The Impact of the Endocrine and Immunological Function of Adipose Tissue on Reproduction in Women with Obesity
Abstract
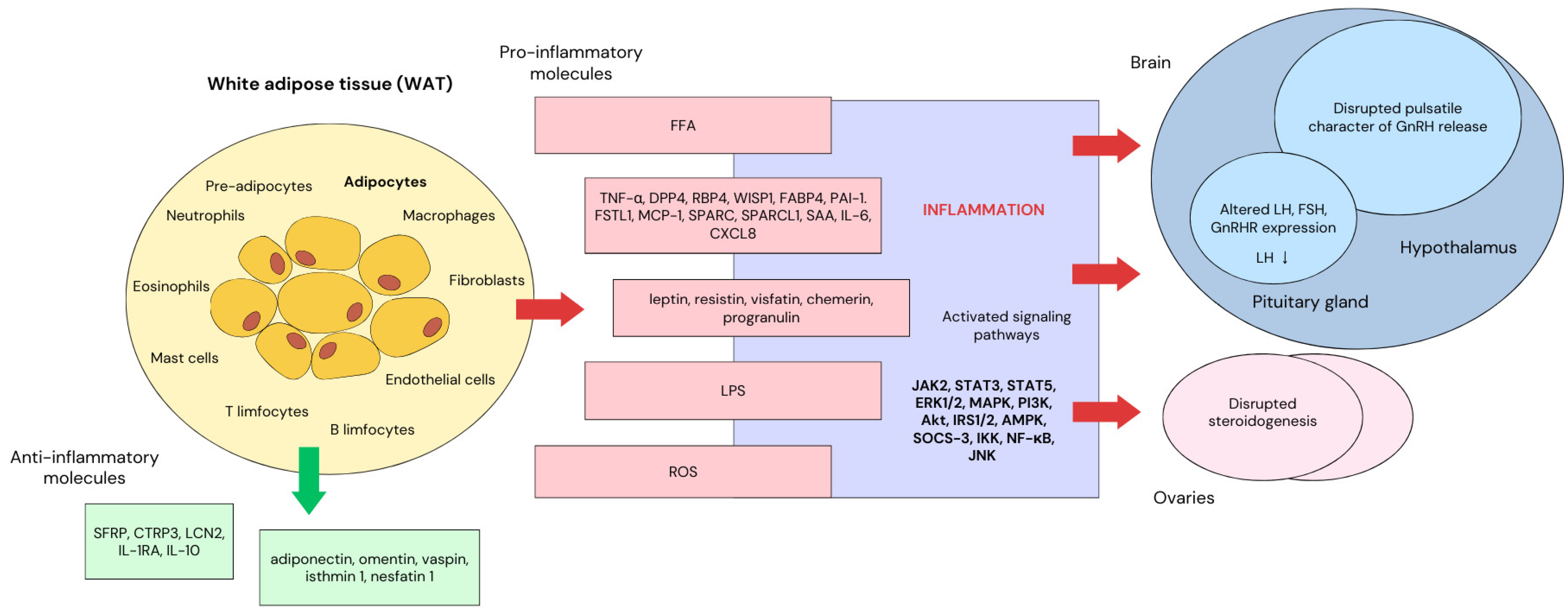
1. Introduction
2. Adipokines
2.1. Leptin
2.2. Adiponectin
2.3. Resistin
2.4. Visfatin
2.5. Omentin
3. Role of Kisspeptin in HPG Axis Regulation
4. Neuroinflammation
5. Hyperinsulinemia and Sex Hormones
Polycystic Ovary Syndrome
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO European Regional. Obesity Report 2022; WHO Regional Office for Europe: Copenhagen, Denmark, 2022. [Google Scholar]
- The Lancet Gastroenterol Hepatol. Obesity: Another ongoing pandemic. Lancet Gastroenterol. Hepatol. 2021, 6, 411. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Farias, M.; Fos-Domenech, J.; Serra, D.; Herrero, L.; Sánchez-Infantes, D. White adipose tissue dysfunction in obesity and aging. Biochem. Pharmacol. 2021, 192, 114723. [Google Scholar] [CrossRef]
- Zhu, Q.; An, Y.A.; Scherer, P.E. Mitochondrial regulation and white adipose tissue homeostasis. Trends Cell Biol. 2022, 32, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, S.S.; Ferreira, T.; Benonisdottir, S.; Rahmioglu, N.; Becker, C.M.; Granne, I.; Zondervan, K.T.; Holmes, M.V.; Lindgren, C.M.; Wittemans, L.B.L. Obesity and risk of female reproductive conditions: A Mendelian randomisation study. PLoS Med. 2022, 19, e1003679. [Google Scholar] [CrossRef]
- Min, Y.I.; Gao, Y.; Anugu, P.; Anugu, A.; Correa, A. Obesity and overall mortality: Findings from the Jackson Heart Study. BMC Public Health 2021, 21, 50. [Google Scholar] [CrossRef] [PubMed]
- Andreasson, A.; Carlsson, A.C.; Önnerhag, K.; Hagström, H. Waist/Hip Ratio Better Predicts Development of Severe Liver Disease within 20 Years Than Body Mass Index: A Population-based Cohort Study. Clin. Gastroenterol. Hepatol. 2017, 15, 1294–1301.e2. [Google Scholar] [CrossRef]
- Khan, I.; Chong, M.; Le, A.; Mohammadi-Shemirani, P.; Morton, R.; Brinza, C.; Kiflen, M.; Narula, S.; Akhabir, L.; Mao, S.; et al. Surrogate Adiposity Markers and Mortality. JAMA Netw. Open 2023, 6, e2334836. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, S.; Zhang, X.; Pei, M. Significance of Cellular Cross-Talk in Stromal Vascular Fraction of Adipose Tissue in Neovascularization. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1034–1044. [Google Scholar] [CrossRef]
- Huh, J.Y.; Park, Y.J.; Ham, M.; Kim, J.B. Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Mol. Cells 2014, 37, 365–371. [Google Scholar] [CrossRef]
- Ren, Y.; Zhao, H.; Yin, C.; Lan, X.; Wu, L.; Du, X.; Griffiths, H.R.; Gao, D. Adipokines, Hepatokines and Myokines: Focus on Their Role and Molecular Mechanisms in Adipose Tissue Inflammation. Front. Endocrinol. 2022, 13, 873699. [Google Scholar] [CrossRef]
- Kirichenko, T.V.; Markina, Y.V.; Bogatyreva, A.I.; Tolstik, T.V.; Varaeva, Y.R.; Starodubova, A.V. The Role of Adipokines in Inflammatory Mechanisms of Obesity. Int. J. Mol. Sci. 2022, 23, 14982. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose tissue and insulin resistance in obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2019, 10, 1607. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhao, M.; Jia, S. Macrophage: Key player in the pathogenesis of autoimmune diseases. Front. Immunol. 2023, 14, 1080310. [Google Scholar] [CrossRef]
- Lainez, N.M.; Coss, D. Obesity, Neuroinflammation, and Reproductive Function. Endocrinology 2019, 160, 2719–2736. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Hermosillo, A.; de Miguel Ibañez, R.; Pérez-Dionisio, E.K.; Villalobos-Mata, K.A. Obesity as a Neuroendocrine Disorder. Arch. Med. Res. 2023, 54, 102896. [Google Scholar] [CrossRef]
- Zheng, L.; Yang, L.; Guo, Z.; Yao, N.; Zhang, S.; Pu, P. Obesity and its impact on female reproductive health: Unraveling the connections. Front. Endocrinol. 2023, 14, 1326546. [Google Scholar] [CrossRef]
- Silvestris, E.; de Pergola, G.; Rosania, R.; Loverro, G. Obesity as disruptor of the female fertility. Reprod. Biol. Endocrinol. 2018, 16, 22. [Google Scholar] [CrossRef]
- Coelho, M.; Oliveira, T.; Fernandes, R. Biochemistry of adipose tissue: An endocrine organ. Arch. Med. Sci. 2013, 9, 191–200. [Google Scholar] [CrossRef]
- Sahu, B.; Bal, N.C. Adipokines from white adipose tissue in regulation of whole body energy homeostasis. Biochimie 2023, 204, 92–107. [Google Scholar] [CrossRef]
- Caër, C.; Rouault, C.; Le Roy, T.; Poitou, C.; Aron-Wisnewsky, J.; Torcivia, A.; Bichet, J.C.; Clément, K.; Guerre-Millo, M.; André, S. Immune cell-derived cytokines contribute to obesity-related inflammation, fibrogenesis and metabolic deregulation in human adipose tissue. Sci. Rep. 2017, 7, 3000. [Google Scholar] [CrossRef] [PubMed]
- Nono Nankam, P.A.; Blüher, M. Retinol-binding protein 4 in obesity and metabolic dysfunctions. Mol. Cell. Endocrinol. 2021, 531, 111312. [Google Scholar] [CrossRef]
- Helfer, G.; Wu, Q.F. Chemerin: A multifaceted adipokine involved in metabolic disorders. J. Endocrinol. 2018, 238, R79–R94. [Google Scholar] [CrossRef] [PubMed]
- Guardado, S.; Ojeda-Juárez, D.; Kaul, M.; Nordgren, T.M. Comprehensive review of lipocalin 2-mediated effects in lung inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2021, 321, L726–L733. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.B. The complex role of adipokines in obesity, inflammation, and autoimmunity. Clin. Sci. 2021, 135, 731–752. [Google Scholar] [CrossRef]
- Pérez-Pérez, A.; Sánchez-Jiménez, F.; Maymó, J.; Dueñas, J.L.; Varone, C.; Sánchez-Margalet, V. Role of leptin in female reproduction. Clin. Chem. Lab. Med. 2015, 53, 15–28. [Google Scholar] [CrossRef]
- Kawwass, J.F.; Summer, R.; Kallen, C.B. Direct effects of leptin and adiponectin on peripheral reproductive tissues: A critical review. Mol. Hum. Reprod. 2015, 21, 617–632. [Google Scholar] [CrossRef]
- Liu, J.; Yang, X.; Yu, S.; Zheng, R. The Leptin Signaling. Adv. Exp. Med. Biol. 2018, 1090, 123–144. [Google Scholar] [CrossRef]
- Gorska, E.; Popko, K.; Stelmaszczyk-Emmel, A.; Ciepiela, O.; Kucharska, A.; Wasik, M. Leptin receptors. Eur. J. Med. Res. 2010, 15 (Suppl. S2), 50–54. [Google Scholar] [CrossRef]
