Using Transcriptomics to Determine the Mechanism for the Resistance to Fusarium Head Blight of a Wheat-Th. elongatum Translocation Line
Abstract
:1. Introduction
2. Results
2.1. Establishment of a Wheat-Th. elongatum 7EL Chromosome Translocation Line YNM158 with FHB Resistance
2.2. RNA-Sequencing (RNA-Seq) Data Quality, Assembly, and Annotation of YNM158 and YM158
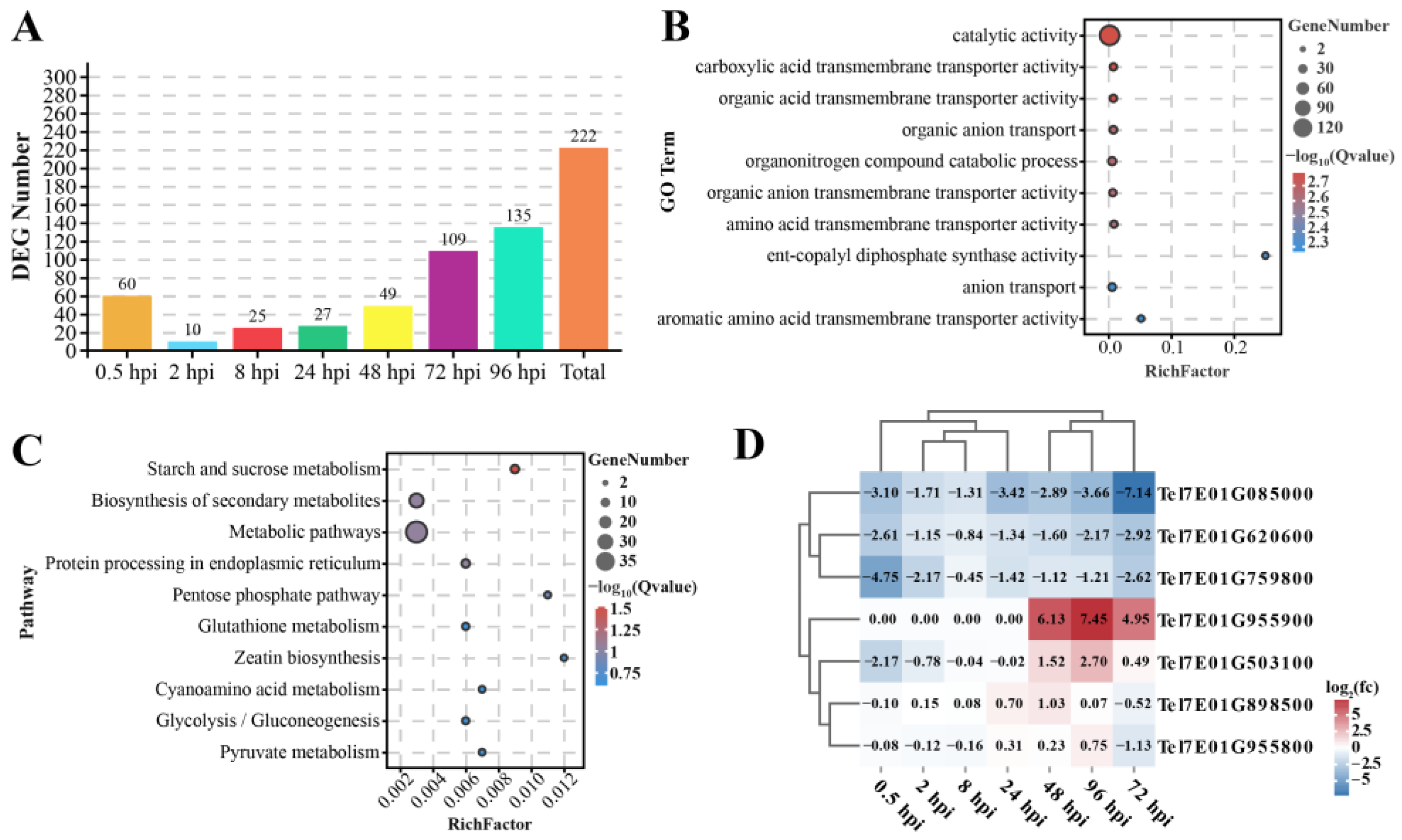
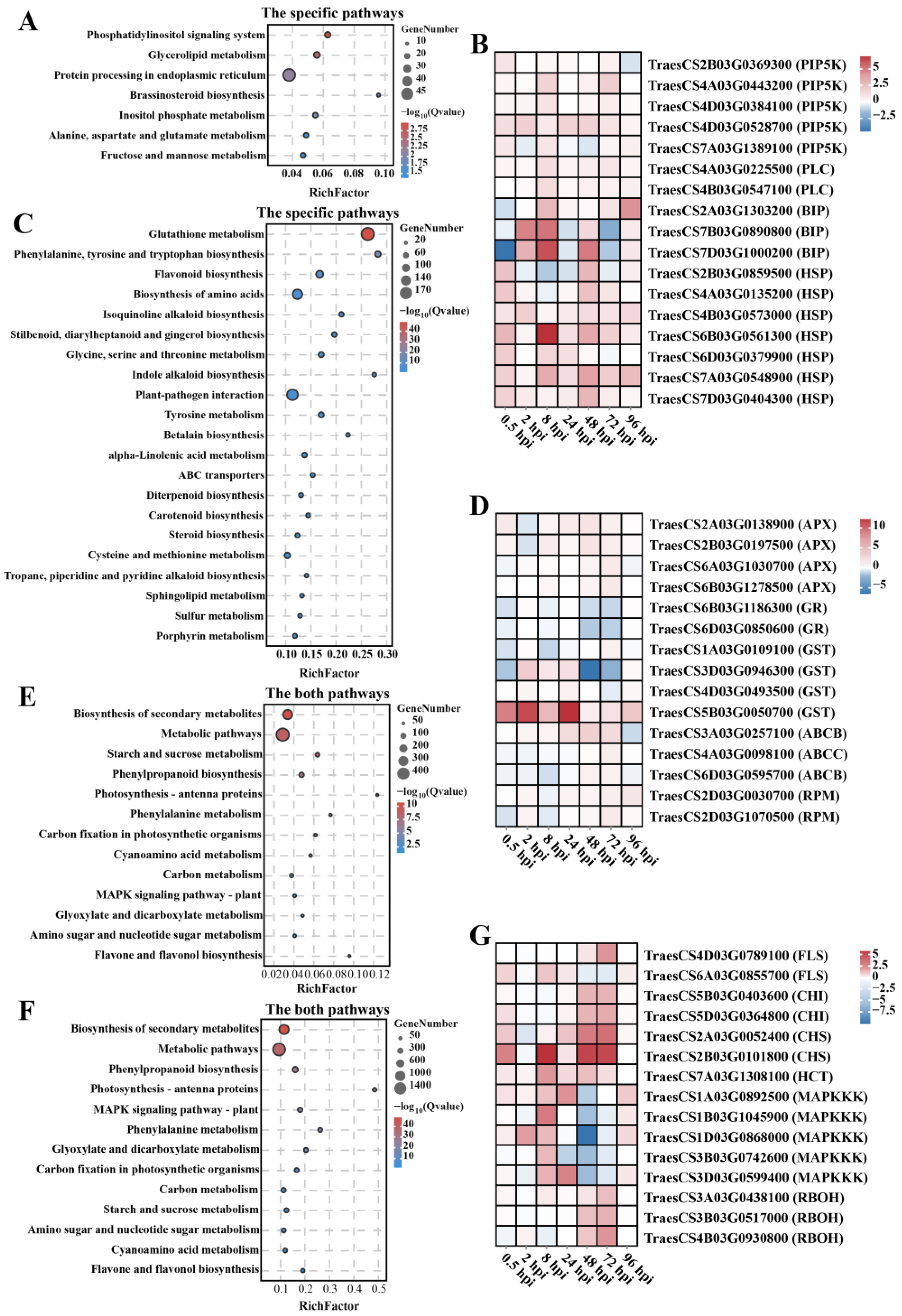
2.3. Identification of the Differentially Expressed Genes (DEGs) on Chromosome 7EL Post Inoculation with F. graminearum
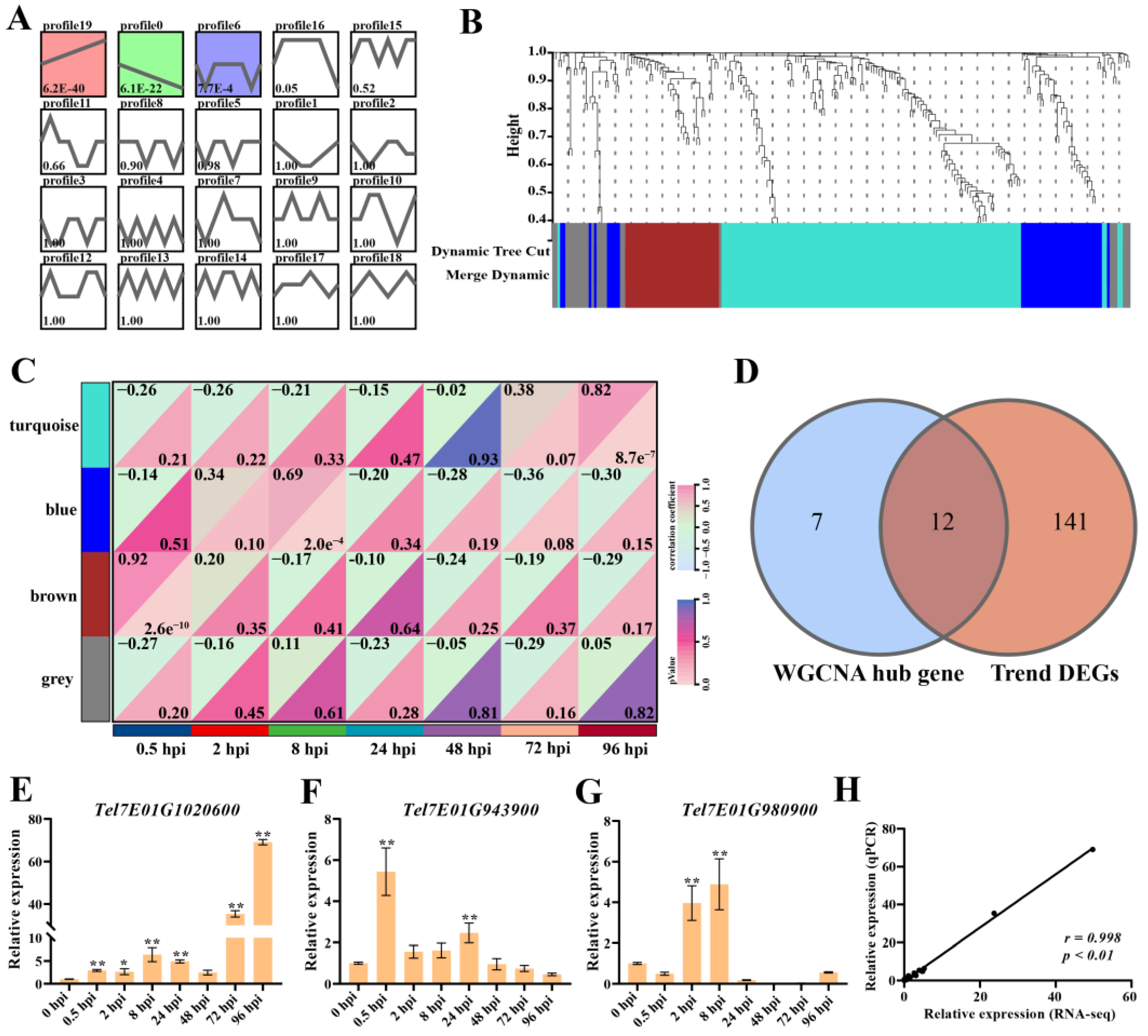
2.4. Gene Expression Patterns Analysis of DGEs on Chromosome 7EL at Different Infection Time Points
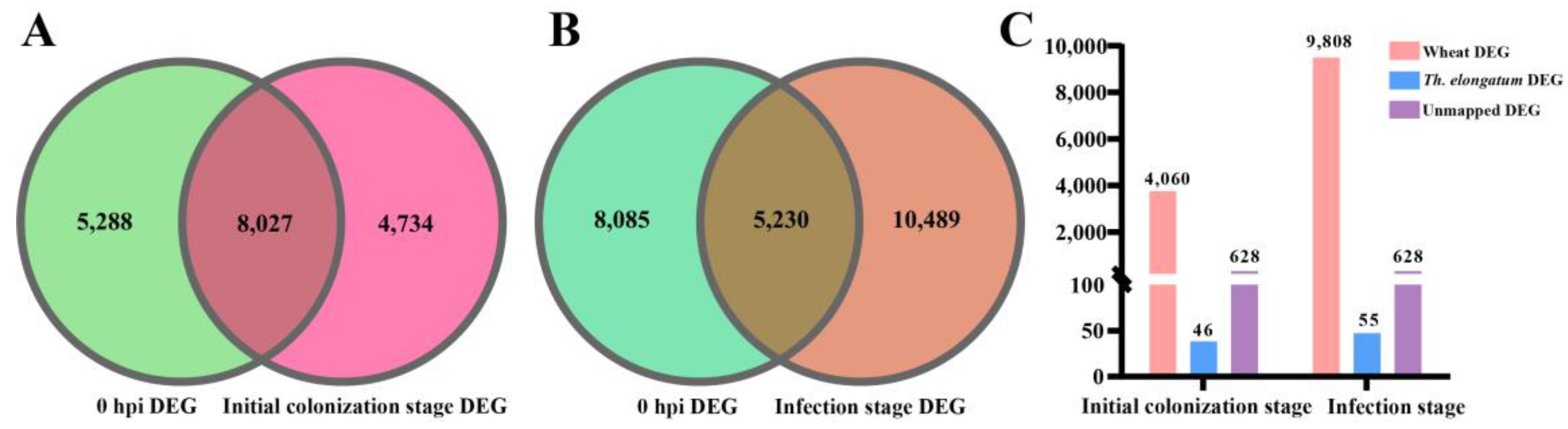
2.5. Extraction of Wheat DEGs between YM158 and YNM158 after F. graminearum Infection
2.6. KEGG Pathway Enrichment Analysis of Wheat DEGs at Different Stages
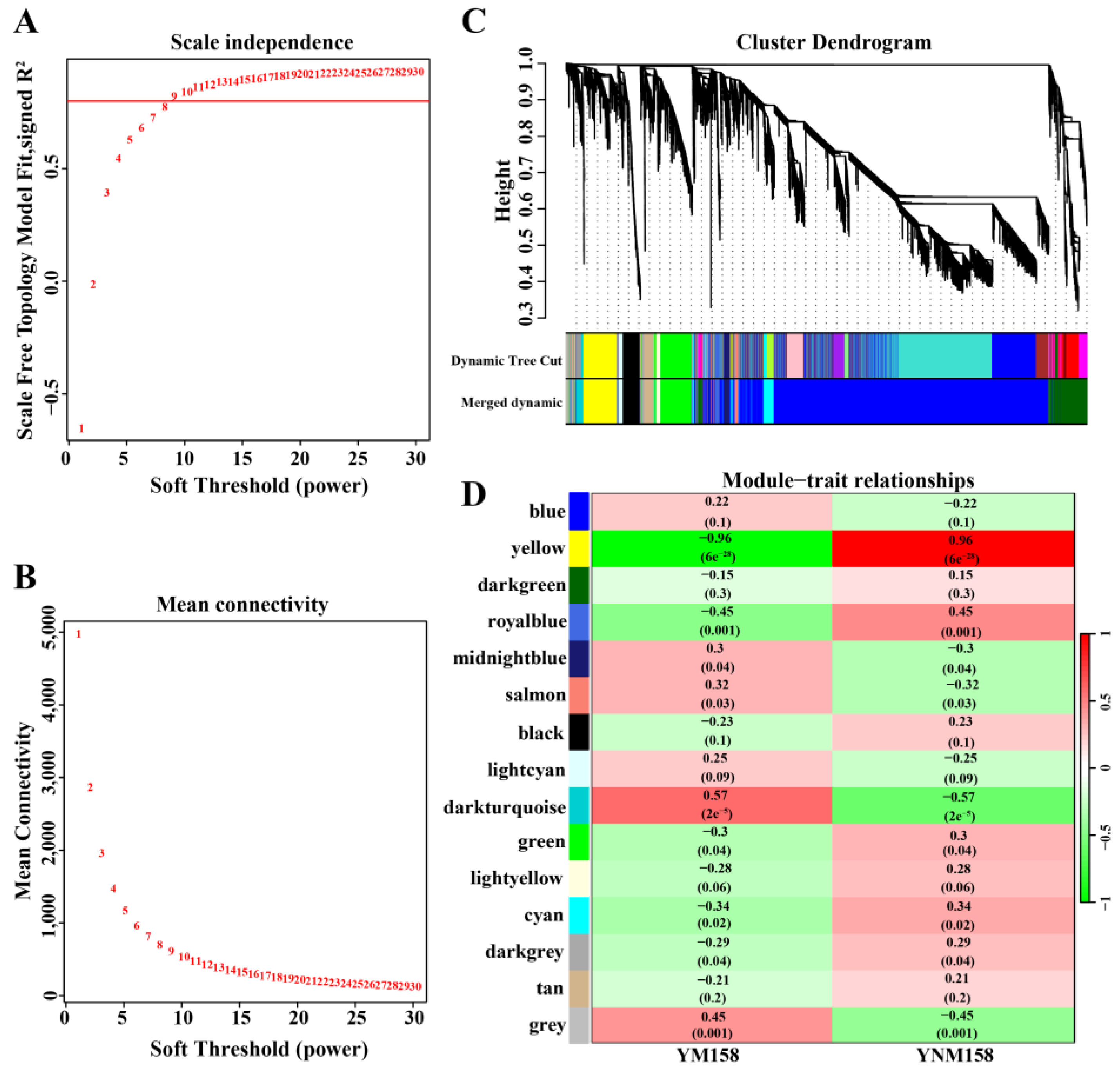
2.7. WGCNA of Wheat DEGs
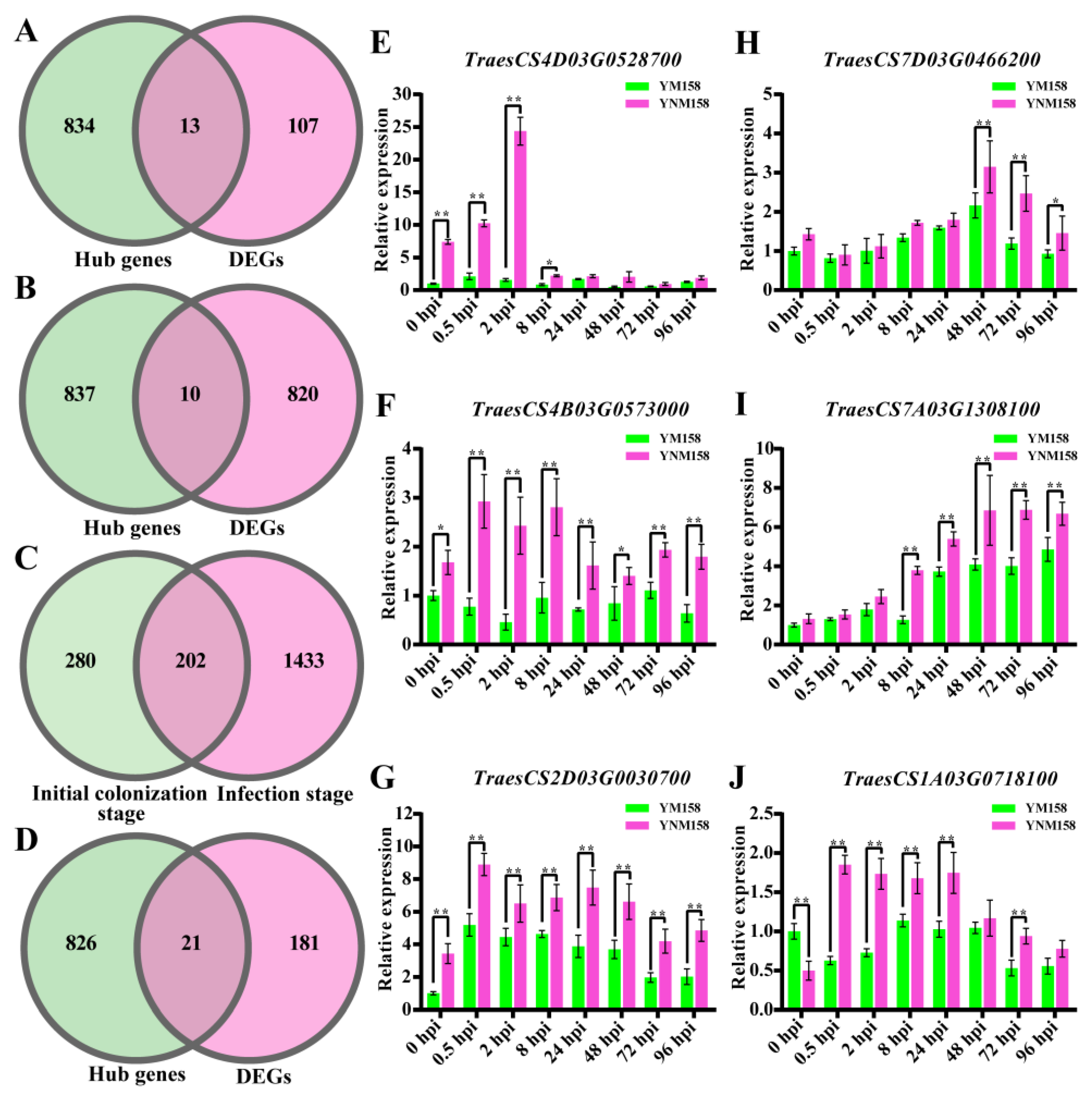
2.8. Core-Gene Screening at Different Infection Stages
3. Discussion
3.1. YNM158 Can Be Effectively Applied in FHB Improvement in Wheat Breeding
3.2. Transcriptome Analysis Validated That Glutathione Is One of the Important Contributors to FHB-Resistance Roles in the Pathogen Infection Stage
3.3. Other Genes from the 7EL Fragment in YNM158 Might Also Be Involved in Increasing FHB Resistance Especially in the Pathogen Initial Colonization Stage
3.4. Introgression of the 7EL Fragment Altered the Gene Expression in Wheat after F. graminearum Inoculation
4. Materials and Methods
4.1. Plant Materials
4.2. Cell Cycle Synchronization and Preparation of Mitotic Chromosomes
4.3. Genomic In Situ Hybridization (GISH) and Fluorescence In Situ Hybridization (FISH) Analysis
4.4. Evaluation of Disease Resistance
4.5. De Novo Assembly of RNA-Seq Reads and Quantifying Gene Expression
4.6. Quantitative Real-Time Polymerase Chain Reaction
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cuthbert, P.A.; Somers, D.J.; Thomas, J.; Cloutier, S.; Brule-Babel, A. Fine mapping Fhb1, a major gene controlling Fusarium head blight resistance in bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2006, 112, 1465–1472. [Google Scholar] [CrossRef]
- Cuthbert, P.A.; Somers, D.J.; Brule-Babel, A. Mapping of Fhb2 on chromosome 6BS: A gene controlling Fusarium head blight field resistance in bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2007, 114, 429–437. [Google Scholar] [CrossRef]
- Xue, S.; Li, G.Q.; Jia, H.Y.; Xu, F.; Lin, F.; Tang, M.Z.; Wang, Y.; An, X.; Xu, H.B.; Zhang, L.X.; et al. Fine mapping Fhb4, a major QTL conditioning resistance to Fusarium infection in bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2010, 121, 147–156. [Google Scholar] [CrossRef]
- Xue, S.L.; Xu, F.; Tang, M.Z.; Zhou, Y.; Li, G.Q.; An, X.; Lin, F.; Xu, H.B.; Jia, H.Y.; Zhang, L.X.; et al. Precise mapping Fhb5, a major QTL conditioning resistance to Fusarium infection in bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2011, 123, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, G.Q.; Jia, H.Y.; Cheng, R.; Zhong, J.K.; Shi, J.X.; Chen, R.T.; Wen, Y.X.; Ma, Z.Q. Breeding evaluation and precise mapping of Fhb8 for Fusarium head blight resistance in wheat (Triticum aestivum). Plant Breed. 2023, 143, 26–33. [Google Scholar] [CrossRef]
