Cloning and Functional Study of AmGDSL1 in Agropyron mongolicum
Abstract
1. Introduction
2. Results
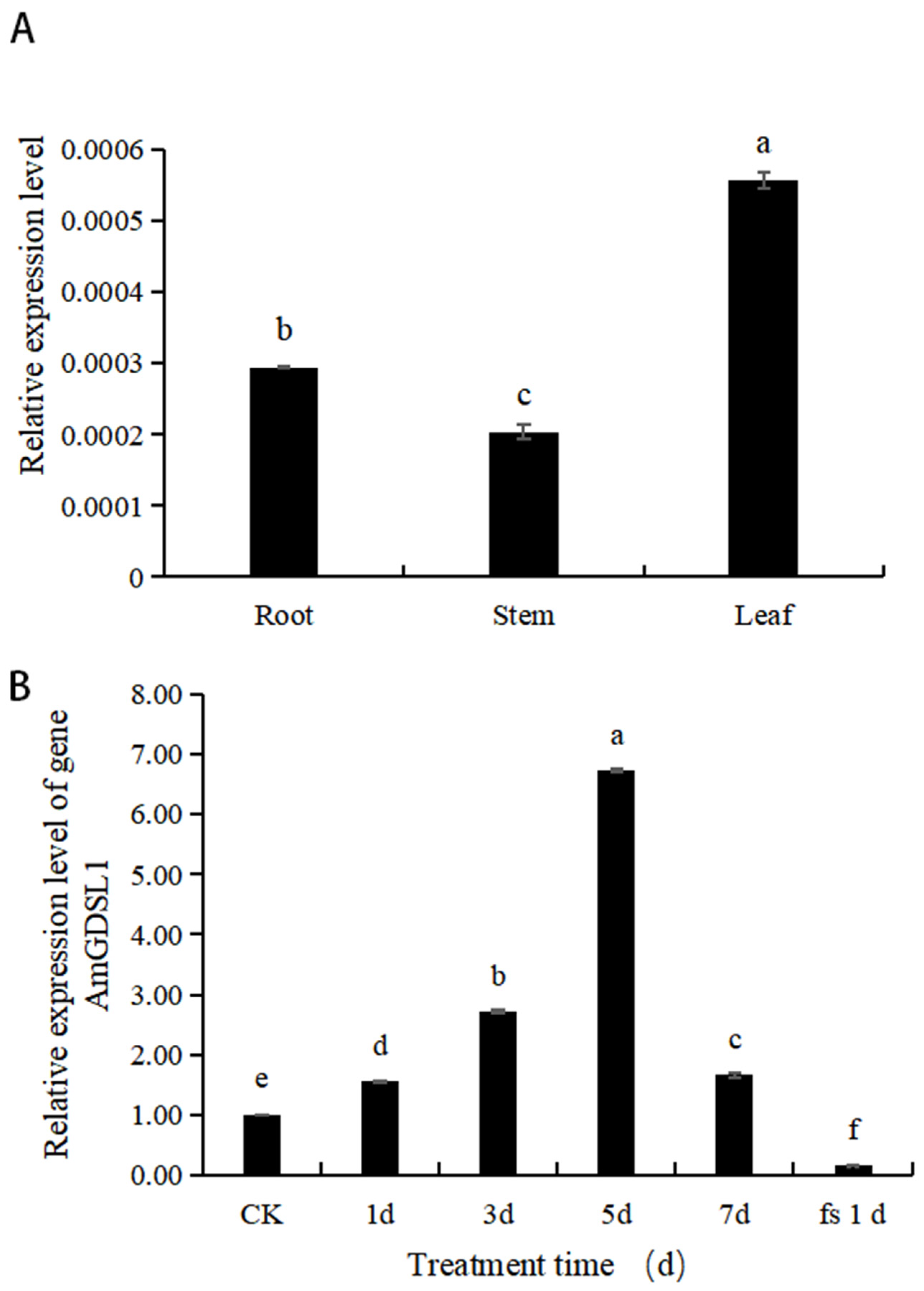
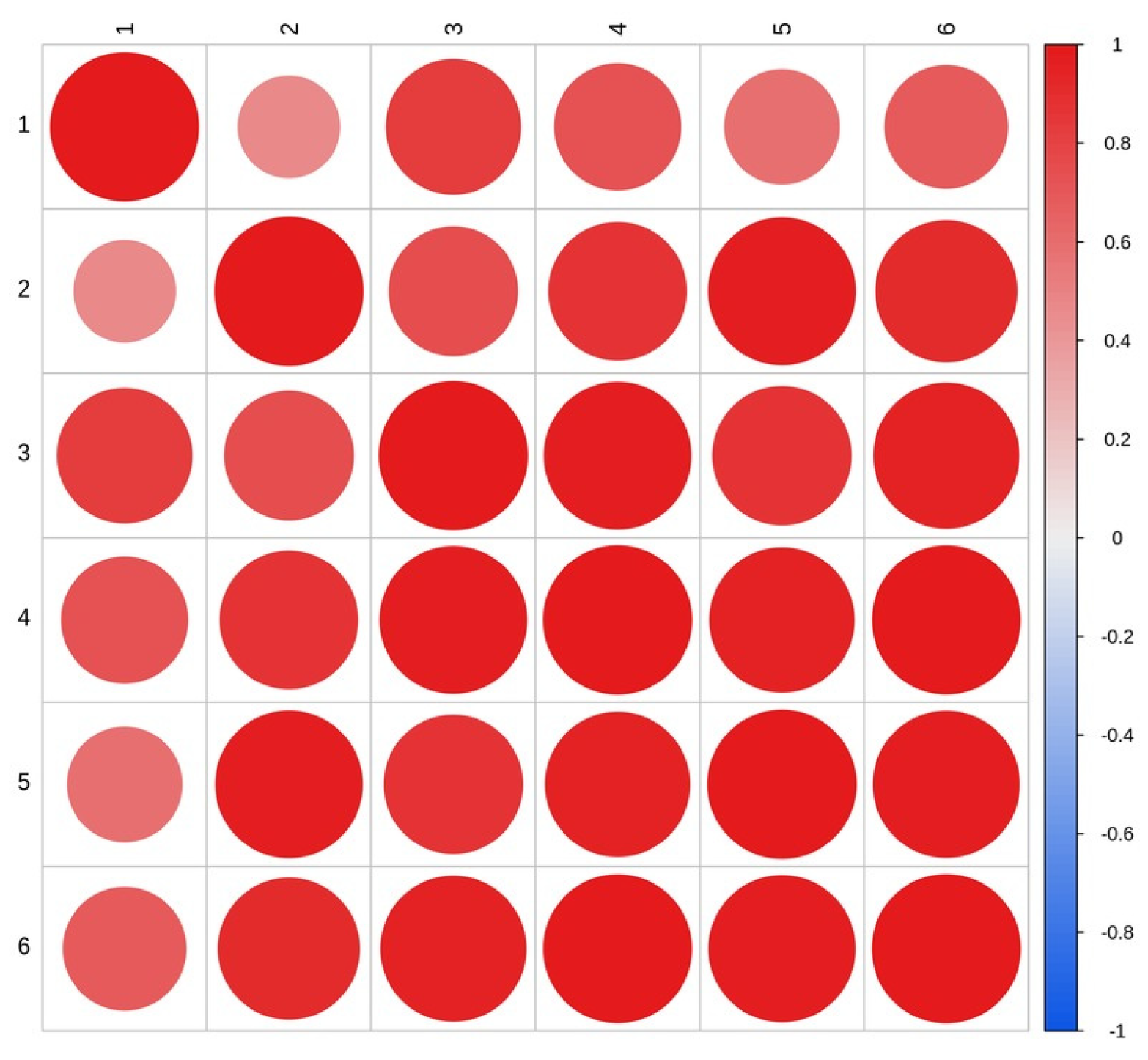
2.1. Analysis of AmGDSL1 Gene Expression in A. mongolicum
2.2. Cloning of AmGDSL1 Gene in A. mongolicum and Overexpression Vector Construction
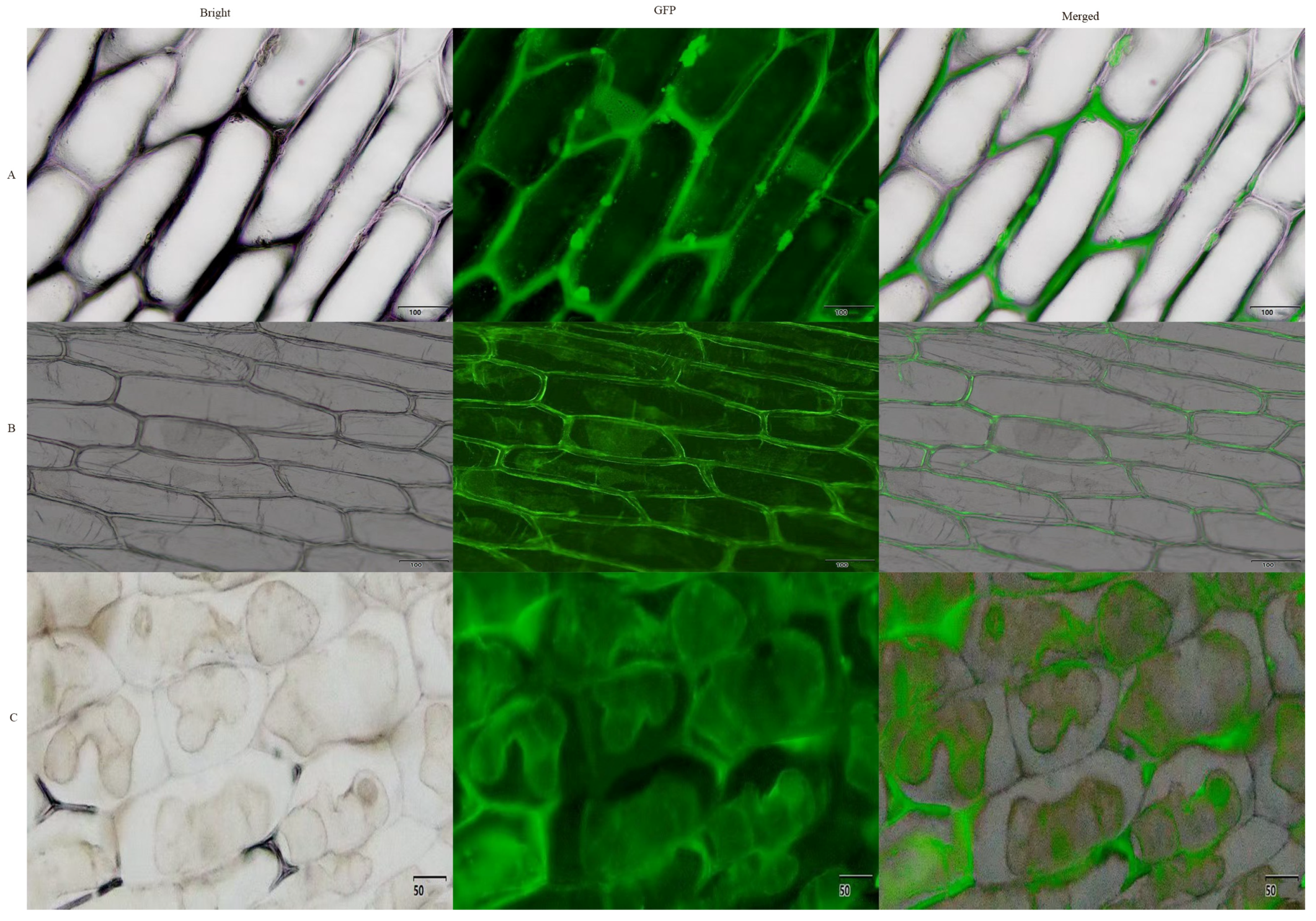
2.3. Subcellular Localization of AmGDSL1 Gene
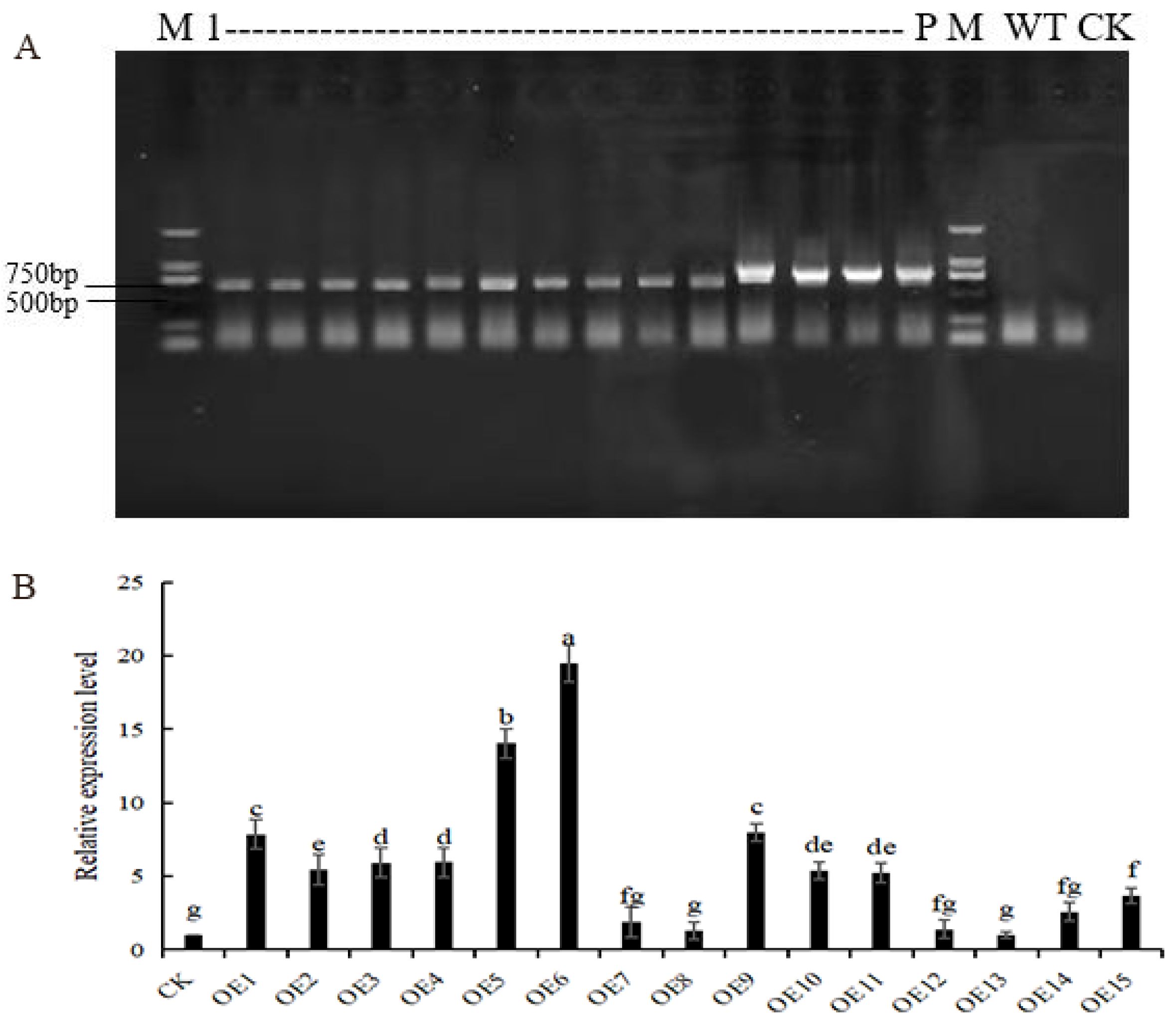
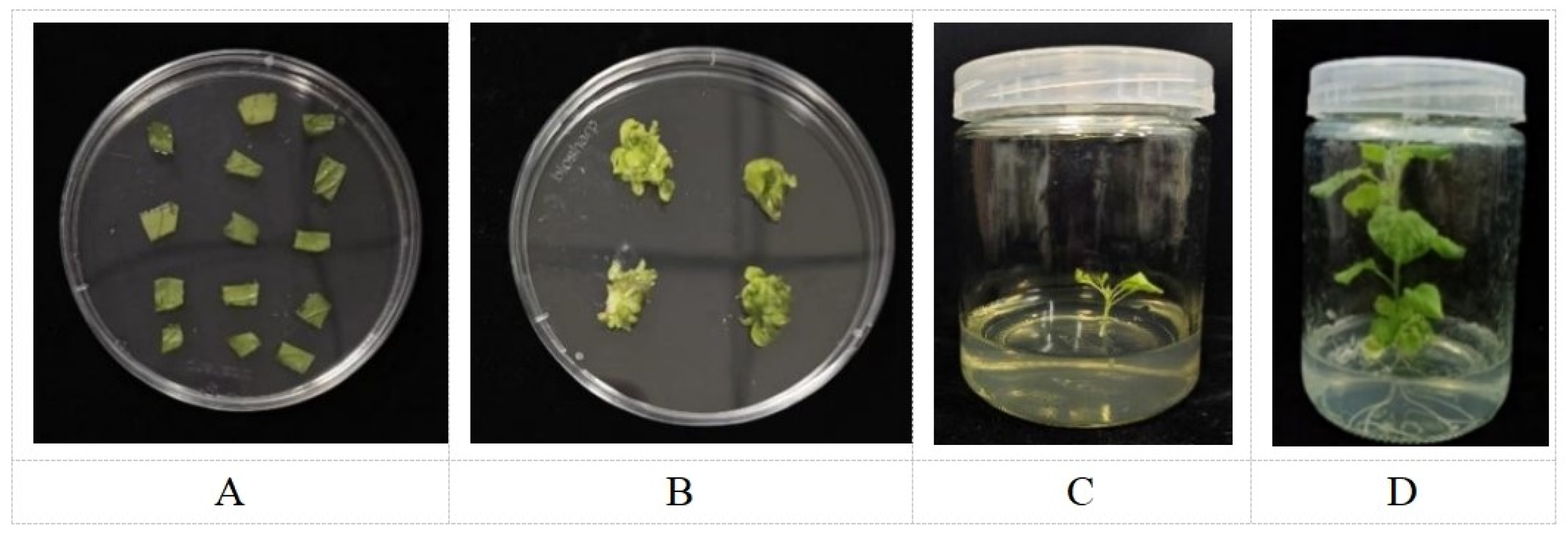
2.4. Production and Identification of AmGDSL1 Transgenic Tobacco
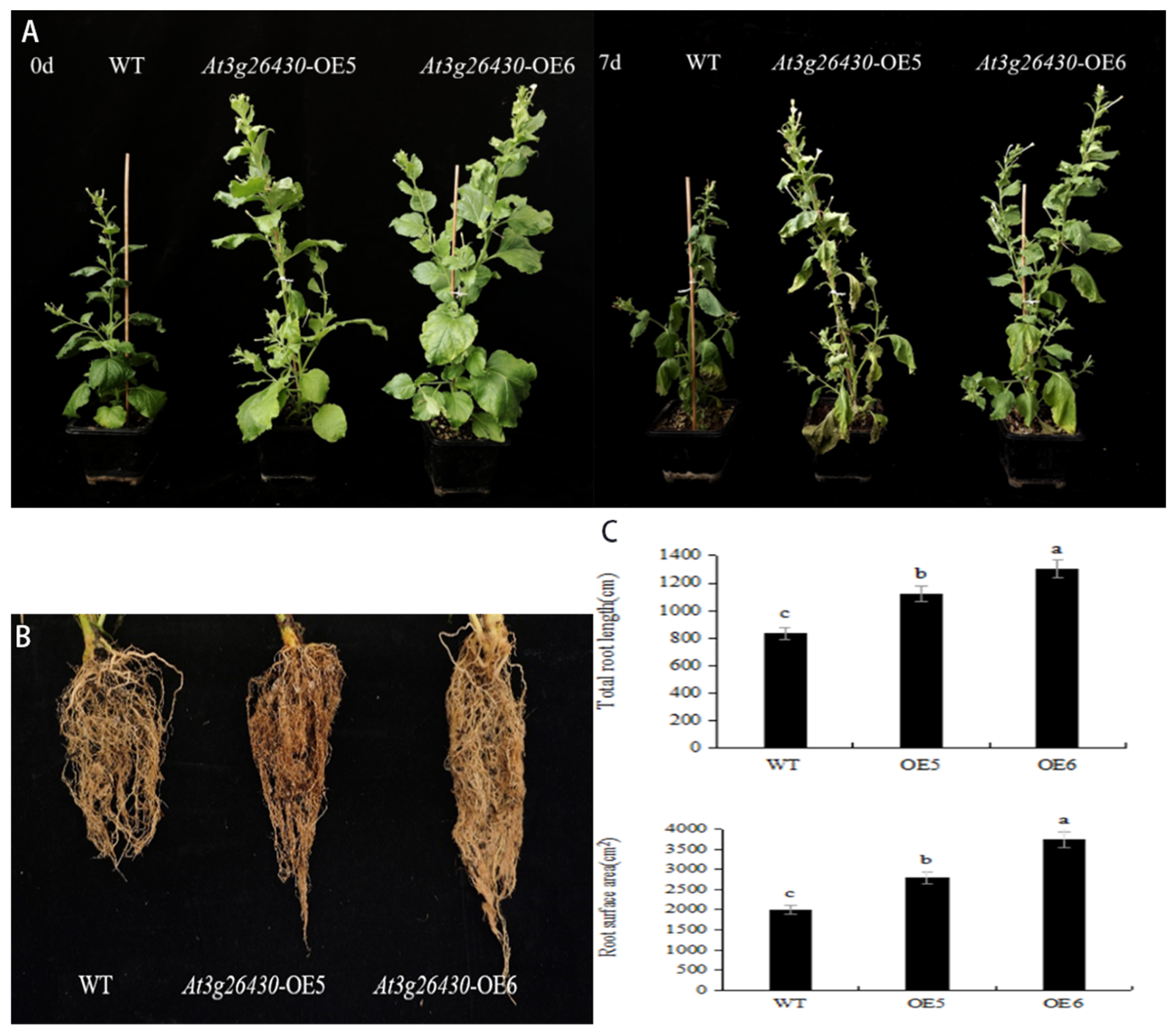
2.5. Phenotype Observation of Transgenic Tobacco under Drought Stress
2.6. Overexpression of AmGDSL1 Enhanced Antioxidant Capacity of Tobacco
2.7. Expression Regulation in AmGDSL1 Transgenic Tobacco
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Sample Preparation
4.2. Cloning of AmGDSL1 Gene and Vector Construction
4.3. Subcellular Localization of AmGDSL1
4.4. Obtaining and Identifying of AmGDSL1 Transgenic Tobacco Plants
4.5. Drought Stress Tolerance Assays of AmGDSL1 Transgenic Tobacco Plant
4.6. Analysis of AmGDSL1 Gene Expression
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2,4-D | 2,4-Dichlorophenoxyacetic acid |
| 6-BA | 6-Benzylaminopurine |
| CAT | Catalase |
| GELP | GDSL-type esterase/lipase |
| IAA | Indole acetic acid |
| Kana | Kanamycin |
