The KDR Gene rs2071559 and the VEGF Gene rs6921438 May Be Associated with Diabetic Nephropathy in Caucasians with Type 2 Diabetes Mellitus
Abstract
:1. Introduction
2. Results
2.1. Clinical Characteristics
2.2. Genetical Data
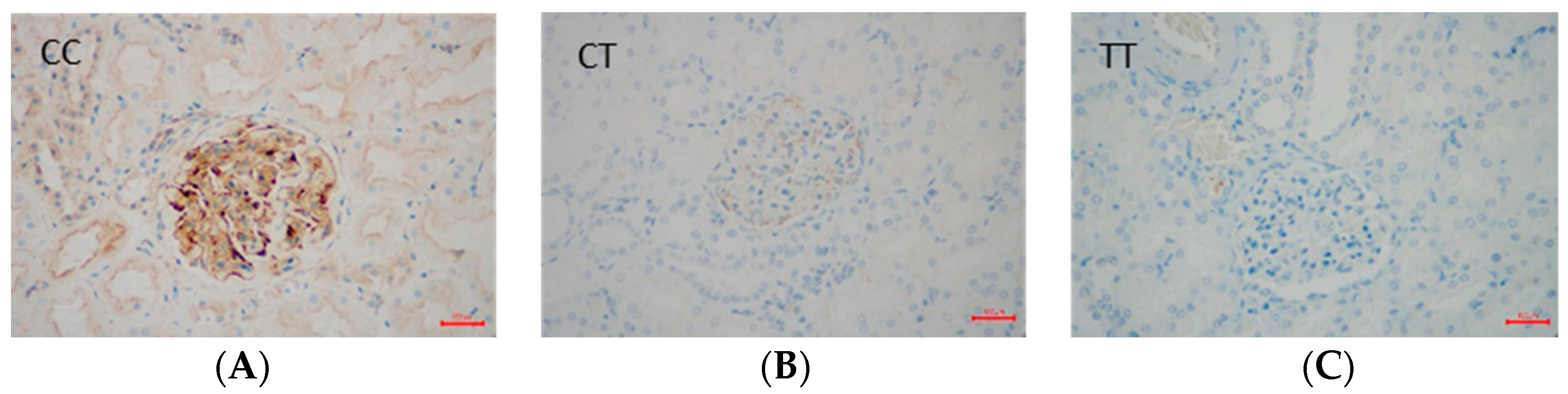
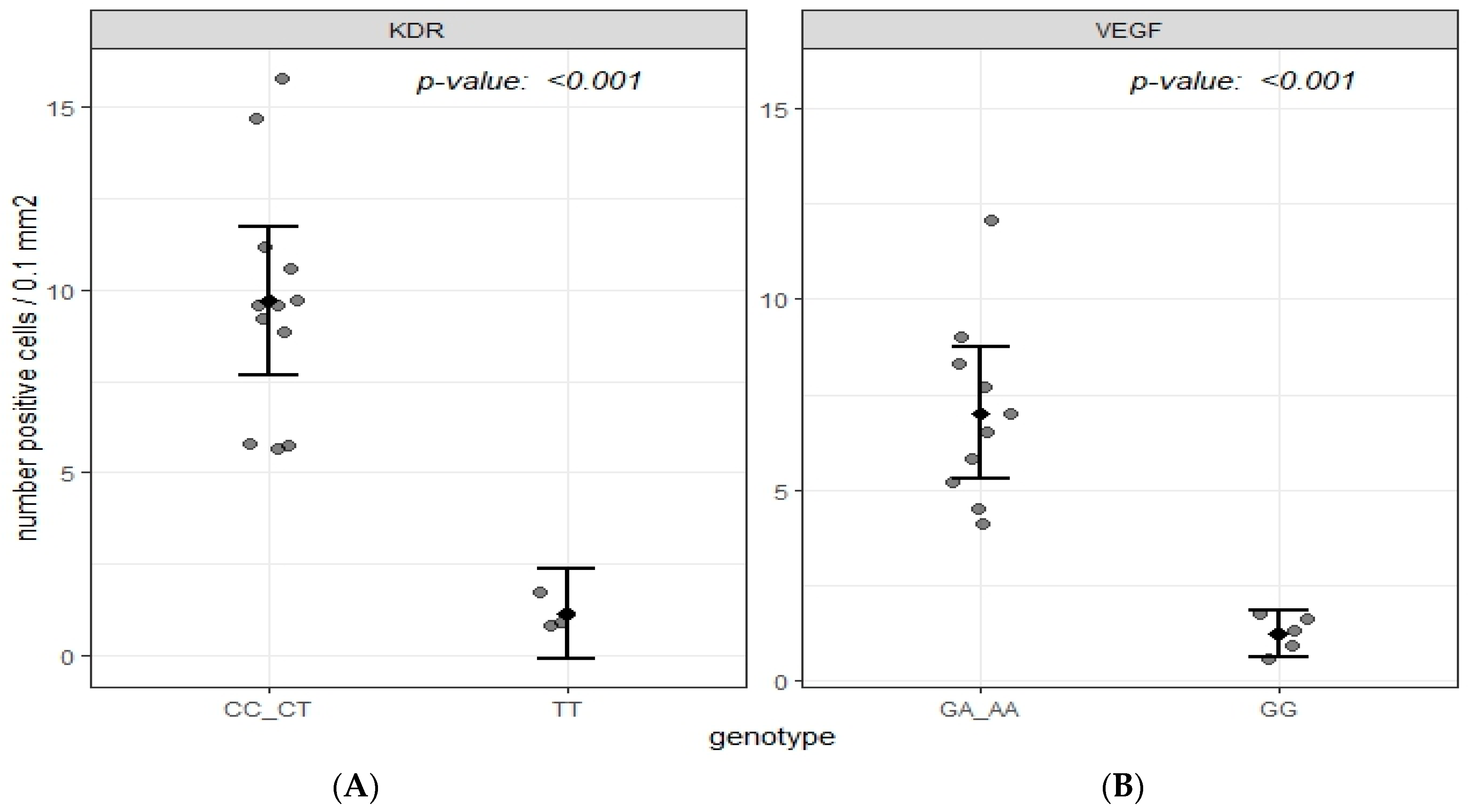
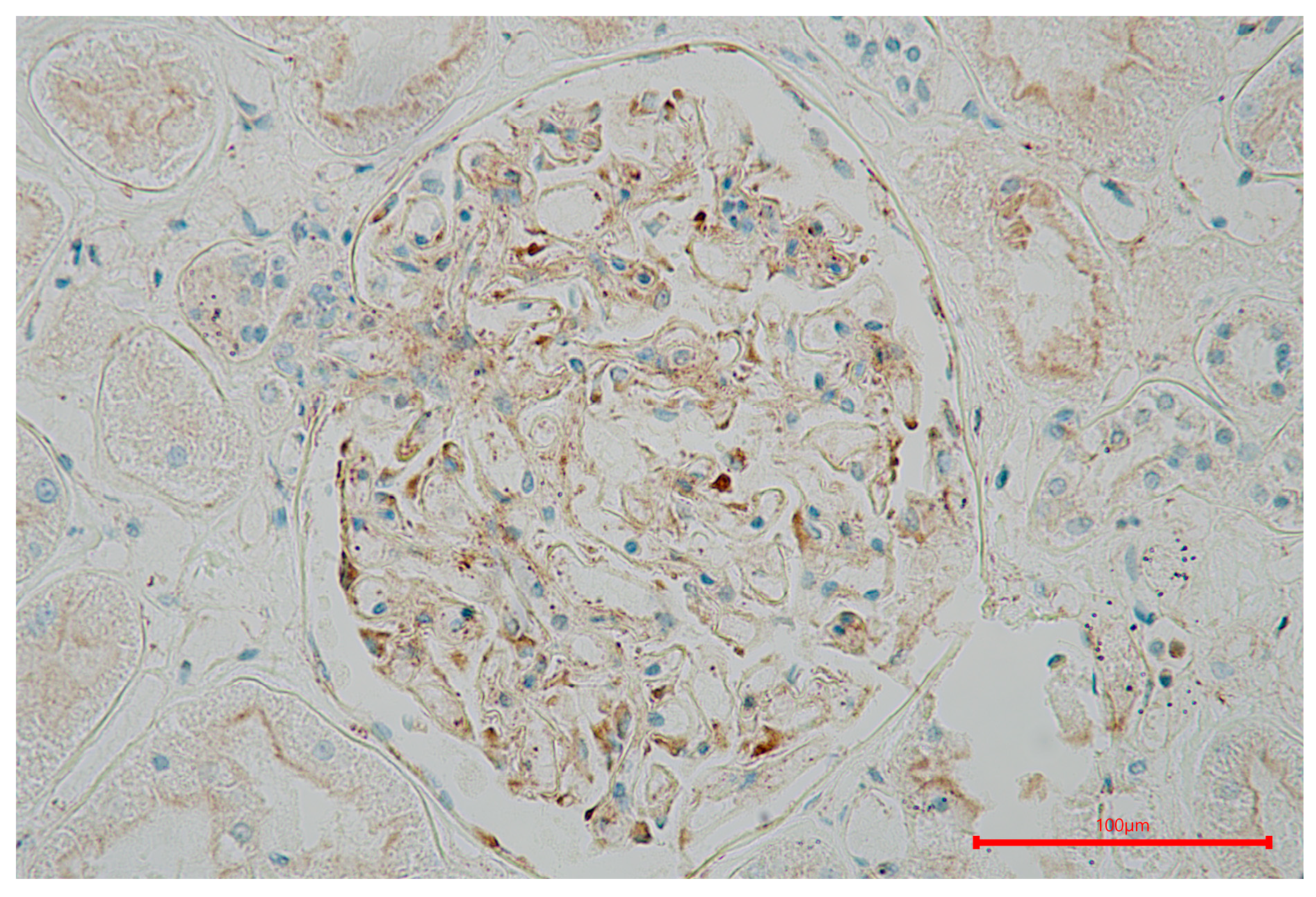
2.3. Immunohistochemical Data
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Biochemical Analyses
4.3. Genotyping
4.4. Immunohistochemistry
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ritz, E.; Orth, S.R. Nephropathy in patients with type 2 diabetes mellitus. N. Engl. J. Med. 1999, 341, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Petrica, L.; Vlad, A.; Gluhovschi, G.; Gadalean, F.; Dumitrascu, V.; Vlad, D.; Popescu, R.; Velciov, S.; Gluhovschi, C.; Bob, F.; et al. Glycated peptides are associated with the variability of endothelial dysfunction in the cerebral vessels and the kidney in type 2 diabetes mellitus patients: A cross-sectional study. J. Diabetes Complic. 2015, 29, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.B.; Florez, J.C. Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 2020, 16, 377–390. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morrish, N.J.; Wang, S.L.; Stevens, L.K.; Fuller, J.H.; Keen, H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia 2001, 44 (Suppl. 2), S14–S21. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, T.; Ninomiya, T.; Jha, V.; Neal, B.; Patrice, H.M.; Okpechi, I.; Zhao, M.H.; Lv, J.; Garg, A.X.; Knight, J.; et al. Worldwide access to treatment for end-stage kidney disease: A systematic review. Lancet 2015, 385, 1975–1982. [Google Scholar] [CrossRef] [PubMed]
- da Rocha Fernandes, J.; Ogurtsova, K.; Linnenkamp, U.; Guariguata, L.; Seuring, T.; Zhang, P.; Cavan, D.; Makaroff, L.E. IDF Diabetes Atlas estimates of 2014 global health expenditures on diabetes. Diabetes Res. Clin. Pract. 2016, 117, 48–54. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Economic Costs of Diabetes in the U.S. in 2017. Diabetes Care 2018, 41, 917–928. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yen, F.S.; Wei, J.C.; Shih, Y.H.; Hsu, C.C.; Hwu, C.M. Impact of individual microvascular disease on the risks of macrovascular complications in type 2 diabetes: A nationwide population-based cohort study. Cardiovasc. Diabetol. 2023, 22, 109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carpena, M.P.; Rados, D.V.; Sortica, D.A.; Souza, B.M.; Reis, A.F.; Canani, L.H.; Crispim, D. Genetics of diabetic nephropathy. Arq. Bras. Endocrinol. Metabol. 2010, 54, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Kohan, D.E.; Rossi, N.F.; Inscho, E.W.; Pollock, D.M. Regulation of blood pressure and salt homeostasis by endothelin. Physiol. Rev. 2011, 91, 1–77. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Conway, B.R.; Maxwell, A.P. Genetics of diabetic nephropathy: Are there clues to the understanding of common kidney diseases? Nephron Clin. Pract. 2009, 112, c213–c221. [Google Scholar] [CrossRef] [PubMed]
