Abstract
A new complex of copper(II) with methyl-5-(trifluoromethyl)pyrazol-3-yl-ketazine (H2L) was synthesized with the composition [Cu2L2]∙C2H5OH (1). Recrystallization of the sample from DMSO yielded a single crystal of the composition [Cu2L2((CH3)2SO)] (2). The coordination compounds were studied by single-crystal X-ray diffraction analysis, IR spectroscopy, and static magnetic susceptibility method. The data obtained indicate that the polydentate ligand is coordinated by both acyclic nitrogen and heterocyclic nitrogen atoms. The cytotoxic activity of the ligand and complex 1 was investigated on human cell lines MCF7 (breast adenocarcinoma), Hep2 (laryngeal carcinoma), A549 (lung carcinoma), HepG2 (hepatocellular carcinoma), and MRC5 (non-tumor lung fibroblasts). The complex was shown to have a pronounced dose-dependent cytotoxicity towards these cell lines with LC50 values in the range of 0.18–4.03 μM.
1. Introduction
Drug discovery based on pyrazole scaffold is one of the popular trends in medicinal chemistry [1,2,3,4,5]. Substituted pyrazole derivatives demonstrate a broad spectrum of biological properties, including antibacterial, cytotoxic, anti-inflammatory, antitubercular, antitumor activities, etc. [6,7,8,9,10,11,12,13,14,15,16,17]. In some cases, pyrazole-bearing pharmacological agents display multiple actions against different targets.
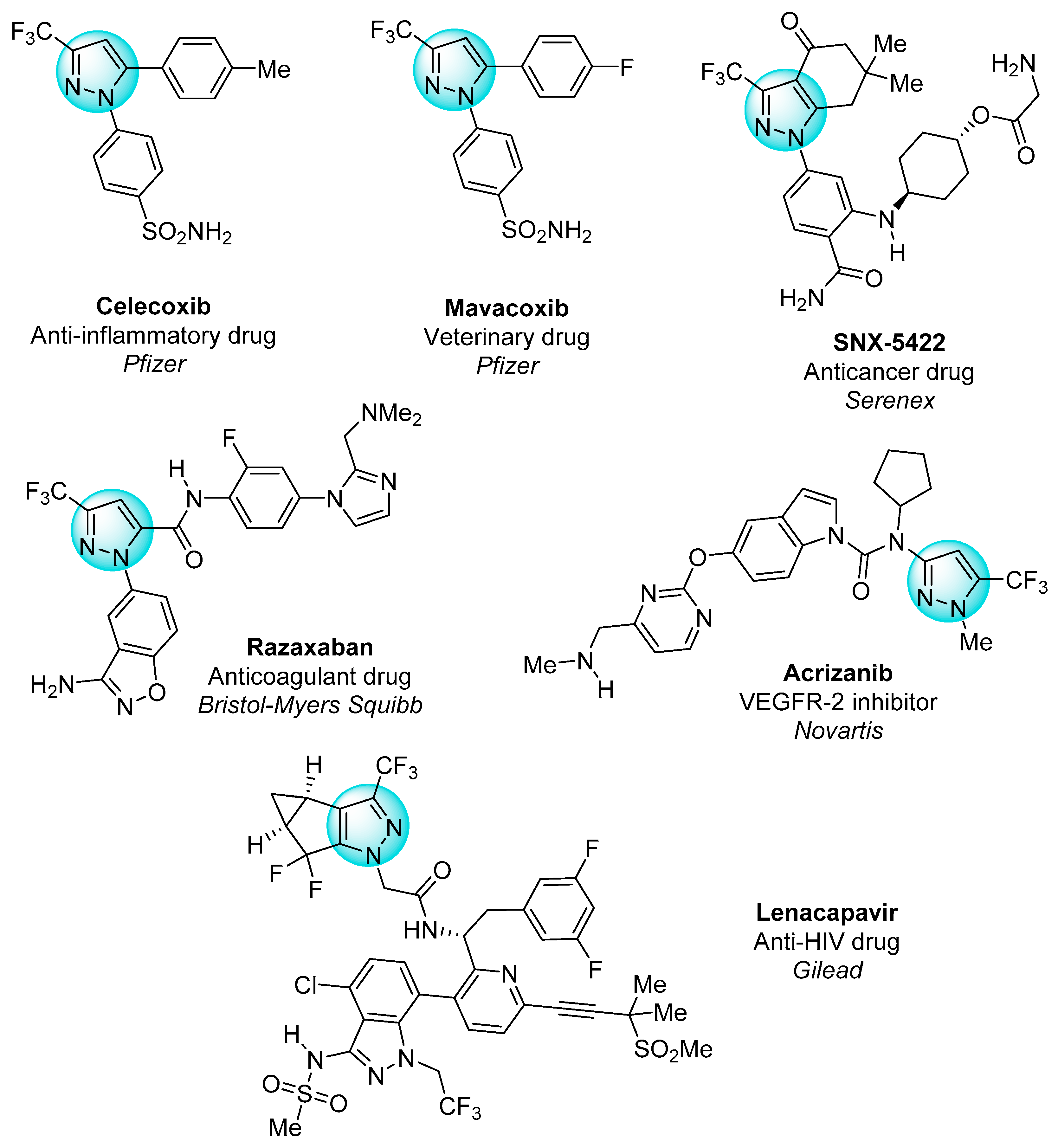
The functionalization of the pyrazole core with fluorinated substituents has led to the development of many bioactive molecules, as shown in Figure 1 [13,15,16,17,18]. Currently, almost a quarter of all drugs in the pharmacological market contain fluorine in their composition. Owing to the high electronegativity, small size, and low polarizability of fluorine, its introduction into the structure of organic compounds leads to an increase in their lipophilicity, membrane permeability, and metabolic stability, which determines pronounced biological activity, including anticancer [19].
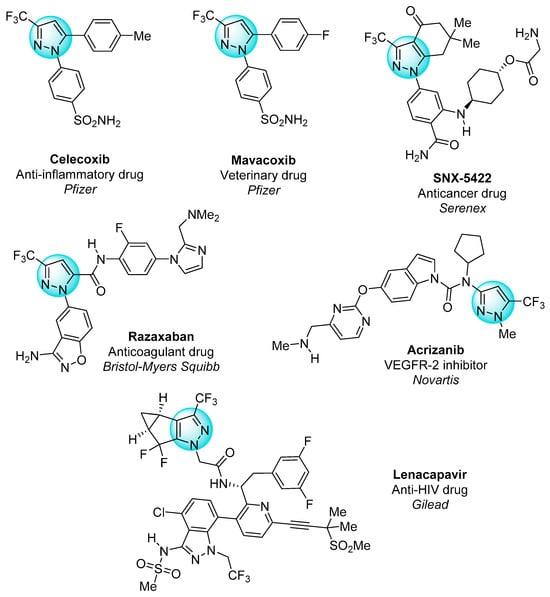
Figure 1.
Pharmaceuticals with the trifluoromethylated pyrazole motif.
The use of pyrazoles in the synthesis of metal complexes represents a promising avenue in the design of novel antitumor agents [20]. This strategy enables the combination of pyrazole bioactivity with their coordination properties as N-ligands [21,22,23,24]. Complexes based on the biocompatible copper(II) ion represent an alternative to existing anticancer metallodrugs [20]. Depending on the nature of substituents and reaction conditions, pyrazoles behave both as mono- and bidentate ligands, forming complexes with copper ions of various oxidation states and nuclearity [20,21,22,23,24,25,26,27,28,29]. In particular, the presence of trifluoromethyl groups in the pyrazole ring leads to an increase in the N–H acidity of the heterocycle and the stability of complexes with transition metals [13,30]. In addition, copper(II) pyrazolates are of interest because of their magnetic, catalytic, and photophysical properties [31,32,33,34,35].
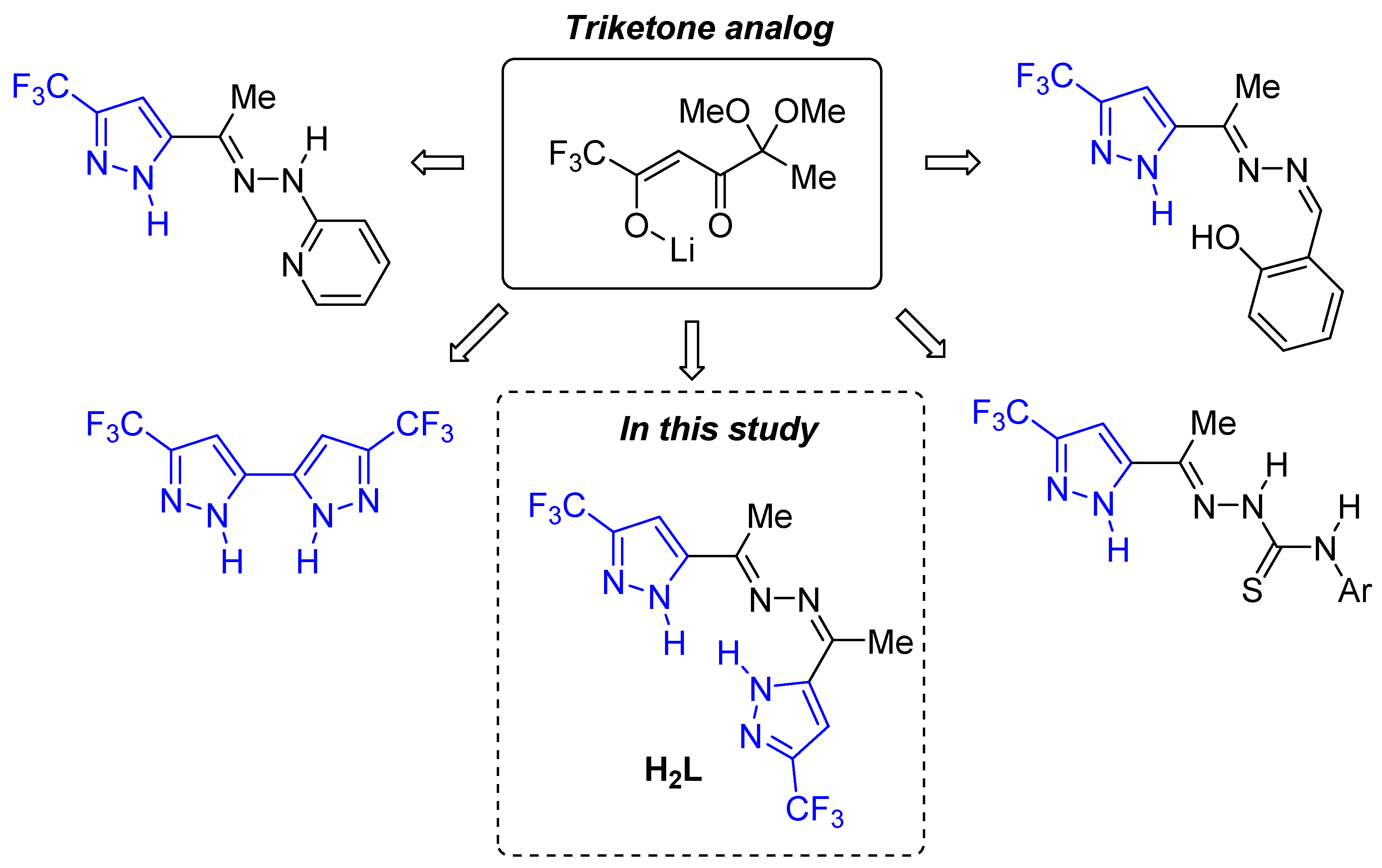
Previously, we have developed an approach to the synthesis of functionalized pyrazoles based on analogs of 1,2,4-triketones [18,33,34,35,36,37,38,39,40] (Figure 2), including fluorine-containing bis-pyrazoles [39,40]. In this work, we have investigated the complexation reactions of copper(II) with methyl-5-(trifluoromethyl)pyrazol-3-yl-ketazine (H2L) (Figure 2). The structure of H2L contains six nitrogen-donor atoms capable of coordination with transition metal ions. Although the potential of pyrazole derivatives in coordination chemistry is well documented, the number of fluorinated bis-pyrazoles used as ligands remains low [13].
