Hepatocyte Growth Factor Modulates Corneal Endothelial Wound Healing In Vitro
Abstract
1. Introduction
2. Results
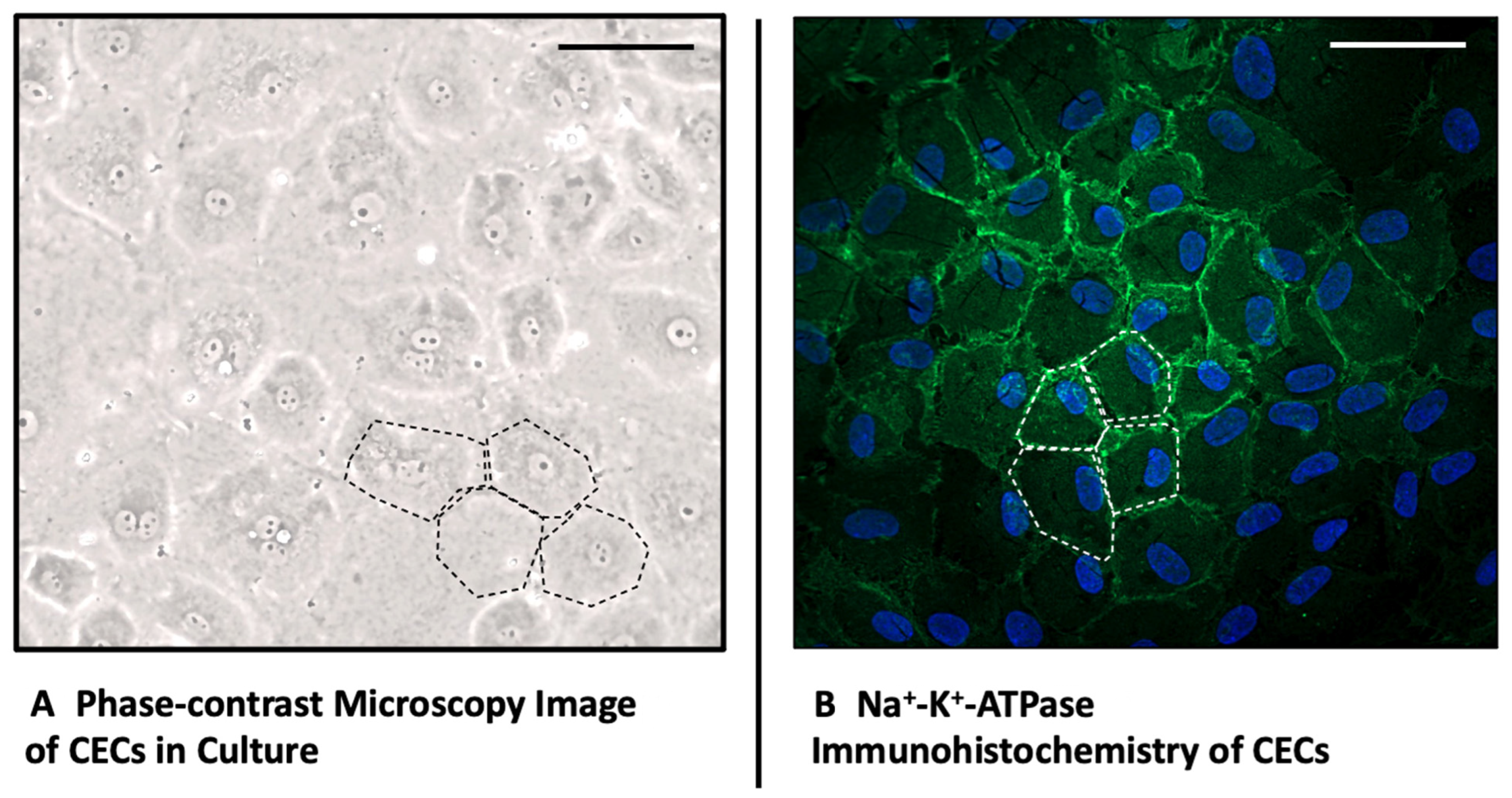
2.1. Morphology and Immunohistochemistry of Corneal Endothelial Cells
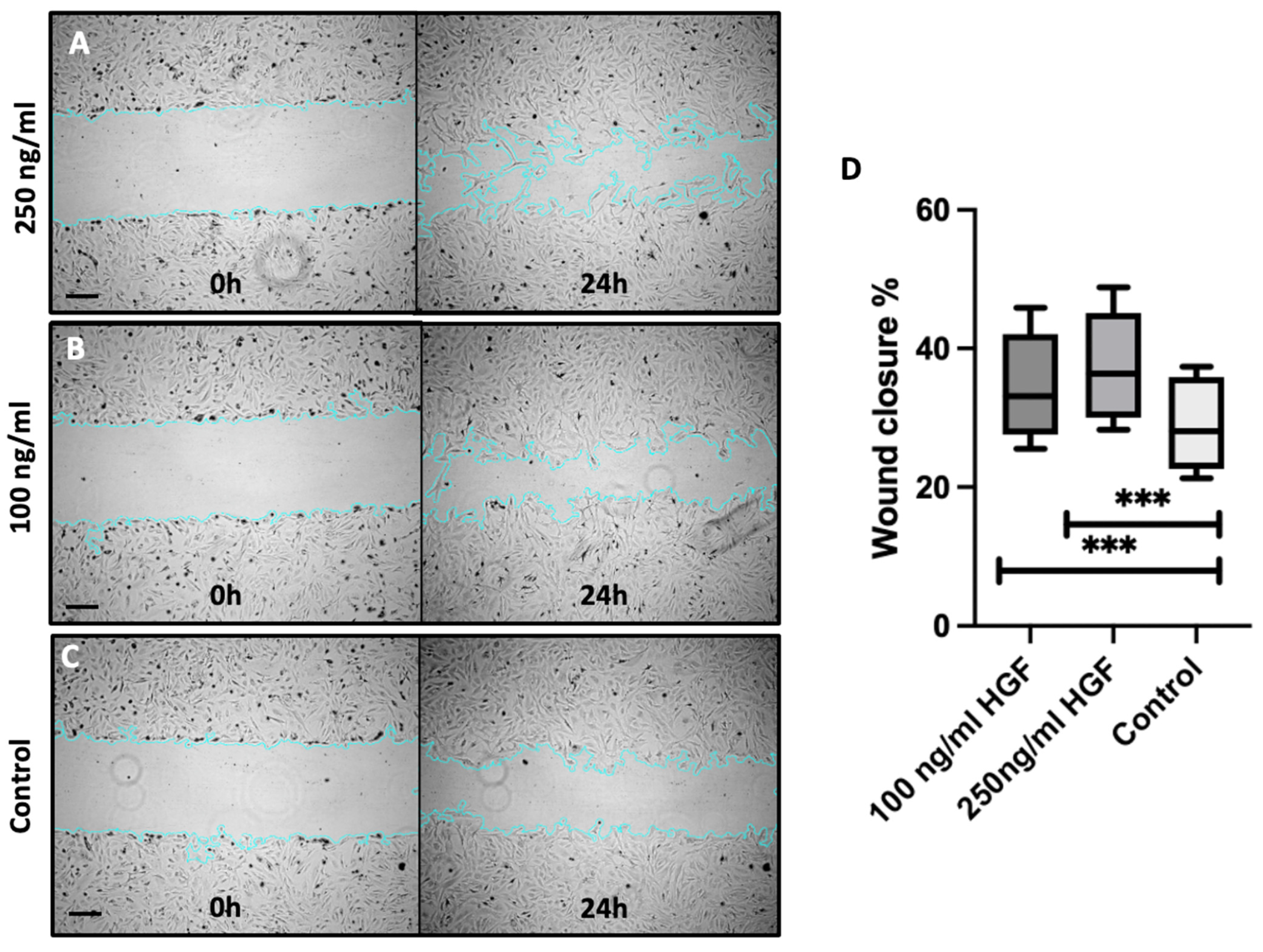
2.2. HGF Enhances Corneal Endothelial Cell Migration
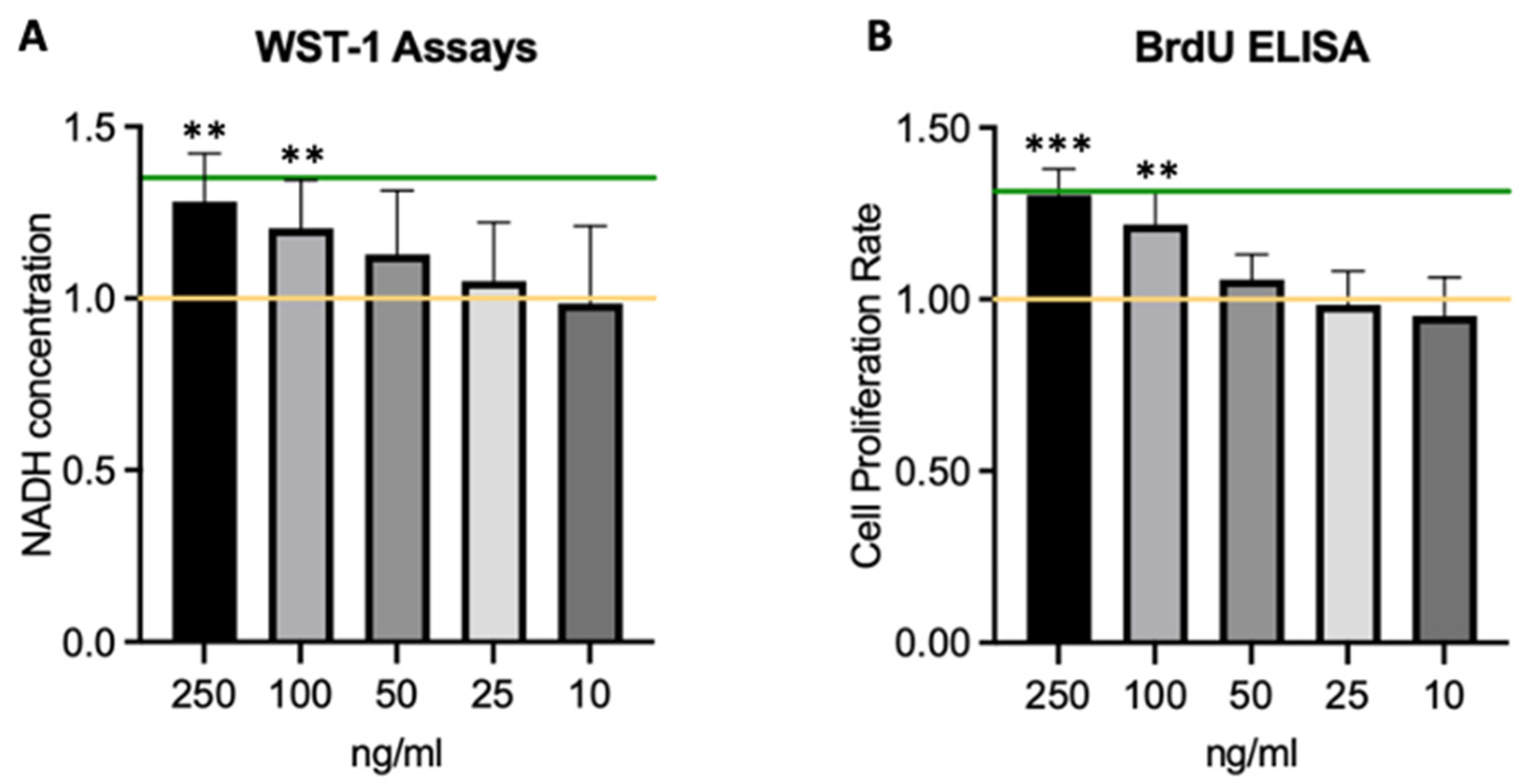
2.3. HGF Increases Corneal Endothelial Cell Viability and Proliferation
2.4. HGF Protects Corneal Endothelial Cells from Oxidative Stress
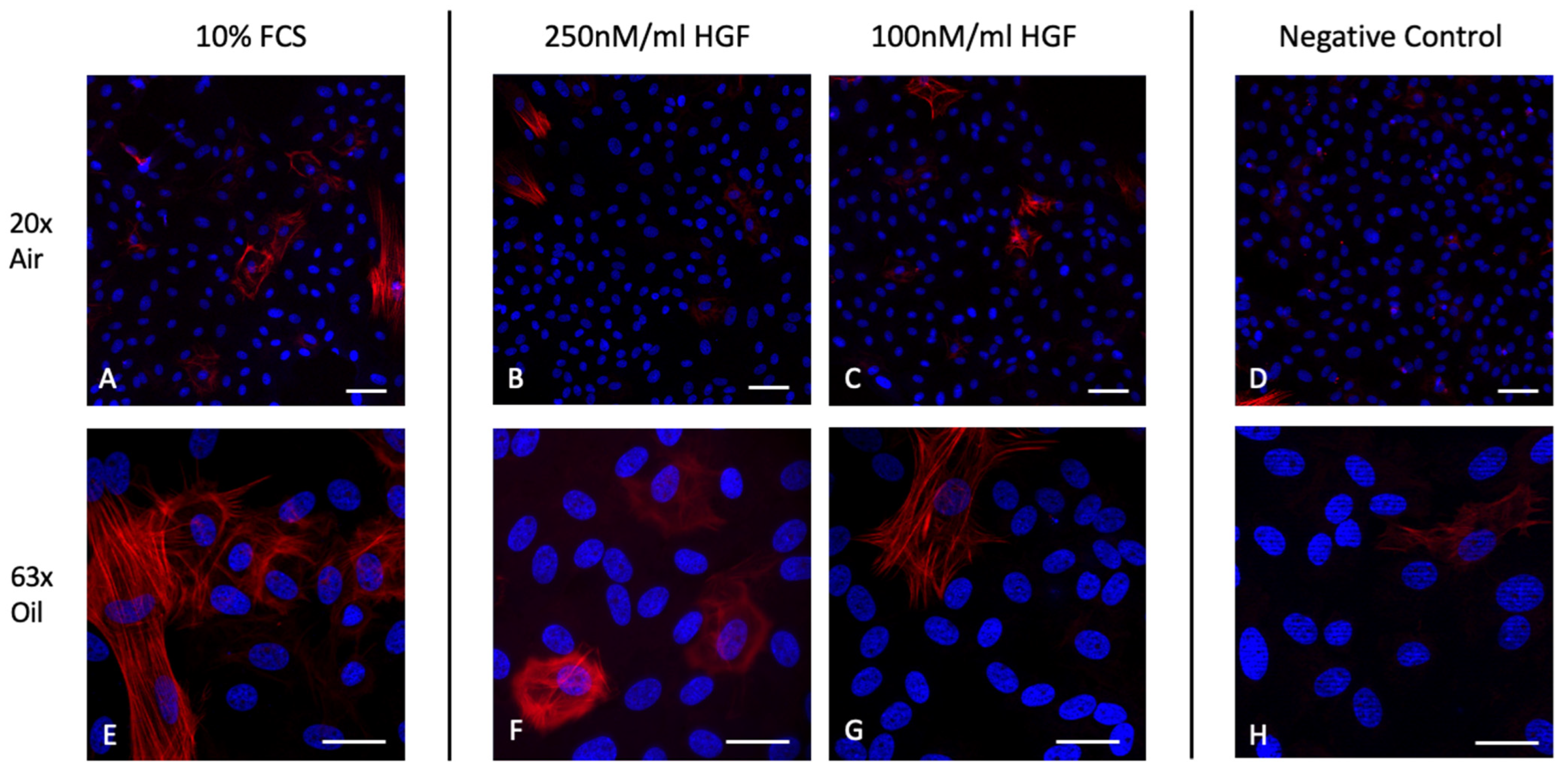
2.5. HGF Does Not Induce α-SMA Expression in Corneal Endothelial Cells
3. Discussion
4. Materials and Methods
4.1. Swine Corneal Endothelial Cells
4.1.1. Animal Tissue and Cell Isolation
4.1.2. Cell Culture
4.2. Immunohistochemistry
4.2.1. Na+-K+-ATPase Staining
4.2.2. Epithelial-Mesenchymal-Transition Staining
4.3. Scratch Migration Assay
4.4. Cell Viability and Cell Proliferation
4.5. Oxidative Stress Model
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, X.; Pinsky, P.M. The Balance of Fluid and Osmotic Pressures across Active Biological Membranes with Application to the Corneal Endothelium. PLoS ONE 2015, 10, e0145422. [Google Scholar] [CrossRef]
- Bonanno, J.A. Identity and regulation of ion transport mechanisms in the corneal endothelium. Prog. Retin. Eye Res. 2003, 22, 69–94. [Google Scholar] [CrossRef] [PubMed]
- Joyce, N.C. Proliferative capacity of the corneal endothelium. Prog. Retin. Eye Res. 2003, 22, 359–389. [Google Scholar] [CrossRef] [PubMed]
- Oie, Y.; Watanabe, S.; Nishida, K. Evaluation of Visual Quality in Patients With Fuchs Endothelial Corneal Dystrophy. Cornea 2016, 35, S55–S58. [Google Scholar] [CrossRef] [PubMed]
- Melles, G.R.; San Ong, T.; Ververs, B.; Van der Wees, J. Preliminary clinical results of Descemet membrane endothelial keratoplasty. Am. J. Ophthalmol. 2008, 145, 222–227. [Google Scholar] [CrossRef]
- Chamberlain, W.; Lin, C.C.; Austin, A.; Schubach, N.; Clover, J.; McLeod, S.D.; Porco, T.C.; Lietman, T.M.; Rose-Nussbaumer, J. Descemet Endothelial Thickness Comparison Trial: A Randomized Trial Comparing Ultrathin Descemet Stripping Automated Endothelial Keratoplasty with Descemet Membrane Endothelial Keratoplasty. Ophthalmology 2019, 126, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Kruse, F.E.; Schrehardt, U.S.; Tourtas, T. Optimizing outcomes with Descemet’s membrane endothelial keratoplasty. Curr. Opin. Ophthalmol. 2014, 25, 325–334. [Google Scholar] [CrossRef]
