Abstract
Determining the estrous cycle stages in mice is essential for optimizing breeding strategies, synchronizing experimental timelines, and facilitating studies in behavior, drug testing, and genetics. It is critical for reducing the production of genetically unmodified offspring in the generation and investigation of genetically modified animal models. An accurate detection of the estrus cycle is particularly relevant in the context of the 3Rs—Replacement, Reduction, and Refinement. The estrous cycle, encompassing the reproductive phases of mice, is key to refining experimental designs and addressing ethical issues related to the use of animals in research. This study presents results from two independent laboratories on the efficacy of the Mouse Estrus Detector (MED) from ELMI Ltd. (Latvia) for the accurate determination of the estrus phase. The female mice of five strains/stocks (CD1, FVB/N, C57Bl6/J, B6D2F1, and Swiss) were used. The results showed that the MEDProTM is a low-traumatic, simple, rapid, and painless method of estrus detection that supports the principles of the 3Rs. The use of the MEDProTM for estrus detection in mice caused minimal stress, enhanced mating efficiency, facilitated an increase in the number of embryos for in vitro fertilization, and allowed the production of the desired number of foster animals.
1. Introduction
The laboratory mouse (Mus musculus, L., 1758) is an important biological object for modern research in various fields of medicine and biology because of its relative similarity with the human genome and its high grade of conservation on molecular and cellular mechanisms associated with basic physiological systems (cardiovascular, nervous, endocrine, immune, etc.) [1,2,3]. The importance of the mouse as an object of biomedical research is also determined by the availability of effective technologies for the modification of the mouse genome, and the short reproductive period [4,5,6].
Identification of the estrous stages is a key step in various mouse genome bioengineering technologies and manipulations with mouse embryos. It is important to accurately stage pregnancies for evaluating prenatal pharmacological treatments and embryonic toxicity [7,8,9,10,11,12]. Additionally, determining the stages of the estrous cycle is of significant importance in diverse biomedical research applications, particularly in the context of pharmacodynamic investigations [13,14,15]. The distinct phases of the female sexual cycle exert a profound influence on the experimental process and outcomes, playing a critical role, especially in neuroscience research [16,17,18]. Efficient detection of estrus in female mice also allows efficient crossbreeding, facilitating the acquisition of pseudo-fertile (foster) mice for embryo transfer procedures [19]. This, in turn, significantly reduces the number of animals required for experiments, an important aspect of biomedical research [13,20,21]. Moreover, precise detection of the estrous cycle in mice refines experimental procedures, minimizing stress associated with unnecessary mating attempts and reducing the discomfort experienced by the animals, contributing to their well-being [19,22]. This approach ensures that experiments are conducted at appropriate times, thereby reducing variability in experimental outcomes. The resulting refinement contributes to the production of more reliable and reproducible data, enhancing the overall quality of scientific research [16,18,23,24]. Therefore, accurate determination of the estrous cycle in mice not only promotes ethical considerations in animal research but also optimizes the efficiency of experimental designs. This alignment with the 3R principles—Replacement, Reduction, and Refinement—underscores the commitment to ethical and efficient animal use in research practices [20,25].
The laboratory mouse is a polyestrous species with a reproductive cycle length of 4–5 days. An estrous cycle typically comprises four main phases, each characterized by specific hormonal changes and physiological alterations: (1) proestrus (PE), (2) estrus (E), (3) metestrus (ME), and (4) diestrus (DE), that are constantly repeated. The regulation is based on the hypothalamic–pituitary–gonadal (HPG) axis, where gonadotropin-releasing hormone (GnRH) from the hypothalamus initiates the cycle by stimulating the biosynthesis and secretion of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) in the anterior lobe of the pituitary gland [26]. During proestrus, increased FSH levels activate the cAMP-protein kinase A (PKA) pathway in granulosa cells, enhancing cell proliferation and estrogen production by increasing aromatase (Cyp19a1) gene expression [27]. Estrogen binds to its receptors (ERα and ERβ), promoting the transcription of genes crucial for follicular development. Elevated estrogen levels trigger a LH surge through positive feedback from the hypothalamus and pituitary gland. The LH surge activates the PI3K-Akt pathway in theca and granulosa cells, leading to ovulation by promoting oocyte maturation and follicular rupture through the activation of proteolytic enzymes during the estrus stage [28]. Post-ovulation, during metestrus, the corpus luteum forms and secretes progesterone, which maintains the uterine lining (endometrium) and prepares it for implantation of a potential embryo. Key regulatory pathways include cAMP-PKA, which induces steroidogenic acute regulatory protein (StAR) gene activation, which mediates progesterone synthesis [29]. Progesterone binds to its receptors (PR-A and PR-B), activating transcription of genes involved in uterine maintenance and immunomodulation, such as the orphan nuclear receptor NR4A1, integrins, and vascular endothelial growth factor (VEGF) [30]. Transforming growth factor-beta (TGF-β) signaling and cytokine regulation are involved in tissue remodeling and immune regulation [31]. In diestrus, elevated progesterone levels continue to maintain the uterine environment and exert negative feedback on the HPG axis, reducing GnRH, FSH, and LH levels to prevent the development of new follicles. The interplay between these hormones and molecular pathways, including cAMP-PKA, PI3K-Akt, estrogen receptor, progesterone receptor, and TGF-β signaling, provides precise regulation of follicular growth, ovulation, and preparation for potential pregnancy [32]. Various methods for determining these estrous stages have been reported [33,34,35], including the following: (1) visual inspection of vaginal opening; (2) cytological analysis of vaginal smears/lavages; (3) quantitative measurement of sex hormones (including estrogen and progesterone) in blood plasma [36]; (4) biochemical analysis of urine and feces [37]; and (5) measurement of the impedance of the vaginal mucosa [33].
Visual examination of the vaginal opening, which varies from maximally dilated during estrus to constricted during diestrus, is a straightforward but subjective and severely limited method. Notably, significant variability exists between various strains of laboratory mice concerning the appearance of the vagina during different estrous cycle stages [34,38]. The analysis of sex hormones in blood plasma and the biochemical examination of urine and feces provide a more precise determination of the estrous cycle stage. However, these methods are time-consuming and require the presence of qualified personnel and expensive equipment. In addition, daily blood sampling for biochemical analysis results in a reduction in circulating blood volume, which is painful and stressful for animals. Therefore, it is not suitable for mass screening and monitoring of animals. The accuracy of detection of the sex hormones in urine and feces is limited and strongly depends on the particular mouse strain employed in the experiment [39]. Cytological examination of the vaginal smear to identify the predominant cell types present during different stages of the estrous cycle is the most accurate method, and it is accepted as the “gold standard” for determining distinct stages of the reproduction cycle in rodents [35,40,41]. Despite its precision, this method is time-consuming and requires the expertise of qualified personnel. Consequently, it is also not suitable for large-scale animal screening and monitoring. Measurement of the impedance of the epithelial cell layer of the vaginal mucosa to determine the estrous cycle stages in rodents is the simplest, most cost-effective method for obtaining data on ovulation [42,43]. Historically, the development of detectors to determine ovulation time by assessing the impedance of the vaginal walls in farm animals traces back to 1961. During this period, the USSR issued a certificate of authorship №736350/30-1 for the “Method for determining the optimal time of inseminating cows”, followed by the reception of patent USSR No. 178602 for a device invented for measuring the electrical resistance of the vaginal walls of cows. The application of vaginal impedance measurements was introduced for the first time in laboratory animals in 1977, specifically rats [44] and guinea pigs [45]. Commercial devices engineered to measure impedance in laboratory rodents were subsequently developed by Muromachi Kikai Co., Ltd. (Chūō, Japan) and designated as rat vaginal impedance checkers MK-11 and MK-12. These devices proved remarkable effectiveness in determining the estrus in rats. However, the measurement applicability in mice has been neither guaranteed nor verified by the manufacturer (muromachi.com).
In this study, we present an integrated and independent comprehensive analysis of a novel Mouse Estrus Detector (MED) MEDProTM device developed by ELMI Ltd. (Riga, Latvia) [46], which is specifically designed for the quantification of vaginal mucosa active resistance in mice.
2. Results
2.1. Comparison of Vaginal Smear Cytology, Vaginal Opening, and AR Value
To evaluate the effectiveness of the MEDProTM and determine an active resistance (AR) value for detecting estrus stage in mouse cycles, we conducted a comprehensive series of experiments involving CD-1, FVB/N, and C57Bl6/J female mice (n = 10 for each strain). We simultaneously performed observational assessments of vaginal opening, AR measurements, and vaginal smear sample cytology on the same animals over an 11-day period, with daily evaluations for each mouse in the respective order (Table S1).
We collected a total of 330 vaginal smear samples from females (11 vaginal smears per animal) of the investigated strains. Notably, each strain demonstrated different estrous cycle regularity, with variations observed in the continuous progression from proestrus to estrus to metestrus and diestrus stages. We observed a cycle regularity of approximately 60% for CD-1, 80% for FVB/N, and 40% for C57Bl6/J strains.
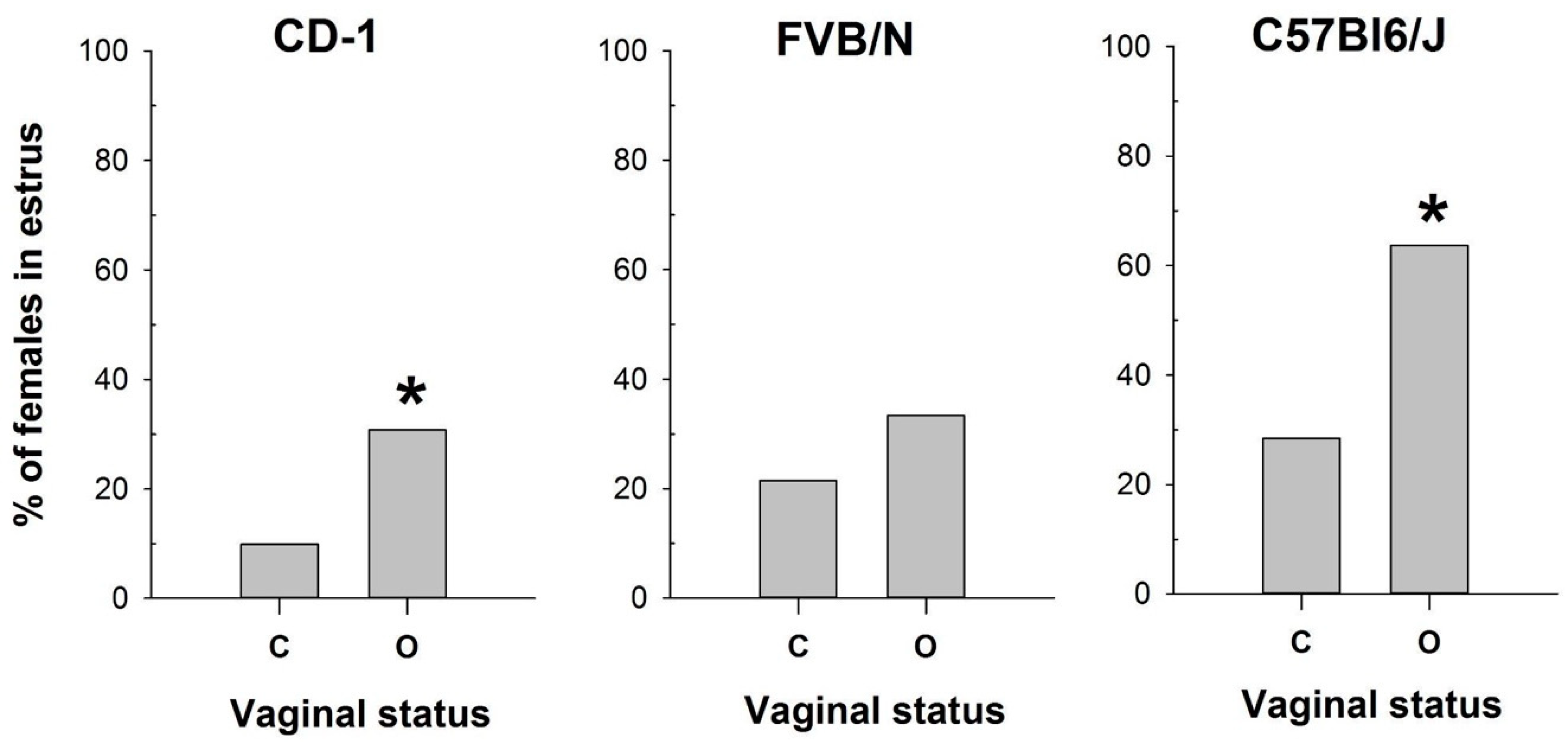
Comparison of vaginal opening assessment with vaginal smear analysis revealed pronounced differences depending on the mouse strain examined (Figure 1). While vaginal opening observations were made at each estrous stage of the investigated strains, the percentages of estrus verification varied. Specifically, vaginal opening correlated with estrus determination in 63% of cases for the CD-1 strain and 39% for the FVB/N and C57Bl6/J strains (Figure S1).