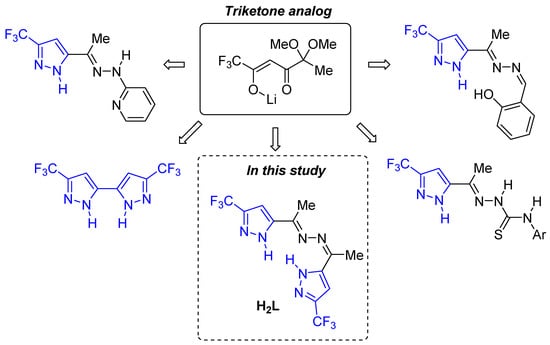
Figure 2.
Functionalized pyrazoles and bis-pyrazoles based on analogs of 1,2,4-triketones.
2. Results and Discussion
2.1. General Procedure for Synthesis of Compounds
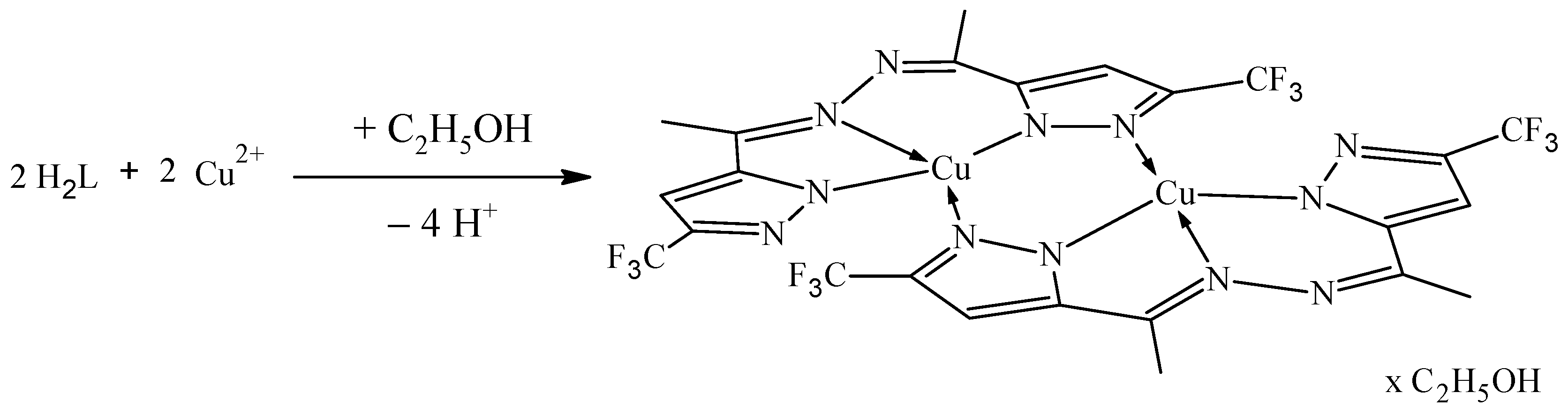
Complex 1 was synthesized by varying the metal: ligand ratios (2:1, 1:1, 1:2, 1:3) and using solutions of different copper(II) salts—nitrate, chloride, and sulfate. In all cases, the same dark green color complex was isolated. Elemental analysis and IR spectra of the complexes obtained with different copper(II) salts showed that they have the same composition [Cu2L2]∙C2H5OH (Scheme 1).
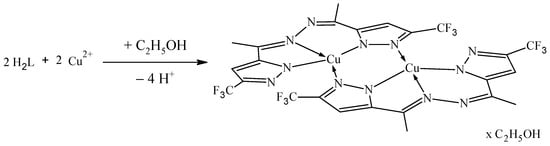
Scheme 1.
Synthesis of complex 1.
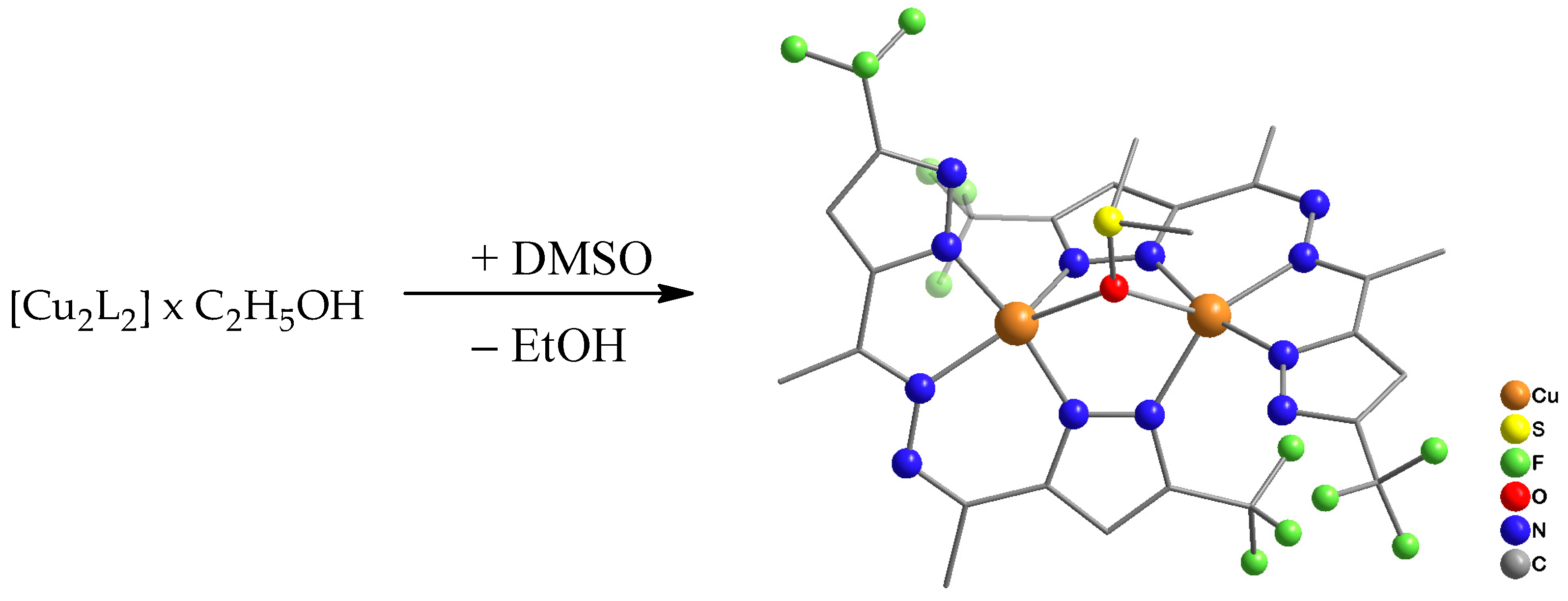
Upon recrystallization of portion 1 from DMSO and prolonged standing of the solution, we were able to obtain a single crystal of the complex [Cu2L2((CH3)2SO)] (2) suitable for X-ray diffraction analysis (Scheme 2).
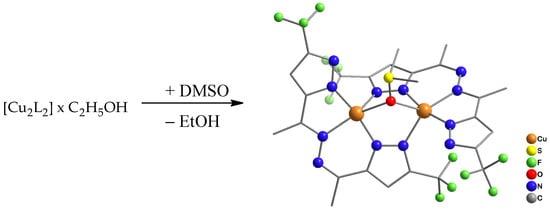
Scheme 2.
Synthesis of complex 2.
2.2. Crystallography
X-ray phase analysis data indicate the crystallinity of powdered sample 1. However, we were unable to grow single crystals of 1 suitable for analysis. To determine the coordination ability of the new ligand, we obtained and studied a single crystal of complex 2. It should be noted that the comparison of diffractograms indicates that (despite the same Cu:ligand ratio) complexes 1 and 2 are not isostructural (Figure S1). The differences should be explained, among other things, by their different composition and packaging of molecules.
