Purinergic Signaling in Non-Parenchymal Liver Cells
Abstract
1. Introduction
2. The Liver and Chronic Disease
2.1. Inflammation
2.2. Fibrosis
2.3. Cirrhosis
2.4. Hepatocellular Carcinoma
3. Purines and Cell Signaling
4. Purines in Liver Pathology
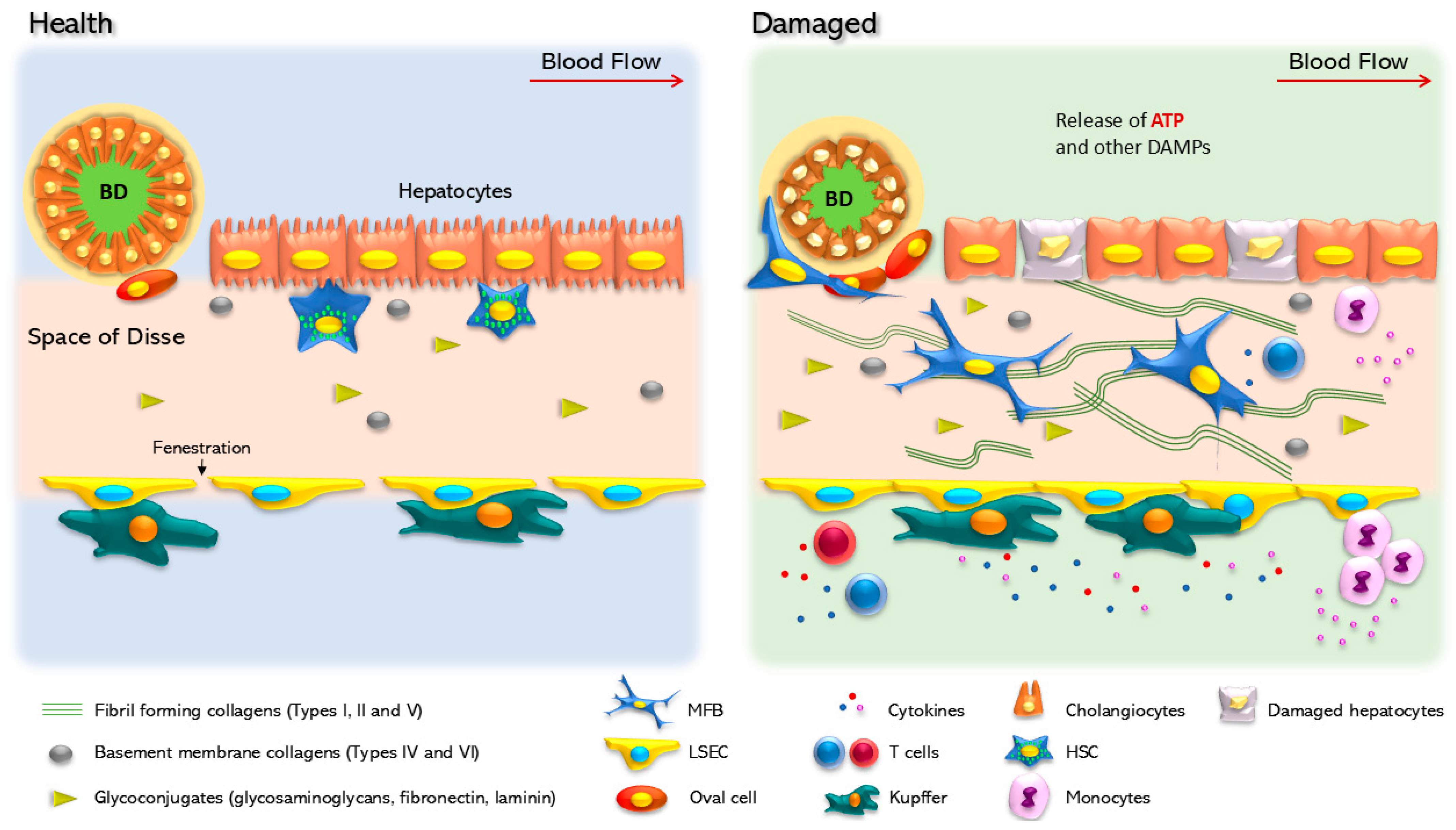
5. Purinergic Signaling in Non-Parenchymal Liver Cells
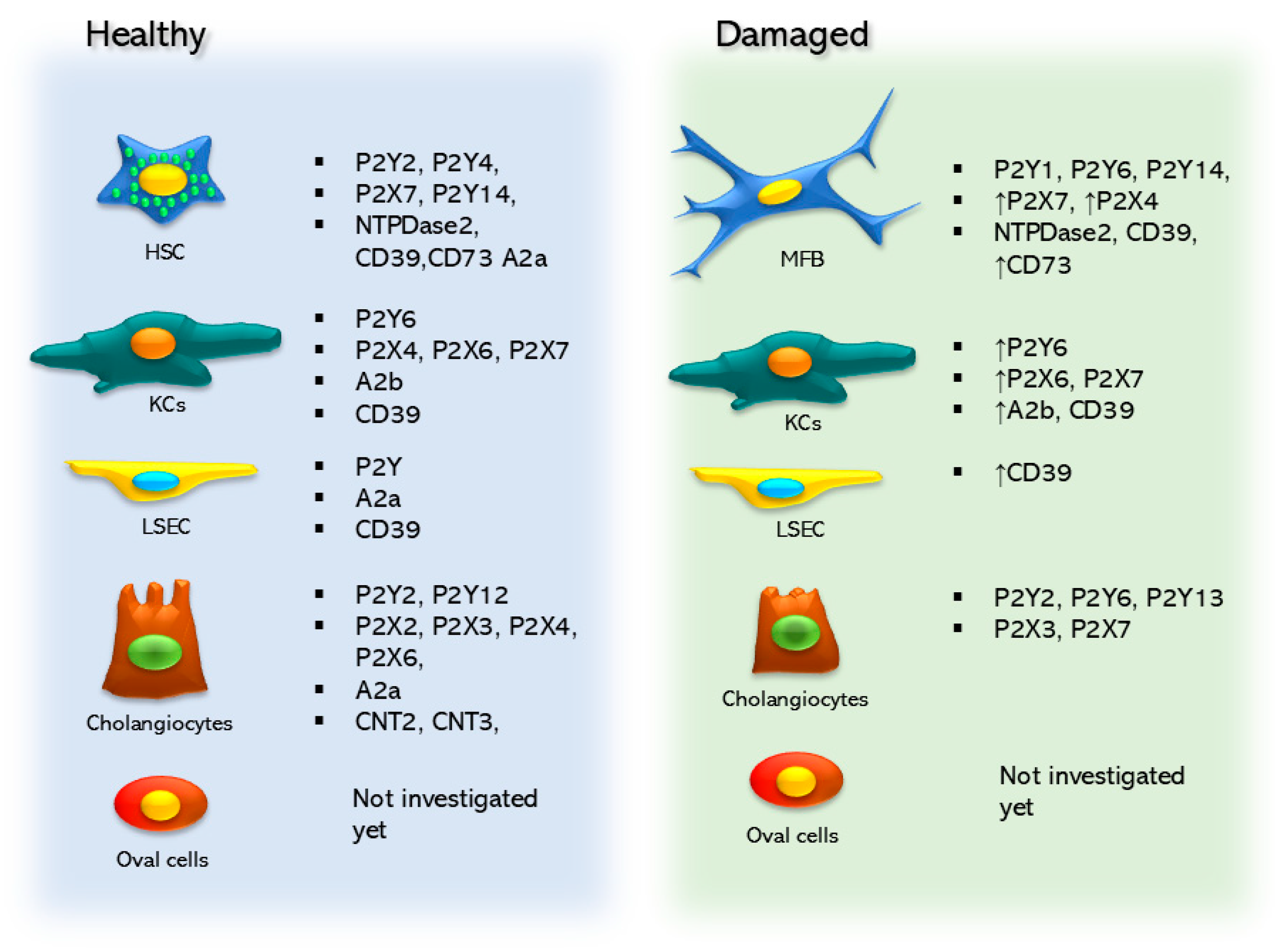
5.1. Hepatic Stellate Cells
5.1.1. Nucleotide Signaling in Hepatic Stellate Cells
5.1.2. Ectonucleotidases in Hepatic Stellate Cells
5.1.3. Adenosine Signaling in Hepatic Stellate Cells
5.2. Kupffer Cells
Nucleotide Signaling in Kupffer Cells
5.3. Purinergic Signaling in Liver Sinusoidal Endothelial Cells
5.4. Purinergic Signaling in Cholangiocytes
5.4.1. Nucleotide Signaling in Cholangiocytes
5.4.2. Adenosine Signaling in Cholangiocytes
6. Therapeutic Targeting of Purinergic Signaling in Non-Parenchymal Liver Cells
7. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Taub, R. Liver Regeneration: From Myth to Mechanism. Nat. Rev. Mol. Cell Biol. 2004, 5, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, R.; Matz-Soja, M. Liver Zonation: Novel Aspects of Its Regulation and Its Impact on Homeostasis. World J. Gastroenterol. 2014, 20, 8491–8504. [Google Scholar] [CrossRef]
- Jungermann, K.; Katz, N. Functional Specialization of Different Hepatocyte Populations. Physiol. Rev. 1989, 69, 708–764. [Google Scholar] [CrossRef] [PubMed]
- Ghafoory, S.; Stengl, C.; Kopany, S.; Mayadag, M.; Mechtel, N.; Murphy, B.; Schattschneider, S.; Wilhelmi, N.; Wölfl, S. Oxygen Gradient Induced in Microfluidic Chips Can Be Used as a Model for Liver Zonation. Cells 2022, 11, 3734. [Google Scholar] [CrossRef]
- Ma, R.; Martínez-Ramírez, A.S.; Borders, T.L.; Gao, F.; Sosa-Pineda, B. Metabolic and Non-Metabolic Liver Zonation Is Established Non-Synchronously and Requires Sinusoidal Wnts. eLife 2020, 9, e46206. [Google Scholar] [CrossRef]
- Michalopoulos, G.K.; Bhushan, B. Liver Regeneration: Biological and Pathological Mechanisms and Implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Yagi, S.; Hirata, M.; Miyachi, Y.; Uemoto, S. Liver Regeneration after Hepatectomy and Partial Liver Transplantation. Int. J. Mol. Sci. 2020, 21, 8414. [Google Scholar] [CrossRef]
- Shaker, M.E. The Contribution of Sterile Inflammation to the Fatty Liver Disease and the Potential Therapies. Biomed. Pharmacother. 2022, 148, 112789. [Google Scholar] [CrossRef]
- Yuen, M.-F.; Chen, D.-S.; Dusheiko, G.M.; Janssen, H.L.A.; Lau, D.T.Y.; Locarnini, S.A.; Peters, M.G.; Lai, C.-L. Hepatitis B Virus Infection. Nat. Rev. Dis. Primers 2018, 4, 18035. [Google Scholar] [CrossRef]
- Mihm, S. Danger-Associated Molecular Patterns (DAMPs): Molecular Triggers for Sterile Inflammation in the Liver. Int. J. Mol. Sci. 2018, 19, 3104. [Google Scholar] [CrossRef]
- Gong, J.; Tu, W.; Liu, J.; Tian, D. Hepatocytes: A Key Role in Liver Inflammation. Front. Immunol. 2022, 13, 1083780. [Google Scholar] [CrossRef]
- Yang Zhou, J. Innate Immunity and Early Liver Inflammation. Front. Immunol. 2023, 14, 1175147. [Google Scholar] [CrossRef]
- Tiegs, G.; Horst, A.K. TNF in the Liver: Targeting a Central Player in Inflammation. Semin. Immunopathol. 2022, 44, 445–459. [Google Scholar] [CrossRef]
- Koyama, Y.; Brenner, D.A. Liver Inflammation and Fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Ju, D. Inflammasome: A Double-Edged Sword in Liver Diseases. Front. Immunol. 2018, 9, 2201. [Google Scholar] [CrossRef]
- Yu, C.; Chen, P.; Miao, L.; Di, G. The Role of the NLRP3 Inflammasome and Programmed Cell Death in Acute Liver Injury. Int. J. Mol. Sci. 2023, 24, 3067. [Google Scholar] [CrossRef] [PubMed]
- Barbier, L.; Ferhat, M.; Salamé, E.; Robin, A.; Herbelin, A.; Gombert, J.-M.; Silvain, C.; Barbarin, A. Interleukin-1 Family Cytokines: Keystones in Liver Inflammatory Diseases. Front. Immunol. 2019, 10, 2014. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, B.; Leszczynska, A.; Reca, A.; Booshehri, L.M.; Onyuru, J.; Tan, Z.; Wree, A.; Friess, H.; Hartmann, D.; Papouchado, B.; et al. NLRP3 Activation in Neutrophils Induces Lethal Autoinflammation, Liver Inflammation, and Fibrosis. EMBO Rep. 2022, 23, e54446. [Google Scholar] [CrossRef] [PubMed]
- Baiocchini, A.; Montaldo, C.; Conigliaro, A.; Grimaldi, A.; Correani, V.; Mura, F.; Ciccosanti, F.; Rotiroti, N.; Brenna, A.; Montalbano, M.; et al. Extracellular Matrix Molecular Remodeling in Human Liver Fibrosis Evolution. PLoS ONE 2016, 11, e0151736. [Google Scholar] [CrossRef]
- Duarte, S.; Baber, J.; Fujii, T.; Coito, A.J. Matrix Metalloproteinases in Liver Injury, Repair and Fibrosis. Matrix Biol. 2015, 44–46, 147–156. [Google Scholar] [CrossRef]
- Elpek, G.Ö. Cellular and Molecular Mechanisms in the Pathogenesis of Liver Fibrosis: An Update. World J. Gastroenterol. 2014, 20, 7260–7276. [Google Scholar] [CrossRef]
- Kisseleva, T.; Brenner, D. Molecular and Cellular Mechanisms of Liver Fibrosis and Its Regression. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151–166. [Google Scholar] [CrossRef]
- Massey, V.L.; Dolin, C.E.; Poole, L.G.; Hudson, S.V.; Siow, D.L.; Brock, G.N.; Merchant, M.L.; Wilkey, D.W.; Arteel, G.E. The Hepatic “Matrisome” Responds Dynamically to Injury: Characterization of Transitional Changes to the Extracellular Matrix in Mice. Hepatology 2017, 65, 969–982. [Google Scholar] [CrossRef]
- Zhang, M.; Serna-Salas, S.; Damba, T.; Borghesan, M.; Demaria, M.; Moshage, H. Hepatic Stellate Cell Senescence in Liver Fibrosis: Characteristics, Mechanisms and Perspectives. Mech. Ageing Dev. 2021, 199, 111572. [Google Scholar] [CrossRef] [PubMed]
- Roehlen, N.; Crouchet, E.; Baumert, T.F. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells 2020, 9, 875. [Google Scholar] [CrossRef]
- Fallowfield, J.A.; Mizuno, M.; Kendall, T.J.; Constandinou, C.M.; Benyon, R.C.; Duffield, J.S.; Iredale, J.P. Scar-Associated Macrophages Are a Major Source of Hepatic Matrix Metalloproteinase-13 and Facilitate the Resolution of Murine Hepatic Fibrosis. J. Immunol. 2007, 178, 5288–5295. [Google Scholar] [CrossRef] [PubMed]
- Kisseleva, T.; Cong, M.; Paik, Y.; Scholten, D.; Jiang, C.; Benner, C.; Iwaisako, K.; Moore-Morris, T.; Scott, B.; Tsukamoto, H.; et al. Myofibroblasts Revert to an Inactive Phenotype during Regression of Liver Fibrosis. Proc. Natl. Acad. Sci. USA 2012, 109, 9448–9453. [Google Scholar] [CrossRef] [PubMed]
- Schnabl, B.; Kweon, Y.O.; Frederick, J.P.; Wang, X.F.; Rippe, R.A.; Brenner, D.A. The Role of Smad3 in Mediating Mouse Hepatic Stellate Cell Activation. Hepatology 2001, 34, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global Burden of Liver Disease: 2023 Update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Liu, Y.; Zhong, J.; Wang, J.; Sun, L.; Yu, L.; Wang, Y.; Li, Q.; Jin, W.; et al. Remodeling Liver Microenvironment by L-Arginine Loaded Hollow Polydopamine Nanoparticles for Liver Cirrhosis Treatment. Biomaterials 2023, 295, 122028. [Google Scholar] [CrossRef]
- Pellicoro, A.; Ramachandran, P.; Iredale, J.P.; Fallowfield, J.A. Liver Fibrosis and Repair: Immune Regulation of Wound Healing in a Solid Organ. Nat. Rev. Immunol. 2014, 14, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Ginès, P.; Krag, A.; Abraldes, J.G.; Solà, E.; Fabrellas, N.; Kamath, P.S. Liver Cirrhosis. Lancet 2021, 398, 1359–1376. [Google Scholar] [CrossRef]
- Trebicka, J.; Macnaughtan, J.; Schnabl, B.; Shawcross, D.L.; Bajaj, J.S. The Microbiota in Cirrhosis and Its Role in Hepatic Decompensation. J. Hepatol. 2021, 75 (Suppl. S1), S67–S81. [Google Scholar] [CrossRef]
- Hasa, E.; Hartmann, P.; Schnabl, B. Liver Cirrhosis and Immune Dysfunction. Int. Immunol. 2022, 34, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, K.; Osawa, Y.; Kimura, K. Wnt/β-Catenin Signaling as a Potential Target for the Treatment of Liver Cirrhosis Using Antifibrotic Drugs. Int. J. Mol. Sci. 2018, 19, 3103. [Google Scholar] [CrossRef]
- Hartke, J.; Johnson, M.; Ghabril, M. The Diagnosis and Treatment of Hepatocellular Carcinoma. Semin. Diagn. Pathol. 2017, 34, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Zajkowska, M.; Mroczko, B. Chemokines in Primary Liver Cancer. Int. J. Mol. Sci. 2022, 23, 8846. [Google Scholar] [CrossRef]