- Evans, M.C.; Lord, R.A.; Anderson, G.M. Multiple Leptin Signalling Pathways in the Control of Metabolism and Fertility: A Means to Different Ends? Int. J. Mol. Sci. 2021, 22, 9210. [Google Scholar] [CrossRef] [PubMed]
- Hileman, S.M.; Tornøe, J.; Flier, J.S.; Bjørbaek, C. Transcellular transport of leptin by the short leptin receptor isoform ObRa in Madin-Darby Canine Kidney cells. Endocrinology 2000, 141, 1955–1961. [Google Scholar] [CrossRef] [PubMed]
- Biernat, W.; Szczęsna, M.; Kirsz, K.; Zieba, D.A. Seasonal and Nutritional Fluctuations in the mRNA Levels of the Short Form of the Leptin Receptor (LRa) in the Hypothalamus and Anterior Pituitary in Resistin-Treated Sheep. Animals 2021, 11, 2451. [Google Scholar] [CrossRef] [PubMed]
- Münzberg, H. Leptin-signaling pathways and leptin resistance. Forum Nutr. 2010, 63, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Childs, G.V.; Odle, A.K.; MacNicol, M.C.; MacNicol, A.M. The Importance of Leptin to Reproduction. Endocrinology 2021, 162, bqaa204. [Google Scholar] [CrossRef]
- Seoane-Collazo, P.; Martínez-Sánchez, N.; Milbank, E.; Contreras, C. Incendiary Leptin. Nutrients 2020, 12, 472. [Google Scholar] [CrossRef]
- Villanueva, E.C.; Myers, M.G., Jr. Leptin receptor signaling and the regulation of mammalian physiology. Int. J. Obes. 2008, 32 (Suppl. S7), S8–S12. [Google Scholar] [CrossRef]
- Socol, C.T.; Chira, A.; Martinez-Sanchez, M.A.; Nuñez-Sanchez, M.A.; Maerescu, C.M.; Mierlita, D.; Rusu, A.V.; Ruiz-Alcaraz, A.J.; Trif, M.; Ramos-Molina, B. Leptin Signaling in Obesity and Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 4713. [Google Scholar] [CrossRef]
- Li, S.; Li, X. Leptin in normal physiology and leptin resistance. Sci. Bull. 2016, 61, 1480–1488. [Google Scholar] [CrossRef]
- Quennell, J.H.; Mulligan, A.C.; Tups, A.; Liu, X.; Phipps, S.J.; Kemp, C.J.; Herbison, A.E.; Grattan, D.R.; Anderson, G.M. Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology 2009, 150, 2805–2812. [Google Scholar] [CrossRef] [PubMed]
- Stamou, M.I.; Georgopoulos, N.A. Kallmann syndrome: Phenotype and genotype of hypogonadotropic hypogonadism. Metabolism 2018, 86, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Cravo, R.M.; Margatho, L.O.; Osborne-Lawrence, S.; Donato, J., Jr.; Atkin, S.; Bookout, A.L.; Rovinsky, S.; Frazão, R.; Lee, C.E.; Gautron, L.; et al. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience 2011, 173, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Donato, J., Jr.; Cravo, R.M.; Frazão, R.; Gautron, L.; Scott, M.M.; Lachey, J.; Castro, I.A.; Margatho, L.O.; Lee, S.; Lee, C.; et al. Leptin’s effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J. Clin. Investig. 2011, 121, 355–368. [Google Scholar] [CrossRef]
- Petrine JC, P.; Franci, C.R.; Del Bianco-Borges, B. Leptin actions through the nitrergic system to modulate the hypothalamic expression of the kiss1 mRNA in the female rat. Brain Res. 2020, 1728, 146574. [Google Scholar] [CrossRef]
- Akhter, N.; CarlLee, T.; Syed, M.M.; Odle, A.K.; Cozart, M.A.; Haney, A.C.; Allensworth-James, M.L.; Beneš, H.; Childs, G.V. Selective deletion of leptin receptors in gonadotropes reveals activin and GnRH-binding sites as leptin targets in support of fertility. Endocrinology 2014, 155, 4027–4042. [Google Scholar] [CrossRef]
- Odle, A.K.; Akhter, N.; Syed, M.M.; Allensworth-James, M.L.; Beneš, H.; Melgar Castillo, A.I.; MacNicol, M.C.; MacNicol, A.M.; Childs, G.V. Leptin Regulation of Gonadotrope Gonadotropin-Releasing Hormone Receptors As a Metabolic Checkpoint and Gateway to Reproductive Competence. Front. Endocrinol. 2017, 8, 367. [Google Scholar] [CrossRef]
- Cella, F.; Giordano, G.; Cordera, R. Serum leptin concentrations during the menstrual cycle in normal-weight women: Effects of an oral triphasic estrogen-progestin medication. Eur. J. Endocrinol. 2000, 142, 174–178. [Google Scholar] [CrossRef]
- Ahrens, K.; Mumford, S.L.; Schliep, K.C.; Kissell, K.A.; Perkins, N.J.; Wactawski-Wende, J.; Schisterman, E.F. Serum leptin levels and reproductive function during the menstrual cycle. Am. J. Obstet. Gynecol. 2014, 210, 248.e1. [Google Scholar] [CrossRef]
- De Biasi, S.N.; Apfelbaum, L.I.; Apfelbaum, M.E. In vitro effect of leptin on LH release by anterior pituitary glands from female rats at the time of spontaneous and steroid-induced LH surge. Eur. J. Endocrinol. 2001, 145, 659–665. [Google Scholar] [CrossRef][Green Version]
- Watanobe, H.; Schiöth, H.B. Nitric oxide mediates leptin-induced preovulatory luteinizing hormone and prolactin surges in rats. Brain Res. 2001, 923, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Tipsmark, C.K.; Strom, C.N.; Bailey, S.T.; Borski, R.J. Leptin stimulates pituitary prolactin release through an extracellular signal-regulated kinase-dependent pathway. J. Endocrinol. 2008, 196, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Kosior-Korzecka, U.; Bobowiec, R. Leptin effect on nitric oxide and GnRH-induced FSH secretion from ovine pituitary cells in vitro. J. Physiol. Pharmacol. 2006, 57, 637–647. [Google Scholar] [PubMed]
- Richards, J.S.; Pangas, S.A. New insights into ovarian function. In Fertility Control; Springer: Berlin/Heidelberg, Germany, 2010; pp. 3–27. [Google Scholar] [CrossRef]
- Salem, A.M. Variation of Leptin during Menstrual Cycle and Its Relation to the Hypothalamic-Pituitary-Gonadal (HPG) Axis: A Systematic Review. Int. J. Women’s Health 2021, 13, 445–458. [Google Scholar] [CrossRef]
- Macedo, T.J.S.; Santos, J.M.S.; Bezerra, M.É.S.; Menezes, V.G.; Gouveia, B.B.; Barbosa, L.M.R.; Lins, T.L.B.G.; Monte, A.P.O.; Barberino, R.S.; Batista, A.M.; et al. Immunolocalization of leptin and its receptor in the sheep ovary and in vitro effect of leptin on follicular development and oocyte maturation. Mol. Cell. Endocrinol. 2019, 495, 110506. [Google Scholar] [CrossRef]
- Hong, K.J.; Lin, J.J.; Lin, L.H.; Lai, T.H. The intrafollicular concentration of leptin as a potential biomarker to predict oocyte maturity in in-vitro fertilization. Sci. Rep. 2022, 12, 19573. [Google Scholar] [CrossRef]