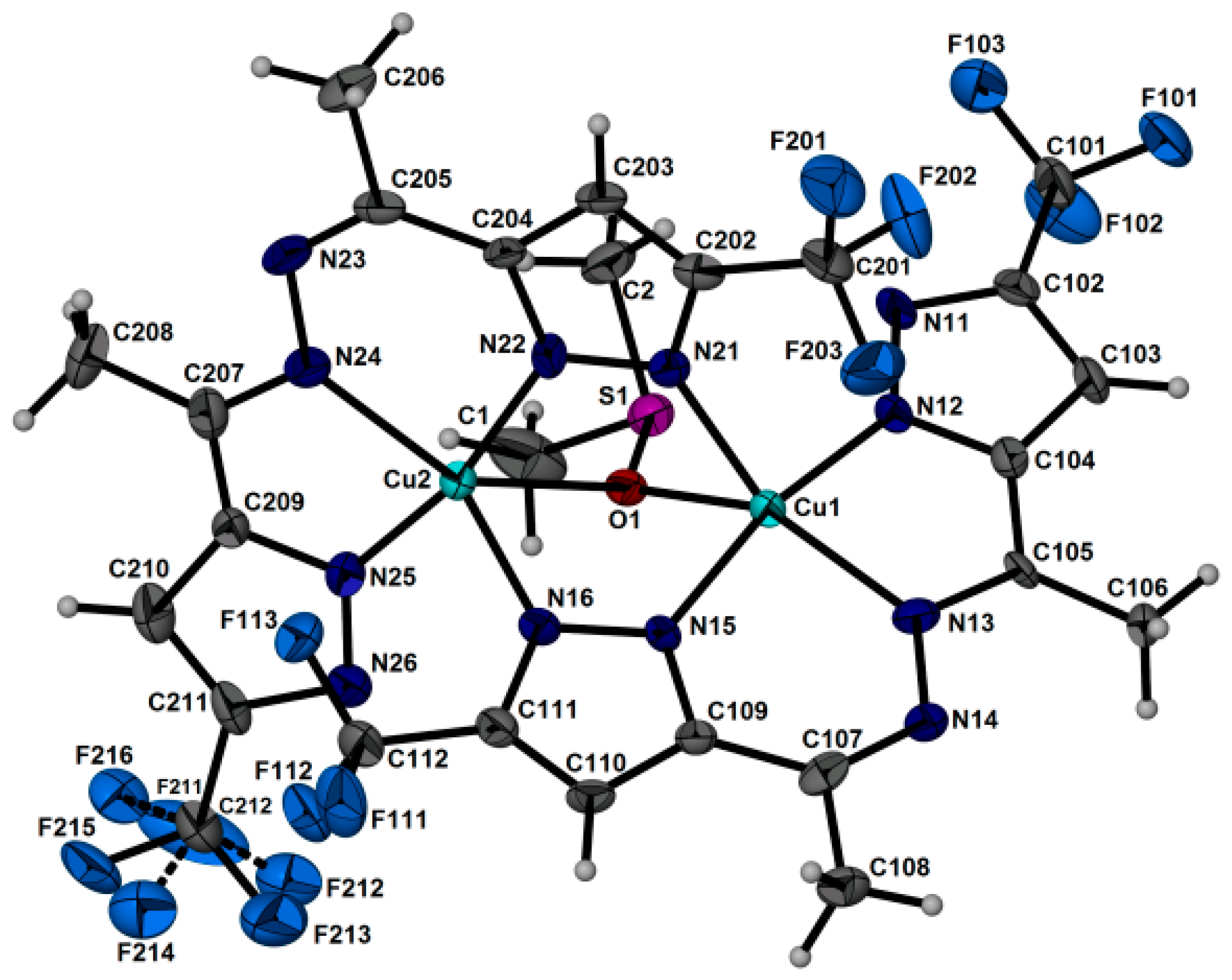
Single-crystal X-ray diffraction analysis of complex 2 indicates that the [Cu2L2((CH3)2SO)] phase crystallizes in a triclinic crystal system. The crystal packing is molecular (Figure 3 and Figure 4). Copper(II) ions replace both protons in the ligand H2L, and the obtained anion L2− acts as a tetradentate ligand, coordinating to two Cu(II) ions by nitrogen atoms; at the same time, the carbon-nitrogen framework of the ligand remains almost planar. The coordination sphere of each Cu(II) can be described as a slightly inclined trigonal bipyramidal geometry with 4N atoms from two ligands and an O atom from DMSO. The equatorial planes of the bipyramids defined by Cu(1), O(1), N(13), N(21) [N(13)-Cu(1)-N(21) = 135.5(2), N(13)-Cu(1)-O(1) = 139.5(2), N(21)-Cu(1)-O(1) = 84.9(2); totally 359.9(6)°] and Cu(2), O(1), N(16), N(24) [N(24)-Cu(2)-N(16) = 141.4(2), N(16)-Cu(2)-O(1) = 84.94(18), N(24)-Cu(2)-O(1) = 133.61(19); totally 359.9(7)°] are absolutely planar. A five-membered CuN2C2 chelate cycle is formed using one nitrogen atom of the first pyrazole ring and one nitrogen of the diazo group. Two nitrogen atoms of the second pyrazole ring form the six-membered metallocycle Cu2N4, acting as a bridge. The O atom of the solvent DMSO molecule coordinates with both Cu(II) ions, serving as an additional stability bridge. The main Cu-N(O) interatomic distances and valence angles are presented in Table 1.
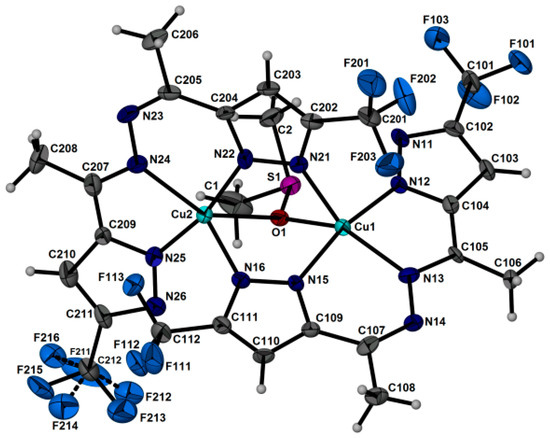
Figure 3.
The molecular complex 2 with the labeling (ellipsoids are plotted with 50% probability).
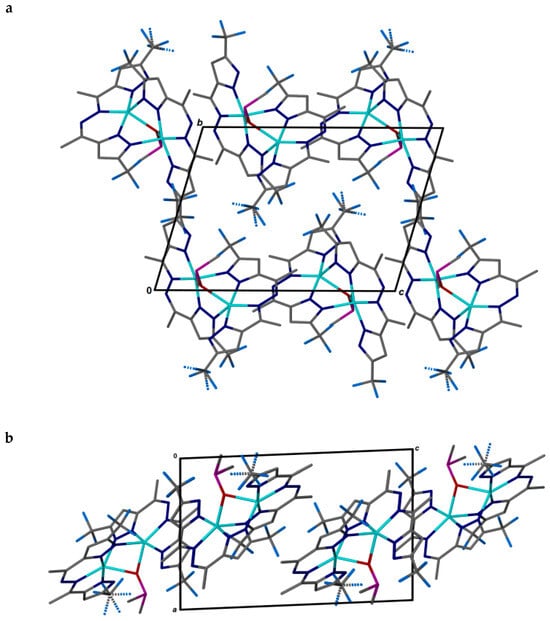
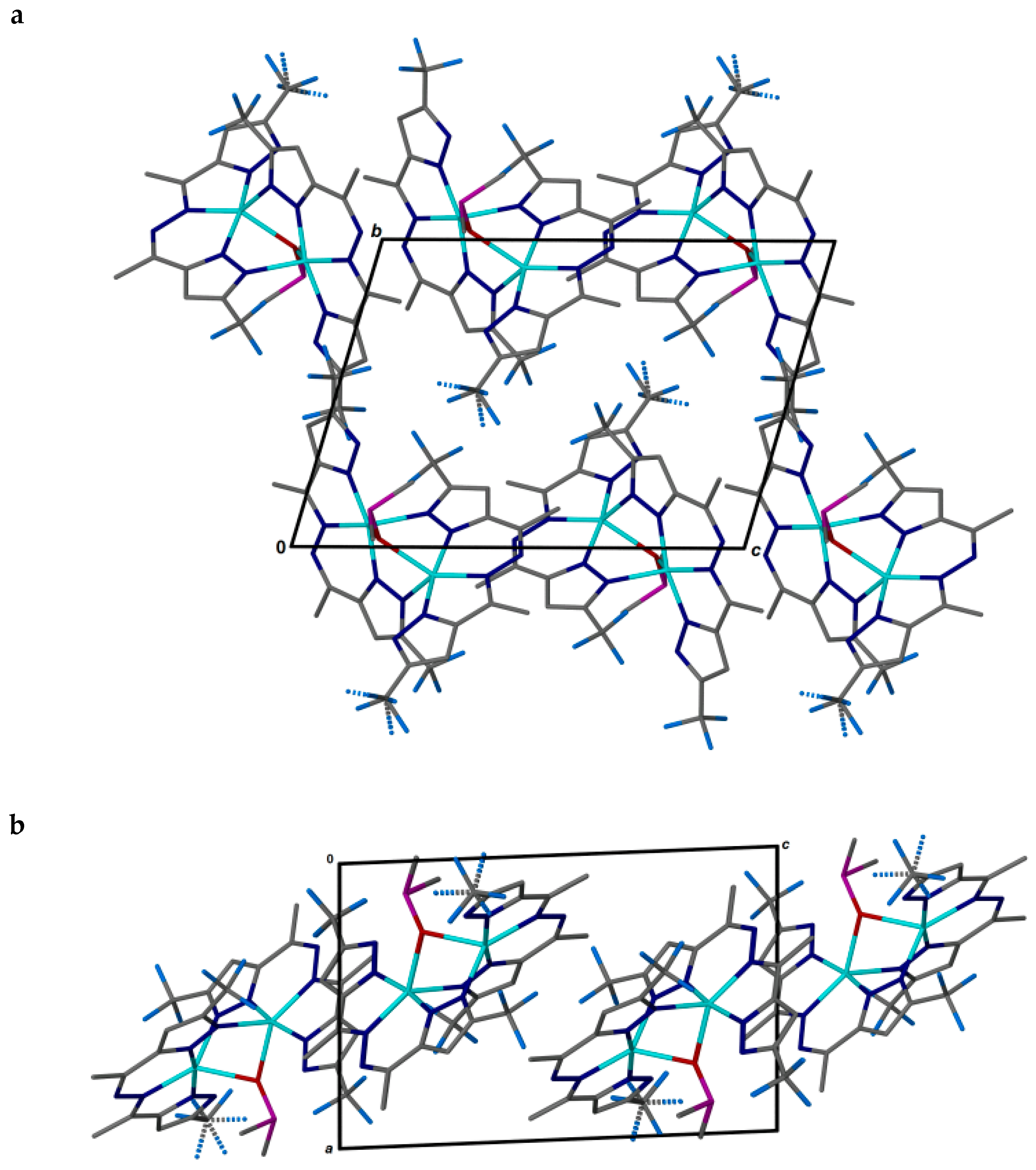
Figure 4.
Two orientations of the pseudo-layer of 2 in the bc plane (a) and along the b-axis (b).

Table 1.
Basic interatomic distances (A) and angles (°) for [Cu2L2((CH3)2SO)] (2).
The corrugated pseudo-layers of molecular binuclear complex particles in the bc plane, distorted according to the elongated shape, could be isolated in the package (Figure 4). Further packing of the layers takes place one above the other without displacement, according to the corrugation.
