Increased Expression of the Neuropeptides PACAP/VIP in the Brain of Mice with CNS Targeted Production of IL-6 Is Mediated in Part by Trans-Signalling
Abstract
:1. Introduction
2. Results
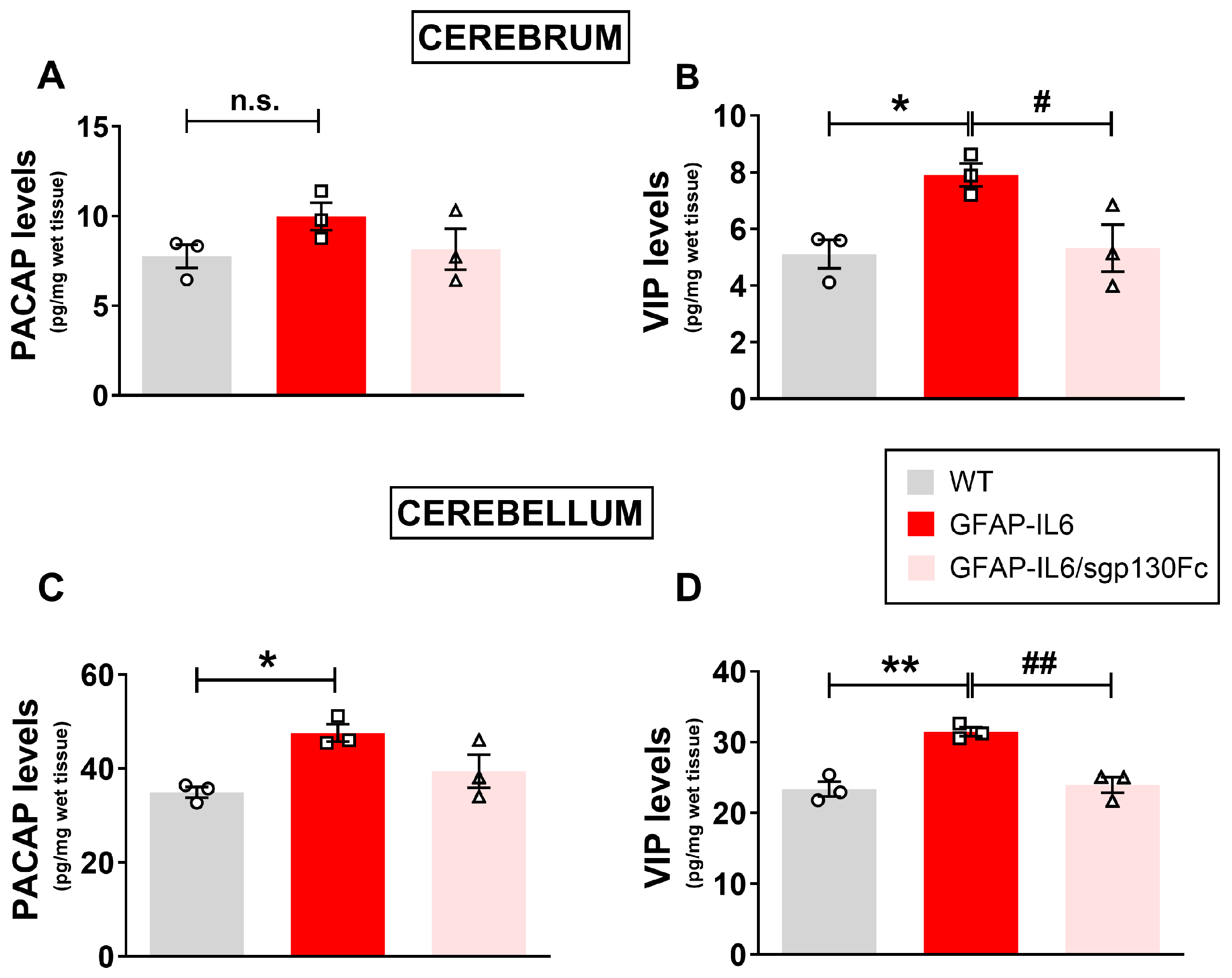
2.1. PACAP and VIP Levels Are Increased in the Brain of GFAP-IL6 Mice and Partly Reduced by Inhibition of Trans-Signalling
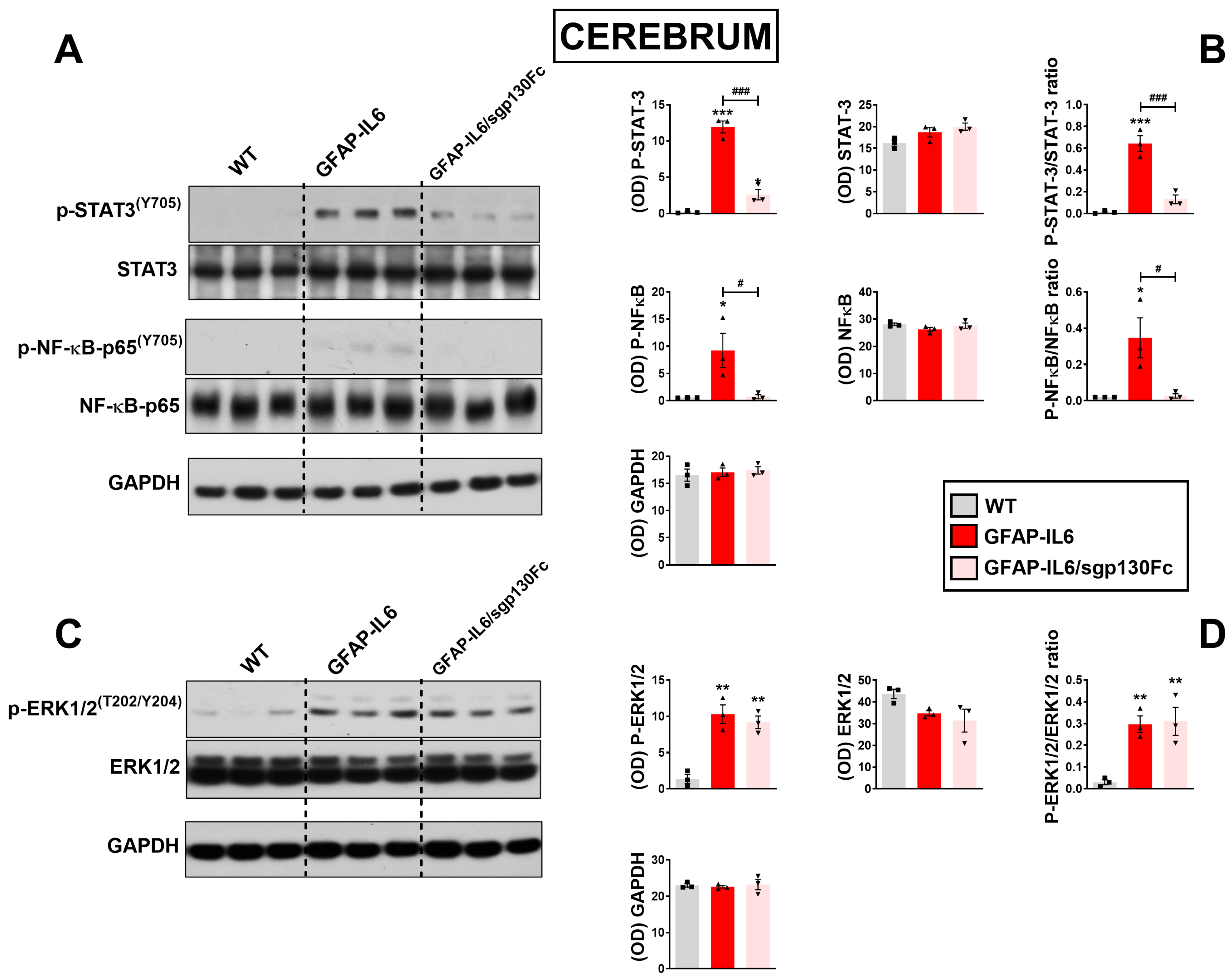
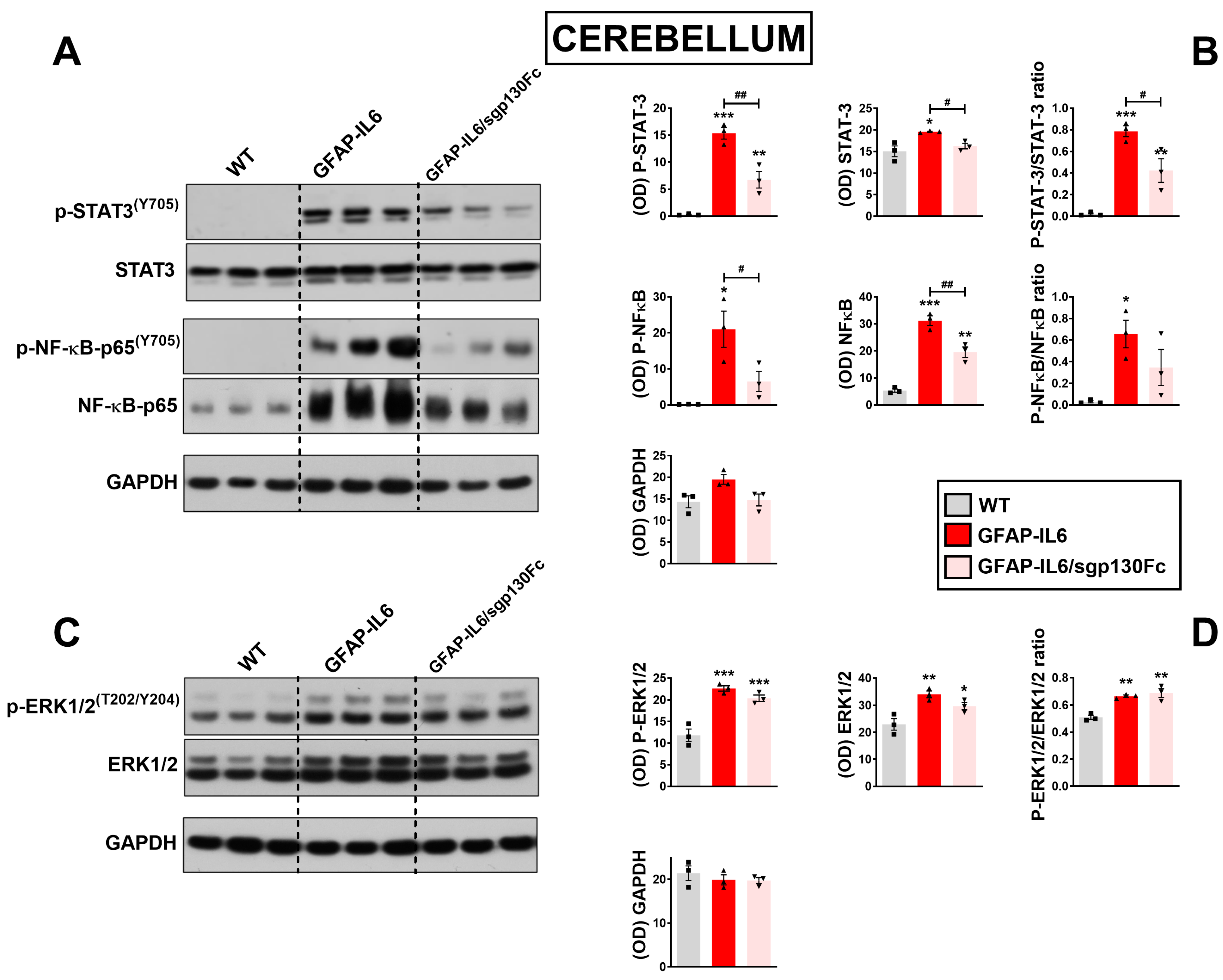
2.2. Trans-Signalling Inhibition Prevents STAT3, NF-κB but Not Erk1/2MAPK Phosphorylation in GFAP-IL6 Mice
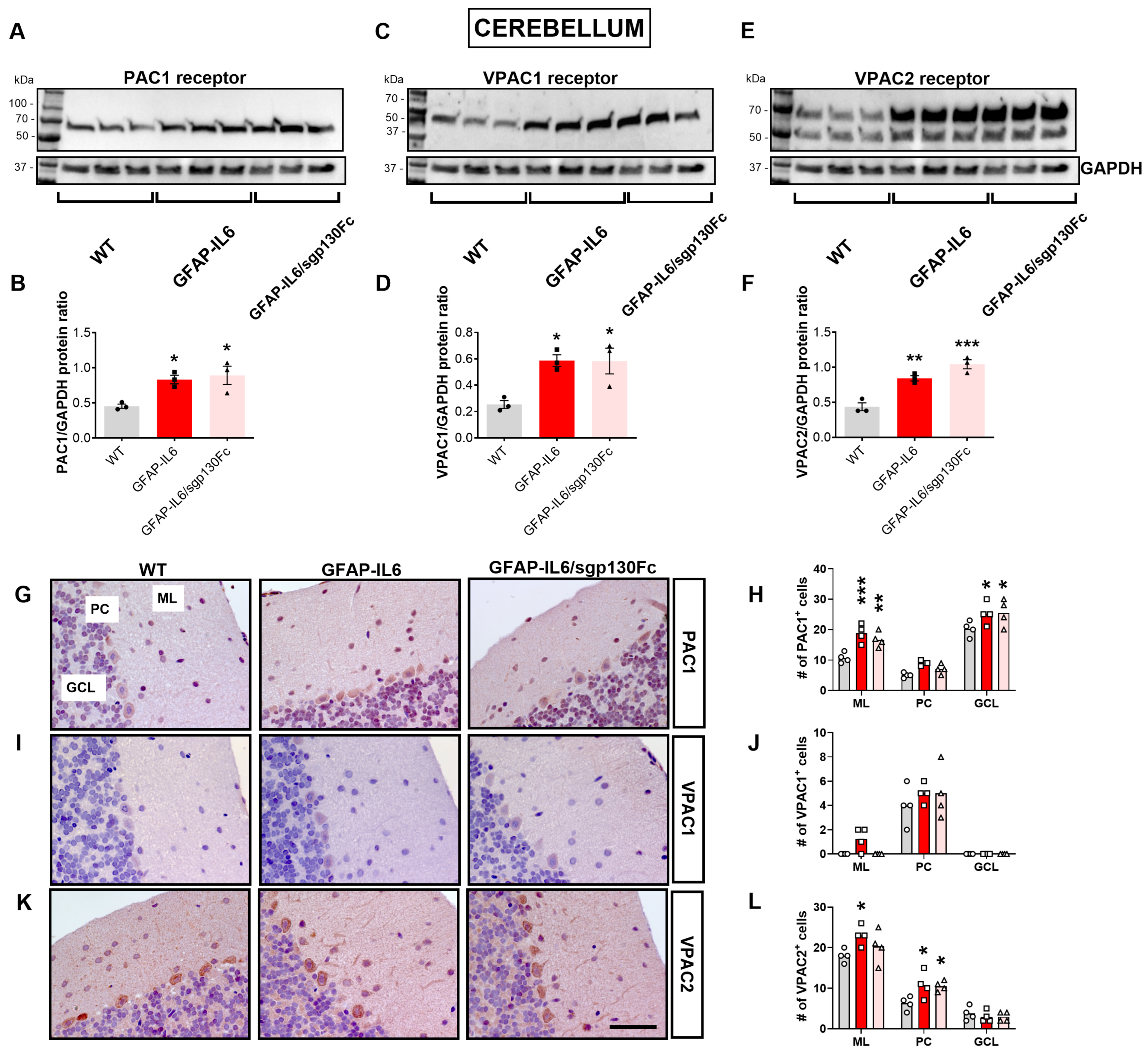
2.3. Forced Astrocyte-Restricted IL-6 Production Increases the PAC1 and VPAC2 Receptors in the Mouse Brain
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Antibodies
4.3. Enzyme-Linked Immunosorbent Assay (ELISA)
4.4. SDS–Polyacrylamide Gel Electrophoresis and Western Blotting
4.5. Immunohistochemistry
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bauer, S.; Kerr, B.J.; Patterson, P.H. The neuropoietic cytokine family in development, plasticity, disease and injury. Nat. Rev. Neurosci. 2007, 8, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, P.C.; Behrmann, I.; Haan, S.; Hermanns, H.M.; Müller-Newen, G.; Schaper, F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003, 374, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lupardus, P.; Laporte, S.L.; Garcia, K.C. Structural biology of shared cytokine receptors. Annu. Rev. Immunol. 2009, 27, 29–60. [Google Scholar] [CrossRef]
- Rothaug, M.; Becker-Pauly, C.; Rose-John, S. The role of interleukin-6 signaling in nervous tissue. Biochim. Biophys. Acta 2016, 1863, 1218–1227. [Google Scholar] [CrossRef]