- Al-Aqbi, M.; Hart, R.; Ajuogu, P.; de Touw, T.V.; McFarlane, J.; Smart, N. Follicular fluid leptin as a marker for pregnancy outcomes in women undergoing IVF treatment: A systematic review and meta-analysis. Hum. Fertil. 2022, 25, 33–42. [Google Scholar] [CrossRef]
- Chou, S.H.; Mantzoros, C. 20 years of leptin: Role of leptin in human reproductive disorders. J. Endocrinol. 2014, 223, T49–T62. [Google Scholar] [CrossRef]
- Cnop, M.; Havel, P.J.; Utzschneider, K.M.; Carr, D.B.; Sinha, M.K.; Boyko, E.J.; Retzlaff, B.M.; Knopp, R.H.; Brunzell, J.D.; Kahn, S.E. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: Evidence for independent roles of age and sex. Diabetologia 2003, 46, 459–469. [Google Scholar] [CrossRef]
- Ahl, S.; Guenther, M.; Zhao, S.; James, R.; Marks, J.; Szabo, A.; Kidambi, S. Adiponectin Levels Differentiate Metabolically Healthy vs Unhealthy Among Obese and Nonobese White Individuals. J. Clin. Endocrinol. Metab. 2015, 100, 4172–4180. [Google Scholar] [CrossRef]
- Nguyen, T.M.D. Adiponectin: Role in Physiology and Pathophysiology. Int. J. Prev. Med. 2020, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted Physiological Roles of Adiponectin in Inflammation and Diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Kim, J.S.; Jo, M.J.; Cho, E.; Ahn, S.Y.; Kwon, Y.J.; Ko, G.J. The Roles and Associated Mechanisms of Adipokines in Development of Metabolic Syndrome. Molecules 2022, 27, 334. [Google Scholar] [CrossRef] [PubMed]
- Rak, A.; Mellouk, N.; Froment, P.; Dupont, J. Adiponectin and resistin: Potential metabolic signals affecting hypothalamo-pituitary gonadal axis in females and males of different species. Reproduction 2017, 153, R215–R226. [Google Scholar] [CrossRef]
- Hopkins, T.A.; Ouchi, N.; Shibata, R.; Walsh, K. Adiponectin actions in the cardiovascular system. Cardiovasc. Res. 2007, 74, 11–18. [Google Scholar] [CrossRef]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Tsatsanis, C.; Zacharioudaki, V.; Androulidaki, A.; Dermitzaki, E.; Charalampopoulos, I.; Minas, V.; Gravanis, A.; Margioris, A.N. Adiponectin induces TNF-alpha and IL-6 in macrophages and promotes tolerance to itself and other pro-inflammatory stimuli. Biochem. Biophys. Res. Commun. 2005, 335, 1254–1263. [Google Scholar] [CrossRef]
- Fang, H.; Judd, R.L. Adiponectin Regulation and Function. Compr. Physiol. 2018, 8, 1031–1063. [Google Scholar] [CrossRef]
- Haugen, F.; Drevon, C.A. Activation of nuclear factor-kappaB by high molecular weight and globular adiponectin. Endocrinology 2007, 148, 5478–5486. [Google Scholar] [CrossRef]
- Yau, S.Y.; Li, A.; Hoo, R.L.; Ching, Y.P.; Christie, B.; Lee, T.M.; Xu, A.; So, K.F. Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin. Proc. Natl. Acad. Sci. USA 2014, 111, 15810–15815. [Google Scholar] [CrossRef]
- Wang, Z.V.; Scherer, P.E. Adiponectin, the past two decades. J. Mol. Cell Biol. 2016, 8, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Gao, Y.; Yao, T.; Huang, Y.; He, Z.; Kong, X.; Yu, K.J.; Wang, R.T.; Guo, H.; Yan, J.; et al. Adiponectin potentiates the acute effects of leptin in arcuate Pomc neurons. Mol. Metab. 2016, 5, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Cheng, K.K.; Hoo, R.L.; Siu, P.M.; Yau, S.Y. The Novel Perspectives of Adipokines on Brain Health. Int. J. Mol. Sci. 2019, 20, 5638. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.P.; Lv, W.S.; Yang, J.; Nie, A.F.; Cheng, X.B.; Yang, Y.; Ge, Y.; Li, X.Y.; Ning, G. Globular adiponectin inhibits GnRH secretion from GT1-7 hypothalamic GnRH neurons by induction of hyperpolarization of membrane potential. Biochem. Biophys. Res. Commun. 2008, 371, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.P.; Liu, C.; Bi, W.K.; Hu, Y.T.; Chen, Q.; Huang, H.; Liang, J.X.; Li, L.T.; Lin, L.X.; Chen, G. Adiponectin inhibits KISS1 gene transcription through AMPK and specificity protein-1 in the hypothalamic GT1-7 neurons. J. Endocrinol. 2012, 214, 177–189. [Google Scholar] [CrossRef]
- Estienne, A.; Bongrani, A.; Reverchon, M.; Ramé, C.; Ducluzeau, P.H.; Froment, P.; Dupont, J. Involvement of Novel Adipokines, Chemerin, Visfatin, Resistin and Apelin in Reproductive Functions in Normal and Pathological Conditions in Humans and Animal Models. Int. J. Mol. Sci. 2019, 20, 4431. [Google Scholar] [CrossRef]
- Fazeli, P.K.; Bredella, M.A.; Pachon-Peña, G.; Zhao, W.; Zhang, X.; Faje, A.T.; Resulaj, M.; Polineni, S.P.; Holmes, T.M.; Lee, H.; et al. The dynamics of human bone marrow adipose tissue in response to feeding and fasting. JCI Insight 2021, 6, e138636. [Google Scholar] [CrossRef]
- Patel, L.; Buckels, A.C.; Kinghorn, I.J.; Murdock, P.R.; Holbrook, J.D.; Plumpton, C.; Macphee, C.H.; Smith, S.A. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem. Biophys. Res. Commun. 2003, 300, 472–476. [Google Scholar] [CrossRef]
- Pestel, J.; Blangero, F.; Watson, J.; Pirola, L.; Eljaafari, A. Adipokines in obesity and metabolic-related-diseases. Biochimie 2023, 212, 48–59. [Google Scholar] [CrossRef]
- Kaminska, B.; Kurowicka, B.; Kiezun, M.; Dobrzyn, K.; Kisielewska, K.; Gudelska, M.; Kopij, G.; Szymanska, K.; Zarzecka, B.; Koker, O.; et al. The Role of Adipokines in the Control of Pituitary Functions. Animals 2024, 14, 353. [Google Scholar] [CrossRef]
- Flores-Cordero, J.A.; Pérez-Pérez, A.; Jiménez-Cortegana, C.; Alba, G.; Flores-Barragán, A.; Sánchez-Margalet, V. Obesity as a Risk Factor for Dementia and Alzheimer’s Disease: The Role of Leptin. Int. J. Mol. Sci. 2022, 23, 5202. [Google Scholar] [CrossRef]
- Morash, B.A.; Ur, E.; Wiesner, G.; Roy, J.; Wilkinson, M. Pituitary resistin gene expression: Effects of age, gender and obesity. Neuroendocrinology 2004, 79, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Maillard, V.; Elis, S.; Desmarchais, A.; Hivelin, C.; Lardic, L.; Lomet, D.; Uzbekova, S.; Monget, P.; Dupont, J. Visfatin and resistin in gonadotroph cells: Expression, regulation of LH secretion and signalling pathways. Reprod. Fertil. Dev. 2017, 29, 2479–2495. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Acquarone, E.; Monacelli, F.; Borghi, R.; Nencioni, A.; Odetti, P. Resistin: A reappraisal. Mech. Ageing Dev. 2019, 178, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Rak-Mardyła, A.; Durak, M.; Lucja Gregoraszczuk, E. Effects of resistin on porcine ovarian follicle steroidogenesis in prepubertal animals: An in vitro study. Reprod. Biol. Endocrinol. 2013, 11, 45. [Google Scholar] [CrossRef]