- Gain, P.; Jullienne, R.; He, Z.; Aldossary, M.; Acquart, S.; Cognasse, F.; Thuret, G. Global Survey of Corneal Transplantation and Eye Banking. JAMA Ophthalmol. 2016, 134, 167–173. [Google Scholar] [CrossRef]
- Gurnani, B.; Kaur, K.; Lalgudi, V.G.; Tripathy, K. Risk Factors for Descemet Membrane Endothelial Keratoplasty Rejection: Current Perspectives- Systematic Review. Clin. Ophthalmol. 2023, 17, 421–440. [Google Scholar] [CrossRef]
- Vij, N.; Sharma, A.; Thakkar, M.; Sinha, S.; Mohan, R.R. PDGF-driven proliferation, migration, and IL8 chemokine secretion in human corneal fibroblasts involve JAK2-STAT3 signaling pathway. Mol. Vis. 2008, 14, 1020–1027. [Google Scholar]
- Wilson, S.E.; Mohan, R.R.; Mohan, R.R.; Ambrosio, R.; Hong, J.; Lee, J. The corneal wound healing response: Cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog. Retin. Eye Res. 2001, 20, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Frati, L.; Daniele, S.; Delogu, A.; Covelli, I. Selective binding of the epidermal growth factor and its specific effects on the epithelial cells of the cornea. Exp. Eye Res. 1972, 14, 135–141. [Google Scholar] [CrossRef]
- Liao, J.K.; Seto, M.; Noma, K. Rho kinase (ROCK) inhibitors. J. Cardiovasc. Pharmacol. 2007, 50, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Neuss, S.; Becher, E.; Wöltje, M.; Tietze, L.; Jahnen-Dechent, W. Functional expression of HGF and HGF receptor/c-met in adult human mesenchymal stem cells suggests a role in cell mobilization, tissue repair, and wound healing. Stem Cells 2004, 22, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Conway, K.; Price, P.; Harding, K.G.; Jiang, W.G. The molecular and clinical impact of hepatocyte growth factor, its receptor, activators, and inhibitors in wound healing. Wound Repair Regen. 2006, 14, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.E.; McGowan, J.A.; Bucher, N.L. Partial characterization of a hepatocyte growth factor from rat platelets. J. Cell. Physiol. 1984, 119, 183–192. [Google Scholar] [CrossRef]
- Papaccio, F.; Della Corte, C.M.; Viscardi, G.; Di Liello, R.; Esposito, G.; Sparano, F.; Ciardiello, F.; Morgillo, F. HGF/MET and the Immune System: Relevance for Cancer Immunotherapy. Int. J. Mol. Sci. 2018, 19, 3595. [Google Scholar] [CrossRef]