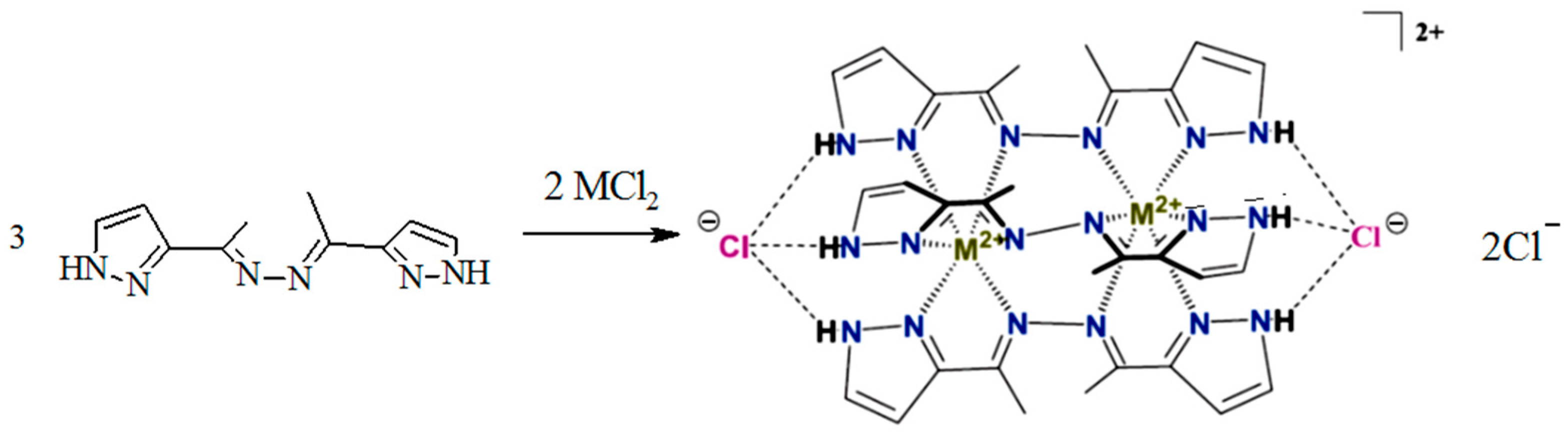
It should be noted that the coordination ability of the fluorinated ligand differs significantly from that assumed for the non-fluorinated analog [41] (Scheme 3).
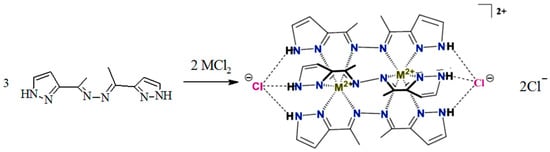
Scheme 3.
The expected type of cage metal complexes for 3D metals with non-fluorinated ligands [41].
2.3. Spectroscopy
In the high-frequency region of the infrared spectra of the ligand (Figure S2), (N-H) vibration bands near 3250 cm−1 are observed. In the spectrum of complex 1 (Figure S3), (O-H) bands are present within the broad range of 3500–3000 cm−1. The (C-H) bands of methyl groups and pyrazole rings are in the interval 3090–2830 cm−1. For H2L, at 1630 cm−1, a band of valence-deformation vibration of the acyclic bond -C=N- is observed, which is very sensitive to coordination. In the spectrum of the complex, this band appears at 1608 cm−1. In the spectrum of H2L in the region of 1550–1500 cm−1, the bands of valence-deformation vibrations of pyrazole rings are observed, which are split or shifted by ~30 cm−1 in the spectrum of the complex (Table 2). The changes in the position and shape of the bands are caused by the coordination of nitrogen atoms to Cu(II) [42].

Table 2.
Wave numbers (frequencies, cm–1) of absorption band maxima in the IR spectra of H2L and complex 1.
2.4. Magnetic Measurements
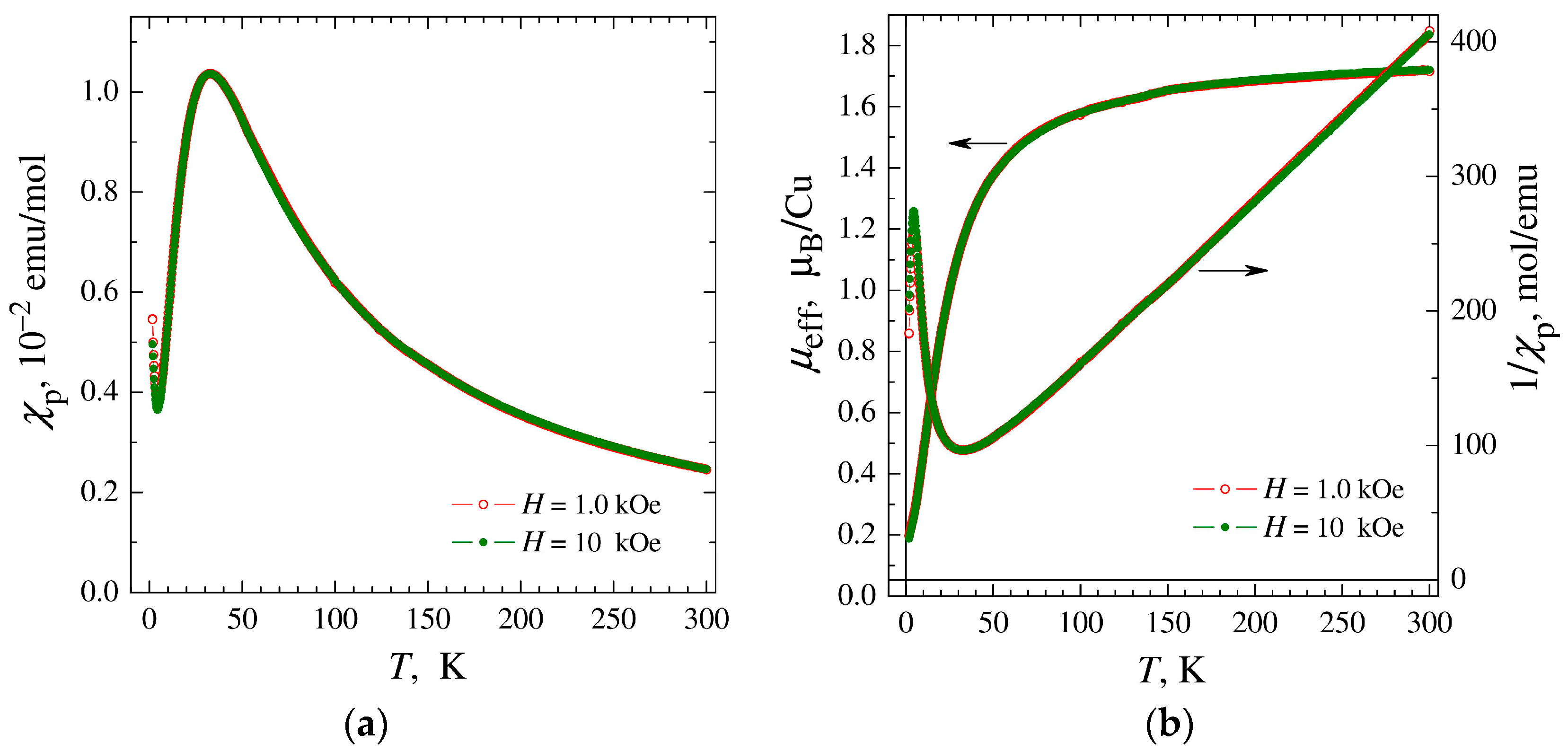
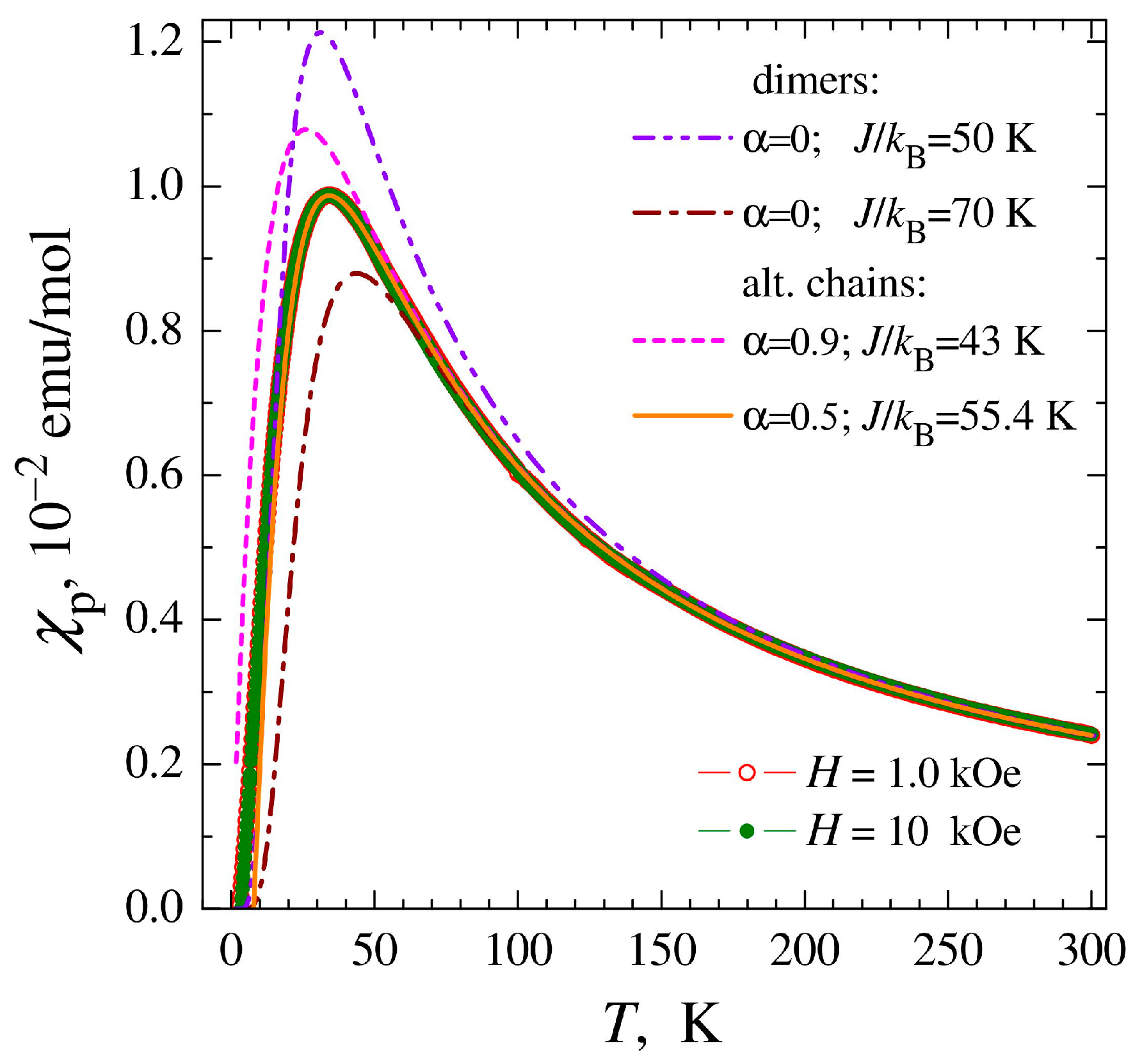
The temperature and field dependences of the magnetic susceptibility were measured for a polycrystalline sample of complex 1 and corrected for temperature-independent Langevin diamagnetism to single out the paramagnetic component of the susceptibility χp(T) associated with copper ions. The resulting χp(T) dependences were insensitive to the thermo-magnetic prehistory and exhibited a broad peak at Tm ≅ 34 K, independent of the magnitude of the applied magnetic field (Figure 5a). The drop in magnetic susceptibility upon cooling below Tm is unambiguous evidence of the predominance of antiferromagnetic (AF) interactions between copper ions, while the absence of phase transition and the smoothness of χp change indicate a reduced dimensionality of the magnetic subsystem, reduced to the level of chains or polynuclear clusters.