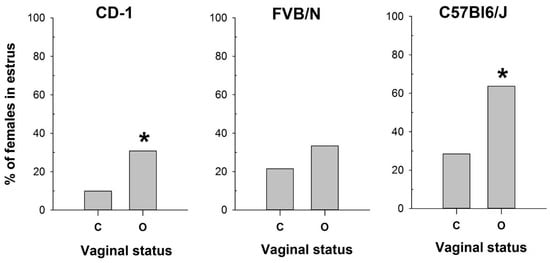
Figure 1.
The proportion of female mice with a cytologically estimated estrus with a closed (C) or open (O) vagina: CD-1 (n = 71 and n = 39 for C and O, respectively); FVB/N (n = 79 and n = 31 for C and O, respectively); C57Bl6/J (n = 88 and n = 22 for C and O, respectively). *—p < 0.05 (Chi-square test).
Conversely, the vaginal opening was detected in 33% of metestrus and 26% of diestrus cycles in CD-1 and in 35% of metestrus and 8% of diestrus cycles in the FVB/N strain (Figure S1). In contrast, C57Bl/6J mice exhibited vaginal opening in only 8% of metestrus cycles and 0% of diestrus cycles (Figure S1). Based on the cytological determination (used as the gold standard) of the estrus stage in the mouse strains tested, we compared the proportions of mice in estrus among females with open and closed vaginas (Figure 1) using the Chi-square test. The comparison showed a significant difference in these proportions for CD-1 (χ2 = 6.133, df = 1, p = 0.013) and C57Bl6/J (χ2 = 8.067, df = 1, p = 0.005), but not for FVB/N (χ2 = 1.611, df = 1, p = 0.204). Thus, this method was ineffective in determining estrus in FVB/N females.
The obtained results highlight the high variability in the efficacy of vaginal opening observation across mouse strains and underscore the importance of utilizing complementary methods for accurate estrus determination.
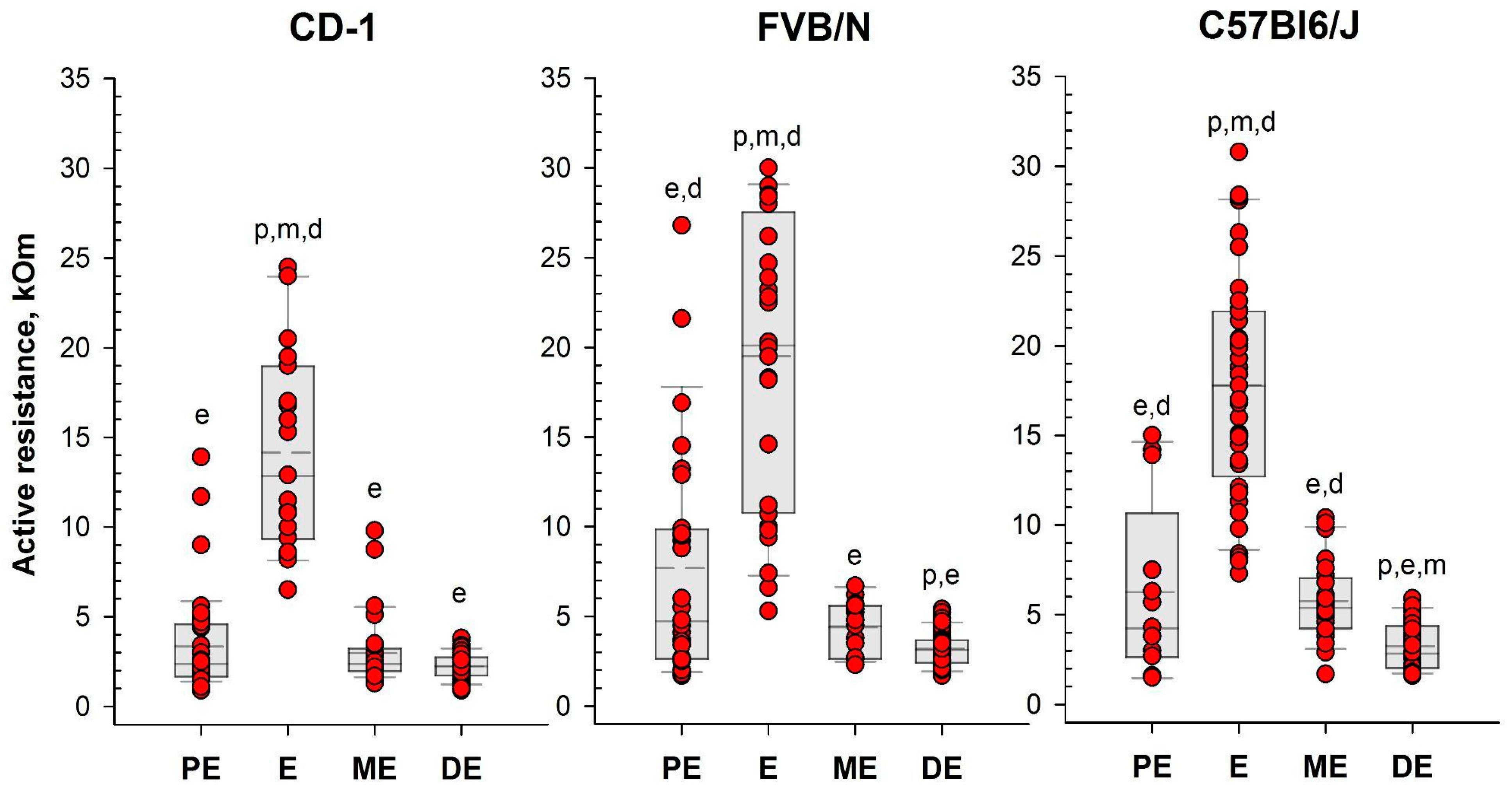
Following the assessment of vaginal opening and prior to vaginal smear sampling, we performed daily measurements of vaginal AR in the investigated mice using the MEDProTM device (Figure 2 and Figure 3, Table S1). A two-way mixed model analysis of variance (ANOVA) was conducted to analyze the data. The results revealed significant differences between estrous cycle stages in the value of AR for each strain: CD-1 (F(3,44) = 13.53; p = 0.000); FVB/N (F(3,39) = 36.62; p = 0.000); and C57Bl6/J (F(3,50) = 48.22; p = 0.000). In female mice of all strains, AR was significantly higher during estrus compared to other stages of the cycle (p < 0.001). Simultaneously, the AR of the vaginal wall was at its lowest values in diestrus (Figure 4 and Figure S2, and Table S1).

Figure 2.
Photo of the mouse estrus detector MEDProTM device (left panel) and a magnified image of its probe (right panel).
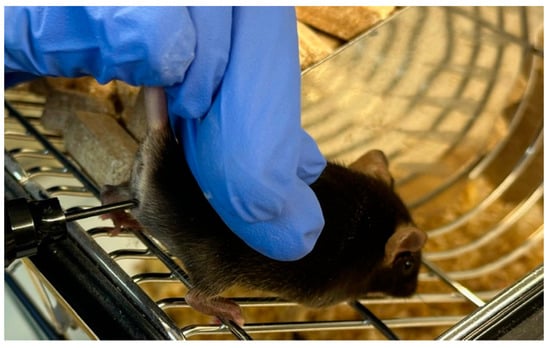
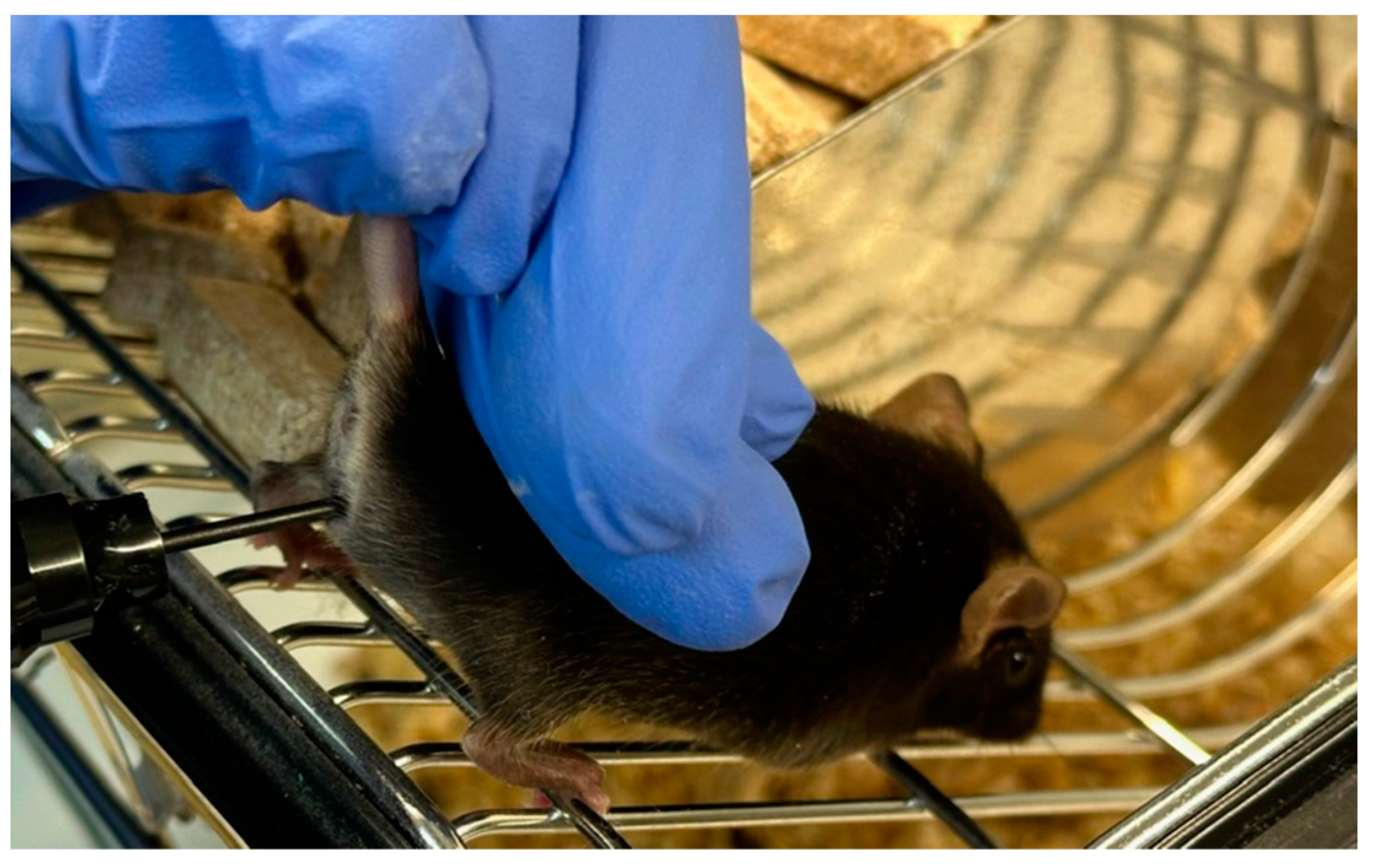
Figure 3.