- Honjo, M.; Tanihara, H.; Inatani, M.; Kido, N.; Sawamura, T.; Yue, B.Y.; Narumiya, S.; Honda, Y. Effects of rho-associated protein kinase inhibitor Y-27632 on intraocular pressure and outflow facility. Investig. Ophthalmol. Vis. Sci. 2001, 42, 137–144. [Google Scholar]
- Jung, K.H.; Park, B.H.; Hong, S.S. Progress in cancer therapy targeting c-Met signaling pathway. Arch. Pharm. Res. 2012, 35, 595–604. [Google Scholar] [CrossRef]
- Wilson, S.E.; Walker, J.W.; Chwang, E.L.; He, Y.G. Hepatocyte growth factor, keratinocyte growth factor, their receptors, fibroblast growth factor receptor-2, and the cells of the cornea. Investig. Ophthalmol. Vis. Sci. 1993, 34, 2544–2561. [Google Scholar]
- Li, Q.; Weng, J.; Mohan, R.R.; Bennett, G.L.; Schwall, R.; Wang, Z.F.; Tabor, K.; Kim, J.; Hargrave, S.; Cuevas, K.H.; et al. Hepatocyte growth factor and hepatocyte growth factor receptor in the lacrimal gland, tears, and cornea. Investig. Ophthalmol. Vis. Sci. 1996, 37, 727–739. [Google Scholar]
- Bussolino, F.; DI Renzo, M.F.; Ziche, M.; Bocchietto, E.; Olivero, M.; Naldini, L.; Gaudino, G.; Tamagnone, L.; Coffer, A.; Comoglio, P. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J. Cell Biol. 1992, 119, 629–641. [Google Scholar] [CrossRef]
- Grierson, I.; Heathcote, L.; Hiscott, P.; Hogg, P.; Briggs, M.; Hagan, S. Hepatocyte growth factor/scatter factor in the eye. Prog. Retin. Eye Res. 2000, 19, 779–802. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, H.; Thomasy, S.M.; Russell, P.; Murphy, C.J. The role of hepatocyte growth factor in corneal wound healing. Exp. Eye Res. 2018, 166, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Kimoto, M.; Shima, N.; Yamaguchi, M.; Amano, S.; Yamagami, S. Role of hepatocyte growth factor in promoting the growth of human corneal endothelial cells stimulated by L-ascorbic acid 2-phosphate. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7583–7589. [Google Scholar] [CrossRef]
- Omoto, M.; Suri, K.; Amouzegar, A.; Li, M.; Katikireddy, K.R.; Mittal, S.K.; Chauhan, S.K. Hepatocyte Growth Factor Suppresses Inflammation and Promotes Epithelium Repair in Corneal Injury. Mol. Ther. 2017, 25, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Jumelle, C.; Sani, E.S.; Taketani, Y.; Yung, A.; Gantin, F.; Chauhan, S.K.; Annabi, N.; Dana, R. Growth factor-eluting hydrogels for management of corneal defects. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111790. [Google Scholar] [CrossRef]