- Reverchon, M.; Cornuau, M.; Ramé, C.; Guerif, F.; Royère, D.; Dupont, J. Resistin decreases insulin-like growth factor I-induced steroid production and insulin-like growth factor I receptor signaling in human granulosa cells. Fertil. Steril. 2013, 100, 247–255.e3. [Google Scholar] [CrossRef]
- Nikanfar, S.; Oghbaei, H.; Rastgar Rezaei, Y.; Zarezadeh, R.; Jafari-Gharabaghlou, D.; Nejabati, H.R.; Bahrami, Z.; Bleisinger, N.; Samadi, N.; Fattahi, A.; et al. Role of adipokines in the ovarian function: Oogenesis and steroidogenesis. J. Steroid Biochem. Mol. Biol. 2021, 209, 105852. [Google Scholar] [CrossRef]
- Yoshino, J.; Mills, K.F.; Yoon, M.J.; Imai, S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011, 14, 528–536. [Google Scholar] [CrossRef]
- Higgins, C.B.; Mayer, A.L.; Zhang, Y.; Franczyk, M.; Ballentine, S.; Yoshino, J.; DeBosch, B.J. SIRT1 selectively exerts the metabolic protective effects of hepatocyte nicotinamide phosphoribosyltransferase. Nat. Commun. 2022, 13, 1074. [Google Scholar] [CrossRef]
- Nielsen, K.N.; Peics, J.; Ma, T.; Karavaeva, I.; Dall, M.; Chubanava, S.; Basse, A.L.; Dmytriyeva, O.; Treebak, J.T.; Gerhart-Hines, Z. NAMPT-mediated NAD(+) biosynthesis is indispensable for adipose tissue plasticity and development of obesity. Mol. Metab. 2018, 11, 178–188. [Google Scholar] [CrossRef]
- Mlyczyńska, E.; Kieżun, M.; Kurowska, P.; Dawid, M.; Pich, K.; Respekta, N.; Daudon, M.; Rytelewska, E.; Dobrzyń, K.; Kamińska, B.; et al. New Aspects of Corpus Luteum Regulation in Physiological and Pathological Conditions: Involvement of Adipokines and Neuropeptides. Cells 2022, 11, 957. [Google Scholar] [CrossRef] [PubMed]
- Saddi-Rosa, P.; Oliveira, C.S.; Giuffrida, F.M.; Reis, A.F. Visfatin, glucose metabolism and vascular disease: A review of evidence. Diabetol. Metab. Syndr. 2010, 2, 21. [Google Scholar] [CrossRef]
- Heo, Y.J.; Choi, S.E.; Jeon, J.Y.; Han, S.J.; Kim, D.J.; Kang, Y.; Lee, K.W.; Kim, H.J. Visfatin Induces Inflammation and Insulin Resistance via the NF-κB and STAT3 Signaling Pathways in Hepatocytes. J. Diabetes Res. 2019, 2019, 4021623. [Google Scholar] [CrossRef]
- Zorena, K.; Jachimowicz-Duda, O.; Ślęzak, D.; Robakowska, M.; Mrugacz, M. Adipokines and Obesity. Potential Link to Metabolic Disorders and Chronic Complications. Int. J. Mol. Sci. 2020, 21, 3570. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Tian, X.; Shang, P.; Chen, H.; Kang, X.; Tian, Y.; Han, R. Characterization of the visfatin gene and its expression pattern and effect on 3T3-L1 adipocyte differentiation in chickens. Gene 2017, 632, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Dahl, T.B.; Holm, S.; Aukrust, P.; Halvorsen, B. Visfatin/NAMPT: A multifaceted molecule with diverse roles in physiology and pathophysiology. Annu. Rev. Nutr. 2012, 32, 229–243. [Google Scholar] [CrossRef]
- Jacques, C.; Holzenberger, M.; Mladenovic, Z.; Salvat, C.; Pecchi, E.; Berenbaum, F.; Gosset, M. Proinflammatory actions of visfatin/nicotinamide phosphoribosyltransferase (Nampt) involve regulation of insulin signaling pathway and Nampt enzymatic activity. J. Biol. Chem. 2012, 287, 15100–15108. [Google Scholar] [CrossRef] [PubMed]
- Romacho, T.; Villalobos, L.A.; Cercas, E.; Carraro, R.; Sánchez-Ferrer, C.F.; Peiró, C. Visfatin as a novel mediator released by inflamed human endothelial cells. PLoS ONE 2013, 8, e78283. [Google Scholar] [CrossRef]
- Kim, S.R.; Bae, Y.H.; Bae, S.K.; Choi, K.S.; Yoon, K.H.; Koo, T.H.; Jang, H.O.; Yun, I.; Kim, K.W.; Kwon, Y.G.; et al. Visfatin enhances ICAM-1 and VCAM-1 expression through ROS-dependent NF-kappaB activation in endothelial cells. Biochim. Biophys. Acta 2008, 1783, 886–895. [Google Scholar] [CrossRef]
- Agbaedeng, T.A.; Iroga, P.E.; Rathnasekara, V.M.; Zacharia, A.L. Adipokines and stroke: A systematic review and meta-analysis of disease risk and patient outcomes. Obes. Rev. 2024, 25, e13684. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Z.; Tao, H.F.; Qi, X.Y.; Wang, W.L.; Yang, L.; Wang, H.; Xu, P. An elevated plasma level of visfatin increases the risk of myocardial infarction. Genet. Mol. Res. 2014, 13, 8586–8595. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Chang, D.M.; Lin, K.C.; Shin, S.J.; Lee, Y.J. Visfatin in overweight/obesity, type 2 diabetes mellitus, insulin resistance, metabolic syndrome and cardiovascular diseases: A meta-analysis and systemic review. Diabetes Metab. Res. Rev. 2011, 27, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, A.; Kopczyńska, E.; Bronisz, A.; Zmudzińska, M.; Bieliński, M.; Borkowska, A.; Tyrakowski, T.; Junik, R. An evaluation of visfatin levels in obese subjects. Endokrynol. Pol. 2010, 61, 169–173. [Google Scholar]
- Chen, J.; Hu, L.; Ling, Y.; Xu, Y.; Yu, X.; Ma, L.; Shou, M. Risk Correlation Analysis between Polycystic Ovary Syndrome (PCOS) and Serum Visfatin Levels in Middle-Aged Women: Systematic Review and Meta-Analysis. Discov. Med. 2023, 35, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Sun, X.; Wang, X.; Wang, H.; Chen, X. Circulating Adipokine Levels in Nonobese Women with Polycystic Ovary Syndrome and in Nonobese Control Women: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2020, 11, 537809. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, K.; Zaobidna, E.; Rytelewska, E.; Mlyczynska, E.; Kurowska, P.; Dobrzyn, K.; Kiezun, M.; Kaminska, B.; Smolinska, N.; Rak, A.; et al. Visfatin in the porcine pituitary gland: Expression and regulation of secretion during the oestrous cycle and early pregnancy. Sci. Rep. 2023, 13, 18253. [Google Scholar] [CrossRef]