- Selby, N.M.; Taal, M.W. An updated overview of diabetic nephropathy: Diagnosis, prognosis, treatment goals and latest guidelines. Diabetes Obes. Metab. 2020, 22 (Suppl. 1), 3–15. [Google Scholar] [CrossRef] [PubMed]
- Logue, O.C.; McGowan, J.W.; George, E.M.; Bidwell, G.L., 3rd. Therapeutic angiogenesis by vascular endothelial growth factor supplementation for treatment of renal disease. Curr. Opin. Nephrol. Hypertens. 2016, 25, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Brennan, E.; McEvoy, C.; Sadlier, D.; Godson, C.; Martin, F. The genetics of diabetic nephropathy. Genes 2013, 4, 596–619. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Palmer, N.D.; Freedman, B.I. Insights into the genetic architecture of diabetic nephropathy. Curr. Diab Rep. 2012, 12, 423–431. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Merlo, S.; Starčević, J.N.; Mankoč, S.; Šantl Letonja, M.; Cokan Vujkovac, A.; Zorc, M.; Petrovič, D. Vascular Endothelial Growth Factor Gene Polymorphism (rs2010963) and Its Receptor, Kinase Insert Domain-Containing Receptor Gene Polymorphism (rs2071559), and Markers of Carotid Atherosclerosis in Patients with Type 2 Diabetes Mellitus. J. Diabetes Res. 2016, 2016, 1482194. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shibuya, M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes. Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cooper, M.E.; Vranes, D.; Youssef, S.; Stacker, S.A.; Cox, A.J.; Rizkalla, B.; Casley, D.J.; Bach, L.A.; Kelly, D.J.; Gilbert, R.E. Increased renal expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 in experimental diabetes. Diabetes 1999, 48, 2229–2239. [Google Scholar] [CrossRef] [PubMed]
- Sajovic, J.; Cilenšek, I.; Mankoč, S.; Tajnšek, Š.; Kunej, T.; Petrovič, D.; Globočnik Petrovič, M. Vascular endothelial growth factor (VEGF)-related polymorphisms rs10738760 and rs6921438 are not risk factors for proliferative diabetic retinopathy (PDR) in patients with type 2 diabetes mellitus (T2DM). Bosn. J. Basic Med. Sci. 2019, 19, 94–100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vincenti, V.; Cassano, C.; Rocchi, M.; Persico, G. Assignment of the vascular endothelial growth factor gene to human chromosome 6p21.3. Circulation 1996, 93, 1493–1495. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Jeon, Y.J.; Kim, H.S.; Chae, K.Y.; Oh, S.H.; Han, I.B.; Kim, H.S.; Kim, W.C.; Kim, O.J.; Kim, T.G.; et al. The role of VEGF and KDR polymorphisms in moyamoya disease and collateral revascularization. PLoS ONE 2012, 7, e47158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Globočnik Petrovič, M.; Korošec, P.; Košnik, M.; Osredkar, J.; Hawlina, M.; Peterlin, B.; Petrovič, D. Local and genetic determinants of vascular endothelial growth factor expression in advanced proliferative diabetic retinopathy. Mol. Vis. 2008, 14, 1382–1387. [Google Scholar]