- Plentz, R.R.; Schlegelberger, B.; Flemming, P.; Gebel, M.; Kreipe, H.; Manns, M.P.; Rudolph, K.L.; Wilkens, L. Telomere Shortening Correlates with Increasing Aneuploidy of Chromosome 8 in Human Hepatocellular Carcinoma. Hepatology 2005, 42, 522–526. [Google Scholar] [CrossRef]
- Marrogi, A.J.; Khan, M.A.; van Gijssel, H.E.; Welsh, J.A.; Rahim, H.; Demetris, A.J.; Kowdley, K.V.; Hussain, S.P.; Nair, J.; Bartsch, H.; et al. Oxidative Stress and p53 Mutations in the Carcinogenesis of Iron Overload-Associated Hepatocellular Carcinoma. J. Natl. Cancer Inst. 2001, 93, 1652–1655. [Google Scholar] [CrossRef]
- Díaz-Muñoz, M.; Salín-Pascual, R. Purine Molecules as Hypnogenic Factors Role of Adenosine, ATP, and Caffeine. Cent. Nerv. Syst. Agents Med. Chem. 2010, 10, 259–268. [Google Scholar] [CrossRef]
- Vultaggio-Poma, V.; Falzoni, S.; Salvi, G.; Giuliani, A.L.; Di Virgilio, F. Signalling by Extracellular Nucleotides in Health and Disease. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2022, 1869, 119237. [Google Scholar] [CrossRef] [PubMed]
- Camici, M.; Garcia-Gil, M.; Tozzi, M.G. The Inside Story of Adenosine. Int. J. Mol. Sci. 2018, 19, 784. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, H. History of Ectonucleotidases and Their Role in Purinergic Signaling. Biochem. Pharmacol. 2021, 187, 114322. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Carlón, N.; Salgado-García, R.L.; Guerrero-Tortolero, D.A.; Kraffe, E.; Campos-Ramos, R.; Racotta, I.S. Biochemical Composition and Adenylate Energy Charge Shifts in Longfin Yellowtail (Seriola rivoliana) Embryos during Development under Different Temperatures. J. Therm. Biol. 2023, 112, 103470. [Google Scholar] [CrossRef]
- Dale, N. Biological Insights from the Direct Measurement of Purine Release. Biochem. Pharmacol. 2021, 187, 114416. [Google Scholar] [CrossRef]
- O’Grady, S.M.; Kita, H. ATP Functions as a Primary Alarmin in Allergen-Induced Type 2 Immunity. Am. J. Physiol. Cell Physiol. 2023, 325, C1369–C1386. [Google Scholar] [CrossRef]
- Alvarez, C.L.; Troncoso, M.F.; Espelt, M.V. Extracellular ATP and Adenosine in Tumor Microenvironment: Roles in Epithelial-Mesenchymal Transition, Cell Migration, and Invasion. J. Cell. Physiol. 2022, 237, 389–400. [Google Scholar] [CrossRef]
- Mohlin, C.; Säve, S.; Nilsson, M.; Persson, K. Studies of the Extracellular ATP-Adenosine Pathway in Human Urinary Tract Epithelial Cells. Pharmacology 2009, 84, 196–202. [Google Scholar] [CrossRef]
- Moreno, E.; Canet, J.; Gracia, E.; Lluís, C.; Mallol, J.; Canela, E.I.; Cortés, A.; Casadó, V. Molecular Evidence of Adenosine Deaminase Linking Adenosine A Receptor and CD26 Proteins. Front. Pharmacol. 2018, 9, 106. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Burnstock, G. Purinergic and Glutamatergic Receptors on Astroglia. Adv. Neurobiol. 2014, 11, 55–79. [Google Scholar]
- Ase, A.R.; Therrien, É.; Séguéla, P. An Allosteric Inhibitory Site Conserved in the Ectodomain of P2X Receptor Channels. Front. Cell. Neurosci. 2019, 13, 121. [Google Scholar] [CrossRef] [PubMed]
- Von Kügelgen, I. Pharmacological Characterization of P2Y Receptor Subtypes—An Update. Purinergic Signal. 2024, 20, 99–108. [Google Scholar] [CrossRef]
- Effendi, W.I.; Nagano, T.; Kobayashi, K.; Nishimura, Y. Focusing on Adenosine Receptors as a Potential Targeted Therapy in Human Diseases. Cells 2020, 9, 785. [Google Scholar] [CrossRef] [PubMed]
- Linden, J.; Koch-Nolte, F.; Dahl, G. Purine Release, Metabolism, and Signaling in the Inflammatory Response. Annu. Rev. Immunol. 2019, 37, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Reklow, R.J.; Alvares, T.S.; Zhang, Y.; Miranda Tapia, A.P.; Biancardi, V.; Katzell, A.K.; Frangos, S.M.; Hansen, M.A.; Toohey, A.W.; Cass, C.E.; et al. The Purinome and the preBötzinger Complex—A Ménage of Unexplored Mechanisms That May Modulate/Shape the Hypoxic Ventilatory Response. Front. Cell. Neurosci. 2019, 13, 365. [Google Scholar] [CrossRef]
- Vaughn, B.P.; Robson, S.C.; Longhi, M.S. Purinergic Signaling in Liver Disease. Dig. Dis. 2014, 32, 516–524. [Google Scholar] [CrossRef]
- Velázquez-Miranda, E.; Díaz-Muñoz, M.; Vázquez-Cuevas, F.G. Purinergic Signaling in Hepatic Disease. Purinergic Signal. 2019, 15, 477–489. [Google Scholar] [CrossRef]
- Jain, S.; Jacobson, K.A. Purinergic Signaling in Liver Pathophysiology. Front. Endocrinol. 2021, 12, 718429. [Google Scholar] [CrossRef]
- Tackett, B.C.; Sun, H.; Mei, Y.; Maynard, J.P.; Cheruvu, S.; Mani, A.; Hernandez-Garcia, A.; Vigneswaran, N.; Karpen, S.J.; Thevananther, S. P2Y2 Purinergic Receptor Activation Is Essential for Efficient Hepatocyte Proliferation in Response to Partial Hepatectomy. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G1073–G1087. [Google Scholar] [CrossRef] [PubMed]
- Besnard, A.; Gautherot, J.; Julien, B.; Tebbi, A.; Garcin, I.; Doignon, I.; Péan, N.; Gonzales, E.; Cassio, D.; Grosse, B.; et al. The P2X4 Purinergic Receptor Impacts Liver Regeneration after Partial Hepatectomy in Mice through the Regulation of Biliary Homeostasis. Hepatology 2016, 64, 941–953. [Google Scholar] [CrossRef]
- Gonzales, E.; Julien, B.; Serrière-Lanneau, V.; Nicou, A.; Doignon, I.; Lagoudakis, L.; Garcin, I.; Azoulay, D.; Duclos-Vallée, J.-C.; Castaing, D.; et al. ATP Release after Partial Hepatectomy Regulates Liver Regeneration in the Rat. J. Hepatol. 2010, 52, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Ohana, G.; Cohen, S.; Rath-Wolfson, L.; Fishman, P. A3 Adenosine Receptor Agonist, CF102, Protects against Hepatic Ischemia/reperfusion Injury Following Partial Hepatectomy. Mol. Med. Rep. 2016, 14, 4335–4341. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Imai, M.; Nowak-Machen, M.; Guckelberger, O.; Enjyoji, K.; Wu, Y.; Khalpey, Z.; Berberat, P.; Munasinghe, J.; Robson, S.C. Liver Damage and Systemic Inflammatory Responses Are Exacerbated by the Genetic Deletion of CD39 in Total Hepatic Ischemia. Purinergic Signal. 2011, 7, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Dranoff, J.A.; Kruglov, E.A.; Abreu-Lanfranco, O.; Nguyen, T.; Arora, G.; Jain, D. Prevention of Liver Fibrosis by the Purinoceptor Antagonist Pyridoxal-Phosphate-6-Azophenyl-2′,4′-Disulfonate (PPADS). In Vivo 2007, 21, 957–965. [Google Scholar] [PubMed]
- Huang, C.; Yu, W.; Cui, H.; Wang, Y.; Zhang, L.; Han, F.; Huang, T. P2X7 Blockade Attenuates Mouse Liver Fibrosis. Mol. Med. Rep. 2014, 9, 57–62. [Google Scholar] [CrossRef][Green Version]
- Jiang, M.; Cui, B.-W.; Wu, Y.-L.; Zhang, Y.; Shang, Y.; Liu, J.; Yang, H.-X.; Qiao, C.-Y.; Zhan, Z.-Y.; Ye, H.; et al. P2X7R Orchestrates the Progression of Murine Hepatic Fibrosis by Making a Feedback Loop from Macrophage to Hepatic Stellate Cells. Toxicol. Lett. 2020, 333, 22–32. [Google Scholar] [CrossRef]
- Velázquez-Miranda, E.; Molina-Aguilar, C.; González-Gallardo, A.; Vázquez-Martínez, O.; Díaz-Muñoz, M.; Vázquez-Cuevas, F.G. Increased Purinergic Responses Dependent on P2Y2 Receptors in Hepatocytes from CCl4-Treated Fibrotic Mice. Int. J. Mol. Sci. 2020, 21, 2305. [Google Scholar] [CrossRef]
- Chan, E.S.L.; Montesinos, M.C.; Fernandez, P.; Desai, A.; Delano, D.L.; Yee, H.; Reiss, A.B.; Pillinger, M.H.; Chen, J.-F.; Schwarzschild, M.A.; et al. Adenosine A2A Receptors Play a Role in the Pathogenesis of Hepatic Cirrhosis. Br. J. Pharmacol. 2006, 148, 1144–1155. [Google Scholar] [CrossRef]
- Yang, P.; Chen, P.; Wang, T.; Zhan, Y.; Zhou, M.; Xia, L.; Cheng, R.; Guo, Y.; Zhu, L.; Zhang, J. Loss of A1 Adenosine Receptor Attenuates Alpha-Naphthylisothiocyanate-Induced Cholestatic Liver Injury in Mice. Toxicol. Sci. 2013, 131, 128–138. [Google Scholar] [CrossRef]
- Yang, P.; Han, Z.; Chen, P.; Zhu, L.; Wang, S.; Hua, Z.; Zhang, J. A Contradictory Role of A1 Adenosine Receptor in Carbon Tetrachloride- and Bile Duct Ligation-Induced Liver Fibrosis in Mice. J. Pharmacol. Exp. Ther. 2010, 332, 747–754. [Google Scholar] [CrossRef]