- Carrington, L.M.; Boulton, M. Hepatocyte growth factor and keratinocyte growth factor regulation of epithelial and stromal corneal wound healing. J. Cataract. Refract. Surg. 2005, 31, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Shukla, M.N.; Rose, J.L.; Ray, R.; Lathrop, K.L.; Ray, A.; Ray, P. Hepatocyte growth factor inhibits epithelial to myofibroblast transition in lung cells via Smad7. Am. J. Respir. Cell Mol. Biol. 2009, 40, 643–653. [Google Scholar] [CrossRef]
- Wahab, N.A.; Mason, R.M. A critical look at growth factors and epithelial-to-mesenchymal transition in the adult kidney. Interrelationships between growth factors that regulate EMT in the adult kidney. Nephron. Exp. Nephrol. 2006, 104, e129–e134. [Google Scholar] [CrossRef]
- Sumioka, T.; Ikeda, K.; Okada, Y.; Yamanaka, O.; Kitano, A.; Saika, S. Inhibitory effect of blocking TGF-beta/Smad signal on injury-induced fibrosis of corneal endothelium. Mol. Vis. 2008, 14, 2272–2281. [Google Scholar]
- Hatou, S.; Higa, K.; Inagaki, E.; Yoshida, S.; Kimura, E.; Hayashi, R.; Tsujikawa, M.; Tsubota, K.; Nishida, K.; Shimmura, S. Validation of Na,K-ATPase pump function of corneal endothelial cells for corneal regenerative medicine. Tissue Eng. Part C Methods. 2013, 19, 901–910. [Google Scholar] [CrossRef]
- Bourne, W.M. Biology of the corneal endothelium in health and disease. Eye 2003, 17, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.M.; Liu, T.; Guevara, O.; Stevens, J.; Fanburg, B.L.; Gaestel, M.; Toksoz, D.; Kayyali, U.S. Smooth muscle alpha-actin expression and myofibroblast differentiation by TGFbeta are dependent upon MK2. J. Cell Biochem. 2007, 100, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Hirata-Tominaga, K.; Nakamura, T.; Okumura, N.; Kawasaki, S.; Kay, E.P.; Barrandon, Y.; Koizumi, N.; Kinoshita, S. Corneal endothelial cell fate is maintained by LGR5 through the regulation of hedgehog and Wnt pathway. Stem Cells 2013, 31, 1396–1407. [Google Scholar] [CrossRef] [PubMed]
- Hara, S.; Hayashi, R.; Soma, T.; Kageyama, T.; Duncan, T.; Tsujikawa, M.; Nishida, K. Identification and potential application of human corneal endothelial progenitor cells. Stem Cells Dev. 2014, 23, 2190–2201. [Google Scholar] [CrossRef]