- Ghazizadeh, H.; Avan, A.; Fazilati, M.; Azimi-Nezhad, M.; Tayefi, M.; Ghasemi, F.; Mehramiz, M.; Moohebati, M.; Ebrahimi, M.; Mirhafez, S.R.; et al. Association of rs6921438 A<G with serum vascular endothelial growth factor concentrations in patients with metabolic syndrome. Gene 2018, 667, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Terzić, R.; Cilenšek, I.; Zorc Pleskovič, R.; Mankoč, S.; Milutinović, A. Vascular endothelial growth factor (VEGF)-related single nucleotide polymorphisms rs10738760 and rs6921438 are not associated with diabetic retinopathy (DR) in Slovenian patients with type 2 diabetes mellitus (T2DM). Bosn. J. Basic Med. Sci. 2017, 17, 328–332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lugano, R.; Huang, H.; Dimberg, A. Vascular Endothelial Growth Factor Receptor (VEGFR). In Encyclopedia of Signaling Molecules; Choi, S., Ed.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Lavoz, C.; Rodrigues-Diez, R.R.; Plaza, A.; Carpio, D.; Egido, J.; Ruiz-Ortega, M.; Mezzano, S. VEGFR2 Blockade Improves Renal Damage in an Experimental Model of Type 2 Diabetic Nephropathy. J. Clin. Med. 2020, 9, 302. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Zheng, Y.; Zhang, W.; Yu, H.; Lou, K.; Zhang, Y.; Qin, Q.; Zhao, B.; Yang, Y.; Hui, R. Polymorphisms of KDR gene are associated with coronary heart disease. J. Am. Coll. Cardiol. 2007, 50, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Kariž, S.; Petrovič, D. Minor association of kinase insert domain-containing receptor gene polymorphism (rs2071559) with myocardial infarction in Caucasians with type 2 diabetes mellitus: Case-control cross-sectional study. Clin. Biochem. 2014, 47, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.Q.; Lee, D.Y.; Gerber, H.P.; Keyt, B.A.; Ferrara, N.; Zioncheck, T.F. Homologous up-regulation of KDR/Flk-1 receptor expression by vascular endothelial growth factor in vitro. J. Biol. Chem. 1998, 273, 29979–29985. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.A.; Abbott, C.R.; Gough, M.J. Vascular endothelial growth factor is associated with histological instability of carotid plaques. Br. J. Surg. 2008, 95, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Cilenšek, I.; Šeruga, M.; Makuc, J.; Završnik, M.; Petrovič, D. The ALOXA5AP gene (rs38022789) is associated with diabetic nephropathy in Slovenian patients with type 2 diabetes mellitus. Gene 2020, 741, 144551. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2022, 65, 1925–1966. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grbić, E.; Globočnik Petrovič, M.; Cilenšek, I.; Petrovič, D. SLC22A3 rs2048327 Polymorphism Is Associated with Diabetic Retinopathy in Caucasians with Type 2 Diabetes Mellitus. Biomedicines 2023, 11, 2303. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yuan, Y.; Shao, C.; Guan, Y.; Lu, H.; Wang, D.; Zhang, S. Association between the VEGFR-2 -604T/C polymorphism (rs2071559) and type 2 diabetic retinopathy. Open Life Sci. 2023, 18, 20220081. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qian, Y.; Ding, L.; Cao, L.; Yu, Z.; Shao, X.; Wang, L.; Zhang, M.; Wang, Q.; Che, X.; Jiang, N.; et al. Gene polymorphisms of VEGF and KDR are associated with initial fast peritoneal solute transfer rate in peritoneal dialysis. BMC Nephrol. 2022, 23, 365. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sallinen, R.; Kaunisto, M.A.; Forsblom, C.; Thomas, M.; Fagerudd, J.; Pettersson-Fernholm, K.; Groop, P.H.; Wessman, M.; Finnish Diabetic Nephropathy Study Group. Association of the SLC22A1, SLC22A2, and SLC22A3 genes encoding organic cation transporters with diabetic nephropathy and hypertension. Ann. Med. 2010, 42, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, S.P.; Reddi, A.S.; Chandran, C.B.; Chevalier, J.M.; Okechukwu, C.N.; Seshan, S.V. Collapsing glomerulopathy superimposed on diabetic nephropathy: Insights into etiology of an under-recognized, severe pattern of glomerular injury. Nephrol. Dial. Transplant. 2014, 29, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Cruz-González, F.; Cieza-Borrella, C.; Cabrillo-Estévez, L.; Cañete-Campos, C.; Escudero-Domínguez, F.; González-Sarmiento, R. VEGF A (rs699947 and rs833061) and VEGFR2 (rs2071559) gene polymorphisms are not associated with AMD susceptibility in a Spanish population. Curr. Eye Res. 2013, 38, 1274–1277. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhu, H.; Wang, X.; Tang, Q.; Huang, H.; Wu, K.; Zhu, J.; Feng, Z.; Shi, G. The patterns and expression of KDR in normal tissues of human internal organs. J. Mol. Histol. 2011, 42, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Bonnefond, A.; Saulnier, P.J.; Stathopoulou, M.G.; Grarup, N.; Ndiaye, N.C.; Roussel, R.; Nezhad, M.A.; Dechaume, A.; Lantieri, O.; Hercberg, S.; et al. What is the contribution of two genetic variants regulating VEGF levels to type 2 diabetes risk and to microvascular complications? PLoS ONE 2013, 8, e55921. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gilbert, R.E.; Vranes, D.; Berka, J.L.; Kelly, D.J.; Cox, A.; Wu, L.L.; Stacker, S.A.; Cooper, M.E. Vascular endothelial growth factor and its receptors in control and diabetic rat eyes. Lab. Investig. 1998, 78, 1017–1027. [Google Scholar] [PubMed]