- Qi, L.L.; Pumphrey, M.O.; Friebe, B.; Chen, P.D.; Gill, B.S. Molecular cytogenetic characterization of alien introgressions with gene Fhb3 for resistance to Fusarium head blight disease of wheat. Theor. Appl. Genet. 2008, 117, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Cainong, J.C.; Bockus, W.W.; Feng, Y.G.; Chen, P.D.; Qi, L.L.; Sehgal, S.K.; Danilova, T.V.; Koo, D.H.; Friebe, B.; Gill, B.S. Chromosome engineering, mapping, and transferring of resistance to Fusarium head blight disease from Elymus tsukushiensis into wheat. Theor. Appl. Genet. 2015, 128, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhang, X.L.; Hou, Y.L.; Cai, J.J.; Shen, X.R.; Zhou, T.T.; Xu, H.H.; Ohm, H.W.; Wang, H.W.; Li, A.F.; et al. High-density mapping of the major FHB resistance gene Fhb7 derived from Thinopyrum ponticum and its pyramiding with Fhb1 by marker-assisted selection. Theor. Appl. Genet. 2015, 128, 2301–2316. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; Sun, S.L.; Ge, W.Y.; Zhao, L.F.; Hou, B.Q.; Wang, K.; Lyu, Z.F.; Chen, L.Y.; Xu, S.S.; Guo, J.; et al. Horizontal gene transfer of Fhb7 from fungus underlies Fusarium head blight resistance in wheat. Science 2020, 368, eaba5435. [Google Scholar] [CrossRef]
- Dai, Y.; Huang, S.; Sun, G.; Li, H.; Chen, J. Origins and chromosome differentiation of Thinopyrum elongatum revealed by PepC, Pgk1 genes and ND-FISH. Genome 2021, 64, 901–913. [Google Scholar] [CrossRef]
- Jauhar, P.P.; Peterson, T.S.; Xu, S.S. Cytogenetic and molecular characterization of a durum alien disomic addition line with enhanced tolerance to Fusarium head blight. Genome 2009, 52, 467–483. [Google Scholar] [CrossRef]
- Jauhar; Prem, P. Durum wheat genetic stocks involving chromosome 1E of diploid wheatgrass: Resistance to Fusarium head blight. Nucleus 2014, 57, 19–23. [Google Scholar]
- Liu, H.P.; Dai, Y.; Chi, D.; Huang, S.; Li, H.F.; Duan, Y.M.; Cao, W.G.; Gao, Y.; Fedak, G.; Chen, J.M. Production and Molecular Cytogenetic Characterization of a Durum Wheat-Thinopyrum elongatum 7E Disomic Addition Line with Resistance to Fusarium Head Blight. Cytogenet. Genome Res. 2017, 153, 165–173. [Google Scholar] [CrossRef]
- Shen, R.; Kong, L.R.; Ohm, H. Fusarium head blight resistance in hexaploid wheat (Triticum aestivum)-Lophopyrum genetic lines and tagging of the alien chromatin by PCR markers. Theor. Appl. Genet. 2004, 108, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Shen, X.R.; Hao, Y.F.; Cai, J.J.; Ohm, H.W.; Kong, L.R. A genetic map of Lophopyrum ponticum chromosome 7E, harboring resistance genes to Fusarium head blight and leaf rust. Theor. Appl. Genet. 2011, 122, 263–270. [Google Scholar] [CrossRef]
- Guo, X.R.; Wang, M.; Kang, H.Y.; Zhou, Y.H.; Han, F.P. Distribution, Polymorphism and Function Characteristics of the GST-Encoding Fhb7 in Triticeae. Plants 2022, 11, 2074. [Google Scholar] [CrossRef]
- Guo, X.R.; Shi, Q.H.; Wang, M.; Yuan, J.; Zhang, J.; Wang, J.; Liu, Y.; Su, H.D.; Wang, Z.; Li, J.B.; et al. Functional analysis of the glutathione S-transferases from Thinopyrum and its derivatives on wheat Fusarium head blight resistance. Plant Biotechnol. J. 2023, 21, 1091–1093. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Ohm, H. Fusarium head blight resistance derived from Lophopyrum elongatum chromosome 7E and its augmentation with Fhb1 in wheat. Plant Breed. 2006, 125, 424–429. [Google Scholar] [CrossRef]