- Chatterjee, S.; Rana, R.; Corbett, J.; Kadiiska, M.B.; Goldstein, J.; Mason, R.P. P2X7 Receptor-NADPH Oxidase Axis Mediates Protein Radical Formation and Kupffer Cell Activation in Carbon Tetrachloride-Mediated Steatohepatitis in Obese Mice. Free Radic. Biol. Med. 2012, 52, 1666–1679. [Google Scholar] [CrossRef]
- Feldbrügge, L.; Jiang, Z.G.; Csizmadia, E.; Mitsuhashi, S.; Tran, S.; Yee, E.U.; Rothweiler, S.; Vaid, K.A.; Sévigny, J.; Schmelzle, M.; et al. Distinct Roles of Ecto-Nucleoside Triphosphate Diphosphohydrolase-2 (NTPDase2) in Liver Regeneration and Fibrosis. Purinergic Signal. 2018, 14, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Amaral, S.S.; Oliveira, A.G.; Marques, P.E.; Quintão, J.L.D.; Pires, D.A.; Resende, R.R.; Sousa, B.R.; Melgaço, J.G.; Pinto, M.A.; Russo, R.C.; et al. Altered Responsiveness to Extracellular ATP Enhances Acetaminophen Hepatotoxicity. Cell Commun. Signal. 2013, 11, 10. [Google Scholar] [CrossRef]
- Abdelaziz, H.A.; Shaker, M.E.; Hamed, M.F.; Gameil, N.M. Repression of Acetaminophen-Induced Hepatotoxicity by a Combination of Celastrol and Brilliant Blue G. Toxicol. Lett. 2017, 275, 6–18. [Google Scholar] [CrossRef]
- Xie, Y.; Williams, C.D.; McGill, M.R.; Lebofsky, M.; Ramachandran, A.; Jaeschke, H. Purinergic Receptor Antagonist A438079 Protects against Acetaminophen-Induced Liver Injury by Inhibiting p450 Isoenzymes, Not by Inflammasome Activation. Toxicol. Sci. 2013, 131, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Hoque, R.; Sohail, M.A.; Salhanick, S.; Malik, A.F.; Ghani, A.; Robson, S.C.; Mehal, W.Z. P2X7 Receptor-Mediated Purinergic Signaling Promotes Liver Injury in Acetaminophen Hepatotoxicity in Mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1171–G1179. [Google Scholar] [CrossRef] [PubMed]
- Ayata, C.K.; Ganal, S.C.; Hockenjos, B.; Willim, K.; Vieira, R.P.; Grimm, M.; Robaye, B.; Boeynaems, J.M.; Di Virgilio, F.; Pellegatti, P.; et al. Purinergic P2Y2 Receptors Promote Neutrophil Infiltration and Hepatocyte Death in Mice with Acute Liver Injury. Gastroenterology 2012, 143, 1620–1629.e4. [Google Scholar] [CrossRef] [PubMed]
- Le Guilcher, C.; Garcin, I.; Dellis, O.; Cauchois, F.; Tebbi, A.; Doignon, I.; Guettier, C.; Julien, B.; Tordjmann, T. The P2X4 Purinergic Receptor Regulates Hepatic Myofibroblast Activation during Liver Fibrogenesis. J. Hepatol. 2018, 69, 644–653. [Google Scholar] [CrossRef]
- Imarisio, C.; Alchera, E.; Sutti, S.; Valente, G.; Boccafoschi, F.; Albano, E.; Carini, R. Adenosine A2a Receptor Stimulation Prevents Hepatocyte Lipotoxicity and Non-Alcoholic Steatohepatitis (NASH) in Rats. Clin. Sci. 2012, 123, 323–332. [Google Scholar] [CrossRef]
- Das, S.; Seth, R.K.; Kumar, A.; Kadiiska, M.B.; Michelotti, G.; Diehl, A.M.; Chatterjee, S. Purinergic Receptor X7 Is a Key Modulator of Metabolic Oxidative Stress-Mediated Autophagy and Inflammation in Experimental Nonalcoholic Steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G950–G963. [Google Scholar] [CrossRef]
- Blasetti Fantauzzi, C.; Menini, S.; Iacobini, C.; Rossi, C.; Santini, E.; Solini, A.; Pugliese, G. Deficiency of the Purinergic Receptor 2X Attenuates Nonalcoholic Steatohepatitis Induced by High-Fat Diet: Possible Role of the NLRP3 Inflammasome. Oxid. Med. Cell. Longev. 2017, 2017, 8962458. [Google Scholar] [CrossRef] [PubMed]
- Baeza-Raja, B.; Goodyear, A.; Liu, X.; Lam, K.; Yamamoto, L.; Li, Y.; Dodson, G.S.; Takeuchi, T.; Kisseleva, T.; Brenner, D.A.; et al. Pharmacological Inhibition of P2RX7 Ameliorates Liver Injury by Reducing Inflammation and Fibrosis. PLoS ONE 2020, 15, e0234038. [Google Scholar] [CrossRef]
- Henao-Mejia, J.; Elinav, E.; Jin, C.; Hao, L.; Mehal, W.Z.; Strowig, T.; Thaiss, C.A.; Kau, A.L.; Eisenbarth, S.C.; Jurczak, M.J.; et al. Inflammasome-Mediated Dysbiosis Regulates Progression of NAFLD and Obesity. Nature 2012, 482, 179–185. [Google Scholar] [CrossRef]
- Dong, Z.; Wei, Y.; Tao, M.; Zhang, L. Activation of the Purinergic Receptor P2X7 Improves Hepatosteatosis by Promoting Lipophagy. FEBS Lett. 2021, 595, 2768–2780. [Google Scholar] [CrossRef] [PubMed]
- Dusabimana, T.; Park, E.J.; Je, J.; Jeong, K.; Yun, S.P.; Kim, H.J.; Kim, H.; Park, S.W. P2Y2R Deficiency Ameliorates Hepatic Steatosis by Reducing Lipogenesis and Enhancing Fatty Acid β-Oxidation through AMPK and PGC-1α Induction in High-Fat Diet-Fed Mice. Int. J. Mol. Sci. 2021, 22, 5528. [Google Scholar] [CrossRef]
- Alchera, E.; Rolla, S.; Imarisio, C.; Bardina, V.; Valente, G.; Novelli, F.; Carini, R. Adenosine A2a Receptor Stimulation Blocks Development of Nonalcoholic Steatohepatitis in Mice by Multilevel Inhibition of Signals That Cause Immunolipotoxicity. Transl. Res. 2017, 182, 75–87. [Google Scholar] [CrossRef]
- Cai, Y.; Li, H.; Liu, M.; Pei, Y.; Zheng, J.; Zhou, J.; Luo, X.; Huang, W.; Ma, L.; Yang, Q.; et al. Disruption of Adenosine 2A Receptor Exacerbates NAFLD through Increasing Inflammatory Responses and SREBP1c Activity. Hepatology 2018, 68, 48–61. [Google Scholar] [CrossRef]
- Fishman, P.; Cohen, S.; Itzhak, I.; Amer, J.; Salhab, A.; Barer, F.; Safadi, R. The A3 Adenosine Receptor Agonist, Namodenoson, Ameliorates Non-alcoholic Steatohepatitis in Mice. Int. J. Mol. Med. 2019, 44, 2256–2264. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.X.; Sheng, X.D.; Wang, Y.L.; Wen Lv, X. Blocking P2X4 Purinergic Receptor Attenuates Alcohol-Related Liver Fibrosis by Inhibiting Hepatic Stellate Cell Activation through PI3K/AKT Signaling Pathway. Int. Immunopharmacol. 2022, 113, 109326. [Google Scholar] [CrossRef]
- Xia, G.-Q.; Cai, J.-N.; Wu, X.; Fang, Q.; Zhao, N.; Lv, X.-W. The Mechanism by Which ATP Regulates Alcoholic Steatohepatitis through P2X4 and CD39. Eur. J. Pharmacol. 2022, 916, 174729. [Google Scholar] [CrossRef]
- Yang, P.; Wang, Z.; Zhan, Y.; Wang, T.; Zhou, M.; Xia, L.; Yang, X.; Zhang, J. Endogenous A1 Adenosine Receptor Protects Mice from Acute Ethanol-Induced Hepatotoxicity. Toxicology 2013, 309, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.-W.; Rothweiler, S.; Wei, G.; Ikenaga, N.; Liu, S.B.; Sverdlov, D.Y.; Vaid, K.A.; Longhi, M.S.; Kuang, M.; Robson, S.C.; et al. The Ectonucleotidase ENTPD1/CD39 Limits Biliary Injury and Fibrosis in Mouse Models of Sclerosing Cholangitis. Hepatol. Commun. 2017, 1, 957–972. [Google Scholar] [CrossRef] [PubMed]
- Savio, L.E.B.; de Andrade Mello, P.; Figliuolo, V.R.; de Avelar Almeida, T.F.; Santana, P.T.; Oliveira, S.D.S.; Silva, C.L.M.; Feldbrügge, L.; Csizmadia, E.; Minshall, R.D.; et al. CD39 Limits P2X7 Receptor Inflammatory Signaling and Attenuates Sepsis-Induced Liver Injury. J. Hepatol. 2017, 67, 716–726. [Google Scholar] [CrossRef]
- Ni, J.; Zhang, Z.; Luo, X.; Xiao, L.; Wang, N. Plasticizer DBP Activates NLRP3 Inflammasome through the P2X7 Receptor in HepG2 and L02 Cells. J. Biochem. Mol. Toxicol. 2016, 30, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Schulien, I.; Hockenjos, B.; van Marck, V.; Ayata, C.K.; Follo, M.; Thimme, R.; Hasselblatt, P. Extracellular ATP and Purinergic P2Y Receptor Signaling Promote Liver Tumorigenesis in Mice by Exacerbating DNA Damage. Cancer Res. 2020, 80, 699–708. [Google Scholar] [CrossRef]
- Fishman, P.; Stemmer, S.M.; Bareket-Samish, A.; Silverman, M.H.; Kerns, W.D. Targeting the A3 Adenosine Receptor to Treat Hepatocellular Carcinoma: Anti-Cancer and Hepatoprotective Effects. Purinergic Signal. 2023, 19, 513–522. [Google Scholar] [CrossRef]
- Kamm, D.R.; McCommis, K.S. Hepatic Stellate Cells in Physiology and Pathology. J. Physiol. 2022, 600, 1825–1837. [Google Scholar] [CrossRef]
- Giampieri, M.P.; Jezequel, A.M.; Orlandi, F. The Lipocytes in Normal Human Liver. A Quantitative Study. Digestion 1981, 22, 165–169. [Google Scholar] [CrossRef]
- Andrews, T.S.; Atif, J.; Liu, J.C.; Perciani, C.T.; Ma, X.-Z.; Thoeni, C.; Slyper, M.; Eraslan, G.; Segerstolpe, A.; Manuel, J.; et al. Single-Cell, Single-Nucleus, and Spatial RNA Sequencing of the Human Liver Identifies Cholangiocyte and Mesenchymal Heterogeneity. Hepatol. Commun. 2022, 6, 821–840. [Google Scholar] [CrossRef]
- Diamanti, K.; Inda Díaz, J.S.; Raine, A.; Pan, G.; Wadelius, C.; Cavalli, M. Single Nucleus Transcriptomics Data Integration Recapitulates the Major Cell Types in Human Liver. Hepatol. Res. 2021, 51, 233–238. [Google Scholar] [CrossRef]