- Campbell, I.L.; Erta, M.; Lim, S.L.; Frausto, R.; May, U.; Rose-John, S.; Scheller, J.; Hidalgo, J. Trans-signaling is a dominant mechanism for the pathogenic actions of interleukin-6 in the brain. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 2503–2513. [Google Scholar] [CrossRef]
- Jostock, T.; Müllberg, J.; Ozbek, S.; Atreya, R.; Blinn, G.; Voltz, N.; Fischer, M.; Neurath, M.F.; Rose-John, S. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur. J. Biochem. 2001, 268, 160–167. [Google Scholar] [CrossRef]
- Erta, M.; Quintana, A.; Hidalgo, J. Interleukin-6, a major cytokine in the central nervous system. Int. J. Biol. Sci. 2012, 8, 1254–1266. [Google Scholar] [CrossRef]
- Vezzani, A.; Viviani, B. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology 2015, 96, 70–82. [Google Scholar] [CrossRef]
- Quintana, A.; Molinero, A.; Borup, R.; Nielsen, F.C.; Campbell, I.L.; Penkowa, M.; Hidalgo, J. Effect of astrocyte-targeted production of IL-6 on traumatic brain injury and its impact on the cortical transcriptome. Dev. Neurobiol. 2008, 68, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.L. Neuropathogenic actions of cytokines assessed in transgenic mice. Int. J. Dev. Neurosci. 1995, 13, 275–284. [Google Scholar] [CrossRef]
- Heyser, C.J.; Masliah, E.; Samimi, A.; Campbell, I.L.; Gold, L.H. Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proc. Natl. Acad. Sci. USA 1997, 94, 1500–1505. [Google Scholar] [CrossRef]