- Nardi, G.M.; Ferrara, E.; Converti, I.; Cesarano, F.; Scacco, S.; Grassi, R.; Gnoni, A.; Grassi, F.R.; Rapone, B. Does Diabetes Induce the Vascular Endothelial Growth Factor (VEGF) Expression in Periodontal Tissues? A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 2765. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hammes, H.P.; Lin, J.; Bretzel, R.G.; Brownlee, M.; Breier, G. Upregulation of the vascular endothelial growth factor/vascular endothelial growth factor receptor system in experimental background diabetic retinopathy of the rat. Diabetes 1998, 47, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Aiello, L.P.; Wong, J.S. Role of vascular endothelial growth factor in diabetic vascular complications. Kidney Int. Suppl. 2000, 77, S113–S119. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cheng, H.; Jiang, S.; Zhang, L.; Liu, X.; Chen, P.; Liu, J.; Li, Y.; Liu, X.; Wang, L.; et al. The relationship between diabetic retinopathy and diabetic nephropathy in type 2 diabetes. Front. Endocrinol. 2024, 15, 1292412. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tung, C.W.; Hsu, Y.C.; Shih, Y.H.; Chang, P.J.; Lin, C.L. Glomerular mesangial cell and podocyte injuries in diabetic nephropathy. Nephrology 2018, 23 (Suppl 4), 32–37. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Gallacher, B.; Patel, H.; Orme, C. Glucose-induced protein kinase C activation regulates vascular permeability factor mRNA expression and peptide production by human vascular smooth muscle cells in vitro. Diabetes 1997, 46, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Hakroush, S.; Moeller, M.J.; Theilig, F.; Kaissling, B.; Sijmonsma, T.P.; Jugold, M.; Akeson, A.L.; Traykova-Brauch, M.; Hosser, H.; Hähnel, B.; et al. Effects of increased renal tubular vascular endothelial growth factor (VEGF) on fibrosis, cyst formation, and glomerular disease. Am. J. Pathol. 2009, 175, 1883–1895. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hasegawa, S.; Nakano, T.; Torisu, K.; Tsuchimoto, A.; Eriguchi, M.; Haruyama, N.; Masutani, K.; Tsuruya, K.; Kitazono, T. Vascular endothelial growth factor-C ameliorates renal interstitial fibrosis through lymphangiogenesis in mouse unilateral ureteral obstruction. Lab. Investig. 2017, 97, 1439–1452. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Sun, W.; Xu, C.; Gu, S.; Li, Y.; Liu, Z.; Chen, J. Genetic variants in KDR transcriptional regulatory region affect promoter activity and intramuscular fat deposition in Erhualian pigs. Anim. Genet. 2014, 45, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Harris, R.C. Renal endothelial dysfunction in diabetic nephropathy. Cardiovasc. Hematol. Disord. Drug Targets. 2014, 14, 22–33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leung, W.K.; Gao, L.; Siu, P.M.; Lai, C.W. Diabetic nephropathy and endothelial dysfunction: Current and future therapies, and emerging of vascular imaging for preclinical renal-kinetic study. Life Sci. 2016, 166, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Rahimi-Madiseh, M.; Malekpour-Tehrani, A.; Bahmani, M.; Rafieian-Kopaei, M. The research and development on the antioxidants in prevention of diabetic complications. Asian Pac. J. Trop. Med. 2016, 9, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Ma, L.