- Mimura, T.; Yamagami, S.; Yokoo, S.; Araie, M.; Amano, S. Comparison of rabbit corneal endothelial cell precursors in the central and peripheral cornea. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3645–3648. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Carlson, E.C.; Funderburgh, M.L.; Birk, D.E.; Pearlman, E.; Guo, N.; Kao, W.W.-Y.; Funderburgh, J.L. Stem cell therapy restores transparency to defective murine corneas. Stem Cells 2009, 27, 1635–1642. [Google Scholar] [CrossRef]
- Auffarth, G.U.; Son, H.-S.; Koch, M.; Weindler, J.; Merz, P.; Daphna, O.; Marcovich, A.L.; Augustin, V.A. Implantation of an Artificial Endothelial Layer for Treatment of Chronic Corneal Edema. Cornea 2021, 40, 1633–1638. [Google Scholar] [CrossRef]
- Ong Tone, S.; Kocaba, V.; Böhm, M.; Wylegala, A.; White, T.L.; Jurkunas, U.V. Fuchs endothelial corneal dystrophy: The vicious cycle of Fuchs pathogenesis. Prog. Retin. Eye Res. 2021, 80, 100863. [Google Scholar] [CrossRef]
- Aouimeur, I.; Sagnial, T.; Coulomb, L.; Maurin, C.; Thomas, J.; Forestier, P.; Ninotta, S.; Perrache, C.; Forest, F.; Gain, P.; et al. Investigating the Role of TGF-beta Signaling Pathways in Human Corneal Endothelial Cell Primary Culture. Cells 2023, 12, 1624. [Google Scholar] [CrossRef]
- Peh, G.S.L.; Bandeira, F.; Neo, D.; Adnan, K.; Hartono, Y.; Ong, H.S.; Naso, S.; Venkatraman, A.; Gomes, J.A.P.; Kocaba, V.; et al. Effects of Rho-Associated Kinase (Rock) Inhibitors (Alternative to Y-27632) on Primary Human Corneal Endothelial Cells. Cells 2023, 12, 1307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yu, F.; Yan, C.; Shao, C.; Gu, P.; Fu, Y.; Sun, H.; Fan, X. PTEN Inhibition Accelerates Corneal Endothelial Wound Healing through Increased Endothelial Cell Division and Migration. Investig. Ophthalmol. Vis. Sci. 2020, 61, 19. [Google Scholar] [CrossRef]
- Tandon, A.; Tovey, J.C.; Sharma, A.; Gupta, R.; Mohan, R.R. Role of transforming growth factor Beta in corneal function, biology and pathology. Curr. Mol. Med. 2010, 10, 565–578. [Google Scholar] [PubMed]
- Miettinen, P.J.; Ebner, R.; Lopez, A.R.; Derynck, R. TGF-beta induced transdifferentiation of mammary epithelial cells to mesenchymal cells: Involvement of type I receptors. J. Cell Biol. 1994, 127, 2021–2036. [Google Scholar] [CrossRef] [PubMed]