| MDA | Malondialdehyde |
| NAA | 1-Naphthaleneacetic acid |
| PCR | Polymerase chain reaction |
| POD | Peroxidase |
| PRO | Proline |
| qRT-PCR | Real-time quantitative PCR |
| Rif | Rifampicin |
| SOD | Superoxide dismutase |
| TMT | Timentin |
| WT | Wild-type tobacco |
References
- Yang, P.; Zhai, X.; Huang, H.; Zhang, Y.; Zhu, Y.; Shi, X.; Zhou, L.; Fu, C. Association and driving factors of meteorological drought and agricultural drought in Ningxia, Northwest China. Atmos. Res. 2023, 289, 106753. [Google Scholar] [CrossRef]
- Nadarajah, K.N. ROS Homeostasis in Abiotic stress tolerance in plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Sun, J.; Li, Z.; Wang, Y.; Yang, A. NtNAC053, A Novel NAC transcription factor, confers drought and salt tolerances in tobacco. Front. Plant. Sci. 2022, 13, 817106. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Yi, R.; Ma, Y.; Dong, Q.; Mao, K.; Ma, F. Apple WRKY transcription factor MdWRKY56 positively modulates drought stress tolerance. Environ. Exp. Bot. 2023, 212, 105400. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Ye, X.; Zhang, Q.; Li, Y.; Chen, M. Genome-wide identification and expression analysis of the WRKY gene family in response to low-temperature and drought stresses in Cucurbita pepo L. Sci. Hortic. 2024, 330, 113048. [Google Scholar] [CrossRef]