-L.; Li, B.-X.; Li, M.-C. Clinical significance of vascular endothelial growth factor and endothelin-1 in serum levels as novel indicators for predicting the progression of diabetic nephropathy. Eur. J. Inflamm. 2023, 21. [Google Scholar] [CrossRef]
- Sato, Y.; Kanno, S.; Oda, N.; Abe, M.; Ito, M.; Shitara, K.; Shibuya, M. Properties of two VEGF receptors, Flt-1 and KDR, in signal transduction. Ann. N. Y. Acad. Sci. 2000, 902, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Piera-Velazquez, S.; Jimenez, S.A. Endothelial to Mesenchymal Transition: Role in Physiology and in the Pathogenesis of Human Diseases. Physiol. Rev. 2019, 99, 1281–1324. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Završnik, M.; Kariž, S.; Makuc, J.; Šeruga, M.; Cilenšek, I.; Petrovič, D. PECAM-1 Leu125Val (rs688) Polymorphism and Diabetic Nephropathy in Caucasians with Type 2 Diabetes Mellitus. Anal. Cell Pathol. 2016, 2016, 3152967. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fadini, G.P.; Albiero, M.; Bonora, B.M.; Avogaro, A. Angiogenic Abnormalities in Diabetes Mellitus: Mechanistic and Clinical Aspects. J. Clin. Endocrinol. Metab. 2019, 104, 5431–5444. [Google Scholar] [CrossRef] [PubMed]
- Letonja, J.; Završnik, M.; Makuc, J.; Šeruga, M.; Peterlin, A.; Cilenšek, I.; Petrovič, D. Sirtuin 1 rs7069102 polymorphism is associated with diabetic nephropathy in patients with type 2 diabetes mellitus. Bosn. J. Basic. Med. Sci. 2021, 21, 642–646. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, E.; Wang, H.; Chakrabarti, S. Endothelial-to-mesenchymal transition: An underappreciated mediator of diabetic complications. Front. Endocrinol. 2023, 14, 1050540. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cha, D.R.; Kim, N.H.; Yoon, J.W.; Jo, S.K.; Cho, W.Y.; Kim, H.K.; Won, N.H. Role of vascular endothelial growth factor in diabetic nephropathy. Kidney Int. Suppl. 2000, 77, S104–S112. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.E.; Tsalamandris, C.; Allen, T.J.; Colville, D.; Jerums, G. Early nephropathy predicts vision-threatening retinal disease in patients with type I diabetes mellitus. J. Am. Soc. Nephrol. 1998, 9, 85–89. [Google Scholar] [CrossRef] [PubMed]

| Cases (N = 344) | Controls (N = 553) | p-Value | |
|---|---|---|---|
| Sex [M] | 201 (58.4%) | 318 (57.5%) | 0.78 |
| Age [years] | 65.35 ± 9.37 | 65.43 ± 8.63 | 0.90 |
| Duration of T2D [years] | 15.69 ± 7.64 | 13.99 ± 7.00 | <0.001 |
| Duration of hypertension [years] | 13.65 ± 8.92 | 12.18 ± 7.83 | 0.020 |
| SBP [mmHg] | 155.08 ± 19.4 | 148.45 ± 19.61 | <0.001 |
| DBP [mmHg] | 84.45 ± 11.55 | 83.09 ± 10.40 | 0.071 |