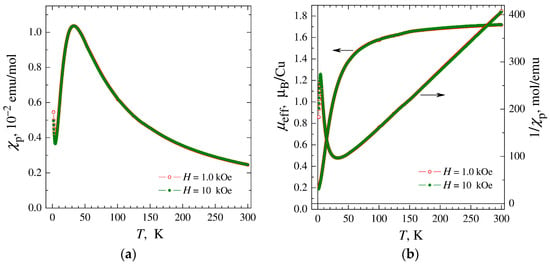
Figure 5.
(a) Temperature dependences of paramagnetic component of magnetic susceptibility χp of complex 1 measured in magnetic fields H = 1, 10 kOe. (b) Temperature dependences of the inverse susceptibility 1/χp and effective magnetic moment μeff calculated in the approximation of non-interacting ions (θ = 0).
In the high-temperature region, the obtained χp(T) data can be formally approximated by the Curie–Weiss dependence with values of the effective momentum μeff ≈ 1.80 μB and the Weiss constant θ ≈ −26 K (Figure 5b). The μeff value is characteristic of copper ions Cu2+ (S = 1/2) and corresponds to an average value of the g-factor g ≈ 2.08, exceeding the spin-only value g ≈ 2 due to the contribution of orbital moments. In turn, in the region of the lowest temperatures, after passing the peak, a susceptibility increase is observed, typical for most samples of chain and polynuclear complexes and associated with the inevitable presence of a small number of mononuclear impurities. To determine the percentage of monomer impurities, their contribution to the temperature dependence of the susceptibility was approximated by the Curie–Weiss dependence and the contribution to the field dependence of the magnetization M(H) was approximated by the Brillouin function. The analysis showed that the monomer fraction includes ~1.2% of copper ions. The magnetic susceptibility of the main phase can be determined by subtracting the contribution of mononuclear impurities from the experimental data.
As can be seen in Figure 6, the magnetic susceptibility of the main phase of complex 1 after passing the peak at Tm decreases almost to zero, which clearly indicates the formation of a gap in the spectrum of spin excitations. The most obvious explanation for this is related to the presence of magnetic dimers in the structure of the complex described by the Hamiltonian [43], where J is the exchange interaction between Cu2+ ions inside the dimer (J < 0 for the AF interaction), which is consistent with the structural data for the related complex 2. However, the model of isolated dimers, in which the interaction between dimers J′ = αJ is assumed to be zero, does not allow us to achieve any acceptable quality description of the experimental data χp(T) (Figure 6). The position of the magnetic susceptibility peak at Tm ~ 34 K in the isolated dimer model corresponds to the exchange interaction within the dimers J/kB ≈ 50 K; however, when such a value of J is chosen, the model calculation significantly exceeds the experimental values of χp (dashed-double dotted curve in Figure 6). In turn, the behavior of the magnetic susceptibility within the range of 80–300 K in the same model requires a value of J/kB ≈ 70 K (dashed-dotted line). This can be explained by the presence of an additional AF interaction between the dimers, J′ = αJ ≠ 0.
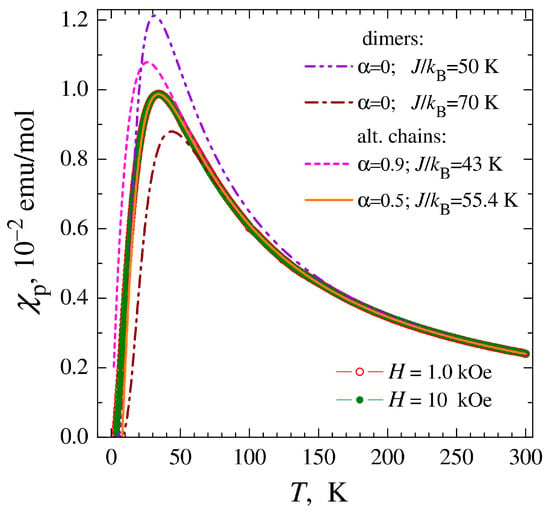
Figure 6.
Temperature dependencies of the magnetic susceptibility χp of complex 1, after subtracting the contribution from the monomer impurity. The lines show approximations of the experimental data by theoretical models: the model of non-interacting dimers (dashed-dotted line, dashed-double dotted line) and the model of dimerized chains (dashed and solid lines) with the interaction parameters within dimers J and between dimers J′ = αJ indicated in the figure. The first curve (dashed-double dotted line) corresponds to the optimization of the model description in the region of the magnetic susceptibility peak; for the other curves, the fitting of the J parameter was carried out in the temperature region 80–300 K.
One of the limiting cases of strong AF interaction between dimers is the homogeneous chain, which also exhibits a broad peak in magnetic susceptibility [44]. However, the homogeneous chain lacks a gap in the magnetic excitation spectrum, and the magnetic susceptibility drops at low temperatures by only ~30% relative to the peak value rather than to zero, as observed experimentally. An intermediate variant is a partially dimerized chain described by the Hamiltonian [44,45,46], when a strong AF interaction in pairs of ions, J, alternates with a weaker J′ = αJ interaction between pairs. The availability of the theory of partially dimerized chains [44,45] allowed us to perform simulations that showed that by adjusting the degree of dimerization α (dashed and solid lines in Figure 6), a reasonably good agreement of the model with the experimental data can be achieved. The optimal fitting result was obtained for α ≈ 0.5, which means a sufficiently strong interaction between dimers, only two times weaker than the AF interaction within the dimer, J′ = J/2.
Detailed structural data (Figure 3) were obtained only for complex 2 since it was possible to grow single crystals for it. However, certain conclusions can be drawn from the structure of complex 1, which differs from complex 2 only by the absence of the (CH3)2SO group coordinated with the copper(II) ion. As can be seen in Figure 3, the two copper ions in the structure of complex 2, and obviously complex 1, are united in a stable metallocycle Cu2N4, which leads to the appearance of magnetic dimers. In the structure of complex 2, dimers are spatially separated by groups (CH3)2SO, while in complex 1, these groups are absent, which leads, according to the magnetic data, to the formation of a chemical bond between the dimers, capable of transferring between them strong enough AF exchange interaction. It should be noted that the magnetic susceptibility data, while demonstrating the presence of interaction between magnetic Cu2+-Cu2+ dimers, still do not allow us to conclude whether linear chains of dimers, ladder, or other similar structures are formed upon the packing of molecules in the crystal structure.