- Zhang, W.; Danilova, T.; Zhang, M.Y.; Ren, S.F.; Zhu, X.W.; Zhang, Q.J.; Zhong, S.B.; Dykes, L.; Fiedler, J.; Xu, S.; et al. Cytogenetic and genomic characterization of a novel tall wheatgrass-derived Fhb7 allele integrated into wheat B genome. Theor. Appl. Genet. 2022, 135, 4409–4419. [Google Scholar] [CrossRef]
- Stephens, A.E.; Gardiner, D.M.; White, R.G.; Munn, A.L.; Manners, J.M. Phases of Infection and Gene Expression of Fusarium graminearum during Crown Rot Disease of Wheat. Mol. Plant-Microbe Interact. 2008, 21, 1571–1581. [Google Scholar] [CrossRef]
- Kong, L.N.; Song, X.Y.; Xiao, J.; Sun, H.J.; Dai, K.L.; Lan, C.X.; Singh, P.W.; Yuan, C.X.; Zhang, S.Z.; Singh, R.; et al. Development and characterization of a complete set of Triticum aestivum-Roegneria ciliaris disomic addition lines. Theor. Appl. Genet. 2018, 131, 1793–1806. [Google Scholar] [CrossRef]
- Gong, B.R.; Zhu, W.; Li, S.Y.; Wang, Y.Q.; Xu, L.L.; Wang, Y.; Zeng, J.; Fan, X.; Sha, L.N.; Zhang, H.Q.; et al. Molecular cytogenetic characterization of wheat-Elymus repens chromosomal translocation lines with resistance to Fusarium head blight and stripe rust. BMC Plant Biol. 2019, 19, 590. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Xu, M.R.; Wang, Y.F.; Cheng, X.F.; Huang, C.X.; Zhang, H.; Li, T.D.; Wang, C.Y.; Chen, C.H.; Wang, Y.J.; et al. Development and Molecular Cytogenetic Identification of Two Wheat-Aegilops geniculata Roth 7M(g) Chromosome Substitution Lines with Resistance to Fusarium Head Blight, Powdery Mildew and Stripe Rust. Int. J. Mol. Sci. 2022, 23, 7056. [Google Scholar] [CrossRef] [PubMed]
- Haldar, A.; Tekieh, F.; Balcerzak, M.; Wolfe, D.; Lim, D.; Joustra, K.; Konkin, D.; Han, F.P.; Fedak, G.; Ouellet, T. Introgression of Thinopyrum elongatum DNA fragments carrying resistance to Fusarium head blight into Triticum aestivum cultivar Chinese Spring is associated with alteration of gene expression. Genome 2021, 64, 1009–1020. [Google Scholar] [CrossRef]
- Zhang, R.Q.; Wang, X.E.; Chen, P.D. Molecular and cytogenetic characterization of a small alien-segment translocation line carrying the softness genes of Haynaldia villosa. Genome 2012, 55, 639–646. [Google Scholar] [CrossRef]
- Li, J.; Bao, Y.; Han, R.; Wang, X.; Xu, W.; Li, G.; Yang, Z.; Zhang, X.; Li, X.; Liu, A.; et al. Molecular and Cytogenetic Identification of Stem Rust Resistant Wheat-Thinopyrum intermedium Introgression Lines. Plant Dis. 2022, 106, 2447–2454. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.D.; You, C.F.; Hu, Y.; Chen, S.W.; Zhou, B.; Cao, A.Z.; Wang, X. Radiation-induced translocations with reduced Haynaldia villosa chromatin at the Pm21 locus for powdery mildew resistance in wheat. Mol. Breed. 2013, 31, 477–484. [Google Scholar] [CrossRef]
- Yang, C.; Liu, R.; Pang, J.; Ren, B.; Zhou, H.; Wang, G.; Wang, E.; Liu, J. Poaceae-specific cell wall-derived oligosaccharides activate plant immunity via OsCERK1 during Magnaporthe oryzae infection in rice. Nat. Commun. 2021, 12, 2178. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Daudi, A.; Butt, V.S.; Bolwell, G.P. Reactive oxygen species and their role in plant defence and cell wall metabolism. Planta 2012, 236, 765–779. [Google Scholar] [CrossRef]