Measurement of vaginal wall active resistance in female mice by using the MEDProTM device.
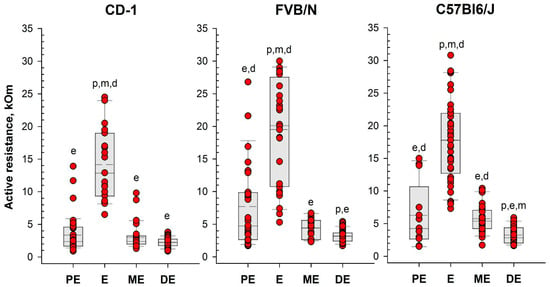
Figure 4.
Vaginal active resistance values as a function of the stage of the estrous cycle (PE—proestrus, E—estrus, ME—metestrus, DE—diestrus), determined by vaginal smear in female mice of different strains. Single-value plots show each observation’s value. The box represents the 25th and 75th percentiles and illustrates the median (solid line) and the mean (dashed line). The whiskers show the 10th and 90th percentiles. Letters indicate a significant difference (p < 0.05, Bonferroni test for multiple comparisons) as follows: p—proestrus, e—estrus, m—metestrus, d—diestrus.
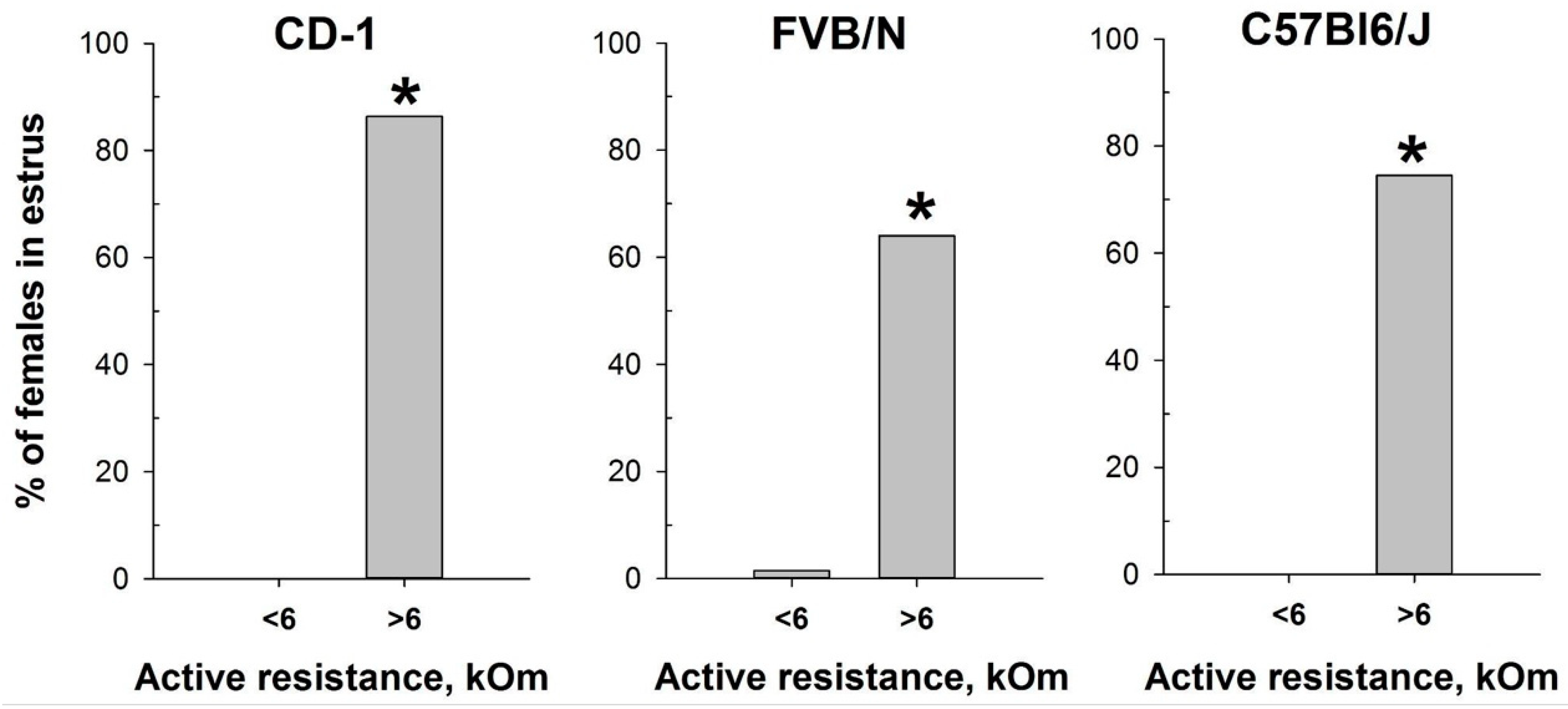
Based on the determination of vaginal wall AR in females from investigated mouse strains, an AR value of 6 kOm was chosen as the threshold for identifying estrus. The actual probability of detecting the estrus in the next round of measurements, given AR values equal to or greater than 6 kOm, was calculated as follows: 86% for CD-1, 64% for FVB/N, and 76% for C57Bl/6J mouse strains. Comparison of the proportion of mice in estrus among females with AR < 6 kOm and AR > 6 kOm (Figure 5) showed highly significant differences in all strains studied: CD-1 (χ2 = 85.924, df = 1, p < 0.001), FVB/N (χ2 = 53.539, df = 1, p < 0.001), and C57Bl6/J (χ2 = 64.744, df = 1, p < 0.001).
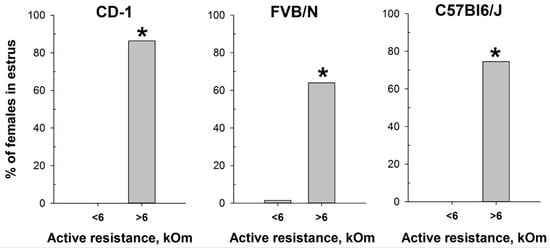
Figure 5.
Proportion of female mice with cytologically estimated estrus with AR < 6 kOm or AR > 6 kOm: CD-1 (n = 88 and n = 22, respectively); FVB/N (n = 68 and n = 42, respectively); C57Bl6/J (n = 55 and n = 55). *—p < 0.001 (Chi-square test).
However, given the substantial interindividual (interstrain) fluctuations observed in vaginal AR (Table S1), it is crucial to emphasize that the determination of the AR threshold for each particular mouse strain or hybrid should be established experimentally before integrating MedProTM into laboratory protocols.
2.2. Estimation of Mating Efficiency for Female Mice with Different Vaginal AR Values
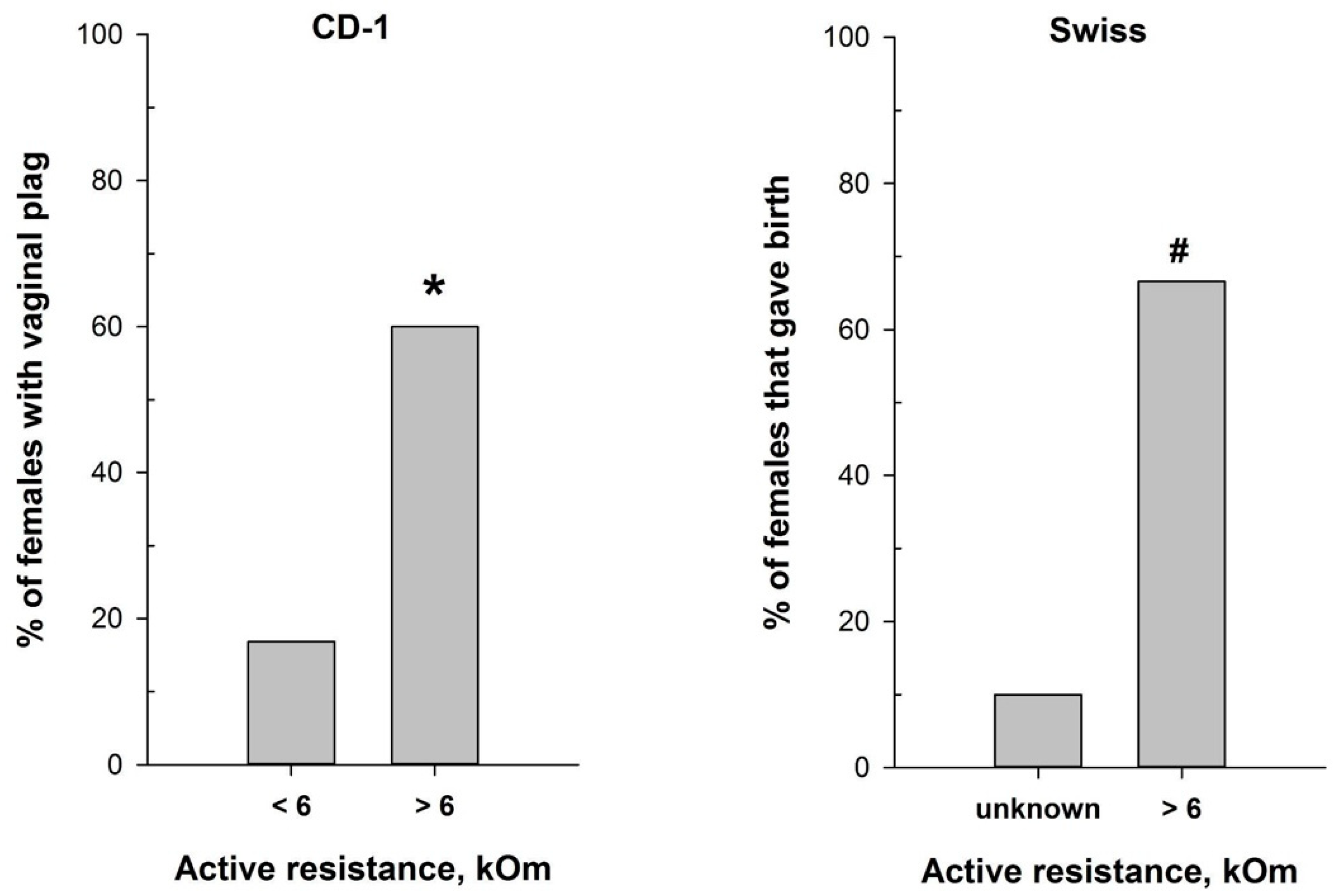
The reliable correlation between the MEDProTM measurements and vaginal smear cytological results in detecting the estrus stage prompted us to test the device for refining mating efficiency. We assessed mating efficiency by examining the presence of the copulatory plug after overnight pairing of CD-1 male mice with females characterized by different values of vaginal AR determined with MEDProTM. As anticipated, the highest presence of the copulatory plug (60%) was detected after overnight pairing of CD-1 male mice with females with AR values exceeding 6 kOm (χ2 = 17.620, df = 1, p < 0.001) (Figure 6). This corresponds with the estrus and, putatively, partly with the proestrus stages, which align with the ovulation phase. Notably, the vaginal plug in mice can sometimes be small and lie deeply in the vagina, or it can dissolve and become not visible [47]. Therefore, it is still possible that mating occurred in a higher number of animals than observed.