- Zhu, C.; Lin, Z.; Liu, Y.; Li, H.; Di, X.; Li, T. A bamboo bHLH transcription factor PeRHL4 has dual functions in enhancing drought and phosphorus starvation tolerance. Plant Cell Environ. 2024, 47, 3015–3029. [Google Scholar] [CrossRef]
- Li, C.; Yan, C.; Sun, Q.; Wang, J.; Yuan, C.; Mou, Y. The bHLH transcription factor AhbHLH112 improves the drought tolerance of peanut. BMC Plant Biol. 2021, 21, 540. [Google Scholar] [CrossRef]
- Zhao, Y.; Cheng, X.Y.; Liu, X.D.; Wu, H.F.; Bi, H.H.; Xu, H.X. The Wheat MYB transcription factor TaMYB31 is involved in drought stress responses in Arabidopsis. Front. Plant Sci. 2018, 9, 1426. [Google Scholar] [CrossRef]
- Li, M.J.; Qiao, Y.; Li, Y.Q.; Shi, Z.L.; Zhang, N.; Bi, C.L.; Guo, J.K. A R2R3-MYB transcription factor gene in common wheat (namely TaMYBsm1) involved in enhancement of drought tolerance in transgenic Arabidopsis. J. Plant Res. 2016, 129, 1097–1107. [Google Scholar] [CrossRef]
- Mao, H.; Li, S.; Chen, B.; Jian, C.; Mei, F.; Zhang, Y. Variation in cis-regulation of a NAC transcription factor contributes to drought tolerance in wheat. Mol. Plant 2022, 15, 276–292. [Google Scholar] [CrossRef]
- Mergby, D.; Hanin, M.; Saidi, M.N. The durum wheat NAC transcription factor TtNAC2A enhances drought stress tolerance in Arabidopsis. Environ. Exp. Bot. 2021, 186, 104439. [Google Scholar] [CrossRef]