- Payen, V.L.; Lavergne, A.; Alevra Sarika, N.; Colonval, M.; Karim, L.; Deckers, M.; Najimi, M.; Coppieters, W.; Charloteaux, B.; Sokal, E.M.; et al. Single-Cell RNA Sequencing of Human Liver Reveals Hepatic Stellate Cell Heterogeneity. JHEP Rep. 2021, 3, 100278. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L. Hepatic Stellate Cells: Protean, Multifunctional, and Enigmatic Cells of the Liver. Physiol. Rev. 2008, 88, 125–172. [Google Scholar] [CrossRef]
- Mederacke, I.; Hsu, C.C.; Troeger, J.S.; Huebener, P.; Mu, X.; Dapito, D.H.; Pradere, J.-P.; Schwabe, R.F. Fate Tracing Reveals Hepatic Stellate Cells as Dominant Contributors to Liver Fibrosis Independent of Its Aetiology. Nat. Commun. 2013, 4, 2823. [Google Scholar] [CrossRef] [PubMed]
- She, H.; Xiong, S.; Hazra, S.; Tsukamoto, H. Adipogenic Transcriptional Regulation of Hepatic Stellate Cells. J. Biol. Chem. 2005, 280, 4959–4967. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, H. Adipogenic Phenotype of Hepatic Stellate Cells. Alcohol. Clin. Exp. Res. 2005, 29, 132S–133S. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Wu, L.; Zhang, W.; Ma, W.-T.; Yang, G.-Y.; Zhang, J.; Xue, D.-Y.; Chen, B.; Liu, C. Peroxisome Proliferator-Activated Receptor γ Inhibits Hepatic Stellate Cell Activation Regulated by miR-942 in Chronic Hepatitis B Liver Fibrosis. Life Sci. 2020, 253, 117572. [Google Scholar] [CrossRef]
- Friedman, S.L. Mechanisms of Hepatic Fibrogenesis. Gastroenterology 2008, 134, 1655–1669. [Google Scholar] [CrossRef]
- Wang, S.-S.; Tang, X.T.; Lin, M.; Yuan, J.; Peng, Y.J.; Yin, X.; Shang, G.; Ge, G.; Ren, Z.; Zhou, B.O. Perivenous Stellate Cells Are the Main Source of Myofibroblasts and Cancer-Associated Fibroblasts Formed After Chronic Liver Injuries. Hepatology 2021, 74, 1578–1594. [Google Scholar] [CrossRef]
- Yang, W.; He, H.; Wang, T.; Su, N.; Zhang, F.; Jiang, K.; Zhu, J.; Zhang, C.; Niu, K.; Wang, L.; et al. Single-Cell Transcriptomic Analysis Reveals a Hepatic Stellate Cell-Activation Roadmap and Myofibroblast Origin During Liver Fibrosis in Mice. Hepatology 2021, 74, 2774–2790. [Google Scholar] [CrossRef]
- Takemura, S.; Kawada, N.; Hirohashi, K.; Kinoshita, H.; Inoue, M. Nucleotide Receptors in Hepatic Stellate Cells of the Rat. FEBS Lett. 1994, 354, 53–56. [Google Scholar] [CrossRef]
- Dranoff, J.A.; Ogawa, M.; Kruglov, E.A.; Gaça, M.D.A.; Sévigny, J.; Robson, S.C.; Wells, R.G. Expression of P2Y Nucleotide Receptors and Ectonucleotidases in Quiescent and Activated Rat Hepatic Stellate Cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G417–G424. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, Y.; Wang, S.; Xu, R.; Lv, X. Purinergic P2X7 Receptor Mediates Acetaldehyde-Induced Hepatic Stellate Cells Activation via PKC-Dependent GSK3β Pathway. Int. Immunopharmacol. 2017, 43, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Mederacke, I.; Filliol, A.; Affo, S.; Nair, A.; Hernandez, C.; Sun, Q.; Hamberger, F.; Brundu, F.; Chen, Y.; Ravichandra, A.; et al. The Purinergic P2Y14 Receptor Links Hepatocyte Death to Hepatic Stellate Cell Activation and Fibrogenesis in the Liver. Sci. Transl. Med. 2022, 14, eabe5795. [Google Scholar] [CrossRef]
- Shuai, C.; Xia, G.-Q.; Yuan, F.; Wang, S.; Lv, X.-W. CD39-Mediated ATP-Adenosine Signalling Promotes Hepatic Stellate Cell Activation and Alcoholic Liver Disease. Eur. J. Pharmacol. 2021, 905, 174198. [Google Scholar] [CrossRef]
- Andrade, C.M.B.; Wink, M.R.; Margis, R.; Borojevic, R.; Battastini, A.M.O.; Guma, F.C.R. Activity and Expression of Ecto-Nucleotide Pyrophosphate/phosphodiesterases in a Hepatic Stellate Cell Line. Mol. Cell. Biochem. 2009, 325, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Fernandez, P.; Wilder, T.; Yee, H.; Chiriboga, L.; Chan, E.S.L.; Cronstein, B.N. Ecto-5′-Nucleotidase (CD73)-Mediated Extracellular Adenosine Production Plays a Critical Role in Hepatic Fibrosis. Nucleosides Nucleotides Nucleic Acids 2008, 27, 821–824. [Google Scholar] [CrossRef] [PubMed]
- Fausther, M.; Sheung, N.; Saiman, Y.; Bansal, M.B.; Dranoff, J.A. Activated Hepatic Stellate Cells Upregulate Transcription of Ecto-5′-nucleotidase/CD73 via Specific SP1 and SMAD Promoter Elements. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G904–G914. [Google Scholar] [CrossRef]
- Jia, W.-Q.; Zhou, T.-C.; Dai, J.-W.; Liu, Z.-N.; Zhang, Y.-F.; Zang, D.-D.; Lv, X.-W. CD73 Regulates Hepatic Stellate Cells Activation and Proliferation through Wnt/β-Catenin Signaling Pathway. Eur. J. Pharmacol. 2021, 890, 173667. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, B.; Liu, X.; Wu, X.; Du, J.; Xia, G.; Cai, J.; Zhu, H.; Sheng, X.; Zhang, M.; et al. CD73/NT5E-Mediated Ubiquitination of AURKA Regulates Alcohol-Related Liver Fibrosis via Modulating Hepatic Stellate Cell Senescence. Int. J. Biol. Sci. 2023, 19, 950–966. [Google Scholar] [CrossRef]
- Yang, L.; Gao, Z.-W.; Wu, X.-N.; Liu, C.; Zhang, J.; Zhang, H.-Z.; Dong, K. CD73 Blockade Alleviated Hepatic Fibrosis via Inhibiting Hepatic Stellate Cells Proliferation and Activation. Curr. Mol. Pharmacol. 2023, 17, e220323214863. [Google Scholar] [CrossRef]
- Wu, X.; Liu, X.-Q.; Liu, Z.-N.; Xia, G.-Q.; Zhu, H.; Zhang, M.; Wu, B.-M.; Lv, X.-W. CD73 Aggravates Alcohol-Related Liver Fibrosis by Promoting Autophagy Mediated Activation of Hepatic Stellate Cells through AMPK/AKT/mTOR Signaling Pathway. Int. Immunopharmacol. 2022, 113, 109229. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.Z.; Hakim, W.; Kruglov, E.A.; Watanabe, A.; Watkins, W.; Dranoff, J.A.; Mehal, W.Z. Adenosine Inhibits Cytosolic Calcium Signals and Chemotaxis in Hepatic Stellate Cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G395–G401. [Google Scholar] [CrossRef][Green Version]
- Che, J.; Chan, E.S.L.; Cronstein, B.N. Adenosine A2A Receptor Occupancy Stimulates Collagen Expression by Hepatic Stellate Cells via Pathways Involving Protein Kinase A, Src, and Extracellular Signal-Regulated Kinases 1/2 Signaling Cascade or p38 Mitogen-Activated Protein Kinase Signaling Pathway. Mol. Pharmacol. 2007, 72, 1626–1636. [Google Scholar] [PubMed]
- Block, E.T.; Cronstein, B.N. Interferon-Gamma Inhibits Adenosine A2A Receptor Function in Hepatic Stellate Cells by STAT1-Mediated Repression of Adenylyl Cyclase. Int. J. Infereron. Cytokine Mediat. Res. 2010, 2010, 113–126. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sohail, M.A.; Hashmi, A.Z.; Hakim, W.; Watanabe, A.; Zipprich, A.; Groszmann, R.J.; Dranoff, J.A.; Torok, N.J.; Mehal, W.Z. Adenosine Induces Loss of Actin Stress Fibers and Inhibits Contraction in Hepatic Stellate Cells via Rho Inhibition. Hepatology 2009, 49, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, M.K.; Mehal, W.Z. Activation of Adenosine Receptor A2A Increases HSC Proliferation and Inhibits Death and Senescence by down-Regulation of p53 and Rb. Front. Pharmacol. 2014, 5, 69. [Google Scholar] [CrossRef]
- Wang, H.; Guan, W.; Yang, W.; Wang, Q.; Zhao, H.; Yang, F.; Lv, X.; Li, J. Caffeine Inhibits the Activation of Hepatic Stellate Cells Induced by Acetaldehyde via Adenosine A2A Receptor Mediated by the cAMP/PKA/SRC/ERK1/2/P38 MAPK Signal Pathway. PLoS ONE 2014, 9, e92482. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Saito, S.-Y.; Nishiyama, R.; Nakamura, M.; Todoroki, K.; Toyo’oka, T.; Ishikawa, T. Caffeine Suppresses the Activation of Hepatic Stellate Cells cAMP-Independently by Antagonizing Adenosine Receptors. Biol. Pharm. Bull. 2017, 40, 658–664. [Google Scholar] [CrossRef]
- Velasco-Loyden, G.; Pérez-Carreón, J.I.; Agüero, J.F.C.; Romero, P.C.; Vidrio-Gómez, S.; Martínez-Pérez, L.; Yáñez-Maldonado, L.; Hernández-Muñoz, R.; Macías-Silva, M.; de Sánchez, V.C. Prevention of In Vitro Hepatic Stellate Cells Activation by the Adenosine Derivative Compound IFC305. Biochem. Pharmacol. 2010, 80, 1690–1699. [Google Scholar] [CrossRef]
- Wang, Q.; Dai, X.; Yang, W.; Wang, H.; Zhao, H.; Yang, F.; Yang, Y.; Li, J.; Lv, X. Caffeine Protects against Alcohol-Induced Liver Fibrosis by Dampening the cAMP/PKA/CREB Pathway in Rat Hepatic Stellate Cells. Int. Immunopharmacol. 2015, 25, 340–352. [Google Scholar] [CrossRef]