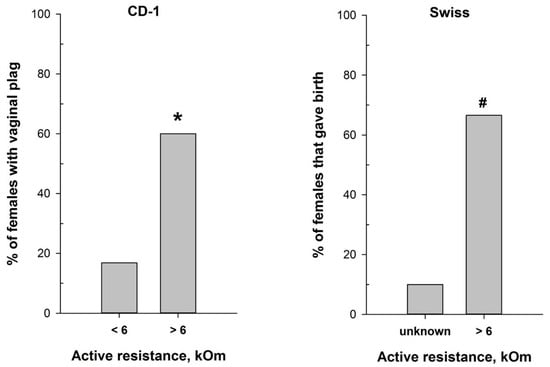
Figure 6.
Mating efficiency in female mice with different values of vaginal active resistance after overnight pairing with male. (left panel)—proportion of CD-1 females with vaginal plug (n = 97 for AR < 6 kOm; n = 25 for AR > 6 kOm); (right panel)—proportion of Swiss females that gave birth (n = 10 for ‘unknown”; n = 9 for AR > 6 kOm). *—p < 0.001 (Chi-square test); #—p < 0.001 (Fisher’s exact test).
Hence, we additionally evaluated the significance of the MEDProTM application for successful timed pregnancies, using male and female Swiss mice. The proportion of Swiss females that gave birth (Figure 6) was significantly higher (p = 0.02, Fisher’s exact test) in the group of females with detected AR > 6 kOm (6 out of 9) compared to the “Undetected females” group (1 out of 10).
2.3. MEDProTM Application for Determining Female Mice Superovulation
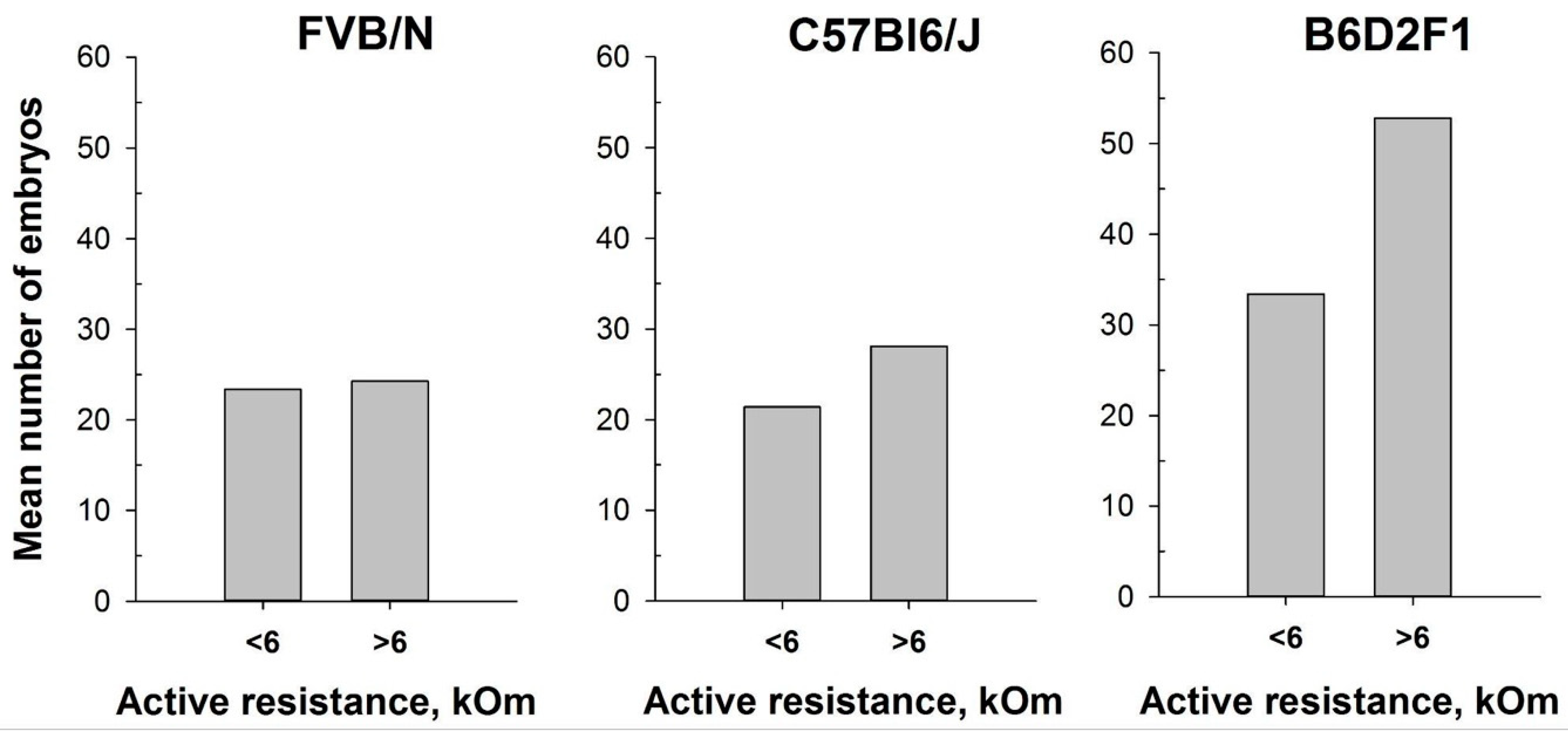
In our attempt to optimize the superovulation protocol, we investigate the impact of the estrous stages on superovulation in mature female mice. The method of mouse superovulation, initially described in 1956 [39], has been employed in transgenesis since the 1980s, enabling the generation of a substantial number of oocytes from a limited number of female mice [48]. While natural ovulation typically yields approximately 6–10 oocytes per female, superovulation can significantly increase the number, often reaching up to 60 oocytes [49]. This process involves the intraperitoneal injection of gonadotropin hormones, which mimic natural mouse hormones and initiate the maturation of numerous oocyte follicles. It is noteworthy that superovulation, akin to normal ovulation, triggers female receptivity and enhances its attraction for males. In this study, we utilized the MEDProTM device to investigate two inbred FVB/N and C57Bl6/J strains and one hybrid strain, B6D2F1 (C57Bl6/J × DBA). Similar to previous experiments, an AR value exceeding 6 kOm was used as the threshold to determine the estrus stage in mice. Our results showed that the intraperitoneal injections of gonadotropin hormones during the estrus stage (AR > 6 kOm) increased the overall number of obtained oocytes. Remarkably, while accurate determination of the estrous cycle with MEDProTM had a less pronounced impact on the FVB/N and C57Bl6/J inbred strains (17% and 33%, respectively), it resulted in an over 50% increase in the number of superovulated oocytes in the B6D2F1 hybrid strain (Figure 7). Notably, the generation of most genetically modified mice in our laboratory is performed by microinjecting CRISPR-Cas9 components into in vitro fertilized one-cell oocytes (zygotes) of the B6D2F1 hybrid strain, which shows high survival rates compared with commonly used inbred strains [50,51].
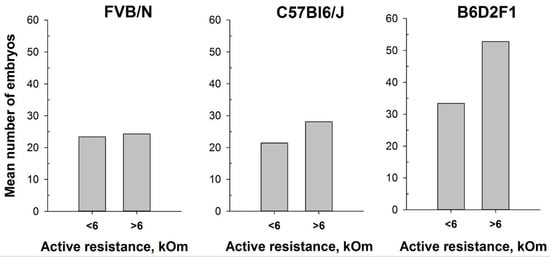
Figure 7.
Number of oocytes per mouse produced after mouse superovulation started at different vaginal AR values—AR < 6 kOm or AR > 6 kOm: FVB/N (n = 19 and n = 5, respectively); C57Bl6/J (n = 29 and n = 15, respectively); B6D2F1 (n = 51 and n = 9, respectively).
2.4. Assessment of Distress Caused by Measuring the AR with MEDProTM
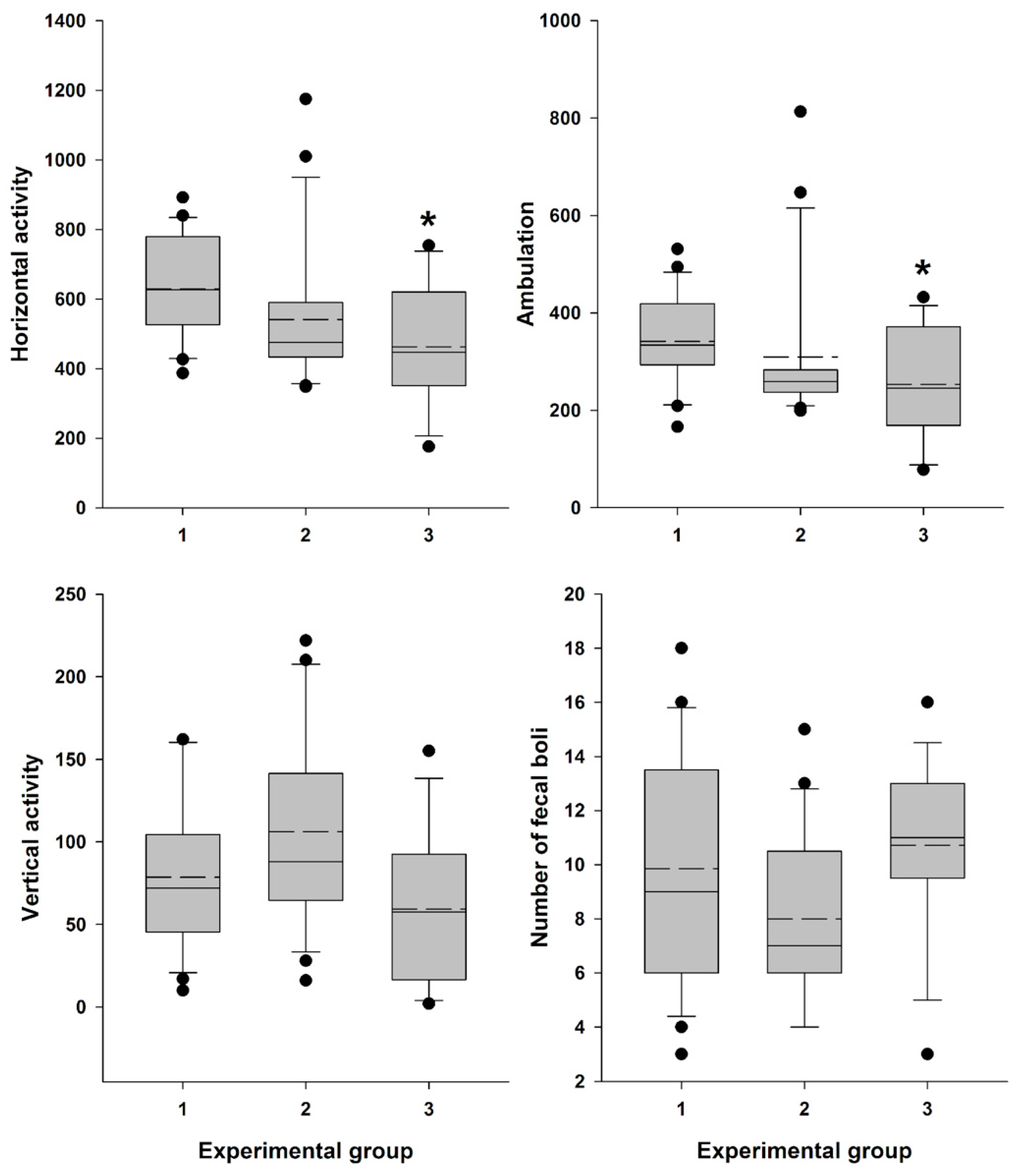
Locomotor activity data were recorded (Supplementary Table S2) and analyzed, confirming a significant influence of the factor “Experimental group” on general horizontal motor activity (H = 9.153; df = 2; p = 0.010) and ambulation (H = 7.687; df = 2; p = 0.021). The influence on vertical activity (H = 5.823; df = 2; p = 0.054) and the number of fecal boli (F (2,53) = 2.759; p = 0.072) was close to significant.