- Yang, Y.; Song, T.; Wang, Y.; Zhang, M.; Li, N.; Yu, M. The wheat WRKY transcription factor TaWRKY1-2D confers drought resistance in transgenic Arabidopsis and wheat (Triticum aestivum L.). Int. J. Biol. Macromol. 2023, 226, 1203–1217. [Google Scholar]
- Wang, D.; Zhang, X.; Cao, Y.; Batool, A.; Xu, Y.; Qiao, Y. TabHLH27 orchestrates root growth and drought tolerance to enhance water use efficiency in wheat. J. Integr. Plant Biol. 2024, 66, 1295–1312. [Google Scholar] [CrossRef]
- Shen, G.; Sun, W.; Chen, Z.; Shi, L.; Hong, J.; Shi, J. Plant GDSL Esterases/Lipases: Evolutionary, physiological and molecular functions in plant development. Plants 2022, 11, 468. [Google Scholar] [CrossRef]
- Vincent, P.M.; Dae, Y.S. The functions and applications of GDSL esterase/lipase proteins in agriculture. JSFA Rep. 2022, 2, 304–312. [Google Scholar]
- Akoh, C.C.; Lee, G.C.; Liaw, Y.C. GDSL family of serine esterase/lipases. Prog. Lipid Res. 2004, 43, 534–552. [Google Scholar] [CrossRef]
- Lee, D.S.; Kim, B.K.; Kwon, S.J. Arabidopsis GDSL lipase 2 plays a role in pathogen defense via negative regulation of auxin signaling. Biochem. Biophys. Res. Commun. 2009, 379, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, H.; Khan, A.; Xu, Y.; Hou, Y.; Wang, Y. Genome wide identification of GDSL gene family explores a novel GhirGDSL26 gene enhancing drought stress tolerance in cotton. BMC Plant Biol. 2023, 23, 1–18. [Google Scholar] [CrossRef]