| BMI | 30.91 ± 4.45 | 30.25 ± 4.52 | 0.031 |
| Active smokers | 32 (9.3%) | 70 (12.7%) | 0.12 |
| CVD | 89 (25.9%) | 165 (29.8%) | 0.20 |
| Family history of CVD | 0.14 | ||
| No | 286 (83.1%) | 449 (81.2%) | |
| Yes (before 55 of age) | 22 (6.4%) | 25 (4.5%) | |
| Yes (after 55 of age) | 36 (10.5%) | 79 (14.3%) | |
| DR | 149 (43.3%) | 115 (20.8%) | <0.001 |
| Duration of DR [years] | 5.00 (4.00–7.00) | 5.00 (5.00–8.00) | 0.23 |
| Dneuropathy | 69 (20.1%) | 39 (7.1%) | <0.001 |
| DF | 62 (18.0%) | 40 (7.2%) | <0.001 |
| S-HbA1c [%] 1 | 7.93 ± 0.28 | 7.65 ± 1.14 | 0.002 |
| S-fasting glucose [mmol/L] | 8.80 (7.25–10.60) | 8.10 (6.80–9.70) | <0.001 |
| S-Hb [g/L] | 138.55 ± 14.17 | 138.98 ± 12.88 | 0.69 |
| S-urea [mmol/L] | 6.40 (5.40–8.20) | 6.00 (4.90–7.60) | <0.001 |
| S-creatinine [µmol/L] | 82.00 (68.00–105.00) | 77.00 (66.00–91.50) | <0.001 |
| Male sex | 93.00 (75.00–109.00) | 84.00 (70.00–98.00) | <0.001 |
| Female sex | 73.00 (57.00–95.25) | 70.00 (61.00–82.00) | 0.041 |
| eGFR [MDRD equation, mL/min] | 75.00 (60.00–90.00) | 76.00 (60.00–90.00) | 0.15 |
| Male sex | 93.00 (75.00–109.00) | 84.00 (70.00–98.00) | <0.001 |
| Female sex | 73.00 (57.00–95.25) | 70.00 (61.00–82.00) | 0.041 |
| S-cystatinC [mg/L] | 0.82 (0.69–1.04) | 0.75 (0.65–0.86) | <0.001 |
| S-Totalcholesterol [mmol/L] | 4.40 (3.80–5.23) | 4.40 (3.90–5.20) | 0.41 |
| S-HDL [mmol/L] | 1.20 (1.00–1.40) | 1.20 (1.00–1.40) | 0.83 |
| S-LDL [mmol/L] | 2.50 (2.00–3.10) | 2.50 (2.00–3.10) | 0.45 |
| S-TGS [mmol/L] | 1.60 (1.10–2.50) | 1.50 (1.00–2.20) | 0.023 |
| U-albumin/creatinine ratio [g/moL], sample no. 1 | 8.14 (3.80–23.60) | 0.99 (0.60–1.58) | <0.001 |
| U-albumin/creatinine ratio [g/moL], sample no. 2 | 8.26 (3.70–25.10) | 1.02 (0.68–1.70) | <0.001 |
| U-albumin/creatinine ratio [g/moL], sample no. 3 | 7.98 (3.82–22.82) | 1.02 (0.68–1.73) | <0.001 |
| Cases (N = 344) | Controls (N = 553) | p-Value | |
|---|---|---|---|
| KDR_rs2071559 | |||
| CC | 88 (25.6%) | 122 (22.1%) | 0.034 |
| CT | 182 (52.9%) | 269 (48.6%) | |
| TT | 74 (21.5%) | 162 (29.3%) | |
| KDR_rs2305948 | |||
| TT | 2 (0.6%) | 11 (2.0%) | 0.16 |
| CT | 73 (21.2%) | 103 (18.6%) | |
| CC | 269 (78.2%) | 439 (79.4%) | |
| HWE | |||
| KDR_rs2071559 | 0.2667 | 0.6048 | |
| KDR_rs2305948 | 0.2104 | 0.0950 | |
| ALLELES | |||
| KDR_rs2071559 | |||
| C (MAF) | 358 (52.0%) | 513 (46.4%) | 0.020 |
| T | 330 (48.0%) | 593 (53.6%) | |
| KDR_rs2305948 | |||
| T (MAF) | 77 (11.2%) | 125 (11.3%) | 0.94 |
| C | 611 (88.8%) | 981 (88.7%) | |
| DOMINANT | |||
| KDR_rs2071559 | |||
| CC + CT | 270 (78.5%) | 391 (70.7%) | 0.010 |
| TT | 74 (21.5%) | 162 (29.3%) | |
| KDR_rs2305948 | |||
| TT + CT | 75 (21.8%) | 114 (20.6%) | 0.67 |
| CC | 269 (78.2%) | 439 (79.4%) | |
| RECESSIVE | |||
| KDR_rs2071559 | |||
| CC | 88 (25.6%) | 122 (22.1%) | 0.23 |
| CT + TT | 256 (74.4%) | 431 (77.9%) | |