- Hachana, S.; Larrivee, B. TGF-beta Superfamily Signaling in the Eye: Implications for Ocular Pathologies. Cells 2022, 11, 2336. [Google Scholar] [CrossRef]
- Tanna, A.P.; Johnson, M. Rho Kinase Inhibitors as a Novel Treatment for Glaucoma and Ocular Hypertension. Ophthalmology 2018, 125, 1741–1756. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, W.; Wang, Y.; Chen, W. HGF/c-Met: A Key Promoter in Liver Regeneration. Front Pharmacol. 2022, 13, 808855. [Google Scholar] [CrossRef]
- Birchmeier, W.; Brinkmann, V.; Niemann, C.; Meiners, S.; DiCesare, S.; Naundorf, H.; Sachs, M. Role of HGF/SF and c-Met in morphogenesis and metastasis of epithelial cells. Ciba. Found Symp. 1997, 212, 230–240. [Google Scholar]
- Matsumoto, K.; Funakoshi, H.; Takahashi, H.; Sakai, K. HGF-Met Pathway in Regeneration and Drug Discovery. Biomedicines 2014, 2, 275–300. [Google Scholar] [CrossRef]
- Pan, F.Y.; Zhang, S.Z.; Xu, N.; Meng, F.L.; Zhang, H.X.; Xue, B.; Han, X.; Li, C.-J. Beta-catenin signaling involves HGF-enhanced HepG2 scattering through activating MMP-7 transcription. Histochem. Cell Biol. 2010, 134, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Tsubaki, M.; Seki, S.; Takeda, T.; Chihara, A.; Arai, Y.; Morii, Y.; Imano, M.; Satou, T.; Shimomura, K. The HGF/Met/NF-kappaB Pathway Regulates RANKL Expression in Osteoblasts and Bone Marrow Stromal Cells. Int. J. Mol. Sci. 2020, 21, 7905. [Google Scholar] [CrossRef]
- Wang, G.; Yang, F.; Zhou, W.; Xiao, N.; Luo, M.; Tang, Z. The initiation of oxidative stress and therapeutic strategies in wound healing. Biomed. Pharmacother. 2023, 157, 114004. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, R.V.; Freitas, M.B.; Esposito, D. Cellular and Molecular Mechanisms of Oxidative Stress in Wound Healing. Oxid. Med. Cell Longev. 2022, 2022, 9785094. [Google Scholar] [CrossRef]
- Salas-Silva, S.; Simoni-Nieves, A.; Razori, M.V.; López-Ramirez, J.; Barrera-Chimal, J.; Lazzarini, R.; Bello, O.; Souza, V.; Miranda-Labra, R.U.; Gutiérrez-Ruiz, M.C.; et al. HGF induces protective effects in alpha-naphthylisothiocyanate-induced intrahepatic cholestasis by counteracting oxidative stress. Biochem. Pharmacol. 2020, 174, 113812. [Google Scholar] [CrossRef]