- García-Caparrós, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative Stress and Antioxidant Metabolism under Adverse Environmental Conditions: A Review. Bot. Rev. 2021, 87, 421–466. [Google Scholar] [CrossRef]
- Pang, C.H.; Wang, B.S. Role of Ascorbate Peroxidase and Glutathione Reductase in Ascorbate-Glutathione Cycle and Stress Tolerance in Plants. In Ascorbate-Glutathione Pathway and Stress Tolerance in Plants; Springer: Dordrecht, The Netherlands, 2010; pp. 91–113. [Google Scholar]
- Lu, C.C.; Jiang, Y.K.; Yue, Y.Z.; Sui, Y.R.; Hao, M.X.; Kang, X.J.; Wang, Q.B.; Chen, D.Y.; Liu, B.Y.; Yin, Z.Y.; et al. Glutathione and neodiosmin feedback sustain plant immunity. J. Exp. Bot. 2023, 74, 976–990. [Google Scholar] [CrossRef] [PubMed]
- Gullner, G.; Kömives, T. The Role of Glutathione and Glutathione-related Enzymes in Plant-pathogen Interactions. In Plant Ecophysiology; Springer: Dordrecht, The Netherlands, 2001; pp. 207–239. [Google Scholar]
- Chen, Y.P.; Xing, L.P.; Wu, G.J.; Wang, H.Z.; Wang, X.E.; Cao, A.Z.; Chen, P.D. Plastidial glutathione reductase from Haynaldia villosa is an enhancer of powdery mildew resistance in wheat (Triticum aestivum). Plant Cell Physiol. 2007, 48, 1702–1712. [Google Scholar] [CrossRef] [PubMed]
- Mueller, L.A.; Goodman, C.D.; Silady, R.A.; Walbot, V. AN9, a petunia glutathione S-transferase required for anthocyanin sequestration, is a flavonoid-binding protein. Plant Physiol. 2000, 123, 1561–1570. [Google Scholar] [CrossRef]
- Gong, H.B.; Jiao, Y.X.; Hu, W.W.; Pua, E.C. Expression of glutathione-S-transferase and its role in plant growth and development in vivo and shoot morphogenesis in vitro. Plant Mol. Biol. 2005, 57, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.G.; Zhang, Y.H.; Meng, Q.L.; Shi, F.M.; Liu, J.; Li, Y.C. Molecular cloning, identification of GSTs family in sunflower and their regulatory roles in biotic and abiotic stress. World J. Microbiol. Biotechnol. 2018, 34, 109. [Google Scholar] [CrossRef]
- Edwards, R.; Dixon, D.P.; Walbot, V. Plant glutathione S-transferases: Enzymes with multiple functions in sickness and in health. Trends Plant Sci. 2000, 5, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.D.; Goodwin, P.H.; Hsiang, T. Induction of glutathione S-transferase genes of Nicotiana benthamiana following infection by Colletotrichum destructivum and C. orbiculare and involvement of one in resistance. J. Exp. Bot. 2005, 56, 1525–1533. [Google Scholar] [CrossRef]
- Pislewska-Bednarek, M.; Nakano, R.T.; Hiruma, K.; Pastorczyk, M.; Sanchez-Vallet, A.; Singkaravanit-Ogawa, S.; Ciesiolka, D.; Takano, Y.; Molina, A.; Schulze-Lefert, P.; et al. Glutathione Transferase U13 Functions in Pathogen-Triggered Glucosinolate Metabolism. Plant Physiol. 2018, 176, 538–551. [Google Scholar] [CrossRef]
- Han, Q.; Chen, R.; Yang, Y.; Cui, X.M.; Ge, F.; Chen, C.Y.; Liu, D.Q. A glutathione S-transferase gene from Lilium regale Wilson confers transgenic tobacco resistance to Fusarium oxysporum. Sci. Hortic. 2016, 198, 370–378. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, J.; Jin, P.F.; Guo, M.Y.; Guo, J.; Cheng, P.; Li, Q.; Wang, B.T. Glutathione S-transferase interactions enhance wheat resistance to powdery mildew but not wheat stripe rust. Plant Physiol. 2022, 190, 1418–1439. [Google Scholar] [CrossRef]