- James Phillips, M. The Liver: An Atlas and Text of Ultrastructural Pathology; Raven Press: New York, NY, USA, 1987; ISBN 9780608058740. [Google Scholar]
- Liaskou, E.; Wilson, D.V.; Oo, Y.H. Innate Immune Cells in Liver Inflammation. Mediat. Inflamm. 2012, 2012, 949157. [Google Scholar] [CrossRef] [PubMed]
- Bilzer, M.; Roggel, F.; Gerbes, A.L. Role of Kupffer Cells in Host Defense and Liver Disease. Liver Int. 2006, 26, 1175–1186. [Google Scholar] [CrossRef]
- Naito, M.; Hasegawa, G.; Takahashi, K. Development, Differentiation, and Maturation of Kupffer Cells. Microsc. Res. Tech. 1997, 39, 350–364. [Google Scholar] [CrossRef]
- Bouwens, L.; Baekeland, M.; De Zanger, R.; Wisse, E. Quantitation, Tissue Distribution and Proliferation Kinetics of Kupffer Cells in Normal Rat Liver. Hepatology 1986, 6, 718–722. [Google Scholar] [CrossRef]
- Kolios, G.; Valatas, V.; Kouroumalis, E. Role of Kupffer Cells in the Pathogenesis of Liver Disease. World J. Gastroenterol. 2006, 12, 7413–7420. [Google Scholar] [CrossRef]
- Klaver, D.; Thurnher, M. Control of Macrophage Inflammation by P2Y Purinergic Receptors. Cells 2021, 10, 1098. [Google Scholar] [CrossRef]
- Del Rey, A.; Renigunta, V.; Dalpke, A.H.; Leipziger, J.; Matos, J.E.; Robaye, B.; Zuzarte, M.; Kavelaars, A.; Hanley, P.J. Knock-out Mice Reveal the Contributions of P2Y and P2X Receptors to Nucleotide-Induced Ca2+ Signaling in Macrophages. J. Biol. Chem. 2006, 281, 35147–35155. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.R.; Chekeni, F.B.; Trampont, P.C.; Lazarowski, E.R.; Kadl, A.; Walk, S.F.; Park, D.; Woodson, R.I.; Ostankovich, M.; Sharma, P.; et al. Nucleotides Released by Apoptotic Cells Act as a Find-Me Signal to Promote Phagocytic Clearance. Nature 2009, 461, 282–286. [Google Scholar] [CrossRef]
- Stober, C.B.; Lammas, D.A.; Li, C.M.; Kumararatne, D.S.; Lightman, S.L.; McArdle, C.A. ATP-Mediated Killing of Mycobacterium Bovis Bacille Calmette-Guérin within Human Macrophages Is Calcium Dependent and Associated with the Acidification of Mycobacteria-Containing Phagosomes. J. Immunol. 2001, 166, 6276–6286. [Google Scholar] [CrossRef]
- De la Rosa, G.; Gómez, A.I.; Baños, M.C.; Pelegrín, P. Signaling Through Purinergic Receptor P2Y Enhances Macrophage IL-1β Production. Int. J. Mol. Sci. 2020, 21, 4686. [Google Scholar] [CrossRef]
- Ren, W.; Rubini, P.; Tang, Y.; Engel, T.; Illes, P. Inherent P2X7 Receptors Regulate Macrophage Functions during Inflammatory Diseases. Int. J. Mol. Sci. 2021, 23, 232. [Google Scholar] [CrossRef] [PubMed]
- Gudipaty, L.; Humphreys, B.D.; Buell, G.; Dubyak, G.R. Regulation of P2X7 Nucleotide Receptor Function in Human Monocytes by Extracellular Ions and Receptor Density. Am. J. Physiol. Cell Physiol. 2001, 280, C943–C953. [Google Scholar] [CrossRef] [PubMed]
- Buisman, H.P.; Steinberg, T.H.; Fischbarg, J.; Silverstein, S.C.; Vogelzang, S.A.; Ince, C.; Ypey, D.L.; Leijh, P.C. Extracellular ATP Induces a Large Nonselective Conductance in Macrophage Plasma Membranes. Proc. Natl. Acad. Sci. USA 1988, 85, 7988–7992. [Google Scholar] [CrossRef] [PubMed]
- Nurkhametova, D.; Siniavin, A.; Streltsova, M.; Kudryavtsev, D.; Kudryavtsev, I.; Giniatullina, R.; Tsetlin, V.; Malm, T.; Giniatullin, R. Does Cholinergic Stimulation Affect the P2X7 Receptor-Mediated Dye Uptake in Mast Cells and Macrophages? Front. Cell. Neurosci. 2020, 14, 548376. [Google Scholar] [CrossRef]
- Bockstiegel, J.; Engelhardt, J.; Weindl, G. P2X7 Receptor Activation Leads to NLRP3-Independent IL-1β Release by Human Macrophages. Cell Commun. Signal. 2023, 21, 335. [Google Scholar] [CrossRef]
- Qu, Y.; Franchi, L.; Nunez, G.; Dubyak, G.R. Nonclassical IL-1 Beta Secretion Stimulated by P2X7 Receptors Is Dependent on Inflammasome Activation and Correlated with Exosome Release in Murine Macrophages. J. Immunol. 2007, 179, 1913–1925. [Google Scholar] [CrossRef]
- Sun, S.; Gong, D.; Liu, R.; Wang, R.; Chen, D.; Yuan, T.; Wang, S.; Xing, C.; Lv, Y.; Du, G.; et al. Puerarin Inhibits NLRP3-Caspase-1-GSDMD-Mediated Pyroptosis via P2X7 Receptor in Cardiomyocytes and Macrophages. Int. J. Mol. Sci. 2023, 24, 13169. [Google Scholar] [CrossRef]
- Mariathasan, S.; Weiss, D.S.; Newton, K.; McBride, J.; O’Rourke, K.; Roose-Girma, M.; Lee, W.P.; Weinrauch, Y.; Monack, D.M.; Dixit, V.M. Cryopyrin Activates the Inflammasome in Response to Toxins and ATP. Nature 2006, 440, 228–232. [Google Scholar] [CrossRef]
- Xiang, Z.; Lv, J.; Jiang, P.; Chen, C.; Jiang, B.; Burnstock, G. Expression of P2X Receptors on Immune Cells in the Rat Liver during Postnatal Development. Histochem. Cell Biol. 2006, 126, 453–463. [Google Scholar] [CrossRef]
- Pei, H.; He, Z.; Du, R.; Han, C.; Sheng, Y.; Wang, J.; Zhou, X.; Li, W.; Cao, C.; Sheng, J.; et al. Imidacloprid Activates Kupffer Cells Pyroptosis to Induce Liver Injury in Mice via P2X7. Int. Immunopharmacol. 2023, 119, 110179. [Google Scholar] [CrossRef]
- Toki, Y.; Takenouchi, T.; Harada, H.; Tanuma, S.-I.; Kitani, H.; Kojima, S.; Tsukimoto, M. Extracellular ATP Induces P2X7 Receptor Activation in Mouse Kupffer Cells, Leading to Release of IL-1β, HMGB1, and PGE2, Decreased MHC Class I Expression and Necrotic Cell Death. Biochem. Biophys. Res. Commun. 2015, 458, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Negishi, Y.; Tsukimoto, M.; Takenouchi, T.; Kitani, H.; Takeda, K. Purinergic Signaling via P2X7 Receptor Mediates IL-1β Production in Kupffer Cells Exposed to Silica Nanoparticle. Toxicology 2014, 321, 13–20. [Google Scholar] [CrossRef]
- Yuan, F.; Cai, J.-N.; Dai, M.; Lv, X. Inhibition of P2Y Receptor Expression in Kupffer Cells Alleviates Alcoholic Steatohepatitis in Mice. Int. Immunopharmacol. 2022, 109, 108909. [Google Scholar] [CrossRef]
- Duarte, F.V.; Amorim, J.A.; Varela, A.T.; Teodoro, J.S.; Gomes, A.P.; Cunha, R.A.; Palmeira, C.M.; Rolo, A.P. Adenosine Receptors: Regulatory Players in the Preservation of Mitochondrial Function Induced by Ischemic Preconditioning of Rat Liver. Purinergic Signal. 2017, 13, 179–190. [Google Scholar] [CrossRef]
- Ben-Ari, Z.; Pappo, O.; Sulkes, J.; Cheporko, Y.; Vidne, B.A.; Hochhauser, E. Effect of Adenosine A2A Receptor Agonist (CGS) on Ischemia/reperfusion Injury in Isolated Rat Liver. Apoptosis 2005, 10, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Reinstein, L.J.; Lichtman, S.N.; Currin, R.T.; Wang, J.; Thurman, R.G.; Lemasters, J.J. Suppression of Lipopolysaccharide-Stimulated Release of Tumor Necrosis Factor by Adenosine: Evidence for A2 Receptors on Rat Kupffer Cells. Hepatology 1994, 19, 1445–1452. [Google Scholar] [PubMed]
- Zhao, N.; Xia, G.; Cai, J.; Li, Z.; Lv, X.-W. Adenosine Receptor A2B Mediates Alcoholic Hepatitis by Regulating cAMP Levels and the NF-KB Pathway. Toxicol. Lett. 2022, 359, 84–95. [Google Scholar] [CrossRef]
- Rothweiler, S.; Feldbrügge, L.; Jiang, Z.G.; Csizmadia, E.; Longhi, M.S.; Vaid, K.; Enjyoji, K.; Popov, Y.V.; Robson, S.C. Selective Deletion of ENTPD1/CD39 in Macrophages Exacerbates Biliary Fibrosis in a Mouse Model of Sclerosing Cholangitis. Purinergic Signal. 2019, 15, 375–385. [Google Scholar] [CrossRef]
- Gao, J.; Zuo, B.; He, Y. Liver Sinusoidal Endothelial Cells as Potential Drivers of Liver Fibrosis (Review). Mol. Med. Rep. 2024, 29, 40. [Google Scholar] [CrossRef]
- DeLeve, L.D. Liver Sinusoidal Endothelial Cells in Hepatic Fibrosis. Hepatology 2015, 61, 1740–1746. [Google Scholar] [CrossRef]
- Sørensen, K.K.; McCourt, P.; Berg, T.; Crossley, C.; Le Couteur, D.; Wake, K.; Smedsrød, B. The Scavenger Endothelial Cell: A New Player in Homeostasis and Immunity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R1217–R1230. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, L.P.; Mates, J.M.; Cheplowitz, A.M.; Avila, C.L.; Zimmerer, J.M.; Yao, Z.; Maiseyeu, A.; Rajaram, M.V.S.; Robinson, J.M.; Anderson, C.L. Scavenger Receptor B1, the HDL Receptor, Is Expressed Abundantly in Liver Sinusoidal Endothelial Cells. Sci. Rep. 2016, 6, 20646. [Google Scholar] [CrossRef]
