goel, R.; Noguera, D.R. Metabolic network analysis reveals microbial community interactions in anammox granules. Nat. Commun. 2017, 8, 15416. [Google Scholar] [CrossRef] [PubMed]


| O3 | SCNF | O3 × SCNF | |||
|---|---|---|---|---|---|
| Flowering period (Z60) | Leaf photosynthetic function | LS | ** | * | NS |
| LMA | ** | NS | NS | ||
| Pn | * | * | NS | ||
| SPAD value | NS | NS | ** | ||
| Soil enzyme activity | SUE | * | * | NS | |
| SNR | NS | NS | NS | ||
| Soil chemistry property | pH | * | NS | ** | |
| OM | NS | * | NS | ||
| NO3−–N | * | ** | ** | ||
| NH4+–N | NS | NS | NS | ||
| AK | * | * | NS | ||
| AP | NS | NS | ** | ||
| Mature period (Z92) | Yield structure | Spike | NS | * | NS |
| Grain per spike | ** | * | * | ||
| TGW | * | * | NS | ||
| Yield | * | ** | NS | ||
| Plant nutrient accumulation | TN | ** | ** | * | |
| TP | NS | * | ** | ||
| TK | ** | NS | NS | ||
| Soil chemistry property | pH | ** | ** | * | |
| OM | * | NS | NS | ||
| NO3−–N | NS | ** | NS | ||
| NH4+–N | NS | NS | NS | ||
| AK | NS | ** | NS | ||
| AP | NS | ** | * |
| Treatment | Chao Index | Shannon Index | Simpson Index | |
|---|---|---|---|---|
| Bacteria | A_CK | 21,435 ± 50 a | 5.70 ± 0.01 b | 0.016 ± 0.000 ab |
| A_S | 21,073 ± 84 a | 5.79 ± 0.01 a | 0.015 ± 0.000 b | |
| E_CK | 20,507 ± 217 a | 5.71 ± 0.01 b | 0.017 ± 0.001 a | |
| E_S | 20,794 ± 199 a | 5.74 ± 0.00 ab | 0.016 ± 0.000 ab | |
| Fungi | A_CK | 157.00 ± 2.33 a | 3.60 ± 0.01 a | 0.061 ± 0.003 a |
| A_S | 148.00 ± 1.86 a | 3.54 ± 0.02 ab | 0.068 ± 0.001 a | |
| E_CK | 134.00 ± 2.91 b | 3.48 ± 0.01 b | 0.069 ± 0.002 a | |
| E_S | 133.33 ± 1.07 b | 3.53 ± 0.02 ab | 0.066 ± 0.002 a | |
| Archaea | A_CK | 629.33 ±2.50 a | 3.83 ± 0.01 a | 0.004 ± 0.001 c |
| A_S | 602.00 ± 4.06 ab | 3.69 ± 0.01 b | 0.060 ± 0.002 bc | |
| E_CK | 587.67 ± 7.56 b | 3.61 ± 0.02 bc | 0.072 ± 0.003 ab | |
| E_S | 594.67 ± 8.70 ab | 3.54 ± 0.03 c | 0.086 ± 0.004 a |
| pH | Organic Matter (g kg−1) | Nitrate Nitrogen (mg kg−1) | Ammonium Nitrogen (mg kg−1) | Available Phosphorus (mg kg−1) | Available Potassium (mg kg−1) |
|---|---|---|---|---|---|
| 6.7 | 30.07 | 43.26 | 2.26 | 17.11 | 60.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, N.; Qian, Y.; Song, L.; Yu, Q.; Sheng, H.; Li, Y.; Zhu, X. Regulating Leaf Photosynthesis and Soil Microorganisms through Controlled-Release Nitrogen Fertilizer Can Effectively Alleviate the Stress of Elevated Ambient Ozone on Winter Wheat. Int. J. Mol. Sci. 2024, 25, 9381. https://doi.org/10.3390/ijms25179381
Zhu N, Qian Y, Song L, Yu Q, Sheng H, Li Y, Zhu X. Regulating Leaf Photosynthesis and Soil Microorganisms through Controlled-Release Nitrogen Fertilizer Can Effectively Alleviate the Stress of Elevated Ambient Ozone on Winter Wheat. International Journal of Molecular Sciences. 2024; 25(17):9381. https://doi.org/10.3390/ijms25179381
Chicago/Turabian StyleZhu, Nanyan, Yinsen Qian, Lingqi Song, Qiaoqiao Yu, Haijun Sheng, Ying Li, and Xinkai Zhu. 2024. "Regulating Leaf Photosynthesis and Soil Microorganisms through Controlled-Release Nitrogen Fertilizer Can Effectively Alleviate the Stress of Elevated Ambient Ozone on Winter Wheat" International Journal of Molecular Sciences 25, no. 17: 9381. https://doi.org/10.3390/ijms25179381
APA StyleZhu, N., Qian, Y., Song, L., Yu, Q., Sheng, H., Li, Y., & Zhu, X. (2024). Regulating Leaf Photosynthesis and Soil Microorganisms through Controlled-Release Nitrogen Fertilizer Can Effectively Alleviate the Stress of Elevated Ambient Ozone on Winter Wheat. International Journal of Molecular Sciences, 25(17), 9381. https://doi.org/10.3390/ijms25179381