Female mice, 30 min after vaginal lavage, exhibited a statistically significant decrease in horizontal activity, including ambulation, compared with the intact control group (Figure 8). The decrease in vertical activity and fecal boli count (indices of emotionality and anxiety) [52] in this group was close to significant. In contrast, animals did not exhibit a statistically significant reduction in either horizontal activity, including ambulation, or in vertical activity and fecal boli counts after AR measurement compared to the intact control group (Figure 8).
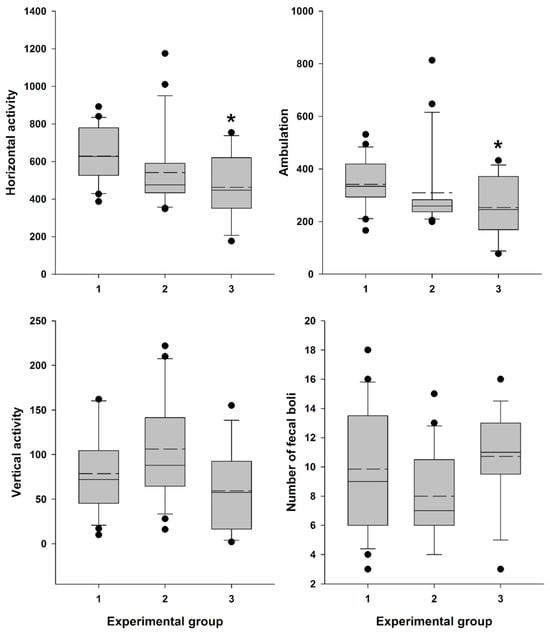
Figure 8.
Locomotor activity and “emotionality” of Swiss female mice. A box-plot and whisker graph show the difference between the three experimental groups: (1) intact female mice (n = 21); (2) female mice 30 min after detector probe insertion into the vagina for measurement of active resistance (n = 21), and (3) female mice 30 min after vaginal lavage (n = 14). The box represents the 25th and 75th percentiles, and illustrates the median (solid line) and the mean (dashed line). The whiskers show the 10th and 90th percentiles and outliers are indicated as dots. * Indicate a significant difference (p < 0.05, Dunn’s test for multiple comparisons) from intact females.
3. Discussion
Understanding the reproductive cycles of animals is essential in various scientific fields, from basic research to toxicological and pharmacological studies. In this context, the accurate determination of estrus, a key phase in the reproductive cycle of female mammals, holds particular significance. Traditional techniques for estrus detection, such as vaginal lavage, can induce stress in animals, potentially biasing experimental results [24,53].
While adopting a new experimental technique, a thorough evaluation of its impact on animal wellbeing is essential. Assessment of stress levels introduced by the technique is important not only for animal wellbeing and ethical consideration but also for ensuring accurate experimental result interpretation and drawing valid conclusions [54]. The measurement of locomotor activity often serves as an indicator of stress and an assessment of the basic status of animals. The decline in locomotor activity in mice may correspond to procedural distress [55]. There has been accumulating evidence showing that a variety of stimuli affect the motor activity of laboratory rodents [52,55,56,57,58]. Such mild stress as grabbing the mice by the tail and neck skin (five times) before placing them in the open field significantly reduced the average travel distance [58]. The results of our study revealed that 30 min after vaginal lavage, female Swiss mice exhibited a statistically significant (but not dramatical) decrease in horizontal activity, including ambulation, and a close to significant decline in vertical activity, along with an increase in defecation level, indicative of heightened emotionality under low levels of novel environmental stress conditions (Figure 8) [52]. In contrast, this pattern was not observed following the insertion of the MEDProTM detector probe and measurement of estrous cycle-related vaginal AR, indicating that this method is a less stressful and more refined approach for estrous stage evaluation.
To assess the efficacy of the MEDPro™ device in detecting estrus stage, we conducted a comparative study across CD-1, FVB/N, and C57Bl6/J mouse strains. We designed a comprehensive series of experiments spanning an 11-day period, wherein we performed daily observational assessments of vaginal opening, measured active resistance (AR), and compared the results with vaginal smear cytology (Figure 9, Table S1). Our study collected a total of 330 vaginal smear samples, revealing different estrous cycle regularities among the investigated strains. When a comparison of vaginal opening with vaginal smear analysis was performed, the rates of estrus confirmation varied significantly, underscoring the variability in the effectiveness of vaginal opening observation across mouse strains and emphasizing the necessity of complementary methodologies for precise estrus determination [34]. To assess differences in vaginal active resistance at different stages of the estrous cycle obtained with the MEDProTM device, we used mixed model rank analysis for each strain of mice. Our results demonstrated a significant (p < 0.01) difference in AR values between stages in all strains (Figure 4, Table S1). Specifically, AR was significantly higher during estrus than during other cycle stages, with the lowest values observed during diestrus. In assessing the likelihood of detecting each estrus cycle in the subsequent round of measurements based on AR values, our data revealed that values equal to or greater than the threshold of 6 kOm resulted in detection probabilities of approximately 76%, 64%, and 76% for CD-1, FVB/N, and C57Bl6/j strains, respectively. Conversely, the probabilities for detecting proestrus and metestrus were comparatively lower. Importantly, values equal to or greater than 6 kOm were not detected for diestrus cycles in any of the investigated mouse strains.
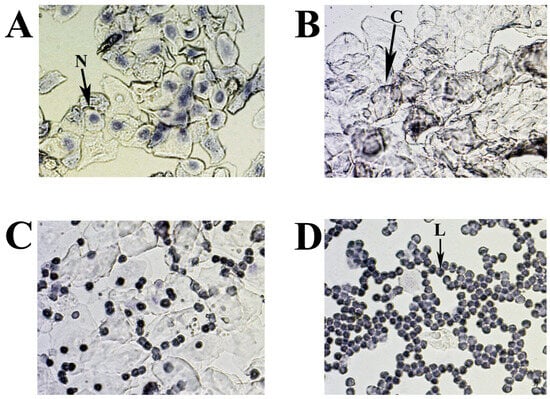
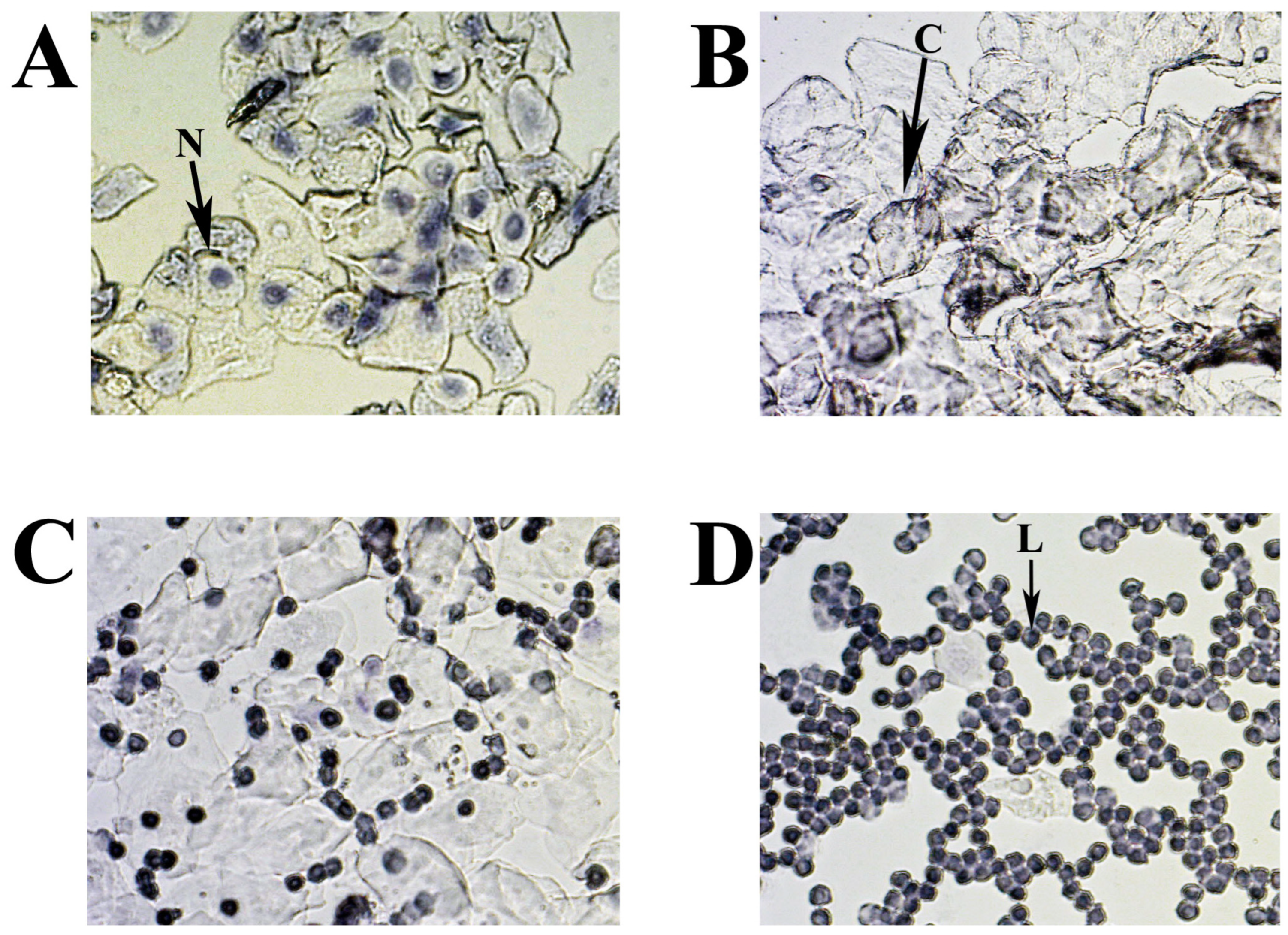
Figure 9.
Representative smears illustrating cytology for each estrous cycle stage: (A) proestrus; (B) estrus; (C) metestrus; (D) diestrus. Three cell types are identified: leukocytes (L), cornified epithelial cells (C), and nucleated epithelial cells (N).
The estrous cycle in mice is regulated by a complex interplay of hormonal and signaling pathways, primarily coordinated by the hypothalamic-pituitary-gonadal (HPG) axis [28,59,60]. This axis functions through the release of gonadotropin-releasing hormone (GnRH) from the hypothalamus, which stimulates the anterior pituitary to secrete follicle-stimulating hormone (FSH) and luteinizing hormone (LH). FSH is pivotal for follicular growth, cellular proliferation, and estrogen production, whereas LH is essential for androgen biosynthesis, oocyte maturation, ovulation, and the differentiation of follicles into the corpus luteum (CL) [28,59,60]. During the proestrus phase, increased FSH levels enhance follicular development and estrogen production. High estrogen levels activate an LH surge that triggers ovulation, marking the transition to the estrus phase and promoting oocyte maturation and follicular rupture. Following ovulation, in the metestrus phase, the corpus luteum forms and secretes progesterone, maintaining the uterine lining for potential embryo implantation. Progesterone remains elevated during diestrus, sustaining the uterine environment and exerting negative feedback on the HPG axis to prevent the development of new follicles. This hormonal interplay, involving FSH, LH, estrogen, and progesterone, precisely regulates follicular growth, ovulation, and preparation for potential pregnancy. Mouse mating typically aligns with the estrus phase, where conditions are optimal for fertilization. Accurate timing of estrus is critical for successful mating and superovulation, which are essential in various scientific disciplines, including reproductive biology, developmental biology, embryology, toxicology, pharmacology, genetics, and biotechnology. These fields often require precise control over reproductive cycles to study processes such as fertilization, embryonic development, the effects of drugs or chemicals on reproduction, and the generation of genetically modified organisms. Traditionally, timed pregnancies are determined by housing male and female mice together overnight and checking for mating signs (“plug”) the following morning. Our findings demonstrate that integrating MEDProTM into the natural breeding protocol significantly enhances mating efficiency by predominantly selecting female mice in the estrus stage of the cycle, offering a streamlined approach to research practices in accordance with the 3Rs principles to refine animal mating (Figure 6) [20,25,54].