2.5. Cytotoxic Activity
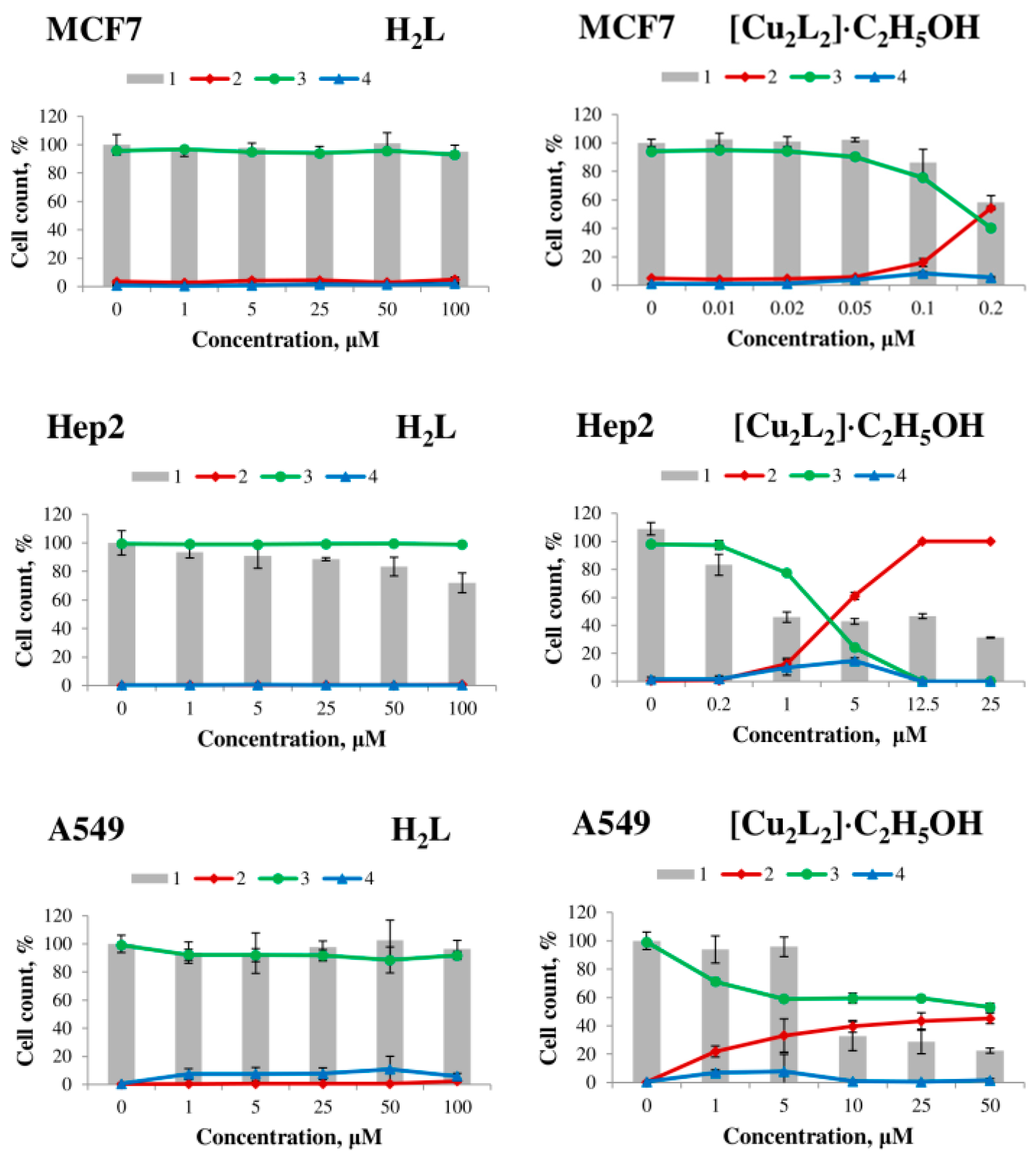
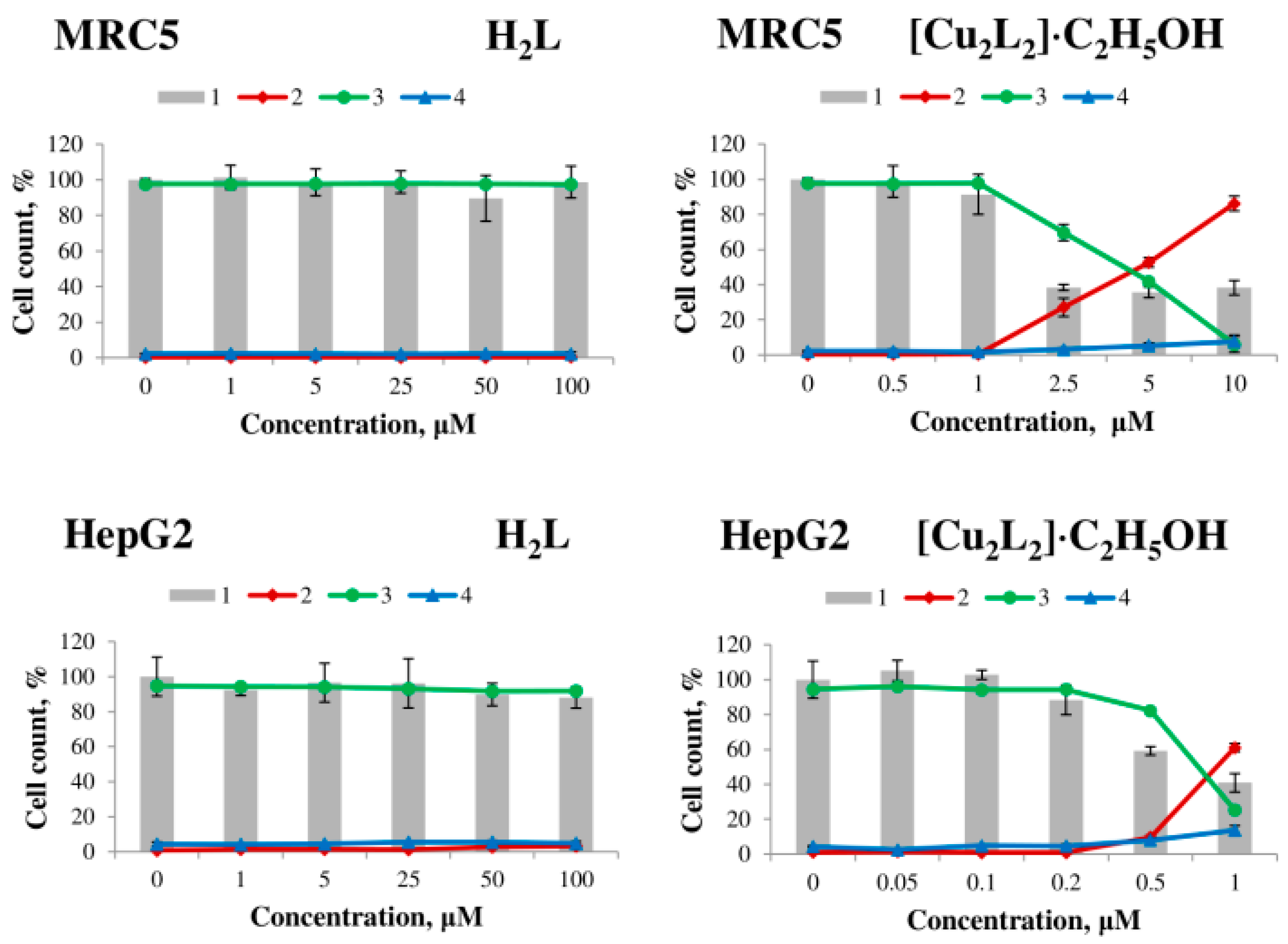
The effect of H2L ligand, Cu(NO3)2, and complex 1 on human cell viability was evaluated on the tumor cell lines MCF7 (breast adenocarcinoma), Hep2 (laryngeal carcinoma), A549 (lung carcinoma), HepG2 (hepatocellular carcinoma) and non-tumor MRC5 lung fibroblasts. The results of the study are presented in Table 3 in the form of the LC50 parameter, which is calculated as the concentration of the substance at which cell death is 50%, and Figure 7 and Figure S4.

Table 3.
Cytotoxic activity of the ligand and complex 1 expressed in terms of LC50 parameter and selectivity index (SI).
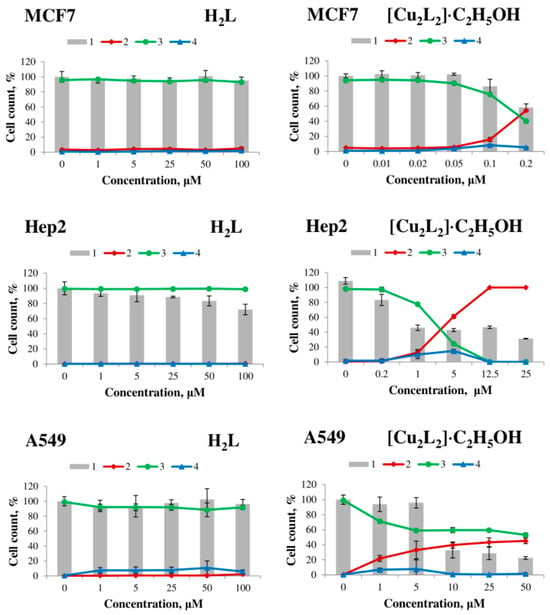
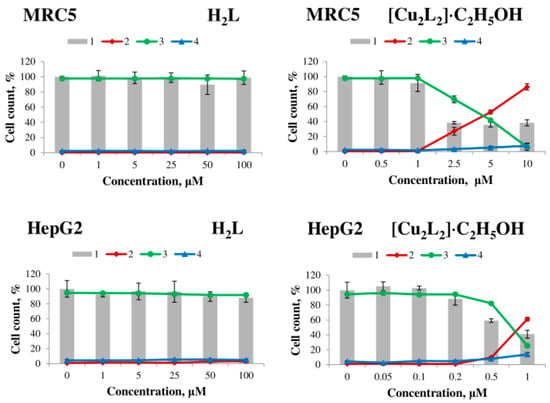
Figure 7.
Effect of H2L ligand (left) and [Cu2L2]∙C2H5OH complex (right) on the viability of MCF7, Hep2, A549 human tumor cells, MRC5 human non-tumor fibroblasts and HepG2 after 48 h exposure. 1—total number of cells, 2—dead cells, 3—live cells, 4—apoptotic cells.
The H2L ligand and copper (II) salt have no cytotoxic effect on all cell lines after 48 h of exposure in the concentration range from 1 to 100 μM, whereas the [Cu2L2]∙C2H5OH complex exhibits strong dose-dependent cytotoxic activity (Table 3, Figure 7). Complex 1 exhibited the highest activity on MCF7 cells (LC50 = 0.18 ± 0.03 μM) and the lowest activity on A549 cells (LC50 = 43.6 ± 2.6 μM).
Based on the obtained data on cytotoxic activity, the selectivity indices (SI, it is a ratio of the LC50 value for non-tumor MRC5 fibroblasts to the LC50 value for tumor cells) of the action of complex 1 against tumor cells were calculated, and the results are presented in Table 3. According to the literature, compounds with SI > 3 (or >9 in some sources) are considered promising for further studies as potential antitumor agents [47]. The investigated copper(II) complex shows high selectivity of action against HepG2 (SI = 5.1) and MCF7 (SI = 22.4) cells; for Hep2 cells, the activity of the complex is comparable to the activity on non-tumor fibroblasts MRC5, and for A549 cells SI < 1.
3. Materials and Methods
3.1. Synthetic Procedures
Synthesis of [Cu2L2]∙C2H5OH (1). A 0.176 g (0.50 mmol) portion of H2L was dissolved on heating in 15 mL of ethanol, and 0.060 g (0.25 mmol) of Cu(NO3)2∙3H2O was dissolved in 1 mL of H2O with the addition of 1 drop of 1N HNO3. The copper(II) solution was added to the hot ligand solution, and a dark green solution was formed, from which a precipitate of the same color quickly formed. The precipitate was filtered off the next day, washed several times with ethanol, and air-dried. The yield was 0.08 g (73% based on Cu).
For 1 found (%): C 35.3, H 2.6, N 18.7, Cu = 14.5.
For C26H22Cu2F12N12O, calculated (%): C 35.7, H 2.8, N 19.2, Cu 14.6.
3.2. Materials and Techniques
Commercially available reagents and solvents were used for the synthesis without additional purification. Methyl-5-(trifluoromethyl)pyrazol-3-yl-ketazine (H2L) was prepared according to the published method [40].
Elemental analysis for C, H, N was performed in the Laboratory of Microanalysis of the N.N. Vorozhtsov Novosibirsk Institute of Organic Chemistry SB RAS. Copper(II) content was analyzed complexometrically with EDTA disodium salt solution and murexide as an indicator after decomposition of the complex samples in concentrated acids (H2SO4 + HClO4).
3.3. Crystallography
X-ray powder diffraction data were collected on a Shimadzu XRD 7000 diffractometer (CuKα radiation (λ = 1.5406 Å), Ni filter, scintillation detector) at room temperature.