- Guoguo, S.; Akaike, T.; Tao, J.; Qi, C.; Nong, Z.; Hui, L. HGF-mediated inhibition of oxidative stress by 8-nitro-cGMP in high glucose-treated rat mesangial cells. Free Radic. Res. 2012, 46, 1238–1248. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.; Hong, Y.; Jingjing, Z.; Yuan, H.; Qi, C.; Nong, Z. HGF suppresses high glucose-mediated oxidative stress in mesangial cells by activation of PKG and inhibition of PKA. Free Radic. Biol. Med. 2010, 49, 467–473. [Google Scholar] [CrossRef]
- Meekins, L.C.; Rosado-Adames, N.; Maddala, R.; Zhao, J.J.; Rao, P.V.; Afshari, N.A. Corneal Endothelial Cell Migration and Proliferation Enhanced by Rho Kinase (ROCK) Inhibitors in In Vitro and In Vivo Models. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6731–6738. [Google Scholar] [CrossRef]
- Miyagi, H.; Kim, S.; Li, J.; Murphy, C.J.; Thomasy, S.M. Topical Rho-Associated Kinase Inhibitor, Y27632, Accelerates Corneal Endothelial Regeneration in a Canine Cryoinjury Model. Cornea 2019, 38, 352–359. [Google Scholar] [CrossRef]
- Organ, S.L.; Tsao, M.S. An overview of the c-MET signaling pathway. Ther. Adv. Med. Oncol. 2011, 3, S7–S19. [Google Scholar] [CrossRef]
- Rahman, S.; Patel, Y.; Murray, J.; Patel, K.V.; Sumathipala, R.; Sobel, M.; Wijelath, E.S. Novel hepatocyte growth factor (HGF) binding domains on fibronectin and vitronectin coordinate a distinct and amplified Met-integrin induced signalling pathway in endothelial cells. BMC Cell Biol. 2005, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.T.; Su, C.C.; Chang, J.S.; Chang, S.W.; Hu, F.R.; Jou, T.S.; Wang, I.J. In Vitro and In Vivo Models to Study Corneal Endothelial-mesenchymal Transition. J. Vis. Exp. 2016, 114, e54329. [Google Scholar]
- Lee, J.; Jung, E.; Heur, M. Injury induces endothelial to mesenchymal transition in the mouse corneal endothelium in vivo via FGF2. Mol. Vis. 2019, 25, 22–34. [Google Scholar] [PubMed]
- Yamashita, K.; Hatou, S.; Inagaki, E.; Higa, K.; Tsubota, K.; Shimmura, S. A Rabbit Corneal Endothelial Dysfunction Model Using Endothelial-Mesenchymal Transformed Cells. Sci. Rep. 2018, 8, 16868. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Yang, K.; Kim, H.J.; Lee, Y.J.; Kim, M.; Choi, Y.H.; Kang, J.L. RhoA-Dependent HGF and c-Met Mediate Gas6-Induced Inhibition of Epithelial-Mesenchymal Transition, Migration, and Invasion of Lung Alveolar Epithelial Cells. Biomolecules 2019, 9, 565. [Google Scholar] [CrossRef]