- Brett, F.M.; Mizisin, A.P.; Powell, H.C.; Campbell, I.L. Evolution of neuropathologic abnormalities associated with blood-brain barrier breakdown in transgenic mice expressing interleukin-6 in astrocytes. J. Neuropathol. Exp. Neurol. 1995, 54, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Giunta, S.; Castorina, A.; Bucolo, C.; Magro, G.; Drago, F.; D’Agata, V. Early changes in pituitary adenylate cyclase-activating peptide, vasoactive intestinal peptide and related receptors expression in retina of streptozotocin-induced diabetic rats. Peptides 2012, 37, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Broome, S.T.; Musumeci, G.; Castorina, A. PACAP and VIP Mitigate Rotenone-Induced Inflammation in BV-2 Microglial Cells. J. Mol. Neurosci. 2022, 72, 2163–2175. [Google Scholar] [CrossRef]
- Castorina, A.; Scuderi, S.; D’Amico, A.G.; Drago, F.; D’Agata, V. PACAP and VIP increase the expression of myelin-related proteins in rat schwannoma cells: Involvement of PAC1/VPAC2 receptor-mediated activation of PI3K/Akt signaling pathways. Exp. Cell Res. 2014, 322, 108–121. [Google Scholar] [CrossRef]
- Karunia, J.; Niaz, A.; Mandwie, M.; Thomas Broome, S.; Keay, K.A.; Waschek, J.A.; Al-Badri, G.; Castorina, A. PACAP and VIP Modulate LPS-Induced Microglial Activation and Trigger Distinct Phenotypic Changes in Murine BV2 Microglial Cells. Int. J. Mol. Sci. 2021, 22, 10947. [Google Scholar] [CrossRef]
- Delgado, M.; Ganea, D. Vasoactive intestinal peptide prevents activated microglia-induced neurodegeneration under inflammatory conditions: Potential therapeutic role in brain trauma. FASEB J. 2003, 17, 1922–1924. [Google Scholar] [CrossRef] [PubMed]