- Suman, P.; Kumari, A.; Harsha, S.; Sapna, G.; Kishor, G. Understanding the structure and function of GDSL-type esterase/lipase genes in pigeon pea for their role under moisture stress conditions. Plant Stress 2023, 10, 100246. [Google Scholar]
- Yun, J.; Zhang, J.; Pan, C. Genome-Wide Analysis of the GDSL Genes in Pecan (Carya illinoensis K. Koch): Phylogeny, structure, promoter cis-elements, co-Expression networks, and response to salt stresses. Genes 2022, 13, 1103. [Google Scholar] [CrossRef]
- Jeum, K.H.; Hyong, W.C.; Sun, H.; Dae, S.; Nak, H.K.; Du, S.C. Function of a novel GDSL-type pepper lipase gene, CaGLIP1, in disease susceptibility and abiotic stress tolerance. Planta 2008, 227, 539–558. [Google Scholar]
- Liu, D.; Zhao, X.; Liu, Y.; Tian, M.; Zhao, J.; Bai, N. The GDSL lipase CpGLIP1 from Chimonanthus praecox improves drought and cold tolerance in Arabidopsis and poplar. Ind. Crop. Prod. 2024, 215, 118636. [Google Scholar] [CrossRef]
- Du, J.; Li, X.; Li, T. Genome-wide transcriptome profiling provides overwintering mechanism of Agropyron mongolicum. BMC Plant Biol. 2017, 17, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.H.; Yu, X.X.; Yu, Z.; Sun, F.C.; Li, X.D.; Li, X.L. RNA_Seq of Agropyron mongolicum Keng in response to drought stress. Grassl. Sci. 2018, 64, 3–15. [Google Scholar] [CrossRef]