The protocol for superovulation in mice is based on the injection of pregnant mare serum gonadotropin (PMSG), a hormone that has the activity of both FSH and LH hormones, followed by an injection of human chorionic gonadotropin (hCG), which has LH-like activity. Hence, the PMSG enhances follicle development and promotes ovulation and luteum formation, whereas the hCG stimulates final follicle maturation and ovulation. This technique aligns with the principles of the 3Rs in animal research, drastically minimizing the number of animals utilized in experiments and enabling the generation of substantial numbers of embryos for various applications [20,61]. In vitro fertilization (IVF), embryo transfer, and embryo cryopreservation are just a few examples of reproductive procedures that rely on superovulated oocytes. Superovulation stands as a crucial step for the generation of genetically modified mouse models through microinjections of zygotes and/or embryonic stem cell delivery into blastocytes. However, it has also been described that different strains of mice and animal weight can potentially influence hormone levels and, therefore, the efficiency of superovulation [62]. Moreover, it was reported that gonadotropin administration in mice should be synchronized with the natural estrous cycle to optimize oocyte quality [63]. Injecting PMSG at diestrus results in a higher percentage of cumulus-free oocytes, oocytes without a polar body, oocytes with intracytoplasmic mitochondrial aggregates, and those with abnormal chromosome distribution and scattering. In contrast, estrus females show the highest percentage of oocytes with normal chromosome distribution and the lowest percentage of oocytes with the aforementioned abnormalities [63]. In addition, the highest pregnancy rates were observed in mice that superovulated during the proestrus and estrus stages [64]. The use of suboptimal mouse strains and/or suboptimal superovulation protocols results in the use of a large number of females to obtain the necessary number of oocytes [61,65,66,67,68]. Given the considerable genetic variations between mouse strains, establishing an optimal superovulation protocol becomes crucial [69]. In this study, we aimed to enhance the superovulation protocol for FVB/N, C57Bl6/J, and B6D2F1 mouse strains that are routinely used for genome editing in our laboratory. Notably, embryonal survival after CRISPR/Cas9 complex microinjection and efficient implantation after embryo-transfer procedures are among the crucial aspects of the generation of genetically modified animals that might directly correlate with the quality of the obtained oocytes after superovulation (Skryabin et al., unpublished). In our analyses, prior to hormonal injections, mice were separated into two groups based on the value of vaginal active resistance. Although, due to technical and ethical limitations, counting the number of oocytes from individual animals was not feasible, our findings suggest that initiating superovulation during the estrus cycle (AR ≥ 6 kOm) consistently resulted in higher oocyte yields across all females, irrespective of mouse strain. Notably, certain strains, such as FVB/N and C57Bl6/J, exhibited slightly enhanced embryo production, while the B6D2F1 hybrid strain displayed a remarkable increase of up to 50 percent more embryos per female animal (Figure 7). This discovery is particularly significant, as the high survival rates of B6D2F1 zygotes make them the primary choice for CRISPR-Cas9 mediated genome editing in our research [50,51]. In summary, using the MEDProTM device allows for a fast, efficient, and minimal impact on the wellness of female mice to determine their estrus cycle. Compared to the other non-traumatic visual method, the MEDProTM device allows for a more accurate detection of the estrus cycle.
4. Materials and Methods
4.1. Animals
In this study, a number of experiments were carried out in female mice of different strains (CD-1, FVB/N, and C57Bl6/J) and hybrid strains, B6D2F1 (C57Bl6/J × DBA); see the detailed description below. Thirty pubertal female mice (ten for each line: CD-1, FVB/N, and C57Bl6/J) were assessed daily for stage of the estrous cycle by assessing vaginal opening status, MEDProTM measurements, and vaginal smear cytology. A two-way mixed model analysis of variance (ANOVA) was performed on the active resistance data after MEDProTM measurements. Animals were kept in specific pathogen-free conditions at the transgenic animals and genetic engineering models (TRAM) core facility of the University Clinic Muenster. All female mice were housed in groups (five animals per cage, with non-coniferous wood-chip bedding, Wilhelm Reckhorn GmbH, Warendorf, Germany) with free access to filtered tap water and food (Altromin, 1324, Lage, Germany). They were maintained under a 12 h light/dark cycle (lights on at 06:00 AM) at an ambient temperature of 20 ± 1 °C and humidity of 50 ± 10%. All mouse procedures with the above-mentioned animals were performed in compliance with the guidelines for the welfare of experimental animals issued by the Federal Government of Germany and approved by the State Agency for Nature, Environment and Consumer Protection North Rhine-Westphalia LANUV (Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen).
In addition, the female (n = 75) and male (n = 19) outbred Swiss mice from a local colony of the Department of Psychopharmacology (Valdman Institute of Pharmacology, St. Petersburg, Russia) were used for behavioral tests and estimation of mating efficiency. Animals were housed in groups of same-sex siblings (three to five mice per TIII cage, Velaz, Czech Republic; with non-coniferous wood-chip bedding, Lignocel, JRS GMBH + Co., Pattensen, Germany) with free access to filtered tap water (filter AQUAPHOR®, St. Petersburg, Russia) and food (standard lab chow, Laboratorkorm, Moscow, Russia). They were maintained under a 12 h light/dark cycle (lights on at 09:00 a.m.) at an ambient temperature of 20 ± 1 °C and humidity of 50 ± 10%. Cages, bedding, and water bottles were changed at regular intervals, i.e., twice a week.
4.2. Vaginal Opening Observation
Vaginal opening observation is a commonly used technique due to its non-invasive nature and simplicity. However, it exhibits significant variability across mouse strains and demonstrates low efficacy in accurately determining estrus stages [34,40,70]. During estrus, the vaginal opening typically appears open, swollen, protruding, and moist with a reddish or pinkish hue, whereas during proestrus, it remains relatively closed and less protruded. Metestrus represents the transitional phase between estrus and diestrus, characterized by a decrease in swelling and moisture and a return to a more closed and constricted appearance. The reddish or pinkish coloration diminishes during this phase, and during diestrus, the vaginal opening appears minimally smaller, dry, and pale.
4.3. General Principles of MEDProTM Design and Measurement
Measuring the impedance of the vaginal walls in mice poses challenges in accurately determining the stage of the estrous cycle. In existing rodent estrous cycle detectors, the electrical resistance of the vaginal mucosa is determined by measuring the electrical impedance (Z), which is the geometric sum of the active resistance of the RS electric circuit and the reactive resistance of the XC: Z2 = RS2 + XC2. RS is an inverse value of tissue conductivity, exhibiting minimal dependence on current frequency, while the reactive component of the XC impedance depends on the frequency of the electric current. In alternating current measurements, Z is influenced by system interfaces in the system, where charge accumulation—polarization—can occur. The properties of interfaces (in a biological object, these are mainly different cell membranes) can be described by taking into account the capacitance C, the resistance of which XC depends on the frequency at which the measurement is made: XC = −1/(ωC), where ω = 2πf; ω—circular frequency; f—frequency in Hz. Measurement of conductivity in biological systems with direct current is challenging due to significant polarization on the surfaces of cell membranes and probe electrodes. Therefore, alternating current is employed for measurements.
In 2015, ELMI Ltd. (Riga, Latvia) initiated the development of the MEDProTM device, primarily focused on determining the vaginal AR in female mice (patent LV15278) [46]. Several versions of the device have been developed and tested, preceding the one presented in this work [42]. These units (Table 1) employ the synchronous detection method, selectively measuring the active resistance of the RS components, thereby eliminating the influence of C as “parasitic” noise on the measurement result. In contrast to the larger probes of MK-11 and MK-12 (Muromachi Kikai Co., Ltd., Japan) estrous cycle detectors, which require time for temperature stabilization of the index, the MEDProTM units feature a miniature probe (Figure 2). This probe comprises two electrodes (∅ = 1.82 mm, 1.2 mm wide, AISI316 material, 1.2 mm electrode spacing) with a low heat capacity, facilitating rapid (<1 s) temperature equalization between probe and tissue. Moreover, the miniature probe (L20xO.D.1.82), when inserted into the mouse vagina (Figure 3), is less traumatic and induces less stress, contributing to improved measurement accuracy.

Table 1.
The specifications of the MedProTM (ELMI Ltd., Latvia).
The device operates in two modes: “Auto” and “Manual” (Figure 2). In “Manual” mode, the sinusoidal voltage can be adjusted manually. In “Auto” mode, the device automatically regulates the current passing through the sensor electrodes of the probe to ensure it does not exceed 1 µA. If the current approaches this threshold during measurement, the device automatically reduces the sinusoidal voltage in real-time. This regulation is necessary because, at low voltages and high resistance, the resulting current is extremely small and noisy, complicating the accurate determination of impedance components RS and XC. All measurements reported in this study were conducted in “Auto” mode.
4.4. Vaginal Smear Cytology for Mouse Estrous Cycle Stage Identification
Estrus cycle monitoring and stage classification were performed using vaginal cytology. A vaginal smear offers a reliable method (“gold standard”) for determining each estrous cycle stage [34,40,41]. A lavage from each animal was then taken after AR detection. Mouse vaginal smears were collected daily for 11 consecutive days in the morning (11:00 a.m.) and were used as a control assessment for comparison with other methods tested (Table S1). The vagina was washed by pipette with 20 µL of 0,9% NaCl to suspend the epithelial and immune cells. The resulting fluid was placed on a slide and allowed to dry. The cells were stained with 0.1% crystal violet for 1 min, washed with ddH2O, dried, evaluated microscopically, and photographed (Figure 9) [40].
The cytology of mouse estrous cycle stages is characterized by regularly repeating combinations of distinct cell types: leucocytes—L, nuclear epithelial cells—N, and cornified epithelial cells—C (Figure 9A–D). The presence of these cell types correlates with the condition of the vaginal mucosa, is related to the state of the ovaries and uterus, and depends on the levels of different sex hormones circulating in the blood. In the standard mouse estrous cycle (4–5 days), the first stage, proestrus, is characterized by abundant nucleated epithelial cells (Figure 9A) and lasts approximately one day, followed by estrus. The estrus is usually identified by large numbers of cornified (keratinized) epithelial cells with irregular margins (Figure 9B). The predominance of keratinized cells in the vagina lasts for one to two days (depending on the 4 or 5 days of the estrus cycle) and continues until the metestrus stage, which occurs after ovulation. Metestrus is a transitional period between estrus and diestrus, characterized by leukocytes, nucleated epithelial cells, and significant numbers of cornified epithelial cells (Figure 9C). The metestrus lasts about one day and is followed by the diestrus stage, where leukocytes are present together with rounded epithelial cells with nuclei in varying concentrations. The vaginal smear can often appear almost exclusively leucocytic during diestrus (Figure 9D).
4.5. Mouse Mating
We assessed mating efficiency by examining the presence of the copulatory plug after overnight pairing (at a 1:1 ratio) of vasectomized CD-1 male mice with females characterized by different values of vaginal AR determined with MEDProTM. Male mice were vasectomized at approximately 12 weeks of age and subsequently housed individually. To evaluate the significance of the MEDProTM application for successful timed pregnancies, sexually experienced male and sexually naive female Swiss mice (4 months old) intended for breeding were used. The study included two experimental groups. The first group of animals (“Undetected females”) was paired with males at a ratio of 1:1 in a TII cage (Tecniplast, Italy) in the afternoon and left overnight without prior estimation of the AR (n = 10). The second group (“Detected females”) consisted of females that were paired with males under the same conditions, but only after detecting an AR > 6 kOm (n = 9). In both groups, the pairing was conducted under the same environmental conditions to ensure consistency.