- Brifault, C.; Gras, M.; Liot, D.; May, V.; Vaudry, D.; Wurtz, O. Delayed pituitary adenylate cyclase-activating polypeptide delivery after brain stroke improves functional recovery by inducing m2 microglia/macrophage polarization. Stroke 2015, 46, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Harmar, A.J.; Fahrenkrug, J.; Gozes, I.; Laburthe, M.; May, V.; Pisegna, J.R.; Vaudry, D.; Vaudry, H.; Waschek, J.A.; Said, S.I. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR review 1. Br. J. Pharmacol. 2012, 166, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Nakamachi, T.; Tsuchida, M.; Kagami, N.; Yofu, S.; Wada, Y.; Hori, M.; Tsuchikawa, D.; Yoshikawa, A.; Imai, N.; Nakamura, K.; et al. IL-6 and PACAP receptor expression and localization after global brain ischemia in mice. J. Mol. Neurosci. 2012, 48, 518–525. [Google Scholar] [CrossRef]
- Delgado, M. Inhibition of interferon (IFN) gamma-induced Jak-STAT1 activation in microglia by vasoactive intestinal peptide: Inhibitory effect on CD40, IFN-induced protein-10, and inducible nitric-oxide synthase expression. J. Biol. Chem. 2003, 278, 27620–27629. [Google Scholar] [CrossRef]
- Quintana, A.; Müller, M.; Frausto, R.F.; Ramos, R.; Getts, D.R.; Sanz, E.; Hofer, M.J.; Krauthausen, M.; King, N.J.; Hidalgo, J.; et al. Site-specific production of IL-6 in the central nervous system retargets and enhances the inflammatory response in experimental autoimmune encephalomyelitis. J. Immunol. 2009, 183, 2079–2088. [Google Scholar] [CrossRef]