- Van Berkel, T.J.; De Rijke, Y.B.; Kruijt, J.K. Different Fate in Vivo of Oxidatively Modified Low Density Lipoprotein and Acetylated Low Density Lipoprotein in Rats. Recognition by Various Scavenger Receptors on Kupffer and Endothelial Liver Cells. J. Biol. Chem. 1991, 266, 2282–2289. [Google Scholar] [CrossRef]
- Pandey, E.; Nour, A.S.; Harris, E.N. Prominent Receptors of Liver Sinusoidal Endothelial Cells in Liver Homeostasis and Disease. Front. Physiol. 2020, 11, 873. [Google Scholar] [CrossRef] [PubMed]
- Boeynaems, J.M.; Pearson, J.D. P2 Purinoceptors on Vascular Endothelial Cells: Physiological Significance and Transduction Mechanisms. Trends Pharmacol. Sci. 1990, 11, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Motte, S.; Communi, D.; Pirotton, S.; Boeynaems, J.M. Involvement of Multiple Receptors in the Actions of Extracellular ATP: The Example of Vascular Endothelial Cells. Int. J. Biochem. Cell Biol. 1995, 27, 1–7. [Google Scholar] [CrossRef]
- Hashimoto, N.; Watanabe, T.; Shiratori, Y.; Ikeda, Y.; Kato, H.; Han, K.; Yamada, H.; Toda, G.; Kurokawa, K. Prostanoid Secretion by Rat Hepatic Sinusoidal Endothelial Cells and Its Regulation by Exogenous Adenosine Triphosphate. Hepatology 1995, 21, 1713–1718. [Google Scholar]
- Beldi, G.; Wu, Y.; Sun, X.; Imai, M.; Enjyoji, K.; Csizmadia, E.; Candinas, D.; Erb, L.; Robson, S.C. Regulated Catalysis of Extracellular Nucleotides by Vascular CD39/ENTPD1 Is Required for Liver Regeneration. Gastroenterology 2008, 135, 1751–1760. [Google Scholar] [CrossRef][Green Version]
- Feng, L.; Sun, X.; Csizmadia, E.; Han, L.; Bian, S.; Murakami, T.; Wang, X.; Robson, S.C.; Wu, Y. Vascular CD39/ENTPD1 Directly Promotes Tumor Cell Growth by Scavenging Extracellular Adenosine Triphosphate. Neoplasia 2011, 13, 206–216. [Google Scholar] [CrossRef]
- Mandili, G.; Alchera, E.; Merlin, S.; Imarisio, C.; Chandrashekar, B.R.; Riganti, C.; Bianchi, A.; Novelli, F.; Follenzi, A.; Carini, R. Mouse Hepatocytes and LSEC Proteome Reveal Novel Mechanisms of Ischemia/reperfusion Damage and Protection by A2aR Stimulation. J. Hepatol. 2015, 62, 573–580. [Google Scholar] [CrossRef]
- Kaczmarek, E.; Koziak, K.; Sévigny, J.; Siegel, J.B.; Anrather, J.; Beaudoin, A.R.; Bach, F.H.; Robson, S.C. Identification and Characterization of CD39/vascular ATP Diphosphohydrolase. J. Biol. Chem. 1996, 271, 33116–33122. [Google Scholar] [CrossRef] [PubMed]
- Goepfert, C.; Sundberg, C.; Sévigny, J.; Enjyoji, K.; Hoshi, T.; Csizmadia, E.; Robson, S. Disordered Cellular Migration and Angiogenesis in cd39-Null Mice. Circulation 2001, 104, 3109–3115. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Huebert, R.C.; Karlsen, T.; Strazzabosco, M.; LaRusso, N.F.; Gores, G.J. Cholangiocyte Pathobiology. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Björkström, N.K. Immunobiology of the Biliary Tract System. J. Hepatol. 2022, 77, 1657–1669. [Google Scholar] [CrossRef]
- Wang, W.; Chen, D.; Wang, J.; Wen, L. Cellular Homeostasis and Repair in the Biliary Tree. Semin. Liver Dis. 2022, 42, 271–282. [Google Scholar] [CrossRef]
- Lanzoni, G.; Cardinale, V.; Carpino, G. The Hepatic, Biliary, and Pancreatic Network of Stem/progenitor Cell Niches in Humans: A New Reference Frame for Disease and Regeneration. Hepatology 2016, 64, 277–286. [Google Scholar] [CrossRef]
- Alpini, G.; McGill, J.M.; Larusso, N.F. The Pathobiology of Biliary Epithelia. Hepatology 2002, 35, 1256–1268. [Google Scholar] [CrossRef]
- Marzioni, M.; Glaser, S.S.; Francis, H.; Phinizy, J.L.; LeSage, G.; Alpini, G. Functional Heterogeneity of Cholangiocytes. Semin. Liver Dis. 2002, 22, 227–240. [Google Scholar] [CrossRef]
- Alpini, G.; Roberts, S.; Kuntz, S.M.; Ueno, Y.; Gubba, S.; Podila, P.V.; LeSage, G.; LaRusso, N.F. Morphological, Molecular, and Functional Heterogeneity of Cholangiocytes from Normal Rat Liver. Gastroenterology 1996, 110, 1636–1643. [Google Scholar] [CrossRef]
- Yoo, K.-S.; Lim, W.T.; Choi, H.S. Biology of Cholangiocytes: From Bench to Bedside. Gut Liver 2016, 10, 687–698. [Google Scholar] [CrossRef]
- Mancinelli, R.; Franchitto, A.; Gaudio, E.; Onori, P.; Glaser, S.; Francis, H.; Venter, J.; Demorrow, S.; Carpino, G.; Kopriva, S.; et al. After Damage of Large Bile Ducts by Gamma-Aminobutyric Acid, Small Ducts Replenish the Biliary Tree by Amplification of Calcium-Dependent Signaling and de Novo Acquisition of Large Cholangiocyte Phenotypes. Am. J. Pathol. 2010, 176, 1790–1800. [Google Scholar] [CrossRef] [PubMed]
- Gouw, A.S.H.; Clouston, A.D.; Theise, N.D. Ductular Reactions in Human Liver: Diversity at the Interface. Hepatology 2011, 54, 1853–1863. [Google Scholar] [CrossRef]
- Maroni, L.; Ninfole, E.; Pinto, C.; Benedetti, A.; Marzioni, M. Gut-Liver Axis and Inflammasome Activation in Cholangiocyte Pathophysiology. Cells 2020, 9, 736. [Google Scholar] [CrossRef] [PubMed]
- Giordano, D.M.; Pinto, C.; Maroni, L.; Benedetti, A.; Marzioni, M. Inflammation and the Gut-Liver Axis in the Pathophysiology of Cholangiopathies. Int. J. Mol. Sci. 2018, 19, 3003. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gao, W.-K.; Shu, Y.-Y.; Ye, J. Mechanisms of Ductular Reaction in Non-Alcoholic Steatohepatitis. World J. Gastroenterol. 2022, 28, 2088–2099. [Google Scholar] [CrossRef]
- Sato, K.; Marzioni, M.; Meng, F.; Francis, H.; Glaser, S.; Alpini, G. Ductular Reaction in Liver Diseases: Pathological Mechanisms and Translational Significances. Hepatology 2019, 69, 420–430. [Google Scholar] [CrossRef]
- Boyer, J.L.; Soroka, C.J. Bile Formation and Secretion: An Update. J. Hepatol. 2021, 75, 190–201. [Google Scholar] [CrossRef]
- Pinzani, M.; Luong, T.V. Pathogenesis of Biliary Fibrosis. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2018, 1864, 1279–1283. [Google Scholar] [CrossRef] [PubMed]
- Fabris, L.; Spirli, C.; Cadamuro, M.; Fiorotto, R.; Strazzabosco, M. Emerging Concepts in Biliary Repair and Fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, G102–G116. [Google Scholar] [CrossRef]
- Carey, E.J.; Ali, A.H.; Lindor, K.D. Primary Biliary Cirrhosis. Lancet 2015, 386, 1565–1575. [Google Scholar] [CrossRef]
- Salter, K.D.; Fitz, J.G.; Roman, R.M. Domain-Specific Purinergic Signaling in Polarized Rat Cholangiocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 278, G492–G500. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Roman, R.; Lidofsky, S.D.; Fitz, J.G. Autocrine Signaling through ATP Release Represents a Novel Mechanism for Cell Volume Regulation. Proc. Natl. Acad. Sci. USA 1996, 93, 12020–12025. [Google Scholar] [CrossRef]
- Taylor, A.L.; Kudlow, B.A.; Marrs, K.L.; Gruenert, D.C.; Guggino, W.B.; Schwiebert, E.M. Bioluminescence Detection of ATP Release Mechanisms in Epithelia. Am. J. Physiol. 1998, 275, C1391–C1406. [Google Scholar] [CrossRef]
- Roman, R.M.; Wang, Y.; Lidofsky, S.D.; Feranchak, A.P.; Lomri, N.; Scharschmidt, B.F.; Fitz, J.G. Hepatocellular ATP-Binding Cassette Protein Expression Enhances ATP Release and Autocrine Regulation of Cell Volume. J. Biol. Chem. 1997, 272, 21970–21976. [Google Scholar] [CrossRef] [PubMed]
- Scoazec, J.Y.; Bringuier, A.F.; Medina, J.F.; Martínez-Ansó, E.; Veissiere, D.; Feldmann, G.; Housset, C. The Plasma Membrane Polarity of Human Biliary Epithelial Cells: In Situ Immunohistochemical Analysis and Functional Implications. J. Hepatol. 1997, 26, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Pasyk, E.A.; Foskett, J.K. Cystic Fibrosis Transmembrane Conductance Regulator-Associated ATP and Adenosine 3′-Phosphate 5’-Phosphosulfate Channels in Endoplasmic Reticulum and Plasma Membranes. J. Biol. Chem. 1997, 272, 7746–7751. [Google Scholar] [CrossRef]
- Krause, U.; Rider, M.H.; Hue, L. Protein Kinase Signaling Pathway Triggered by Cell Swelling and Involved in the Activation of Glycogen Synthase and Acetyl-CoA Carboxylase in Isolated Rat Hepatocytes. J. Biol. Chem. 1996, 271, 16668–16673. [Google Scholar] [CrossRef]
- Feranchak, A.P.; Roman, R.M.; Schwiebert, E.M.; Fitz, J.G. Phosphatidylinositol 3-Kinase Contributes to Cell Volume Regulation through Effects on ATP Release. J. Biol. Chem. 1998, 273, 14906–14911. [Google Scholar] [CrossRef]
- Feranchak, A.P.; Roman, R.M.; Doctor, R.B.; Salter, K.D.; Toker, A.; Fitz, J.G. The Lipid Products of Phosphoinositide 3-Kinase Contribute to Regulation of Cholangiocyte ATP and Chloride Transport. J. Biol. Chem. 1999, 274, 30979–30986. [Google Scholar] [CrossRef]