- Chen, Y.; Kistler, H.C.; Ma, Z.H. Fusarium graminearum Trichothecene Mycotoxins: Biosynthesis, Regulation, and Management. Annu. Rev. Phytopathol. 2019, 57, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Song, R.R.; Cheng, Y.F.; Wen, M.X.; Song, X.Y.; Wang, T.; Xia, M.S.; Sun, H.J.; Cheng, M.H.; Cui, H.M.; Yuan, C.X.; et al. Transferring a new Fusarium head blight resistance locus FhbRc1 from Roegneria ciliaris into wheat by developing alien translocation lines. Theor. Appl. Genet. 2023, 136, 36. [Google Scholar] [CrossRef] [PubMed]
- Ilgen, P.; Hadeler, B.; Maier, F.J.; Schäfer, W. Developing Kernel and Rachis Node Induce the Trichothecene Pathway of Fusarium graminearum during Wheat Head Infection. Mol. Plant-Microbe Interact. 2009, 22, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Boenisch, M.J.; Schäfer, W. Fusarium graminearum forms mycotoxin producing infection structures on wheat. BMC Plant Biol. 2011, 11, 110. [Google Scholar] [CrossRef]
- Mudge, A.M.; Dill-Macky, R.; Dong, Y.H.; Gardiner, D.M.; White, R.G.; Manners, J.M. A role for the mycotoxin deoxynivalenol in stem colonisation during crown rot disease of wheat caused by Fusarium graminearum and Fusarium pseudograminearum. Physiol. Mol. Plant Pathol. 2006, 69, 73–85. [Google Scholar] [CrossRef]
- Sun, Y.C.; Wang, X.J.; Liu, F.Y.; Guo, H.Y.; Wang, J.F.; Wei, Z.T.; Kang, Z.S.; Tang, C.L. A Leucine-Rich Repeat Receptor-like Kinase TaBIR1 Contributes to Wheat Resistance against Puccinia striiformis f. sp. tritici. Int. J. Mol. Sci. 2023, 24, 6438. [Google Scholar] [CrossRef]
- Guo, F.L.; Wu, T.C.; Shen, F.D.; Xu, G.B.A.; Qi, H.J.; Zhang, Z.Y. The cysteine-rich receptor-like kinase TaCRK3 contributes to defense against Rhizoctonia cerealis in wheat. J. Exp. Bot. 2021, 72, 6904–6919. [Google Scholar] [CrossRef]
- Thapa, G.; Gunupuru, L.R.; Hehir, J.G.; Kahla, A.; Mullins, E.; Doohan, F.M. A Pathogen-Responsive Leucine Rich Receptor Like Kinase Contributes to Fusarium Resistance in Cereals. Front. Plant Sci. 2018, 9, 867. [Google Scholar] [CrossRef]
- Coleman, A.D.; Maroschek, J.; Raasch, L.; Takken, F.L.W.; Ranf, S.; Hückelhoven, R. The Arabidopsis leucine-rich repeat receptor-like kinase MIK2 is a crucial component of early immune responses to a fungal-derived elicitor. New Phytol. 2021, 229, 3453–3466. [Google Scholar] [CrossRef]
- Ruan, Y.L. Sucrose Metabolism: Gateway to Diverse Carbon Use and Sugar Signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Liu, Y.H.; Offler, C.E.; Ruan, Y.L. Regulation of fruit and seed response to heat and drought by sugars as nutrients and signals. Front. Plant Sci. 2013, 4, 282. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Le Roy, K.; Bolouri-Moghaddam, M.R.; Vanhaecke, M.; Lammens, W.; Rolland, F.; Van den Ende, W. Exploring the neutral invertase-oxidative stress defence connection in Arabidopsis thaliana. J. Exp. Bot. 2011, 62, 3849–3862. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulos, V.; Gilbert, M.J.; Pittman, J.K.; Marvier, A.C.; Buchanan, A.J.; Sauer, N.; Hall, J.L.; Williams, L.E. The monosaccharide transporter gene, AtSTP4, and the cell-wall invertase, Atβfruct1, are induced in Arabidopsis during infection with the fungal biotroph Erysiphe cichoracearum. Plant Physiol. 2003, 132, 821–829. [Google Scholar] [CrossRef]