- Heyen, J.R.; Ye, S.; Finck, B.N.; Johnson, R.W. Interleukin (IL)-10 inhibits IL-6 production in microglia by preventing activation of NF-kappaB. Brain Res. Mol. Brain Res. 2000, 77, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.D.; Harrison, S.C. Structure of an IkappaBalpha/NF-kappaB complex. Cell 1998, 95, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Vaudry, D.; Falluel-Morel, A.; Bourgault, S.; Basille, M.; Burel, D.; Wurtz, O.; Fournier, A.; Chow, B.K.; Hashimoto, H.; Galas, L.; et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol. Rev. 2009, 61, 283–357. [Google Scholar] [CrossRef] [PubMed]
- Waschek, J. VIP and PACAP: Neuropeptide modulators of CNS inflammation, injury, and repair. Br. J. Pharmacol. 2013, 169, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Jozwiak-Bebenista, M.; Kowalczyk, E. Neuroleptic Drugs and PACAP Differentially Affect the mRNA Expression of Genes Encoding PAC1/VPAC Type Receptors. Neurochem. Res. 2017, 42, 943–952. [Google Scholar] [CrossRef]
- Brenneman, D.E.; Phillips, T.M.; Hauser, J.; Hill, J.M.; Spong, C.Y.; Gozes, I. Complex array of cytokines released by vasoactive intestinal peptide. Neuropeptides 2003, 37, 111–119. [Google Scholar] [CrossRef]
- Dejda, A.; Sokolowska, P.; Nowak, J.Z. Neuroprotective potential of three neuropeptides PACAP, VIP and PHI. Pharmacol. Rep. 2005, 57, 307–320. [Google Scholar]
- Zusev, M.; Gozes, I. Differential regulation of activity-dependent neuroprotective protein in rat astrocytes by VIP and PACAP. Regul. Pept. 2004, 123, 33–41. [Google Scholar] [CrossRef]