- Zhang, X.F.; Ma, Y.H.; Fan, B.B.; Sun, F.C.; Zhai, Y.Q.; Zhao, Y. The Agropyron mongolicum bHLH Gene AmbHLH148 Positively Involved in Transgenic Nicotiana benthamiana Adaptive Response to Drought Stress. Agronomy 2023, 13, 2918. [Google Scholar] [CrossRef]
- Ao, T.G.B.Y.; Gao, L.J.; Wang, L.C.; Li, Y.Q.; Zhao, Y.; Lang, M.L.; Yang, X.J. Cloning and expression analysis of AP2/EREBP transcription factor gene (MwAP2/EREBP) in Agropyron mongolicum Keng. Nanosci. Nanotechnol. Lett. 2017, 9, 2031–2038. [Google Scholar] [CrossRef]
- Zhang, X.T.; Fan, B.B.; Yu, Z.; Nie, L.Z.; Zhao, Y.; Yu, X.X. Functional analysis of three miRNAs in Agropyron mongolicum Keng under drought stress. Agronomy 2019, 9, 661. [Google Scholar] [CrossRef]
- Fan, B.B.; Sun, F.C.; Yu, Z.; Zhang, X.F.; Yu, X.X.; Wu, J. Integrated analysis of small RNAs, transcriptome and degradome sequencing reveal the drought stress network in Agropyron mongolicum Keng. Front. Plant Sci. 2022, 13, 976684. [Google Scholar] [CrossRef]
- Chepyshko, H.; Lai, C.P.; Huang, L.M. Multifunctionality and diversity of GDSL esterase/lipase gene family in rice (Oryza sativa L. japonica) genome: New insights from bioinformatics analysis. BMC Genom. 2012, 13, 309. [Google Scholar]
- Ren, R.; Yang, X.; Xu, J.H. Genome-wide identification and analysis of promising GDSL-type lipases related to gummy stem blight resistance in watermelon (Citrullus lanatus). Sci. Hortic. 2021, 289, 110461. [Google Scholar] [CrossRef]