- Chang, Q.; Lin, X.H.; Yao, M.H.; Liu, P.; Guo, J.; Huang, L.L.; Voegele, R.T.; Kang, Z.S.; Liu, J. Hexose transporter PsHXT1-mediated sugar uptake is required for pathogenicity of wheat stripe rust. Plant Biotechnol. J. 2020, 18, 2367–2369. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Q.; Qu, X.Q.; Hou, B.H.; Sosso, D.; Osorio, S.; Fernie, A.R.; Frommer, W.B. Sucrose Efflux Mediated by SWEET Proteins as a Key Step for Phloem Transport. Science 2012, 335, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Sutton, P.N.; Gilbert, M.J.; Williams, L.E.; Hall, J.L. Powdery mildew infection of wheat leaves changes host solute transport and invertase activity. Physiol. Plant. 2010, 129, 787–795. [Google Scholar] [CrossRef]
- Harewood, L.; Fraser, P. The impact of chromosomal rearrangements on regulation of gene expression. Hum. Mol. Genet. 2014, 23, R76–R82. [Google Scholar] [CrossRef]
- Spielmann, M.; Lupianez, D.G.; Mundlos, S. Structural variation in the 3D genome. Nat. Rev. Genet. 2018, 19, 453–467. [Google Scholar] [CrossRef]
- Perk, E.A.; Di Palma, A.A.; Colman, S.; Mariani, O.; Cerrudo, I.; D’Ambrosio, J.M.; Robuschi, L.; Pombo, M.A.; Rosli, H.G.; Villareal, F.; et al. CRISPR/Cas9-mediated phospholipase C 2 knock-out tomato plants are more resistant to Botrytis cinerea. Planta 2023, 257, 117. [Google Scholar] [CrossRef]
- Gonorazky, G.; Guzzo, M.C.; Abd-El-Haliem, A.M.; Joosten, M.; Laxalt, A.M. Silencing of the tomato phosphatidylinositol-phospholipase C2 (SlPLC2) reduces plant susceptibility to Botrytis cinerea. Mol. Plant Pathol. 2016, 17, 1354–1363. [Google Scholar] [CrossRef]
- Laxalt, A.M.; Munnik, T. Phospholipid signalling in plant defence. Curr. Opin. Plant Biol. 2002, 5, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Vossen, J.H.; Abd-El-Haliem, A.; Fradin, E.F.; van den Berg, G.C.M.; Ekengren, S.K.; Meijer, H.J.G.; Seifi, A.; Bai, Y.L.; ten Have, A.; Munnik, T.; et al. Identification of tomato phosphatidylinositol-specific phospholipase-C (PI-PLC) family members and the role of PLC4 and PLC6 in HR and disease resistance. Plant J. 2010, 62, 224–239. [Google Scholar] [CrossRef] [PubMed]
- Song, F.M.; Goodman, R.M. Molecular cloning and characterization of a rice phosphoinositide-specific phospholipase C gene, OsPI-PLC1, that is activated in systemic acquired resistance. Physiol. Mol. Plant Pathol. 2002, 61, 31–40. [Google Scholar] [CrossRef]
- Shimada, T.L.; Betsuyaku, S.; Inada, N.; Ebine, K.; Fujimoto, M.; Uemura, T.; Takano, Y.; Fukuda, H.; Nakano, A.; Ueda, T. Enrichment of Phosphatidylinositol 4,5-Bisphosphate in the Extra-Invasive Hyphal Membrane Promotes Colletotrichum Infection of Arabidopsis thaliana. Plant Cell Physiol. 2019, 60, 1514–1524. [Google Scholar] [CrossRef]
- Park, C.J.; Seo, Y.S. Heat Shock Proteins: A Review of the Molecular Chaperones for Plant Immunity. Plant Pathol. J. 2015, 31, 323–333. [Google Scholar] [CrossRef]