- Schlosser, S.F.; Burgstahler, A.D.; Nathanson, M.H. Isolated Rat Hepatocytes Can Signal to Other Hepatocytes and Bile Duct Cells by Release of Nucleotides. Proc. Natl. Acad. Sci. USA 1996, 93, 9948–9953. [Google Scholar] [CrossRef]
- Roman, R.M.; Feranchak, A.P.; Salter, K.D.; Wang, Y.; Fitz, J.G. Endogenous ATP Release Regulates Cl− Secretion in Cultured Human and Rat Biliary Epithelial Cells. Am. J. Physiol. 1999, 276, G1391–G1400. [Google Scholar] [CrossRef] [PubMed]
- Roman, R.M.; Fitz, J.G. Emerging Roles of Purinergic Signaling in Gastrointestinal Epithelial Secretion and Hepatobiliary Function. Gastroenterology 1999, 116, 964–979. [Google Scholar] [CrossRef] [PubMed]
- Gatof, D.; Kilic, G.; Fitz, J.G. Vesicular Exocytosis Contributes to Volume-Sensitive ATP Release in Biliary Cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 286, G538–G546. [Google Scholar] [CrossRef]
- Schlenker, T.; Romac, J.M.; Sharara, A.I.; Roman, R.M.; Kim, S.J.; LaRusso, N.; Liddle, R.A.; Fitz, J.G. Regulation of Biliary Secretion through Apical Purinergic Receptors in Cultured Rat Cholangiocytes. Am. J. Physiol. 1997, 273, G1108–G1117. [Google Scholar] [CrossRef]
- Dranoff, J.A.; Masyuk, A.I.; Kruglov, E.A.; LaRusso, N.F.; Nathanson, M.H. Polarized Expression and Function of P2Y ATP Receptors in Rat Bile Duct Epithelia. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G1059–G1067. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.; Sathe, M.; Kresge, C.; Esser, V.; Ueno, Y.; Venter, J.; Glaser, S.S.; Alpini, G.; Feranchak, A.P. Adenosine Triphosphate Release and Purinergic (P2) Receptor-Mediated Secretion in Small and Large Mouse Cholangiocytes. Hepatology 2010, 52, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Masyuk, A.I.; Masyuk, T.V.; LaRusso, N.F. Cholangiocyte Primary Cilia in Liver Health and Disease. Dev. Dyn. 2008, 237, 2007–2012. [Google Scholar] [CrossRef]
- Masyuk, A.I.; Gradilone, S.A.; Banales, J.M.; Huang, B.Q.; Masyuk, T.V.; Lee, S.-O.; Splinter, P.L.; Stroope, A.J.; Larusso, N.F. Cholangiocyte Primary Cilia Are Chemosensory Organelles That Detect Biliary Nucleotides via P2Y12 Purinergic Receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G725–G734. [Google Scholar] [CrossRef]
- Doctor, R.B.; Matzakos, T.; McWilliams, R.; Johnson, S.; Feranchak, A.P.; Fitz, J.G. Purinergic Regulation of Cholangiocyte Secretion: Identification of a Novel Role for P2X Receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G779–G786. [Google Scholar] [CrossRef]
- Dutta, A.K.; Woo, K.; Khimji, A.-K.; Kresge, C.; Feranchak, A.P. Mechanosensitive Cl− Secretion in Biliary Epithelium Mediated through TMEM16A. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G87–G98. [Google Scholar] [CrossRef]
- Godoy, V.; Banales, J.M.; Medina, J.F.; Pastor-Anglada, M. Functional Crosstalk between the Adenosine Transporter CNT3 and Purinergic Receptors in the Biliary Epithelia. J. Hepatol. 2014, 61, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Maynard, J.P.; Lee, J.-S.; Sohn, B.H.; Yu, X.; Lopez-Terrada, D.; Finegold, M.J.; Goss, J.A.; Thevananther, S. P2X3 Purinergic Receptor Overexpression Is Associated with Poor Recurrence-Free Survival in Hepatocellular Carcinoma Patients. Oncotarget 2015, 6, 41162–41179. [Google Scholar] [CrossRef] [PubMed]
- Lertsuwan, J.; Ruchirawat, M. Inhibitory Effects of ATP and Adenosine on Cholangiocarcinoma Cell Proliferation and Motility. Anticancer Res. 2017, 37, 3553–3561. [Google Scholar]
- Burnstock, G. Purinergic Signalling: Therapeutic Developments. Front. Pharmacol. 2017, 8, 661. [Google Scholar]
- Burnstock, G. The Therapeutic Potential of Purinergic Signalling. Biochem. Pharmacol. 2018, 151, 157–165. [Google Scholar] [CrossRef]
- Chiang, D.J.; Roychowdhury, S.; Bush, K.; McMullen, M.R.; Pisano, S.; Niese, K.; Olman, M.A.; Pritchard, M.T.; Nagy, L.E. Adenosine 2A Receptor Antagonist Prevented and Reversed Liver Fibrosis in a Mouse Model of Ethanol-Exacerbated Liver Fibrosis. PLoS ONE 2013, 8, e69114. [Google Scholar] [CrossRef][Green Version]
- Khalid, M.; Brisson, L.; Tariq, M.; Hao, Y.; Guibon, R.; Fromont, G.; Mortadza, S.A.S.; Mousawi, F.; Manzoor, S.; Roger, S.; et al. Carcinoma-Specific Expression of P2Y11 Receptor and Its Contribution in ATP-Induced Purinergic Signalling and Cell Migration in Human Hepatocellular Carcinoma Cells. Oncotarget 2017, 8, 37278–37290. [Google Scholar] [CrossRef]
- Cohen, S.; Stemmer, S.M.; Zozulya, G.; Ochaion, A.; Patoka, R.; Barer, F.; Bar-Yehuda, S.; Rath-Wolfson, L.; Jacobson, K.A.; Fishman, P. CF102 an A3 Adenosine Receptor Agonist Mediates Anti-Tumor and Anti-Inflammatory Effects in the Liver. J. Cell. Physiol. 2011, 226, 2438–2447. [Google Scholar] [CrossRef]
- Suresh, R.R.; Jain, S.; Chen, Z.; Tosh, D.K.; Ma, Y.; Podszun, M.C.; Rotman, Y.; Salvemini, D.; Jacobson, K.A. Design and In Vivo Activity of A Adenosine Receptor Agonist Prodrugs. Purinergic Signal. 2020, 16, 367–377. [Google Scholar] [CrossRef]
- Saito, T.; Muramatsu, M.; Ishii, Y.; Saigo, Y.; Konuma, T.; Toriniwa, Y.; Miyajima, K.; Ohta, T. Pathophysiological Analysis of the Progression of Hepatic Lesions in STAM Mice. Physiol. Res. 2017, 66, 791–799. [Google Scholar] [CrossRef]
- Stemmer, S.M.; Benjaminov, O.; Medalia, G.; Ciuraru, N.B.; Silverman, M.H.; Bar-Yehuda, S.; Fishman, S.; Harpaz, Z.; Farbstein, M.; Cohen, S.; et al. CF102 for the Treatment of Hepatocellular Carcinoma: A Phase I/II, Open-Label, Dose-Escalation Study. Oncologist 2013, 18, 25–26. [Google Scholar] [CrossRef]
- Pérez-Carreón, J.I.; Martínez-Pérez, L.; Loredo, M.L.; Yañez-Maldonado, L.; Velasco-Loyden, G.; Vidrio-Gómez, S.; Ramírez-Salcedo, J.; Hernández-Luis, F.; Velázquez-Martínez, I.; Suárez-Cuenca, J.A.; et al. An Adenosine Derivative Compound, IFC305, Reverses Fibrosis and Alters Gene Expression in a Pre-Established CCl4-Induced Rat Cirrhosis. Int. J. Biochem. Cell Biol. 2010, 42, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cabeza de Vaca, R.; Domínguez-López, M.; Guerrero-Celis, N.; Rodríguez-Aguilera, J.R.; Chagoya de Sánchez, V. Inflammation Is Regulated by the Adenosine Derivative Molecule, IFC-305, during Reversion of Cirrhosis in a CCl Rat Model. Int. Immunopharmacol. 2018, 54, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Sandoval, G.; Meza-Toledo, S.E.; Aguirre-Alvarado, C.; Zaragoza-Martinéz, F.; Rodríguez-Páez, L.; Nogueda-Torres, B.; Baeza-Ramírez, I.; Wong-Ramírez, C.; Chagoya-de Sánchez, V.; Suárez-Cuenca, J.A.; et al. New drugs development in Mexico. Gac. Med. Mex. 2007, 143, 33–59. [Google Scholar]
- Menzies, R.I.; Tam, F.W.; Unwin, R.J.; Bailey, M.A. Purinergic Signaling in Kidney Disease. Kidney Int. 2017, 91, 315–323. [Google Scholar] [CrossRef]
- Mishra, S.; Shelke, V.; Dagar, N.; Lech, M.; Gaikwad, A.B. Molecular insights into P2X signalling cascades in acute kidney injury. Purinergic Signal. 2024, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Praetorius, H. The Bacteria and the Host: A Story of Purinergic Signaling in Urinary Tract Infections. Am. J. Physiol. Cell Physiol. 2021, 321, C134–C146. [Google Scholar] [CrossRef]
- Dwyer, K.M. Burnstock Oration—Purinergic Signalling in Kidney Transplantation. Purinergic Signal. 2022, 18, 387–393. [Google Scholar] [CrossRef]
| HSCs | ||
|---|---|---|
| Ligand, Receptor or Enzyme | Effect | Reference |
| Not determined | IP3 production and cytosolic Ca2+ mobilization and induction of cell contraction | [110] |
| P2Y2 and P2Y4 in HSCs and P2Y1 and P2Y6 in MFB | Cytosolic Ca2+ mobilization. Stimulation of activated HSCs with UDP increases the expression level of procollagen-1 by threefold. | [111] |
| P2X7 | Increases expression level by acetaldehyde. Receptor stimulation with BzATP in HSCs induces upregulation of cell proliferation, inflammation and activation markers. | [112] |
| P2X4 | The subunit was overexpressed in hepatotoxic-induced fibrotic liver and in the cell line HSC-T16 treated with acetaldehyde. Blocking of the receptor with 5-BDBD prevents activation of HSC-T16. | [89] |