4.6. Female Mice Superovulation
Superovulation in female mice was induced using two hormones: pregnant mare’s serum gonadotrophin (PMSG), which has follicle-stimulating activity, and human chorionic gonadotrophin (hCG). Both hormones were administered intraperitoneally. PMSG (7.5 U) was injected at 18:00, followed by an injection of hCG (7.5 U) 44–48 h later. The next day, unfertilized oocytes were isolated from the superovulated female mice.
4.7. Behavioral Test
Female Swiss mice (n = 56) were used in this study. The mice were divided into three groups: the MEDProTM group (n = 21), the vaginal lavage group (n = 14), and the intact control group (n = 21). Locomotor activity was assessed 30 min after the AR measurement or vaginal lavage. Tests were conducted between 14:00 and 18:00 h in Plexiglas boxes (internal dimensions: 35 × 24 × 33 cm) with transparent walls and an opaque floor. These boxes were housed in sound- and light-attenuating ventilated cubicles with dim illumination and a fan for masking noise, classified as “low-stress environments”. Locomotor activity was measured using three pairs of photobeams placed 2 cm above the floor. Consecutive crossings of different photobeams were counted as ambulation, while crossings of any photobeam were calculated as general horizontal activity. Additionally, rearing behavior (vertical activity) was scored using eight pairs of photobeams placed 10 cm above the floor. Behavioral measures were collected and recorded using IBM-compatible computers equipped with specialized hardware and software from Med Associates, Inc. (East Fairfield, VT, USA) (Table S2). Mice were placed in the chambers for a 30-min duration. After each session, fecal boli were counted and removed. The testing surface was cleaned with 3% hydrogen peroxide and dried with paper towels before the next test.
4.8. Statistical Analysis
Statistical analyses were performed using SPSS for Windows (ver. 22, SPSS Inc., Chicago, IL, USA) and SigmaPlot (ver. 12.5; Systat Software Inc., Chicago, IL, USA). The data were checked for normality of distribution using the Shapiro–Wilk test. Where necessary, due to conditions of non-normality, the data were transformed prior to analysis. A two-way mixed-model analysis of variance (ANOVA) was performed on the active resistance data. Degrees of freedom are presented rounded down to the nearest integer. Bonferroni’s test was applied for post hoc comparisons whenever indicated by the ANOVA results. Categorical data were analyzed using the Chi-square or Fisher’s exact test and reported as frequency (percentage). The data for locomotor activity were subjected to a Kruskal–Wallis one-way analysis of variance by rank test. Dunn’s tests were applied for post hoc comparisons whenever indicated by the Kruskal–Wallis analysis results. The level of significance was set at 0.05.
5. Conclusions
Our results emphasize the importance of employing advanced techniques, such as the MEDProTM device, in animal research, especially in advancing the principles of refinement, reduction, and replacement (3Rs). By reducing stress-induced bias in experimental results and optimizing reproductive procedures such as superovulation and animal mating, MEDProTM improves research practices while reducing animal requirements. Furthermore, our study demonstrated its efficacy in determining estrus stages in different strains of mice, highlighting the possibility of its use as a complementary technique for accurate estrus detection. We believe that the integration of MEDProTM into research protocols has the potential to improve the wellbeing of laboratory animals and enhance the reliability and ethical integrity of scientific research.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms25179429/s1.
Author Contributions
I.V.B., D.D.M. and B.V.S. conceived the project. I.V.B., B.V.S. and H.K. designed the study and participated in experimental work. I.V.B., D.D.M., B.V.S. and T.S.R. participated in the preparation of the figures. I.V.B., B.V.S. and T.S.R. analyzed the data and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
Core facility TRAM is an institution of the Medical Faculty of the University of Münster. The support of the medical faculty is thankfully recognized.
Institutional Review Board Statement
All mouse studies conducted at the transgenic animals and genetic engineering models (TRAM) core facility of the University Clinic Muenster were performed in compliance with the guidelines for the welfare of experimental animals issued by the Federal Government of Germany and approved by the State Agency for Nature, Environment and Consumer Protection North Rhine-Westphalia LANUV (Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen) (protocol code: AZ: 84.02.04.2016.A546 date of approval of 29.08.2018 until 31.05.2022; protocol code: AZ: 81-02.04.2022.A085 date of approval of 09.06.2022 until 31.05.2027). All mouse studies conducted at the Valdman Institute of Pharmacology (Pavlov Medical University, St. Petersburg, Russia) were performed in compliance with guidelines for the use of laboratory animals for scientific and educational purposes at the I. P. Pavlov St. Petersburg State Medical University (Belozertseva, I.V., Dravolina, O.A., Tur, M.A./ed. E. E. Zvartau/SPB., Publishing House of St. Petersburg State Medical University, 2014, 80 p. (In Russ)) and approved by the IACUC (protocol code: 010_ИФ1_012019/3_300).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We would like to offer special thanks to Vekovischeva O. Yu, who performed the mixed-model analysis.
Conflicts of Interest
Dr. Dmitriy D. Merkulovs is co-inventor of patent LV15278B. The other co-authors have no conflict of interest to declare. ELMI Ltd. (Latvia) had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Dominguez-Oliva, A.; Hernandez-Avalos, I.; Martinez-Burnes, J.; Olmos-Hernandez, A.; Verduzco-Mendoza, A.; Mota-Rojas, D. The Importance of Animal Models in Biomedical Research: Current Insights and Applications. Animals 2023, 13, 1223. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.Y.; Zhang, J. Low rates of expression profile divergence in highly expressed genes and tissue-specific genes during mammalian evolution. Mol. Biol. Evol. 2006, 23, 1119–1128. [Google Scholar] [CrossRef]
- Monaco, G.; van Dam, S.; Casal Novo Ribeiro, J.L.; Larbi, A.; de Magalhaes, J.P. A comparison of human and mouse gene co-expression networks reveals conservation and divergence at the tissue, pathway and disease levels. BMC Evol. Biol. 2015, 15, 259. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, A.; Lu, A.; Humphreys, B.D. Gene Editing: Powerful New Tools for Nephrology Research and Therapy. J. Am. Soc. Nephrol. 2016, 27, 2940–2947. [Google Scholar] [CrossRef] [PubMed]
- Mou, H.; Kennedy, Z.; Anderson, D.G.; Yin, H.; Xue, W. Precision cancer mouse models through genome editing with CRISPR-Cas9. Genome Med. 2015, 7, 53. [Google Scholar] [CrossRef]
- Sommer, D.; Peters, A.E.; Baumgart, A.K.; Beyer, M. TALEN-mediated genome engineering to generate targeted mice. Chromosome Res. 2015, 23, 43–55. [Google Scholar] [CrossRef]
- Broestl, L.; Worden, K.; Moreno, A.J.; Davis, E.J.; Wang, D.; Garay, B.; Singh, T.; Verret, L.; Palop, J.J.; Dubal, D.B. Ovarian Cycle Stages Modulate Alzheimer-Related Cognitive and Brain Network Alterations in Female Mice. eNeuro 2018, 5, ENEURO.0132-17.2018. [Google Scholar] [CrossRef]
- Cordeira, J.; Kolluru, S.S.; Rosenblatt, H.; Kry, J.; Strecker, R.E.; McCarley, R.W. Learning and memory are impaired in the object recognition task during metestrus/diestrus and after sleep deprivation. Behav. Brain Res. 2018, 339, 124–129. [Google Scholar] [CrossRef]
- Goldman, J.M.; Murr, A.S.; Cooper, R.L. The rodent estrous cycle: Characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res. B Dev. Reprod. Toxicol. 2007, 80, 84–97. [Google Scholar] [CrossRef]
- McHenry, J.A.; Otis, J.M.; Rossi, M.A.; Robinson, J.E.; Kosyk, O.; Miller, N.W.; McElligott, Z.A.; Budygin, E.A.; Rubinow, D.R.; Stuber, G.D. Hormonal gain control of a medial preoptic area social reward circuit. Nat. Neurosci. 2017, 20, 449–458. [Google Scholar] [CrossRef]
- Milad, M.R.; Igoe, S.A.; Lebron-Milad, K.; Novales, J.E. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience 2009, 164, 887–895. [Google Scholar] [CrossRef]
- Rodrigues, A.C.C.; Moreira, C.V.L.; Prado, C.C.; Silva, L.S.B.; Costa, R.F.; Arikawe, A.P.; Pedrino, G.R.; Costa, E.A.; Silva, O.N.; Napolitano, H.B.; et al. A comparative analysis of depressive-like behavior: Exploring sex-related differences and insights. PLoS ONE 2023, 18, e0294904. [Google Scholar] [CrossRef]
- Feldwisch-Drentrup, H. Germany weighs whether culling excess lab animals is a crime. Science 2022, 376, 567–568. [Google Scholar] [CrossRef]
- Mahjabeen, S.; Hatipoglu, M.K.; Benbrook, D.M.; Kosanke, S.D.; Garcia-Contreras, D.; Garcia-Contreras, L. Influence of the estrus cycle of the mouse on the disposition of SHetA2 after vaginal administration. Eur. J. Pharm. Biopharm. 2018, 130, 272–280. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, H.; Liu, W.; Hu, C.; Zhou, Y.; Sun, R.; Tang, Y.; Zheng, C.; Yang, J.; Cui, Q. A multi-tissue transcriptomic landscape of female mice in estrus and diestrus provides clues for precision medicine. Front. Cell Dev. Biol. 2022, 10, 983712. [Google Scholar] [CrossRef]
- Chari, T.; Griswold, S.; Andrews, N.A.; Fagiolini, M. The Stage of the Estrus Cycle Is Critical for Interpretation of Female Mouse Social Interaction Behavior. Front. Behav. Neurosci. 2020, 14, 113. [Google Scholar] [CrossRef]
- Karigo, T.; Deutsch, D. Flexibility of neural circuits regulating mating behaviors in mice and flies. Front. Neural Circuits 2022, 16, 949781. [Google Scholar] [CrossRef]
- Rocks, D.; Cham, H.; Kundakovic, M. Why the estrous cycle matters for neuroscience. Biol. Sex. Differ. 2022, 13, 62. [Google Scholar] [CrossRef]
- Hasegawa, A.; Mochida, K.; Ogonuki, N.; Hirose, M.; Tomishima, T.; Inoue, K.; Ogura, A. Efficient and scheduled production of pseudopregnant female mice for embryo transfer by estrous cycle synchronization. J. Reprod. Dev. 2017, 63, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Hubrecht, R.C.; Carter, E. The 3Rs and Humane Experimental Technique: Implementing Change. Animals 2019, 9, 754. [Google Scholar] [CrossRef]