- Kurowska, P.; Mlyczyńska, E.; Dawid, M.; Sierpowski, M.; Estienne, A.; Dupont, J.; Rak, A. Adipokines change the balance of proliferation/apoptosis in the ovarian cells of human and domestic animals: A comparative review. Anim. Reprod. Sci. 2021, 228, 106737. [Google Scholar] [CrossRef]
- Kaminski, T.; Kiezun, M.; Zaobidna, E.; Dobrzyn, K.; Wasilewska, B.; Mlyczynska, E.; Rytelewska, E.; Kisielewska, K.; Gudelska, M.; Bors, K.; et al. Plasma level and expression of visfatin in the porcine hypothalamus during the estrous cycle and early pregnancy. Sci. Rep. 2021, 11, 8698. [Google Scholar] [CrossRef]
- Hallschmid, M.; Randeva, H.; Tan, B.K.; Kern, W.; Lehnert, H. Relationship between cerebrospinal fluid visfatin (PBEF/Nampt) levels and adiposity in humans. Diabetes 2009, 58, 637–640. [Google Scholar] [CrossRef]
- Reverchon, M.; Cornuau, M.; Cloix, L.; Ramé, C.; Guerif, F.; Royère, D.; Dupont, J. Visfatin is expressed in human granulosa cells: Regulation by metformin through AMPK/SIRT1 pathways and its role in steroidogenesis. Mol. Hum. Reprod. 2013, 19, 313–326. [Google Scholar] [CrossRef]
- Estienne, A.; Brossaud, A.; Reverchon, M.; Ramé, C.; Froment, P.; Dupont, J. Adipokines Expression and Effects in Oocyte Maturation, Fertilization and Early Embryo Development: Lessons from Mammals and Birds. Int. J. Mol. Sci. 2020, 21, 3581. [Google Scholar] [CrossRef]
- Reverchon, M.; Rame, C.; Bunel, A.; Chen, W.; Froment, P.; Dupont, J. VISFATIN (NAMPT) Improves In Vitro IGF1-Induced Steroidogenesis and IGF1 Receptor Signaling Through SIRT1 in Bovine Granulosa Cells. Biol. Reprod. 2016, 94, 54. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Choi, K.M. Newly Discovered Adipokines: Pathophysiological Link between Obesity and Cardiometabolic Disorders. Front. Physiol. 2020, 11, 568800. [Google Scholar] [CrossRef]
- Dupont, J.; Pollet-Villard, X.; Reverchon, M.; Mellouk, N.; Levy, R. Adipokines in human reproduction. Horm. Mol. Biol. Clin. Investig. 2015, 24, 11–24. [Google Scholar] [CrossRef]
- Cătoi, A.F.; Suciu, Ş.; Pârvu, A.E.; Copăescu, C.; Galea, R.F.; Buzoianu, A.D.; Vereşiu, I.A.; Cătoi, C.; Pop, I.D. Increased chemerin and decreased omentin-1 levels in morbidly obese patients are correlated with insulin resistance, oxidative stress and chronic inflammation. Clujul Med. 2014, 87, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.A.; Mohamed, S.A. Serum levels of visfatin and omentin-1 in patients with psoriasis and their relation to disease severity. Br. J. Dermatol. 2012, 167, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Hou, P.; Wu, Z.; Nie, Y. Decreased levels of serum omentin-1 in patients with inflammatory bowel disease. Med. Sci. Monit. 2015, 21, 118–122. [Google Scholar] [CrossRef]
- Yang, R.Z.; Lee, M.J.; Hu, H.; Pray, J.; Wu, H.B.; Hansen, B.C.; Shuldiner, A.R.; Fried, S.K.; McLenithan, J.C.; Gong, D.W. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: Possible role in modulating insulin action. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1253–E1261. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Chan, L.; Zhou, S.W. Omentin: Linking metabolic syndrome and cardiovascular disease. Curr. Vasc. Pharmacol. 2014, 12, 136–143. [Google Scholar] [CrossRef]
- Watanabe, K.; Watanabe, R.; Konii, H.; Shirai, R.; Sato, K.; Matsuyama, T.A.; Ishibashi-Ueda, H.; Koba, S.; Kobayashi, Y.; Hirano, T.; et al. Counteractive effects of omentin-1 against atherogenesis†. Cardiovasc. Res. 2016, 110, 118–128. [Google Scholar] [CrossRef]
- Lin, X.; Sun, Y.; Yang, S.; Yu, M.; Pan, L.; Yang, J.; Yang, J.; Shao, Q.; Liu, J.; Liu, Y.; et al. Omentin-1 Modulates Macrophage Function via Integrin Receptors αvβ3 and αvβ5 and Reverses Plaque Vulnerability in Animal Models of Atherosclerosis. Front. Cardiovasc. Med. 2021, 8, 757926. [Google Scholar] [CrossRef]
- Leandro, A.; Queiroz, M.; Azul, L.; Seiça, R.; Sena, C.M. Omentin: A novel therapeutic approach for the treatment of endothelial dysfunction in type 2 diabetes. Free Radic. Biol. Med. 2021, 162, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Li, X.; Liu, F.; Tan, H.; Shang, D. Omentin inhibits TNF-α-induced expression of adhesion molecules in endothelial cells via ERK/NF-κB pathway. Biochem. Biophys. Res. Commun. 2012, 425, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Yamawaki, H.; Kuramoto, J.; Kameshima, S.; Usui, T.; Okada, M.; Hara, Y. Omentin, a novel adipocytokine inhibits TNF-induced vascular inflammation in human endothelial cells. Biochem. Biophys. Res. Commun. 2011, 408, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, L.; Orlando, G.; Ferrante, C.; Recinella, L.; Leone, S.; Chiavaroli, A.; Di Nisio, C.; Shohreh, R.; Manippa, F.; Ricciuti, A.; et al. Orexigenic effects of omentin-1 related to decreased CART and CRH gene expression and increased norepinephrine synthesis and release in the hypothalamus. Peptides 2013, 44, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Respekta, N.; Pich, K.; Mlyczyńska, E.; Dobrzyń, K.; Ramé, C.; Kamiński, T.; Smolińska, N.; Dupont, J.; Rak, A. Plasma level of omentin-1, its expression, and its regulation by gonadotropin-releasing hormone and gonadotropins in porcine anterior pituitary cells. Sci. Rep. 2023, 13, 19325. [Google Scholar] [CrossRef] [PubMed]
- Schäffler, A.; Neumeier, M.; Herfarth, H.; Fürst, A.; Schölmerich, J.; Büchler, C. Genomic structure of human omentin, a new adipocytokine expressed in omental adipose tissue. Biochim. Biophys. Acta 2005, 1732, 96–102. [Google Scholar] [CrossRef]
- Cloix, L.; Reverchon, M.; Cornuau, M.; Froment, P.; Ramé, C.; Costa, C.; Froment, G.; Lecomte, P.; Chen, W.; Royère, D.; et al. Expression and Regulation of INTELECTIN1 in Human Granulosa-Lutein Cells: Role in IGF-1-Induced Steroidogenesis Through NAMPT1. Biol. Reprod. 2014, 91, 50–51. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Fabová, Z.; Loncová, B.; Bauerová, M.; Halim Harrath, A. The adipokines progranulin and omentin—Novel regulators of basic ovarian cell functions. Reprod. Biol. Endocrinol. 2024, 22, 38. [Google Scholar] [CrossRef]