| KDR_rs2305948 | |||
| TT | 2 (0.6%) | 11 (2.0%) | 0.086 |
| CT + CC | 342 (99.4%) | 542 (98.0%) | |
| CT + CC | 342 (99.4%) | 542 (98.0%) |
| VEGF_rs6921438 | Cases (N = 344) | Controls (N = 553) | p-Value |
|---|---|---|---|
| GG | 82 (23.8%) | 180 (32.5%) | 0.007 |
| AG | 176 (51.2%) | 271 (49.0%) | |
| AA | 86 (25.0%) | 102 (18.4%) | |
| ALLELES | |||
| G (MAF) | 340 (49.4%) | 631 (57.1%) | 0.002 |
| A | 348 (50.6%) | 475 (42.9%) | |
| HWE (p-value) | 0.2667 | 0.6048 | |
| DOMINANT | |||
| GG + AG | 258 (75.0%) | 451 (81.6%) | 0.019 |
| AA | 86 (25.0%) | 102 (18.4%) | |
| RECESSIVE | |||
| GG | 82 (23.8%) | 180 (32.5%) | 0.005 |
| AG + AA | 262 (76.2%) | 373 (67.5%) |
| KDR_rs20715599 | Count | OR (95% CI) | p-Value for OR |
|---|---|---|---|
| co-dominant | |||
| CC vs. TT | 88/122 vs. 74/162 | 1.61 (1.02–2.56) | 0.042 |
| CT vs. TT | 182/269 vs. 74/162 | 1.60 (1.08–2.39) | 0.021 |
| dominant | |||
| [CC + CT] vs. TT | 270/391 vs. 74/162 | 1.61 (1.11–2.35) | 0.013 |
| Recessive | |||
| CC vs. [CT + TT] | 88/122 vs. 256/431 | 1.16 (0.79–1.68) | 0.45 |
| KDR_rs2305948 | count | OR (95% CI) | p-value for OR |
| co-dominant | |||
| TT vs. CC | 2/11 vs. 269/439 | 0.21 (0.01–1.38) | 0.17 |
| CT vs. CC | 73/103 vs. 269/439 | 1.53 (0.80–2.93) | 0.20 |
| dominant | |||
| [TT + CT] vs. CC | 75/114 vs. 269/439 | 1.27 (0.69–2.35) | 0.44 |
| recessive | |||
| TT vs. [CT + CC] | 2/11 vs. 342/542 | 0.21 (0.01–1.32) | 0.16 |
| VEGF _rs6921438 | Count | OR (95% CI) | p-Value for OR |
|---|---|---|---|
| co-dominant | |||
| GG vs. AA | 82/180 vs. 86/102 | 0.51 (0.32–0.8) | 0.004 |
| AG vs. AA | 176/271 vs. 86/102 | 0.76 (0.5–1.13) | 0.17 |
| dominant | |||
| [GG + AG] vs. AA | 258/451 vs. 86/102 | 0.66 (0.45–0.96) | 0.030 |
| Recessive | |||
| GG vs. [AG + AA] | 82/180 vs. 262/373 | 0.61 (0.43–0.88) | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nussdorfer, P.; Petrovič, D.; Alibegović, A.; Cilenšek, I.; Petrovič, D. The KDR Gene rs2071559 and the VEGF Gene rs6921438 May Be Associated with Diabetic Nephropathy in Caucasians with Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2024, 25, 9439. https://doi.org/10.3390/ijms25179439
Nussdorfer P, Petrovič D, Alibegović A, Cilenšek I, Petrovič D. The KDR Gene rs2071559 and the VEGF Gene rs6921438 May Be Associated with Diabetic Nephropathy in Caucasians with Type 2 Diabetes Mellitus. International Journal of Molecular Sciences. 2024; 25(17):9439. https://doi.org/10.3390/ijms25179439
Chicago/Turabian StyleNussdorfer, Petra, David Petrovič, Armin Alibegović, Ines Cilenšek, and Danijel Petrovič. 2024. "The KDR Gene rs2071559 and the VEGF Gene rs6921438 May Be Associated with Diabetic Nephropathy in Caucasians with Type 2 Diabetes Mellitus" International Journal of Molecular Sciences 25, no. 17: 9439. https://doi.org/10.3390/ijms25179439