- Volokita, M.; Rosilio, T.; Rivkin, N. Combining comparative sequence and genomic data to ascertain phylogenetic relationships and explore the evolution of the large GDSL-lipase family in land plants. Mol. Biol. Evol. 2011, 28, 551–565. [Google Scholar] [CrossRef]
- Cao, Y.; Han, Y.; Meng, D. Expansion and evolutionary patterns of GDSL-type esterases/lipases in Rosaceae genomes. Funct. Integr. Genom. 2018, 18, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Wang, H.; Xin, X. BrEXL6, a GDSL lipase gene of Brassica rapa, functions in pollen development. Biol. Plant. 2017, 61, 685–692. [Google Scholar] [CrossRef]
- Zhang, P.L.; Zhang, H.; Du, J.K. Genome-wide identification and coexpression analysis of GDSI. genes related to suberin formation during fruit russeting in pear. Hortic. Plant J. 2022, 8, 153–170. [Google Scholar] [CrossRef]
- Su, H.G.; Zhang, X.H.; Wang, T.T. Genome-wide identification, evolution, and expression of GDSL-type esterase/lipase gene family in soybean. Front. Plant Sci. 2020, 11, 726. [Google Scholar] [CrossRef] [PubMed]
- Simone, L.; Jean, F.H.; Gea, G. Abiotic stress: Focus on drought and salt stress, recent insights and perspectives. Front. Plant Sci. 2017, 8, 1214. [Google Scholar]