- Tang, Y.L.; Yu, J.; Zeng, Z.G.; Liu, Y.; Liu, J.Y.; Xu, J.X. Circulating omentin-1 levels in women with polycystic ovary syndrome: A meta-analysis. Gynecol. Endocrinol. 2017, 33, 244–249. [Google Scholar] [CrossRef]
- Mahde, A.; Shaker, M.; Al-Mashhadani, Z. Study of Omentin1 and Other Adipokines and Hormones in PCOS Patients. Oman Med. J. 2009, 24, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Shaker, M.; Mashhadani, Z.I.; Mehdi, A.A. Effect of Treatment with Metformin on Omentin-1, Ghrelin and other Biochemical, Clinical Features in PCOS Patients. Oman Med. J. 2010, 25, 289–293. [Google Scholar] [CrossRef]
- Tan, B.K.; Adya, R.; Farhatullah, S.; Chen, J.; Lehnert, H.; Randeva, H.S. Metformin treatment may increase omentin-1 levels in women with polycystic ovary syndrome. Diabetes 2010, 59, 3023–3031. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Li, L.; Yang, M.; Liu, D.; Liu, H.; Boden, G.; Yang, G. Effects of the long-acting human glucagon-like peptide-1 analog liraglutide on plasma omentin-1 levels in patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2011, 92, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Franik, G.; Sadlocha, M.; Madej, P.; Owczarek, A.; Skrzypulec-Plinta, V.; Plinta, R.; Chudek, J.; Olszanecka-Glinianowicz, M. Circulating omentin-1 levels and inflammation in polycystic ovary syndrome. Ginekol. Pol. 2020, 91, 308–312. [Google Scholar] [CrossRef]
- Rudnicka, E.; Suchta, K.; Grymowicz, M.; Calik-Ksepka, A.; Smolarczyk, K.; Duszewska, A.M.; Smolarczyk, R.; Meczekalski, B. Chronic Low Grade Inflammation in Pathogenesis of PCOS. Int. J. Mol. Sci. 2021, 22, 3789. [Google Scholar] [CrossRef]
- Villa, P.A.; Ruggiero-Ruff, R.E.; Jamieson, B.B.; Campbell, R.E.; Coss, D. Obesity alters POMC and kisspeptin neuron crosstalk leading to reduced luteinizing hormone in male mice. J. Neurosci. 2024, 44, e0222242024. [Google Scholar] [CrossRef]
- Gao, M.; Tao, X.; Zhang, Q.; He, W.; Zhao, T.; Yuan, T. Correlation between kisspeptin and biochemical markers in obese and non-obese women with polycystic ovary syndrome. Gynecol. Endocrinol. 2023, 39, 2215869. [Google Scholar] [CrossRef]
- Ohtaki, T.; Shintani, Y.; Honda, S.; Matsumoto, H.; Hori, A.; Kanehashi, K.; Terao, Y.; Kumano, S.; Takatsu, Y.; Masuda, Y.; et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 2001, 411, 613–617. [Google Scholar] [CrossRef]
- Kotani, M.; Detheux, M.; Vandenbogaerde, A.; Communi, D.; Vanderwinden, J.M.; Le Poul, E.; Brézillon, S.; Tyldesley, R.; Suarez-Huerta, N.; Vandeput, F.; et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J. Biol. Chem. 2001, 276, 34631–34636. [Google Scholar] [CrossRef]
- Manfredi-Lozano, M.; Roa, J.; Ruiz-Pino, F.; Piet, R.; Garcia-Galiano, D.; Pineda, R.; Zamora, A.; Leon, S.; Sanchez-Garrido, M.A.; Romero-Ruiz, A.; et al. Defining a novel leptin-melanocortin-kisspeptin pathway involved in the metabolic control of puberty. Mol. Metab. 2016, 5, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Avendaño, M.S.; Vazquez, M.J.; Tena-Sempere, M. Disentangling puberty: Novel neuroendocrine pathways and mechanisms for the control of mammalian puberty. Hum. Reprod. Update 2017, 23, 737–763. [Google Scholar] [CrossRef]
- Navarro, V.M. Metabolic regulation of kisspeptin—The link between energy balance and reproduction. Nat. Rev. Endocrinol. 2020, 16, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Lehman, M.N.; Coolen, L.M.; Goodman, R.L. Minireview: Kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: A central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 2010, 151, 3479–3489. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Kang, Y.; Zhang, C.; Xie, Y.; Wang, C.; Liu, J.; Yu, C.; Zhao, H.; Huang, D. The Role of Kisspeptin in the Control of the Hypothalamic-Pituitary-Gonadal Axis and Reproduction. Front. Endocrinol. 2022, 13, 925206. [Google Scholar] [CrossRef]
- Zhang, C.; Bosch, M.A.; Qiu, J.; Rønnekleiv, O.K.; Kelly, M.J. 17β-Estradiol increases persistent Na(+) current and excitability of AVPV/PeN Kiss1 neurons in female mice. Mol. Endocrinol. 2015, 29, 518–527. [Google Scholar] [CrossRef]
- Cheng, G.; Coolen, L.M.; Padmanabhan, V.; Goodman, R.L.; Lehman, M.N. The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: Sex differences and effects of prenatal testosterone in sheep. Endocrinology 2010, 151, 301–311. [Google Scholar] [CrossRef]
- Novaira, H.J.; Sonko, M.L.; Radovick, S. Kisspeptin Induces Dynamic Chromatin Modifications to Control GnRH Gene Expression. Mol. Neurobiol. 2016, 53, 3315–3325. [Google Scholar] [CrossRef]
- Abbara, A.; Eng, P.C.; Phylactou, M.; Clarke, S.A.; Richardson, R.; Sykes, C.M.; Phumsatitpong, C.; Mills, E.; Modi, M.; Izzi-Engbeaya, C.; et al. Kisspeptin receptor agonist has therapeutic potential for female reproductive disorders. J. Clin. Investig. 2020, 130, 6739–6753. [Google Scholar] [CrossRef]
- Abbara, A.; Ufer, M.; Voors-Pette, C.; Berman, L.; Ezzati, M.; Wu, R.; Lee, T.Y.; Ferreira, J.C.A.; Migoya, E.; Dhillo, W.S. Endocrine profile of the kisspeptin receptor agonist MVT-602 in healthy premenopausal women with and without ovarian stimulation: Results from 2 randomized, placebo-controlled clinical tricals. Fertil. Steril. 2024, 121, 95–106. [Google Scholar] [CrossRef]
- Cejudo Roman, A.; Pinto, F.M.; Dorta, I.; Almeida, T.A.; Hernández, M.; Illanes, M.; Tena-Sempere, M.; Candenas, L. Analysis of the expression of neurokinin B, kisspeptin, and their cognate receptors NK3R and KISS1R in the human female genital tract. Fertil. Steril. 2012, 97, 1213–1219. [Google Scholar] [CrossRef]