- Chen, L.T.; Hamada, S.; Fujiwara, M.; Zhu, T.H.; Thao, N.P.; Wong, H.L.; Krishna, P.; Ueda, T.; Kaku, H.; Shibuya, N.; et al. The Hop/Sti1-Hsp90 Chaperone Complex Facilitates the Maturation and Transport of a PAMP Receptor in Rice Innate Immunity. Cell Host Microbe 2010, 7, 185–196. [Google Scholar] [CrossRef]
- Kim, N.H.; Hwang, B.K. Pepper Heat Shock Protein 70a Interacts with the Type III Effector AvrBsT and Triggers Plant Cell Death and Immunity. Plant Physiol. 2015, 167, 307–322. [Google Scholar] [CrossRef]
- Liu, J.Z.; Whitham, S.A. Overexpression of a soybean nuclear localized typeIII DnaJ domain-containing HSP40 reveals its roles in cell death and disease resistance. Plant J. 2013, 74, 110–121. [Google Scholar] [CrossRef]
- Widiastuti, A.; Arofatullah, N.A.; Kharisma, A.D.; Sato, T. Upregulation of heat shock transcription factors, Hsp70, and defense-related genes in heat shock-induced resistance against powdery mildew in cucumber. Physiol. Mol. Plant Pathol. 2021, 116, 101730. [Google Scholar] [CrossRef]
- Wei, Y.X.; Zeng, H.Q.; Liu, W.; Cheng, X.; Zhu, B.B.; Guo, J.R.; Shi, H.T. Autophagy-related genes serve as heat shock protein 90 co-chaperones in disease resistance against cassava bacterial blight. Plant J. 2021, 107, 925–937. [Google Scholar] [CrossRef]
- Jelitto-Van Dooren, E.; Vidal, S.; Denecke, J. Anticipating endoplasmic reticulum stress: A novel early response before pathogenesis-related gene induction. Plant Cell 1999, 11, 1935–1943. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Qi, F.; Liang, Y. Fuels for ROS signaling in plant immunity. Trends Plant Sci. 2023, 28, 1124–1131. [Google Scholar] [CrossRef]
- Torres, M.A.; Dangl, J.L.; Jones, J.D.G. Arabidopsis gp91phoxhomologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc. Natl. Acad. Sci. USA 2002, 99, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, H.; Numata, N.; Nakajima, K.; Katou, S.; Kawakita, K.; Rowland, O.; Jones, J.D.G.; Doke, N. Nicotiana benthamiana gp91phox homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans. Plant Cell 2003, 15, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Adachi, H.; Yoshioka, H. Kinase-mediated orchestration of NADPH oxidase in plant immunity. Brief. Funct. Genom. 2015, 14, 253–259. [Google Scholar] [CrossRef]
- Yamamizo, C.; Doke, N.; Yoshioka, H.; Kawakita, K. Involvement of mitogen-activated protein kinase in the induction of StrbohC and StrbohD genes in response to pathogen signals in potato. J. Gen. Plant Pathol. 2007, 73, 304–313. [Google Scholar] [CrossRef]
- Asai, S.; Ohta, K.; Yoshioka, H. MAPK signaling regulates nitric oxide and NADPH oxidase-dependent oxidative bursts in Nicotiana benthamiana. Plant Cell 2008, 20, 1390–1406. [Google Scholar] [CrossRef]
- Zhang, L.L.; Chen, S.Q.; Li, H.F.; Liu, H.P.; Dai, Y.; Gao, Y.; Chen, J.M. Development of wheat-Thinopyrum elongatum translocation lines resistant to Fusarium head blight. Sci. Agric. Sin. 2016, 49, 3477–3488. (In Chinese) [Google Scholar]
- Lei, J.; Zhou, J.; Sun, H.; Wan, W.; Xiao, J.; Yuan, C.; Karafiátová, M.; Doleel, J.; Wang, H.; Wang, X. Development of oligonucleotide probes for FISH karyotyping in Haynaldia villosa, a wild relative of common wheat. Crop J. 2020, 8, 676–681. [Google Scholar] [CrossRef]
- Tang, Z.; Yang, Z.; Fu, S. Oligonucleotides replacing the roles of repetitive sequences pAs1, pSc119.2, pTa-535, pTa 71, CCS1, and PA WRC.1 for FISH analysis. J. Appl. Genet. 2014, 55, 313–318. [Google Scholar] [CrossRef]