| P2Y14 | High expression in HSCs and their ligands, UDP-glucose and UDP-galactose, are released by dying hepatocytes. P2Y14 activation mediates HSC activation. | [113] |
| NTPDase2 | It is expressed through the HSC activation process. | [111] |
| NTPDase1 (CD39) and NTPDase2 | Expressed by HSCs cultured for 1 (quiescent), 4, and 7 (activated) days. In livers with fibrosis induced by administration of CCl4 for 6 weeks, NTPDase2 co-localizes with activated HSCs. | [111] |
| NTPDase1 (CD39) | Pharmacological or genetic inhibition of its expression in the T6 HSC line abolishes acetaldehyde-dependent proliferation and expression of fibrosis markers. | [114] |
| ENPP2-3 | The enzymes were detected in the mouse GRX HSC line. Its activity is higher in the quiescent phenotype than in the activated one, and the expression level of NPP2 increased while NPP3 expression decreased. | [115] |
| CD73 | CD73 KO mice are resistant to experimental fibrosis. | [116] |
| CD73 | Its expression level increases throughout the HSC activation process; this regulation is mediated by the TGF-b pathway. | [117] |
| CD73 | Plays a role in HSC activation, including when the process is mediated by alcohol-dependent damage models. | [118,119,120,121] |
| A2a | In the human HSC line LX2, A2a receptor activation inhibited the PDGF-induced chemotactic response by modulating a pathway that increased cAMP and upregulated the production of TGF-β and collagen I RNA. | [122] |
| A2a | Induces collagen type I and type III overexpression, mediated by a pathway that involves protein kinase A, src kinase, and mitogen-activated protein kinases ERK and p38 in the LX2 HSC line. | [123] |
| A2a | Interferon-g regulates A2a receptor function via the transcriptional regulation of AC by STAT-1 in LX2 cells. | [123,124] |
| A2a | Mediates loss of actin stress fibers throughout a pathway that inhibits cAMP, PKA, and Rho and suppresses endothelin-1 and lysophosphatidic acid-induced cell contraction in the LX2 HSC line. | [125] |
| A2a | Increased cell proliferation and inhibited senescence entry in the LX2 HSC line. | [126] |
| A2a | In the HSC-T6 line, the antagonists caffeine and ZM241385 inhibit the acetaldehyde-dependent expression of fibrotic markers by a cAMP-dependent pathway. | [126,127] |
| A2a | Caffeine inhibits the activation of primary mouse HSCs by Akt1 signaling. | [128] |
| Not determined | Adenosine aspartate prevents the activation of primary rat HSCs and inhibits the proliferative effect of platelet-derived growth factor. | [128,129] |
| Kupffer Cells | ||
|---|---|---|
| Ligand, Receptor or Enzyme | Effect | Reference |
| P2X4 and P2X6 | Both subunits were detected in ED1+ KC by immunofluorescence. Intraperitoneal injection of LPS upregulated P2X6. | [149] |
| P2X7 | Induces NLRP3 inflammasome assembly and, consequently, IL-1β release in primary KC. | [76] |
| P2X7 | The environmental contaminant imidacloprid, a hepatotoxic compound, induces P2X7-dependent pyroptosis in primary KCs. | [151] |
| P2X7 | In the KUP5 mouse line primed with LPS, stimulation with 3 mM of ATP induced channel activation and triggered the release of inflammatory messengers, such as prostaglandin E2, IL-1β, and high mobility group box-1 protein (HMGB1). | [152] |
| P2X7 | The exposure of KUP5 cells to silica nanoparticles (30 nm in diameter) induced ATP release, activating the P2X7 receptor that induces IL-1ẞ production. | [153] |
| P2Y6 | The receptor is overexpressed in the KCs of mice with alcohol-induced steatohepatitis. In the liver of these animals, the administration of UDP elevated the damage marker levels and the release of the proinflammatory cytokines. | [154] |
| ADO | ADO administration in ischemic preconditioning, a transitory occlusion of the portal triad and reperfusion prior to ischemia, protects liver graft viability by modulating KC-dependent inflammation. | [155,156] |
| A2 | Alcohol feeding in mice induced A2b overexpression in KCs. This was reproduced in vitro in the RAW264.7 cell line treated with ethanol. In this model, the A2b receptor KO induces the expression of proinflammatory cytokines while overexpression of this receptor weakens the inflammatory response. | [157] |
| A2b | Alcohol feeding in mice induced A2b overexpression in KCs. This was reproduced in vitro in the RAW264.7 line treated with ethanol. In this last model, A2b KO boosts proinflammatory cytokine expression while overexpression weakens inflammatory response. | [158] |
| CD39 | Myeloid cell-specific deletion of this enzyme increased mouse sensibility to pharmacologically induced biliary fibrosis, together with an increment in the expression levels of the transcripts of the inflammation markers. | [158,159] |
| CD39 | CD39−/− mice show higher inflammation levels and liver damage in response to sepsis, probably due to macrophage activity by deficient extracellular ATP scavenging. | [93] |
| Liver Sinusoidal Endothelial Cells | ||
|---|---|---|
| Ligand, Receptor or Enzyme | Effect | Reference |
| ATP and ADP | Stimulates the release of prostacyclin (PGI2) and nitric oxide (NO), inhibitors of platelet aggregation, through a mechanism involving Ca2+ mobilization. | [166,167] |
| P2Y and/or ADO receptors | Prostaglandin E2 (PGE2) is the main prostanoid secreted by primary cultures of LSECs in response to extracellular nucleotides. | [168] |
| CD39 | Partial hepatectomy induces a specific increment in the expression level of CD39 in LSECs. In CD39−/− mice, hepatic regeneration was impaired. Throughout the regenerative process, angiogenesis decreased while hepatic damage increased. | [168,169] |
| CD39 | Co-culture of mouse LSECs expressing CD39 boosts the proliferation of tumor cells and limits extracellular ATP-dependent cell death induction. | [170] |
| A2a | The A2a agonist CGS21680 protected LSECs from the damage induced by ischemia reperfusion. | [170,171] |
| Cholangiocytes | ||
|---|---|---|
| Ligand, Receptor, or Enzyme | Effect | Reference |
| P2Y2 | In cholangiocytes, UTP stimulates secretion. | [205,206] |
| P2Y12 | The expression of the receptor in the apical membrane of cholangiocytes appears to be restricted to the cilium, a mechano-, osmo-, and chemo-sensory organelle that senses and transmits different stimuli from the bile into the cellular interior, modifying cell functions. | [208,209] |
| P2X: 2, 3, 4, and 6 | In cultured rat cholangiocytes, they modulated biliary secretion. | [210] |
| P2X4 | In Mz-ChA-1 cells, BzATP elicited robust Cl- secretory responses. | [210] |
| A2a | In polarized rat cholangiocytes, it modulates the activity of apical CNT3. | [212] |
| P2Y6, P2Y13, and P2X7 | In cholangiocarcinoma cells, they induce proliferation and motility. | [213,214] |
| P2Y2 and P2X3 | In MMNK-1, an immortalized cholangiocarcinoma cell line, this receptor conferred resistance to cell proliferation and cell motility inhibition. | [213,214] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mata-Martínez, E.; Ramírez-Ledesma, M.G.; Vázquez-Victorio, G.; Hernández-Muñoz, R.; Díaz-Muñoz, M.; Vázquez-Cuevas, F.G. Purinergic Signaling in Non-Parenchymal Liver Cells. Int. J. Mol. Sci. 2024, 25, 9447. https://doi.org/10.3390/ijms25179447
Mata-Martínez E, Ramírez-Ledesma MG, Vázquez-Victorio G, Hernández-Muñoz R, Díaz-Muñoz M, Vázquez-Cuevas FG. Purinergic Signaling in Non-Parenchymal Liver Cells. International Journal of Molecular Sciences. 2024; 25(17):9447. https://doi.org/10.3390/ijms25179447
Chicago/Turabian StyleMata-Martínez, Esperanza, María Guadalupe Ramírez-Ledesma, Genaro Vázquez-Victorio, Rolando Hernández-Muñoz, Mauricio Díaz-Muñoz, and Francisco G. Vázquez-Cuevas. 2024. "Purinergic Signaling in Non-Parenchymal Liver Cells" International Journal of Molecular Sciences 25, no. 17: 9447. https://doi.org/10.3390/ijms25179447
APA StyleMata-Martínez, E., Ramírez-Ledesma, M. G., Vázquez-Victorio, G., Hernández-Muñoz, R., Díaz-Muñoz, M., & Vázquez-Cuevas, F. G. (2024). Purinergic Signaling in Non-Parenchymal Liver Cells. International Journal of Molecular Sciences, 25(17), 9447. https://doi.org/10.3390/ijms25179447