- Rai, J.; Kaushik, K. Reduction of Animal Sacrifice in Biomedical Science & Research through Alternative Design of Animal Experiments. Saudi Pharm. J. 2018, 26, 896–902. [Google Scholar] [CrossRef]
- Jennings, K.J.; de Lecea, L. Neural and Hormonal Control of Sexual Behavior. Endocrinology 2020, 161, bqaa150. [Google Scholar] [CrossRef]
- Jaric, I.; Rocks, D.; Greally, J.M.; Suzuki, M.; Kundakovic, M. Chromatin organization in the female mouse brain fluctuates across the oestrous cycle. Nat. Commun. 2019, 10, 2851. [Google Scholar] [CrossRef]
- Lovick, T.A.; Zangrossi, H., Jr. Effect of Estrous Cycle on Behavior of Females in Rodent Tests of Anxiety. Front. Psychiatry 2021, 12, 711065. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen & Co, Ltd.: London, UK, 1959. [Google Scholar]
- Moenter, S.M.; Starrett, J.R. Estradiol action in the female hypothalamo-pituitary-gonadal axis. J. Neuroendocrinol. 2024, e13390. [Google Scholar] [CrossRef]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar] [CrossRef]
- Makker, A.; Goel, M.M.; Mahdi, A.A. PI3K/PTEN/Akt and TSC/mTOR signaling pathways, ovarian dysfunction, and infertility: An update. J. Mol. Endocrinol. 2014, 53, R103–R118. [Google Scholar] [CrossRef]
- Dyson, M.T.; Kowalewski, M.P.; Manna, P.R.; Stocco, D.M. The differential regulation of steroidogenic acute regulatory protein-mediated steroidogenesis by type I and type II PKA in MA-10 cells. Mol. Cell Endocrinol. 2009, 300, 94–103. [Google Scholar] [CrossRef]
- Goddard, L.M.; Murphy, T.J.; Org, T.; Enciso, J.M.; Hashimoto-Partyka, M.K.; Warren, C.M.; Domigan, C.K.; McDonald, A.I.; He, H.; Sanchez, L.A.; et al. Progesterone receptor in the vascular endothelium triggers physiological uterine permeability preimplantation. Cell 2014, 156, 549–562. [Google Scholar] [CrossRef]
- Ingman, W.V.; Robertson, S.A. The essential roles of TGFB1 in reproduction. Cytokine Growth Factor. Rev. 2009, 20, 233–239. [Google Scholar] [CrossRef]
- DeWitt, N.A.; Whirledge, S.; Kallen, A.N. Updates on molecular and environmental determinants of luteal progesterone production. Mol. Cell Endocrinol. 2020, 515, 110930. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, A.F.; Akhigbe, R.E. Staging of the estrous cycle and induction of estrus in experimental rodents: An update. Fertil. Res. Pract. 2020, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Byers, S.L.; Wiles, M.V.; Dunn, S.L.; Taft, R.A. Mouse estrous cycle identification tool and images. PLoS ONE 2012, 7, e35538. [Google Scholar] [CrossRef]
- Cora, M.C.; Kooistra, L.; Travlos, G. Vaginal Cytology of the Laboratory Rat and Mouse: Review and Criteria for the Staging of the Estrous Cycle Using Stained Vaginal Smears. Toxicol. Pathol. 2015, 43, 776–793. [Google Scholar] [CrossRef] [PubMed]
- Wood, G.A.; Fata, J.E.; Watson, K.L.; Khokha, R. Circulating hormones and estrous stage predict cellular and stromal remodeling in murine uterus. Reproduction 2007, 133, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Achiraman, S.; Archunan, G.; Sankarganesh, D.; Rajagopal, T.; Rengarajan, R.L.; Kokilavani, P.; Kamalakkannan, S.; Kannan, S. Biochemical analysis of female mice urine with reference to endocrine function: A key tool for estrus detection. Zoolog. Sci. 2011, 28, 600–605. [Google Scholar] [CrossRef]
- Mosci, P.; Pietrella, D.; Ricci, G.; Pandey, N.; Monari, C.; Pericolini, E.; Gabrielli, E.; Perito, S.; Bistoni, F.; Vecchiarelli, A. Mouse strain-dependent differences in estrogen sensitivity during vaginal candidiasis. Mycopathologia 2013, 175, 1–11. [Google Scholar] [CrossRef]
- DeLeon, D.D.; Zelinski-Wooten, M.B.; Barkley, M.S. Hormonal basis of variation in oestrous cyclicity in selected strains of mice. J. Reprod. Fertil. 1990, 89, 117–126. [Google Scholar] [CrossRef]
- Ekambaram, G.; Sampath Kumar, S.K.; Joseph, L.D. Comparative Study on the Estimation of Estrous Cycle in Mice by Visual and Vaginal Lavage Method. J. Clin. Diagn. Res. 2017, 11, AC05–AC07. [Google Scholar] [CrossRef]
- Gonzalez, G. Determining the Stage of the Estrous Cycle in Female Mice by Vaginal Smear. Cold Spring Harb. Protoc. 2016, 2016, pdb-rot094474. [Google Scholar] [CrossRef]
- Belozertseva, I.V.; Merkulovs, D.D.; Vilitis, O.J.; Skryabin, B.V. Instrumental methods for determining theestrous cycle stages in small laboratory rodents. Lab. Anim. Sci. 2018, 4, 125–135. [Google Scholar] [CrossRef]
- Chesney, K.L.; Chang, C.; Bryda, E.C. Using Vaginal Impedance Measurement to Identify Proestrus in Rats Given Luteinizing Hormone Releasing Hormone (LHRH) Agonist. J. Am. Assoc. Lab. Anim. Sci. 2020, 59, 282–287. [Google Scholar] [CrossRef]
- Bartos, L. Vaginal impedance measurement used for mating in the rat. Lab. Anim. 1977, 11, 53–55. [Google Scholar] [CrossRef]
- Bartos, L.; Sedlacek, J. Vaginal impedance measurement used for mating in the guinea-pig. Lab. Anim. 1977, 11, 57–58. [Google Scholar] [CrossRef]
- Vilitis, O.; Merkulovs, D.; Mironovs, I.; Mironovs, A.; Merkulova, V. Estrous Cycle Detector. Patent 19 LV15278B, 20 May 2018. Available online: https://patents.google.com/patent/LV15278B/en (accessed on 20 May 2018).
- Behringer, R.; Gertsenstein, M.; Nagy, K.V.; Nagy, A. Selecting Female Mice in Estrus and Checking Plugs. Cold Spring Harb. Protoc. 2016, 2016, 729–731. [Google Scholar] [CrossRef]
- Saunders, T.L. The History of Transgenesis. Methods Mol. Biol. 2020, 2066, 1–26. [Google Scholar] [CrossRef]
- Hasegawa, A.; Mochida, K.; Inoue, H.; Noda, Y.; Endo, T.; Watanabe, G.; Ogura, A. High-Yield Superovulation in Adult Mice by Anti-Inhibin Serum Treatment Combined with Estrous Cycle Synchronization. Biol. Reprod. 2016, 94, 21. [Google Scholar] [CrossRef]
- Brugmans, A.K.; Walter, C.; Moreno, N.; Gobel, C.; Holdhof, D.; de Faria, F.W.; Hotfilder, M.; Jeising, D.; Fruhwald, M.C.; Skryabin, B.V.; et al. A Carboxy-terminal Smarcb1 Point Mutation Induces Hydrocephalus Formation and Affects AP-1 and Neuronal Signalling Pathways in Mice. Cell Mol. Neurobiol. 2023, 43, 3511–3526. [Google Scholar] [CrossRef]
- Skryabin, B.V.; Kummerfeld, D.M.; Gubar, L.; Seeger, B.; Kaiser, H.; Stegemann, A.; Roth, J.; Meuth, S.G.; Pavenstadt, H.; Sherwood, J.; et al. Pervasive head-to-tail insertions of DNA templates mask desired CRISPR-Cas9-mediated genome editing events. Sci. Adv. 2020, 6, eaax2941. [Google Scholar] [CrossRef]
- Seibenhener, M.L.; Wooten, M.C. Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. J. Vis. Exp. 2015, e52434. [Google Scholar] [CrossRef]
- Varol, A.B.; Esen, E.C.; Kocak, E.E. Repeated Collection of Vaginal Smear Causes Stress in Mice. Noro Psikiyatr. Ars. 2022, 59, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Rinwa, P.; Eriksson, M.; Cotgreave, I.; Backberg, M. 3R-Refinement principles: Elevating rodent well-being and research quality. Lab. Anim. Res. 2024, 40, 11. [Google Scholar] [CrossRef] [PubMed]
- Jansen van’t Land, C.; Hendriksen, C.F. Change in locomotor activity pattern in mice: A model for recognition of distress? Lab. Anim. 1995, 29, 286–293. [Google Scholar] [CrossRef]
- Kim, H.J.J.; Zagzoog, A.; Black, T.; Baccetto, S.L.; Ezeaka, U.C.; Laprairie, R.B. Impact of the mouse estrus cycle on cannabinoid receptor agonist-induced molecular and behavioral outcomes. Pharmacol. Res. Perspect. 2022, 10, e00950. [Google Scholar] [CrossRef]
- Klein, C.; Budiman, T.; Homberg, J.R.; Verma, D.; Keijer, J.; van Schothorst, E.M. Measuring Locomotor Activity and Behavioral Aspects of Rodents Living in the Home-Cage. Front. Behav. Neurosci. 2022, 16, 877323. [Google Scholar] [CrossRef]
- Zhang, C.; Li, H.; Han, R. An open-source video tracking system for mouse locomotor activity analysis. BMC Res. Notes 2020, 13, 48. [Google Scholar] [CrossRef]
- Bertolin, K.; Murphy, D.B. 7—Reproductive Tract Changes during the Mouse Estrous Cycle. In The Guide to Investigation of Mouse Pregnancy; Anne Croy, B., Francesco, A.T.Y., DeMayo, J., Lee Adamson, S., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 85–94. [Google Scholar] [CrossRef]
- Matvere, A.; Teino, I.; Varik, I.; Kuuse, S.; Tiido, T.; Kristjuhan, A.; Maimets, T. FSH/LH-Dependent Upregulation of Ahr in Murine Granulosa Cells Is Controlled by PKA Signaling and Involves Epigenetic Regulation. Int. J. Mol. Sci. 2019, 20, 3068. [Google Scholar] [CrossRef]
- Nagy, A.G.M.; Vintersten, K.; Behringer, R. Inducing superovulation. In Manipulating the Mouse Embryo: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2003; pp. 148–150. [Google Scholar]
- Luo, C.; Zuniga, J.; Edison, E.; Palla, S.; Dong, W.; Parker-Thornburg, J. Superovulation strategies for 6 commonly used mouse strains. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 471–478. [Google Scholar]
- Tarin, J.J.; Perez-Albala, S.; Cano, A. Stage of the estrous cycle at the time of pregnant mare’s serum gonadotropin injection affects the quality of ovulated oocytes in the mouse. Mol. Reprod. Dev. 2002, 61, 398–405. [Google Scholar] [CrossRef]
- Inyawilert, W.; Liao, Y.J.; Tang, P.C. Superovulation at a specific stage of the estrous cycle determines the reproductive performance in mice. Reprod. Biol. 2016, 16, 279–286. [Google Scholar] [CrossRef]
- Auerbach, A.B.; Norinsky, R.; Ho, W.; Losos, K.; Guo, Q.; Chatterjee, S.; Joyner, A.L. Strain-dependent differences in the efficiency of transgenic mouse production. Transgenic Res. 2003, 12, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Byers, S.L.; Payson, S.J.; Taft, R.A. Performance of ten inbred mouse strains following assisted reproductive technologies (ARTs). Theriogenology 2006, 65, 1716–1726. [Google Scholar] [CrossRef]
- Suzuki, O.; Asano, T.; Yamamoto, Y.; Takano, K.; Koura, M. Development in vitro of preimplantation embryos from 55 mouse strains. Reprod. Fertil. Dev. 1996, 8, 975–980. [Google Scholar] [CrossRef]
- Vergara, G.J.; Irwin, M.H.; Moffatt, R.J.; Pinkert, C.A. In vitro fertilization in mice: Strain differences in response to superovulation protocols and effect of cumulus cell removal. Theriogenology 1997, 47, 1245–1252. [Google Scholar] [CrossRef]
- Spearow, J.L.; Barkley, M. Genetic control of hormone-induced ovulation rate in mice. Biol. Reprod. 1999, 61, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Caligioni, C.S. Assessing reproductive status/stages in mice. Curr. Protoc. Neurosci. 2009, 48, A.4I.1–A.4I.8. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).