- Castorina, A.; Giunta, S.; Scuderi, S.; D’Agata, V. Involvement of PACAP/ADNP signaling in the resistance to cell death in malignant peripheral nerve sheath tumor (MPNST) cells. J. Mol. Neurosci. 2012, 48, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, M. PACAP signaling to DREAM: A cAMP-dependent pathway that regulates cortical astrogliogenesis. Mol. Neurobiol. 2009, 39, 90–100. [Google Scholar] [CrossRef]
- Lu, J.; Piper, S.J.; Zhao, P.; Miller, L.J.; Wootten, D.; Sexton, P.M. Targeting VIP and PACAP Receptor Signaling: New Insights into Designing Drugs for the PACAP Subfamily of Receptors. Int. J. Mol. Sci. 2022, 23, 8069. [Google Scholar] [CrossRef]
- Grimaldi, M.; Cavallaro, S. Expression and coupling of PACAP/VIP receptors in cortical neurons and type I astrocytes. Ann. N. Y. Acad. Sci. 2000, 921, 312–316. [Google Scholar] [CrossRef]
- Bakalar, D.; Gavrilova, O.; Jiang, S.Z.; Zhang, H.Y.; Roy, S.; Williams, S.K.; Liu, N.; Wisser, S.; Usdin, T.B.; Eiden, L.E. Constitutive and conditional deletion reveals distinct phenotypes driven by developmental versus neurotransmitter actions of the neuropeptide PACAP. J. Neuroendocrinol. 2023, 35, e13286. [Google Scholar] [CrossRef]
- Gomariz, R.P.; Juarranz, Y.; Abad, C.; Arranz, A.; Leceta, J.; Martinez, C. VIP-PACAP system in immunity: New insights for multitarget therapy. Ann. N. Y. Acad. Sci. 2006, 1070, 51–74. [Google Scholar] [CrossRef]
- Castorina, A.; Giunta, S.; Mazzone, V.; Cardile, V.; D’Agata, V. Effects of PACAP and VIP on hyperglycemia-induced proliferation in murine microvascular endothelial cells. Peptides 2010, 31, 2276–2283. [Google Scholar] [CrossRef] [PubMed]