- Petroll, W.M.; Barry-Lane, P.A.; Cavanagh, H.; Jester, J.V. ZO-1 reorganization and myofibroblast transformation of corneal endothelial cells after freeze injury in the cat. Exp. Eye Res. 1997, 64, 257–267. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Zhu, R.T.; Sun, Y.L. Epithelial-mesenchymal transition in liver fibrosis. Biomed. Rep. 2016, 4, 269–274. [Google Scholar] [CrossRef]
- Rockey, D.C.; Weymouth, N.; Shi, Z. Smooth muscle alpha actin (Acta2) and myofibroblast function during hepatic wound healing. PLoS ONE. 2013, 8, e77166. [Google Scholar] [CrossRef]
- Ohlmann, A.; Seitz, R.; Braunger, B.; Seitz, D.; Bösl, M.R.; Tamm, E.R. Norrin promotes vascular regrowth after oxygen-induced retinal vessel loss and suppresses retinopathy in mice. J. Neurosci. 2010, 30, 183–193. [Google Scholar] [CrossRef]
- Rojkind, M.; Domínguez-Rosales, J.-A.; Nieto, N.; Greenwel, P. Role of hydrogen peroxide and oxidative stress in healing responses. Cell Mol. Life Sci. 2002, 59, 1872–1891. [Google Scholar] [PubMed]
- Jeang, L.J.; Margo, C.E.; Espana, E.M. Diseases of the corneal endothelium. Exp. Eye Res. 2021, 205, 108495. [Google Scholar] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tratnig-Frankl, M.; Luft, N.; Magistro, G.; Priglinger, S.; Ohlmann, A.; Kassumeh, S. Hepatocyte Growth Factor Modulates Corneal Endothelial Wound Healing In Vitro. Int. J. Mol. Sci. 2024, 25, 9382. https://doi.org/10.3390/ijms25179382
Tratnig-Frankl M, Luft N, Magistro G, Priglinger S, Ohlmann A, Kassumeh S. Hepatocyte Growth Factor Modulates Corneal Endothelial Wound Healing In Vitro. International Journal of Molecular Sciences. 2024; 25(17):9382. https://doi.org/10.3390/ijms25179382
Chicago/Turabian StyleTratnig-Frankl, Merle, Nikolaus Luft, Guiseppe Magistro, Siegfried Priglinger, Andreas Ohlmann, and Stefan Kassumeh. 2024. "Hepatocyte Growth Factor Modulates Corneal Endothelial Wound Healing In Vitro" International Journal of Molecular Sciences 25, no. 17: 9382. https://doi.org/10.3390/ijms25179382
APA StyleTratnig-Frankl, M., Luft, N., Magistro, G., Priglinger, S., Ohlmann, A., & Kassumeh, S. (2024). Hepatocyte Growth Factor Modulates Corneal Endothelial Wound Healing In Vitro. International Journal of Molecular Sciences, 25(17), 9382. https://doi.org/10.3390/ijms25179382