Single-crystal X-ray diffraction data were collected using the graphite monochromatized MoKα-radiation (λ = 0.71073 Å) at 150(2) K on an X8APEX Bruker Nonius diffractometer equipped with a 4K CCD area detector. The φ-scan technique was employed to measure intensities. Absorption corrections were applied empirically using the SADABS program [48]. Structures were solved by the direct methods of the difference Fourier synthesis and further refined by the full-matrix least squares method using the SHELXTL package(Veesion 2014/7) [49]. Atomic thermal parameters for non-hydrogen atoms were refined anisotropically (Table 4). The positions of hydrogen atoms were calculated corresponding to their geometrical conditions and refined using the riding model. The disordering of the CF3 group was introduced statistically with the restriction of C-F bonds with a length of 1.32(1) Å and a population of 80.8/19.2%. The introduction of the second position of the fluorine atoms also required a slight limitation of the thermal displacement parameters (ISOR).

Table 4.
Crystallographic data and conditions of the diffraction experiment for [Cu2L2((CH3)2SO)] (2).
3.4. Spectroscopy
IR absorption spectra were taken on an IRAfinity-1S spectrometer (Shimadzu, Kyoto, Japan) 4000–400 cm−1 at room temperature, and samples were prepared in KBr.
3.5. Magnetic Measurements
Magnetic properties were measured on a Quantum Design MPMS-XL SQUID magnetometer within the temperature range of 1.77–300 K at magnetic fields H = 0–10 kOe. To determine the paramagnetic component of the molar magnetic susceptibility χp(T), the contributions of core diamagnetism χd and possible ferromagnetism of micro-impurities χFM were subtracted from the measured values of the total susceptibility χ = M/H (M = magnetization). The temperature-independent contribution χd was calculated according to Pascal’s additive scheme. To check the presence of ferromagnetic contribution χFM, the field dependences M(H) and temperature dependences M(T) at different values of the magnetic field were measured, after which the total magnetization of the sample was decomposed into ferromagnetic and paramagnetic components. For the studied sample, the ferromagnetic contribution to the magnetization at H = 10 kOe did not exceed 2%.
3.6. Cytotoxic Activity
The human lung carcinoma cell line A549 was purchased from Biolot (Russia), non-tumor human lung fibroblasts MRC5, breast adenocarcinoma MCF7, laryngeal carcinoma Hep2, hepatocellular carcinoma HepG2—from State Scientific Center of Virology and Biotechnology “Vector”. Cell viability was assessed by double staining Hoechst 33342/propidium iodide (PI) [50]. Cells were seeded onto 96-well plates and cultured in DMEM (MRC5, MCF7, Hep2, HepG2) or DMEM/F12 (A549) medium in a CO2 incubator at 37 °C. After 24 h, drugs dissolved in ethanol were added at a concentration range of 0.01–100 μM and incubated for 48 h. Cells were stained with fluorescent dyes Hoechst 33342 (Sigma-Aldrich) and propidium iodide (Invitrogen) for 30 min at 37 °C. Imaging was performed on an IN CellAnalyzer 2200 instrument (GE Healthcare UK Limited Amersham Place Little Chalfont Buckinghamshire HP7 9NA UK) in automatic mode at 4 fields per well. The obtained images were analyzed using “InCellInvestigator” software(Version 1.5). The result is presented as the percentage of live, dead, and apoptotic cells in the whole population from three independent experiments ± standard deviation. The experimental dependence of the percentage of live cells on the concentration of the compound was approximated by a nonlinear function; the LC50 parameter was calculated as the concentration of the compound at which cell death is 50%.
4. Conclusions
Thus, this pioneering work showed that N-donor ligands based on bis(pyrazolyl)hydrazone containing fluorinated substituents are promising for the synthesis of Cu(II) molecular complexes with significant magnetic and biological activities. It has been shown that the system is not sensitive to the stoichiometry of synthesis and counterions but is sensitive to solvent.
A new copper(II) complex with ligand L2-, which is formed by the loss of two protons from methyl-5-(trifluoromethyl)pyrazol-3-yl-ketazine, was synthesized with the composition [Cu2L2]∙C2H5OH. Recrystallization of the isolated complex from DMSO yielded a single crystal of the complex of the composition [Cu2L2((CH3)2SO)]. Single-crystal X-ray diffraction data showed that the complex [Cu2L2((CH3)2SO)] has a binuclear structure. Ligand L2- is polydentate and is coordinated by both heterocyclic and acyclic nitrogen atoms.
Magnetochemical studies of the [Cu2L2]∙C2H5OH complex have shown that copper(II) ions arranged in Cu2N4 metallocycles form Cu2+-Cu2+ AF dimers with a relatively strong, J/kB ≈ 50 K exchange interaction. When the molecules of the complex are packed into a crystal structure, chemical bonds are formed between them, which are also capable of transferring the AF exchange interaction between the dimers.
The [Cu2L2]∙C2H5OH complex has a strong cytotoxic effect on MCF7, Hep2, HepG2, and MRC5 human cells and exhibits high selectivity for HepG2 (SI = 5.1) and MCF7 (SI = 22.4) tumor cell lines. The nature of this impact requires further research, which will be continued.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25179414/s1.
Author Contributions
O.G.S.: IR spectroscopy, data analysis, writing, and original draft preparation. T.D.M.: synthesis of complexes. D.N.B. and Y.S.K.: synthesis of ligand. N.V.K.: X-ray analysis. L.S.K.: cytotoxic activity study. A.N.L.: study of magnetic properties. L.G.L.: manuscript conception, writing and original draft preparation, data analysis, and interpretation. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was supported by the Ministry of Science and Higher Education of the Russian Federation (projects Nos. 121031700313-8, 121031700314-5). The investigation of biological properties was performed on the equipment of the Center for Collective Use “Proteomic Analysis” (FRC FTM) and supported by the Ministry of Science and Higher Education of the Russian Federation (project No. 122032200236-1).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors express their gratitude to the Ministry of Science and Higher Education of the Russian Federation for financial support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sharma, S.; Singh, V.; Vaishali; Kumar, R.; Jamra, R.; Banyal, N.; Jyoti. From 2011 to 2022: The development of pyrazole derivatives through the α,β-unsaturated carbonyl compounds. Heterocycl. Chem. 2024, 61, 232. [Google Scholar] [CrossRef]
- Zhao, X.; Verma, R.; Sridhara, M.B.; Kumar, K.S.S. Fluorinated azoles as effective weapons in fight against methicillin-resistance staphylococcus aureus (MRSA) and its SAR studies. Bioorg. Chem. 2024, 143, 106975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, C.; Zhang, N.; Fan, R.; Ye, Y.; Xu, J. Recent Advances in the Development of Pyrazole Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2023, 24, 12724. [Google Scholar] [CrossRef] [PubMed]
- Lusardi, M.; Spallarossa, A.; Brullo, C. Amino-Pyrazoles in Medicinal Chemistry: A Review. Int. J. Mol. Sci. 2023, 24, 7834. [Google Scholar] [CrossRef] [PubMed]
- Vahora, M.S.; Boruah, J.J.; Das, S.P. Synthesis and Pharmacological Activities of Pyrazole and Oxadiazole Derivatives: A Review. Russ. J. Org. Chem. 2023, 59, 846–869. [Google Scholar] [CrossRef]
- Ebenezer, O.; Shapi, M.; Tuszynski, J.A. A Review of the Recent Development in the Synthesis and Biological Evaluations of Pyrazole Derivatives. Biomedicines 2022, 10, 1124. [Google Scholar] [CrossRef]
- Karrouchi, K.; Radi, S.; Ramli, Y.; Taoufik, J.; Mabkhot, Y.N.; Al-aizari, F.A.; Ansar, M. Synthesis and Pharmacological Activities of Pyrazole Derivatives: A Review. Molecules 2018, 23, 134. [Google Scholar] [CrossRef]
- Faria, J.V.; Vegi, P.F.; Miguita, A.G.C.; Dos Santos, M.S.; Boechat, N.; Bernardino, A.M.R. Recently reported biological activities of pyrazole compounds. Bioorg. Med. Chem. 2017, 25, 5891–5903. [Google Scholar] [CrossRef]
- Ansari, A.; Ali, A.; Asif, M.; Shamsuzzaman, S. Review: Biologically active pyrazole derivatives. New J. Chem. 2017, 41, 16–41. [Google Scholar] [CrossRef]
- Bennani, F.E.; Doudach, L.; Cherrah, Y.; Ramli, Y.; Karrouchi, K.; Ansar, M.; Faouzi, M.E.A. Overview of recent developments of pyrazole derivatives as an anticancer agent in different cell line. Bioorg. Chem. 2020, 97, 103470. [Google Scholar] [CrossRef]
- Khan, M.F.; Alam, M.M.; Verma, G.; Akhtar, W.; Akhter, M.; Shaquiquzzaman, M. The therapeutic voyage of pyrazole and its analogs: A review. Eur. J. Med. Chem. 2016, 120, 170–201. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Verma, S.K.; Rakesh, K.P.; Girish, Y.R.; Ashrafizadeh, M.; Sharath Kumar, K.S.; Rangappa, K.S. Pyrazole-based analogs as potential antibacterial agents against methicillin-resistance staphylococcus aureus (MRSA) and its SAR elucidation. Eur. J. Med. Chem. 2021, 212, 113134. [Google Scholar] [CrossRef] [PubMed]
- Mykhailiuk, P.K. Fluorinated Pyrazoles: From Synthesis to Applications. Chem. Rev. 2021, 121, 1670–1715. [Google Scholar] [CrossRef] [PubMed]