- Xiao, C.L.; Guo, H.M.; Tang, J. Expression pattern and functional analyses of Arabidopsis guard cell-enriched GDSL lipases. Front. Plant Sci. 2021, 12, 748543. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Yang, X.; Xiao, C.L. GDSL lipase occluded stomatal pore1 is required for wax biosynthesis and stomatal cuticular ledge formation. New Phytol. 2020, 228, 1880–1896. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Wu, X.; Sun, F.; Nie, H.; Du, X.; Li, X.; Fang, Y.; Zhai, Y.; Zhao, Y.; Fan, B.; et al. Cloning and Functional Study of AmGDSL1 in Agropyron mongolicum. Int. J. Mol. Sci. 2024, 25, 9467. https://doi.org/10.3390/ijms25179467
Yan X, Wu X, Sun F, Nie H, Du X, Li X, Fang Y, Zhai Y, Zhao Y, Fan B, et al. Cloning and Functional Study of AmGDSL1 in Agropyron mongolicum. International Journal of Molecular Sciences. 2024; 25(17):9467. https://doi.org/10.3390/ijms25179467
Chicago/Turabian StyleYan, Xiuxiu, Xiaojuan Wu, Fengcheng Sun, Hushuai Nie, Xiaohong Du, Xiaolei Li, Yongyu Fang, Yongqing Zhai, Yan Zhao, Bobo Fan, and et al. 2024. "Cloning and Functional Study of AmGDSL1 in Agropyron mongolicum" International Journal of Molecular Sciences 25, no. 17: 9467. https://doi.org/10.3390/ijms25179467
APA StyleYan, X., Wu, X., Sun, F., Nie, H., Du, X., Li, X., Fang, Y., Zhai, Y., Zhao, Y., Fan, B., & Ma, Y. (2024). Cloning and Functional Study of AmGDSL1 in Agropyron mongolicum. International Journal of Molecular Sciences, 25(17), 9467. https://doi.org/10.3390/ijms25179467