- Owens, L.A.; Abbara, A.; Lerner, A.; O’floinn, S.; Christopoulos, G.; Khanjani, S.; Islam, R.; Hardy, K.; Hanyaloglu, A.C.; Lavery, S.A.; et al. The direct and indirect effects of kisspeptin-54 on granulosa lutein cell function. Hum. Reprod. 2018, 33, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Song, C.; Gao, H.; Ma, T.; Li, T.; Ma, Q.; Yao, T.; Wang, M.; Li, J.; Yi, X.; et al. Leptin and inflammatory factors play a synergistic role in the regulation of reproduction in male mice through hypothalamic kisspeptin-mediated energy balance. Reprod. Biol. Endocrinol. 2021, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, T.; Matsuzaki, T.; Tungalagsuvd, A.; Munkhzaya, M.; Kawami, T.; Niki, H.; Kato, T.; Kuwahara, A.; Uemura, H.; Yasui, T.; et al. Hypothalamic Kiss1 and RFRP gene expressions are changed by a high dose of lipopolysaccharide in female rats. Horm. Behav. 2014, 66, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Sarchielli, E.; Comeglio, P.; Squecco, R.; Ballerini, L.; Mello, T.; Guarnieri, G.; Idrizaj, E.; Mazzanti, B.; Vignozzi, L.; Gallina, P.; et al. Tumor Necrosis Factor-α Impairs Kisspeptin Signaling in Human Gonadotropin-Releasing Hormone Primary Neurons. J. Clin. Endocrinol. Metab. 2017, 102, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Wahab, F.; Bano, R.; Jabeen, S.; Irfan, S.; Shahab, M. Effect of peripheral kisspeptin administration on adiponectin, leptin, and resistin secretion under fed and fasting conditions in the adult male rhesus monkey (Macaca mulatta). Horm. Metab. Res. 2010, 42, 570–574. [Google Scholar] [CrossRef]
- Negi, N.; Das, B.K. CNS: Not an immunoprivilaged site anymore but a virtual secondary lymphoid organ. Int. Rev. Immunol. 2018, 37, 57–68. [Google Scholar] [CrossRef]
- Léon, S.; Nadjar, A.; Quarta, C. Microglia-Neuron Crosstalk in Obesity: Melodious Interaction or Kiss of Death? Int. J. Mol. Sci. 2021, 22, 5243. [Google Scholar] [CrossRef]
- Boleti, A.P.D.A.; de O Cardoso, P.H.; Frihling, B.E.; E Silva, P.S.; de Moraes, L.F.R.N.; Migliolo, L. Adipose tissue, systematic inflammation, and neurodegenerative diseases. Neural Regen. Res. 2023, 18, 38–46. [Google Scholar] [CrossRef]
- Valdearcos, M.; Myers, M.G., Jr.; Koliwad, S.K. Hypothalamic microglia as potential regulators of metabolic physiology. Nat. Metab. 2019, 1, 314–320. [Google Scholar] [CrossRef]
- Dorfman, M.D.; Thaler, J.P. Hypothalamic inflammation and gliosis in obesity. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Paolicelli, R.C.; Bisht, K.; Tremblay, M. Fractalkine regulation of microglial physiology and consequences on the brain and behavior. Front. Cell. Neurosci. 2014, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Dorfman, M.D.; Krull, J.E.; Douglass, J.D.; Fasnacht, R.; Lara-Lince, F.; Meek, T.H.; Shi, X.; Damian, V.; Nguyen, H.T.; Matsen, M.E.; et al. Sex differences in microglial CX3CR1 signalling determine obesity susceptibility in mice. Nat. Commun. 2017, 8, 14556. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.E.; Lainez, N.M.; Nair, M.G.; Coss, D. Visceral adipose tissue imparts peripheral macrophage influx into the hypothalamus. J. Neuroinflamm. 2021, 18, 140. [Google Scholar] [CrossRef] [PubMed]
- Lainez, N.M.; Jonak, C.R.; Nair, M.G.; Ethell, I.M.; Wilson, E.H.; Carson, M.J.; Coss, D. Diet-Induced Obesity Elicits Macrophage Infiltration and Reduction in Spine Density in the Hypothalami of Male but Not Female Mice. Front. Immunol. 2018, 9, 1992. [Google Scholar] [CrossRef]
- Chang, E.H.; Chavan, S.S.; Pavlov, V.A. Cholinergic Control of Inflammation, Metabolic Dysfunction, and Cognitive Impairment in Obesity-Associated Disorders: Mechanisms and Novel Therapeutic Opportunities. Front. Neurosci. 2019, 13, 263. [Google Scholar] [CrossRef]
- Barabás, K.; Szabó-Meleg, E.; Ábrahám, I.M. Effect of Inflammation on Female Gonadotropin-Releasing Hormone (GnRH) Neurons: Mechanisms and Consequences. Int. J. Mol. Sci. 2020, 21, 529. [Google Scholar] [CrossRef]
- Wojtulewicz, K.; Krawczyńska, A.; Tomaszewska-Zaremba, D.; Wójcik, M.; Herman, A.P. Effect of Acute and Prolonged Inflammation on the Gene Expression of Proinflammatory Cytokines and Their Receptors in the Anterior Pituitary Gland of Ewes. Int. J. Mol. Sci. 2020, 21, 6939. [Google Scholar] [CrossRef]
- Navarro, G.; Allard, C.; Xu, W.; Mauvais-Jarvis, F. The role of androgens in metabolism, obesity, and diabetes in males and females. Obesity 2015, 23, 713–719. [Google Scholar] [CrossRef]
- Lonardo, M.S.; Cacciapuoti, N.; Guida, B.; Di Lorenzo, M.; Chiurazzi, M.; Damiano, S.; Menale, C. Hypothalamic-Ovarian axis and Adiposity Relationship in Polycystic Ovary Syndrome: Physiopathology and Therapeutic Options for the Management of Metabolic and Inflammatory Aspects. Curr. Obes. Rep. 2024, 13, 51–70. [Google Scholar] [CrossRef]
- Biernacka-Bartnik, A.; Kocełak, P.; Owczarek, A.J.; Choręza, P.; Puzianowska-Kuźnicka, M.; Markuszewski, L.; Madej, P.; Chudek, J.; Olszanecka-Glinianowicz, M. Prediction of Insulin Resistance and Impaired Fasting Glucose Based on Sex Hormone-Binding Globulin (SHBG) Levels in Polycystic Ovary Syndrome. Int. J. Endocrinol. 2022, 2022, 6498768. [Google Scholar] [CrossRef] [PubMed]
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef] [PubMed]
- Malini, N.A.; Roy, G.K. Influence of Insulin on LH, Testosterone and SHBG in various PCOS Categories based on the Mode of Secretion of LH in relation to FSH Levels. Acta Endocrinol. 2021, 17, 313–318. [Google Scholar] [CrossRef]
- Das, D.; Arur, S. Conserved insulin signaling in the regulation of oocyte growth, development, and maturation. Mol. Reprod. Dev. 2017, 84, 444–459. [Google Scholar] [CrossRef] [PubMed]
- Sakumoto, T.; Tokunaga, Y.; Tanaka, H.; Nohara, M.; Motegi, E.; Shinkawa, T.; Nakaza, A.; Higashi, M. Insulin resistance/hyperinsulinemia and reproductive disorders in infertile women. Reprod. Med. Biol. 2010, 9, 185–190. [Google Scholar] [CrossRef]
- Tay, C.T.; Garrad, R.; Mousa, A.; Bahri, M.; Joham, A.; Teede, H. Polycystic ovary syndrome (PCOS): International collaboration to translate evidence and guide future research. J. Endocrinol. 2023, 257, e220232. [Google Scholar] [CrossRef]
- Teede, H.J.; Tay, C.T.; Laven, J.J.E.; Dokras, A.; Moran, L.J.; Piltonen, T.T.; Costello, M.F.; Boivin, J.; Redman, L.M.; Boyle, J.A.; et al. Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Eur. J. Endocrinol. 2023, 189, G43–G64. [Google Scholar] [CrossRef]
- Garad, R.; Shorakae, S.; Teede, H. Assessment and management of women with polycystic ovary syndrome (PCOS). In Advanced Practice in Endocrinology Nursing; Springer: Berlin/Heidelberg, Germany, 2019; pp. 753–769. [Google Scholar]