- Clemett, D.; Goa, K.L. Celecoxib: A Review of its Use in Osteoarthritis, Rheumatoid Arthritis and Acute Pain. Drugs 2000, 59, 957–980. [Google Scholar] [CrossRef]
- Abdelhaleem, E.F.; Kassab, A.E.; El-Nassan, H.B.; Khalil, O.M. Recent advances in the development of celecoxib analogs as anticancer agents: A review. Arch. Pharm. 2022, 355, e2200326. [Google Scholar] [CrossRef]
- Link, J.O.; Rhee, M.S.; Tse, W.C.; Zheng, J.; Somoza, J.R.; Rowe, W.; Begley, R.; Chiu, A.; Mulato, A.; Hansen, D.; et al. Clinical targeting of HIV capsid protein with a long-acting small molecule. Nature 2020, 584, 614–618. [Google Scholar] [CrossRef]
- Liu, H.-N.; Zhu, Y.; Chi, Y.; Sun, F.-F.; Shan, L.-S.; Wang, Y.-T.; Dai, B. Synthetic approaches and application of representative clinically approved fluorine-enriched anti-cancer medications. Eur. J. Med. Chem. 2024, 276, 116722. [Google Scholar] [CrossRef]
- Bazhin, D.N.; Kudyakova, Y.S.; Onoprienko, A.Y.; Slepukhin, P.A.; Burgart, Y.V.; Saloutin, V.I. Synthesis and tuberculostatic activity of functionalized pyrazoles derived from (trifluoromethyl)pyrazole containing a hydrazone group. Chem. Heterocycl. Compd. 2017, 53, 1324–1329. [Google Scholar] [CrossRef]
- Johnson, B.M.; Shu, Y.-Z.; Zhuo, X.; Meanwell, N.A. Metabolic and Pharmaceutical Aspects of Fluorinated Compounds. J. Med. Chem. 2020, 63, 6315–6386. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Shayanfar, A.; Soltani, B. Copper pyrazole complexes as potential anticancer agents: Evaluation of cytotoxic response against cancer cells and their mechanistic action at the molecular level. Coord. Chem. Rev. 2024, 498, 215459. [Google Scholar] [CrossRef]
- Castroa, I.; Barrosa, W.P.; Calatayuda, M.L.; Lloret, F.; Marinod, N.; De Munnod, G.; Stumpfc, H.O.; Ruiz-Garcíaa, R.; Julve, M. Dicopper(II) pyrazolenophanes: Ligand effects on their structures and magnetic properties. Coord. Chem. Rev. 2016, 315, 135–152. [Google Scholar] [CrossRef]
- Rasika Dias, H.V.; Lovely, C.J. Carbonyl and Olefin Adducts of Coinage Metals Supported by Poly(pyrazolyl)borate and Poly(pyrazolyl)alkane Ligands and Silver Mediated Atom Transfer Reactions. Chem. Rev. 2008, 108, 3223–3238. [Google Scholar] [CrossRef]
- Pettinari, C.; Tabacaru, A.; Galli, S. Coordination polymers and metal–organic frameworks based on poly(pyrazole)-containing ligands. Coord. Chem. Rev. 2016, 307, 1–31. [Google Scholar] [CrossRef]
- Doidge, E.D.; Roebuck, J.W.; Healy, M.R.; Tasker, P.A. Phenolic pyrazoles: Versatile polynucleating ligands. Coord. Chem. Rev. 2015, 288, 98–117. [Google Scholar] [CrossRef]
- Halcrow, M.A. Spin-Сrossover Materials Properties and Applications; John Wiley & Sons Ltd.: Chichester, UK, 2013; 562p. [Google Scholar]
- Pandolfo, L.; Pettinari, C. Trinuclear copper(ii) pyrazolate compounds: A long story of serendipitous discoveries and rational design. CrystEngComm. 2017, 19, 1701–1720. [Google Scholar] [CrossRef]
- Dias, H.V.R.; Singh, S.; Campana, C.F. Toluene-Sandwiched Trinuclear Copper(I) and Silver(I) Triazolates and Phosphine Adducts of Dinuclear Copper(I) and Silver(I) Triazolates. Inorg. Chem. 2008, 47, 3943–3945. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-W.; He, L.-H.; Ju, P.; Chen, J.-L.; Liu, S.-J.; Wen, H.-R. Mechanochromic luminescent materials of bimetallic Cu(i) complexes showing thermally activated delayed fluorescence. J. Mater. Chem. C 2020, 8, 16160–16167. [Google Scholar] [CrossRef]
- Scatena, R.; Massignani, S.; Lanza, A.E.; Zorzi, F.; Monari, M.; Nestola, F.; Pettinari, C.; Pandolfo, L. Synthesis of Coordination Polymers and Discrete Complexes from the Reaction of Copper(II) Carboxylates with Pyrazole: Role of Carboxylates Basicity. Cryst. Growth Des. 2022, 22, 1032–1044. [Google Scholar] [CrossRef]
- Elguero, J.; Yranzo, G.I.; Laynez, J.; Jimenez, P.; Menendez, M.; Catalan, J.; De Paz, J.L.G.; Anvia, F.; Taft, R.W. Effect of the replacement of a methyl by a trifluoromethyl group on the acid-base properties of pyrazoles. Org. J. Chem. 1991, 56, 3942–3947. [Google Scholar] [CrossRef]
- Wang, J.-G.; Liu, Y.; Liu, C.-M.; Chen, J.-H.; Yang, G. An efficient mixed-valence copper pyrazolate catalyst for the conversion of carbon dioxide and epoxides into cyclic carbonates. Dalton Trans. 2023, 52, 9275–9281. [Google Scholar] [CrossRef]
- Mathivatanan, L.; Rogez, G.; Amor, N.B.; Robert, V.; Raptis, R.G.; Boudalis, A. Origin of Ferromagnetism and Magnetic Anisotropy in a Family of Copper(II) Triangles. Chem. Eur. J. 2020, 26, 12769–12784. [Google Scholar] [CrossRef]
- Bazhin, D.N.; Kudyakova, Y.S.; Slepukhin, P.A.; Burgart, Y.V.; Malysheva, N.N.; Kozitsina, A.N.; Ivanova, A.V.; Bogomyakov, A.S.; Saloutin, V.I. Dinuclear copper(II) complex with novel N,N’,N”,O-tetradentate Schiff base ligand containing trifluoromethylpyrazole and hydrazone moieties. Mendeleev Commun. 2018, 28, 202–204. [Google Scholar] [CrossRef]
- Nicola, C.D.; Marchetti, F.; Tombesi, A.; Xhafa, S.; Campitelli, P.; Moroni, M.; Galli, S.; Pettinari, R.; Pettinari, C. Antibacterial activity of copper pyrazolate coordination polymers. New J. Chem. 2023, 47, 19047–19056. [Google Scholar] [CrossRef]
- Xhafa, S.; Olivieri, L.; Di Nicola, C.; Pettinari, R.; Pettinari, C.; Tombesi, A.; Marchetti, F. Copper and Zinc Metal–Organic Frameworks with Bipyrazole Linkers Display Strong Antibacterial Activity against Both Gram+ and Gram− Bacterial Strains. Molecules 2023, 28, 6160. [Google Scholar] [CrossRef] [PubMed]
- Bazhin, D.N.; Kudyakova, Y.S.; Edilova, Y.O.; Burgart, Y.V.; Saloutin, V.I. Fluorinated 1,2,4-triketone analogs: New prospects for heterocyclic and coordination chemistry. Russ. Chem. Bull. 2022, 71, 1321–1341. [Google Scholar] [CrossRef]
- Kudyakova, Y.S.; Onoprienko, A.Y.; Slepukhin, P.A.; Burgart, Y.V.; Saloutin, V.I.; Bazhin, D.N. Fluorine-Containing Furan-3(2Н)-Ones in Reactions with Binucleophiles: CF3 vs C2F5. Chem. Heterocycl. Compd. 2019, 55, 517–522. [Google Scholar] [CrossRef]
- Edilova, Y.O.; Kudyakova, Y.S.; Kiskin, M.A.; Burgart, Y.V.; Saloutin, V.I.; Bazhin, D.N. Expanding 1,2,4-triketone toolbox for use as fluorinated building blocks in the synthesis of pyrazoles, pyridazinones and β-diketohydrazones. J. Fluor. Chem. 2022, 253, 109932. [Google Scholar] [CrossRef]
- Bazhin, D.N.; Chizhov, D.L.; Röschenthaler, G.-V.; Kudyakova, Y.S.; Burgart, Y.V.; Slepukhin, P.A.; Saloutin, V.I.; Charushin, V.N. A concise approach to CF3-containing furan-3-ones, (bis)pyrazoles from novel fluorinated building blocks based on 2,3-butanedione. Tetrahedron Lett. 2014, 55, 5714–5717. [Google Scholar] [CrossRef]
- Bazhin, D.N.; Kudyakova, Y.S.; Röschenthaler, G.-V.; Burgart, Y.V.; Slepukhin, P.A.; Isenov, M.L.; Saloutin, V.I.; Charushin, V.N. A Convenient Approach to CF3-Containing N-Heterocycles Based on 2-Methoxy-2-methyl-5-(trifluoromethyl)furan-3(2H)-one. Eur. J. Org. Chem. 2015, 23, 5236–5245. [Google Scholar] [CrossRef]
- Kats, S.V.; Varzatskii, O.A.; Penkova, L.V.; Vologzhanina, A.V.; Novikov, V.V.; Lebed, E.G.; Voloshin, Y.Z. On a Way to New Types of the Hybrid Polyazomethine-Pyrazolate Metal Pseudomacrobicyclic Complexes: The Synthesis and Structure of Their Ligand Synthones. Macroheterocycles 2014, 7, 34–39. [Google Scholar] [CrossRef][Green Version]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Bleaney, B.; Bowers, K.D. Anomalous paramagnetism of copper acetate. Proc. R. Soc. Lond. 1952, A214, 451–465. [Google Scholar] [CrossRef]
- Hatfield, W.E. New magnetic and structural results for uniformly spaced, alternatingly spaced, and ladder-like copper (II) linear chain compounds. Appl. J. Phys. 1981, 52, 1985–1990. [Google Scholar] [CrossRef]
- Hall, J.W.; Marsh, W.E.; Weller, R.R.; Hatfield, W.E. Exchange coupling in the alternating-chain compounds catena-di-mu-chloro-bis(4-methylpyridine)copper(II), catena-di-mu-bromobis(N-methylimidazole)copper(II), catena-[hexanedione)bis(thiosemicarbazonato)]copper(II), and catena-[octanedione-bis(thiosemicarbazonato)]copper(II). Inorg. Chem. 1981, 20, 1033–1037. [Google Scholar] [CrossRef]
- Lavrenova, L.G.; Sukhikh, T.S.; Glinskaya, L.A.; Trubina, S.V.; Zvereva, V.V.; Lavrov, A.N.; Klyushova, L.S.; Artem’ev, A.V. Synthesis, Structure, and Magnetic and Biological Properties of Copper(II) Complexes with 1,3,4-Thiadiazole Derivatives. Int. J. Mol. Sci. 2023, 24, 13024. [Google Scholar] [CrossRef] [PubMed]
- Indrayanto, G.; Putra, G.S.; Suhud, F. Chapter Six—Validation of in-vitro bioassay methods: Application in herbal drug research. Profiles Drug Subst. Excip. Relat. Methodol. 2021, 46, 273–307. [Google Scholar] [CrossRef]
- APEX2 (version 2012.2-0), SAINT (version 8.18c), and SADABS (version 2008/1). In Bruker Advanced X-ray Solutions; Bruker AXS Inc.: Madison, WI, USA, 2000–2012.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Eremina, J.A.; Lider, E.V.; Kuratieva, N.V.; Samsonenko, D.G.; Klyushova, L.S.; Sheven’, D.G.; Trifonov, R.E.; Ostrovskii, V.A. Synthesis and crystal structures of cytotoxic mixed-ligand copper(II) complexes with alkyl tetrazole and polypyridine derivatives. Inorganica Chim. Acta 2021, 516, 120169. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).