- Dai, Y.; Tabassum, M.A.; Chen, L.; Pan, Z.; Song, L. Physiological and transcriptomic response of soybean seedling roots to variable nitrate levels. Agron. J. 2021, 113, 3639–3652. [Google Scholar] [CrossRef]
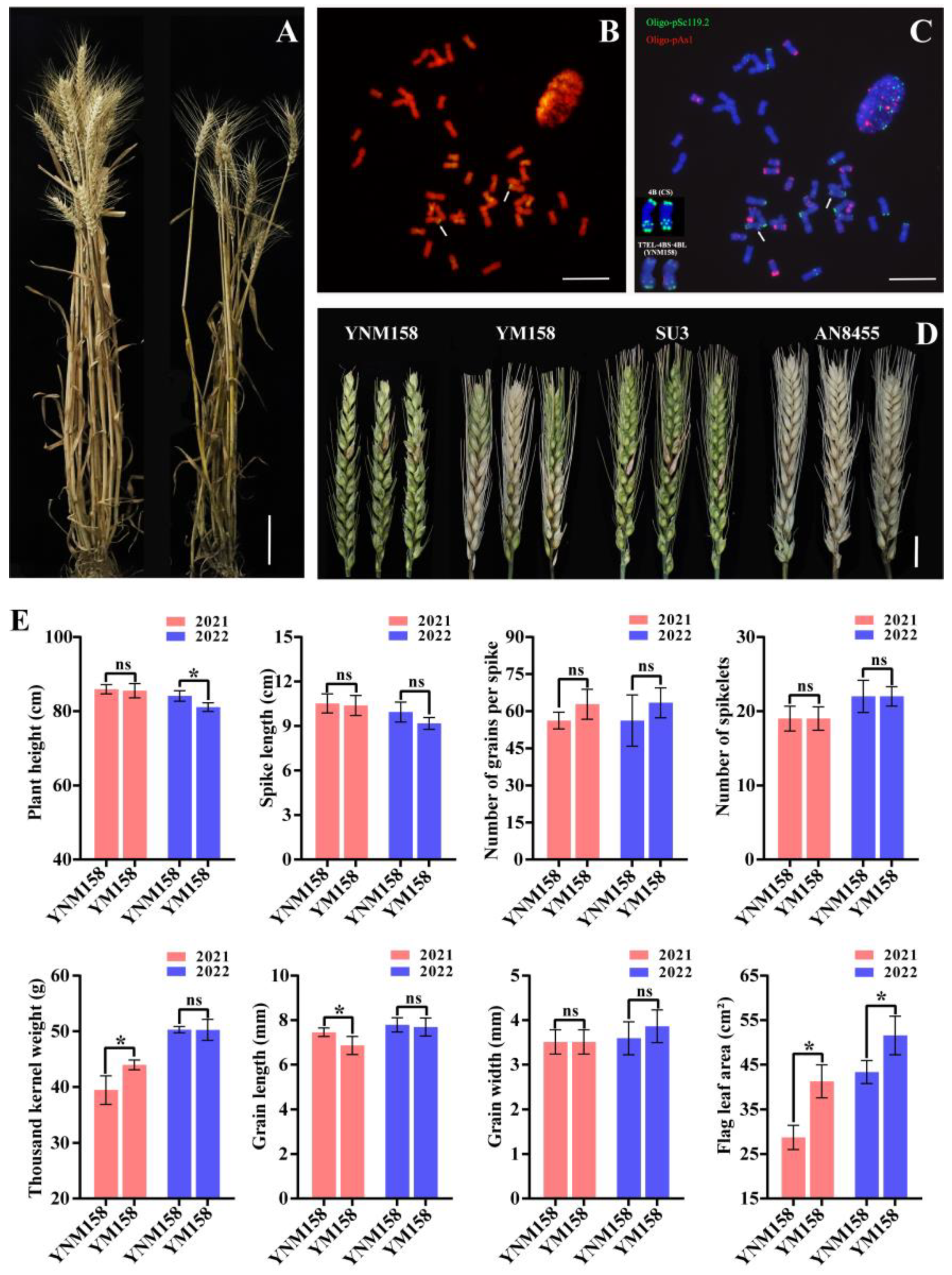
| Lines | 2020–2021 | 2021–2022 | ||
|---|---|---|---|---|
| Field | Greenhouse | Field | Greenhouse | |
| YNM158 | 4.81% ± 0.19 d | 5.18% ± 0.37 c | 5.21% ± 0.23 d | 5.27% ± 0.20 d |
| SU3 | 6.49% ± 1.79 d | 7.38% ± 2.89 c | 4.36% ± 0.22 d | 5.06% ± 0.21 d |
| AN8455 | 83.42% ± 10.36 a | 75.83% ± 14.69 a | 87.88% ± 5.51 a | 60.19% ± 6.24 a |
| YM23 | 28.79% ± 6.41 c | 53.69% ± 5.15 b | 15.54% ± 3.54 c | 21.87% ± 7.05 c |
| YM158 | 47.01% ± 11.92 b | - | 31.80% ± 12.91 b | 45.09% ± 8.21 b |
| Gene ID | Gene Description |
|---|---|
| Tel7E01G1002700 | Lysine ketoglutarate reductase trans-splicing-like protein (DUF707) |
| Tel7E01G1013700 | Galactoside 2-alpha-L-fucosyltransferase |
| Tel7E01G1020600 | Glutathione S-transferase |
| Tel7E01G211400 | Protein kinase |
| Tel7E01G899900 | NF-X1-type zinc finger protein NFXL1 |
| Tel7E01G905000 | Disease resistance protein (NBS-LRR class) family |
| Tel7E01G934300 | Carbonic anhydrase |
| Tel7E01G939300 | Receptor-like kinase |
| Tel7E01G941500 | Carboxypeptidase |
| Tel7E01G943900 | Receptor-like kinase |
| Tel7E01G946300 | Blue copper binding protein |
| Tel7E01G980900 | Monosaccharide-sensing protein 2 |
| Gene ID | Pathway | Gene Description |
|---|---|---|
| Initial colonization stage | ||
| TraesCS4D03G0528700 | Phosphatidylinositol signaling system | Phosphatidylinositol-4-phosphate 5-kinase family protein |
| TraesCS4B03G0573000 | Protein processing in endoplasmic reticulum | 70 kDa heat shock protein |
| Infection stage | ||
| TraesCS2D03G0030700 | Plant–pathogen interaction | NBS-LRR disease resistance protein |
| TraesCS7D03G0466200 | Plant–pathogen interaction | 3-ketoacyl–CoA synthase |
| Both stage | ||
| TraesCS7A03G1308100 | Biosynthesis of secondary metabolites | Hydroxycinnamoyl–CoA |
| TraesCS1A03G0718100 | MAPK signaling pathway–plant | Respiratory burst oxidase-like protein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Y.; Fei, W.; Chen, S.; Shi, J.; Ma, H.; Li, H.; Li, J.; Wang, Y.; Gao, Y.; Zhu, J.; et al. Using Transcriptomics to Determine the Mechanism for the Resistance to Fusarium Head Blight of a Wheat-Th. elongatum Translocation Line. Int. J. Mol. Sci. 2024, 25, 9452. https://doi.org/10.3390/ijms25179452
Dai Y, Fei W, Chen S, Shi J, Ma H, Li H, Li J, Wang Y, Gao Y, Zhu J, et al. Using Transcriptomics to Determine the Mechanism for the Resistance to Fusarium Head Blight of a Wheat-Th. elongatum Translocation Line. International Journal of Molecular Sciences. 2024; 25(17):9452. https://doi.org/10.3390/ijms25179452
Chicago/Turabian StyleDai, Yi, Wenlin Fei, Shiqiang Chen, Juntao Shi, Haigang Ma, Haifeng Li, Jinfeng Li, Yonggang Wang, Yujiao Gao, Jinghuan Zhu, and et al. 2024. "Using Transcriptomics to Determine the Mechanism for the Resistance to Fusarium Head Blight of a Wheat-Th. elongatum Translocation Line" International Journal of Molecular Sciences 25, no. 17: 9452. https://doi.org/10.3390/ijms25179452