- Aggarwal, M.; Chakole, S. Prevalence of Polycystic Ovarian Syndrome and Its Link to Obesity in Adolescent Girls. Cureus 2023, 15, e45405. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, Z.; Kraft, P.; Deng, Q.; Stener-Victorin, E.; Jiang, X. Genomic correlation, shared loci, and causal relationship between obesity and polycystic ovary syndrome: A large-scale genome-wide cross-trait analysis. BMC Med. 2022, 20, 66. [Google Scholar] [CrossRef]
- Barrea, L.; Muscogiuri, G.; Pugliese, G.; de Alteriis, G.; Colao, A.; Savastano, S. Metabolically Healthy Obesity (MHO) vs. Metabolically Unhealthy Obesity (MUO) Phenotypes in PCOS: Association with Endocrine-Metabolic Profile, Adherence to the Mediterranean Diet, and Body Composition. Nutrients 2021, 13, 3925. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, M.; Dilimulati, D.; Lin, Z.; Sun, H.; Cui, R.; Fei, H.; Gao, X.; Zeng, Q.; Shao, X.; et al. Correlation between Serum Uric Acid and Body Fat Distribution in Patients with Polycystic Ovary Syndrome. Front. Endocrinol. 2021, 12, 782808. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, B.O.; Bozdag, G.; Yapici, Z.; Esinler, I.; Yarali, H. Prevalence, phenotype and cardiometabolic risk of polycystic ovary syndrome under different diagnostic criteria. Hum. Reprod. 2012, 27, 3067–3073. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Blasco, F.; Botella-Carretero, J.I.; San Millán, J.L.; Escobar-Morreale, H.F. Prevalence and characteristics of the polycystic ovary syndrome in overweight and obese women. Arch. Intern. Med. 2006, 166, 2081–2086. [Google Scholar] [CrossRef] [PubMed]
- Walters, K.A.; Allan, C.M.; Handelsman, D.J. Rodent models for human polycystic ovary syndrome. Biol. Reprod. 2012, 86, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, A.S.; Middleton, L.J.; Jimenez, M.; Desai, R.; McMahon, A.C.; Allan, C.M.; Handelsman, D.J.; Walters, K.A. Characterization of reproductive, metabolic, and endocrine features of polycystic ovary syndrome in female hyperandrogenic mouse models. Endocrinology 2014, 155, 3146–3159. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, J.; Cheng, X.; Nie, X.; He, B. Insulin resistance in polycystic ovary syndrome across various tissues: An updated review of pathogenesis, evaluation, and treatment. J. Ovarian Res. 2023, 16, 9. [Google Scholar] [CrossRef]
- Chang, H.M.; Wu, H.C.; Sun, Z.G.; Lian, F.; Leung, P.C.K. Neurotrophins and glial cell line-derived neurotrophic factor in the ovary: Physiological and pathophysiological implications. Hum. Reprod. Update 2019, 25, 224–242. [Google Scholar] [CrossRef]
- Urbanek, M.; Du, Y.; Silander, K.; Collins, F.S.; Steppan, C.M.; Strauss, J.F., 3rd; Dunaif, A.; Spielman, R.S.; Legro, R.S. Variation in resistin gene promoter not associated with polycystic ovary syndrome. Diabetes 2003, 52, 214–217. [Google Scholar] [CrossRef]
- Pizzuti, A.; Argiolas, A.; Di Paola, R.; Baratta, R.; Rauseo, A.; Bozzali, M.; Vigneri, R.; Dallapiccola, B.; Trischitta, V.; Frittitta, L. An ATG repeat in the 3′-untranslated region of the human resistin gene is associated with a decreased risk of insulin resistance. J. Clin. Endocrinol. Metab. 2002, 87, 4403–4406. [Google Scholar] [CrossRef][Green Version]
- Majuri, A.; Santaniemi, M.; Rautio, K.; Kunnari, A.; Vartiainen, J.; Ruokonen, A.; Kesäniemi, Y.A.; Tapanainen, J.S.; Ukkola, O.; Morin-Papunen, L. Rosiglitazone treatment increases plasma levels of adiponectin and decreases levels of resistin in overweight women with PCOS: A randomized placebo-controlled study. Eur. J. Endocrinol. 2007, 156, 263–269. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Phan, J.D.; Leung, K.L.; Grogan, T.R.; Ding, X.; Li, X.; Hoyos, L.R.; Abbott, D.H.; Chazenbalk, G.D. Adipose Insulin Resistance in Normal-Weight Women with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2019, 104, 2171–2183. [Google Scholar] [CrossRef] [PubMed]
- Dhurandhar, E.J.; Keith, S.W. The aetiology of obesity beyond eating more and exercising less. Best. Pract. Res. Clin. Gastroenterol. 2014, 28, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Lopez-Tello, J.; Sferruzzi-Perri, A.N. Developmental programming of the female reproductive system-a review. Biol. Reprod. 2021, 104, 745–770. [Google Scholar] [CrossRef] [PubMed]
- Sobrino, V.; Avendaño, M.S.; Perdices-López, C.; Jimenez-Puyer, M.; Tena-Sempere, M. Kisspeptins and the neuroendocrine control of reproduction: Recent progress and new frontiers in kisspeptin research. Front. Neuroendocrinol. 2022, 65, 100977. [Google Scholar] [CrossRef]
- Jensterle, M.; Janez, A.; Fliers, E.; DeVries, J.H.; Vrtacnik-Bokal, E.; Siegelaar, S.E. The role of glucagon-like peptide-1 in reproduction: From physiology to therapeutic perspective. Hum. Reprod. Update 2019, 25, 504–517. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mączka, K.; Stasiak, O.; Przybysz, P.; Grymowicz, M.; Smolarczyk, R. The Impact of the Endocrine and Immunological Function of Adipose Tissue on Reproduction in Women with Obesity. Int. J. Mol. Sci. 2024, 25, 9391. https://doi.org/10.3390/ijms25179391
Mączka K, Stasiak O, Przybysz P, Grymowicz M, Smolarczyk R. The Impact of the Endocrine and Immunological Function of Adipose Tissue on Reproduction in Women with Obesity. International Journal of Molecular Sciences. 2024; 25(17):9391. https://doi.org/10.3390/ijms25179391
Chicago/Turabian StyleMączka, Katarzyna, Olga Stasiak, Paulina Przybysz, Monika Grymowicz, and Roman Smolarczyk. 2024. "The Impact of the Endocrine and Immunological Function of Adipose Tissue on Reproduction in Women with Obesity" International Journal of Molecular Sciences 25, no. 17: 9391. https://doi.org/10.3390/ijms25179391
APA StyleMączka, K., Stasiak, O., Przybysz, P., Grymowicz, M., & Smolarczyk, R. (2024). The Impact of the Endocrine and Immunological Function of Adipose Tissue on Reproduction in Women with Obesity. International Journal of Molecular Sciences, 25(17), 9391. https://doi.org/10.3390/ijms25179391