- Tatsuno, I.; Morio, H.; Tanaka, T.; Uchida, D.; Hirai, A.; Tamura, Y.; Saito, Y. Pituitary adenylate cyclase-activating polypeptide (PACAP) is a regulator of astrocytes: PACAP stimulates proliferation and production of interleukin 6 (IL-6), but not nerve growth factor (NGF), in cultured rat astrocyte. Ann. N. Y. Acad. Sci. 1996, 805, 482–488. [Google Scholar] [CrossRef]
- Armstrong, B.D.; Hu, Z.; Abad, C.; Yamamoto, M.; Rodriguez, W.I.; Cheng, J.; Lee, M.; Chhith, S.; Gomariz, R.P.; Waschek, J.A. Induction of neuropeptide gene expression and blockade of retrograde transport in facial motor neurons following local peripheral nerve inflammation in severe combined immunodeficiency and BALB/C mice. Neuroscience 2004, 129, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, L.M.; Dahlin, L.B.; Danielsen, N. Changes in expression of PACAP in rat sensory neurons in response to sciatic nerve compression. Eur. J. Neurosci. 2004, 20, 1838–1848. [Google Scholar] [CrossRef]
- Tsumuraya, T.; Ohtaki, H.; Song, D.; Sato, A.; Watanabe, J.; Hiraizumi, Y.; Nakamachi, T.; Xu, Z.; Dohi, K.; Hashimoto, H.; et al. Human mesenchymal stem/stromal cells suppress spinal inflammation in mice with contribution of pituitary adenylate cyclase-activating polypeptide (PACAP). J. Neuroinflamm. 2015, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, R.E. gp130 cytokines are positive signals triggering changes in gene expression and axon outgrowth in peripheral neurons following injury. Front. Mol. Neurosci. 2011, 4, 62. [Google Scholar] [CrossRef]
- Jones, E.A.; Conover, J.; Symes, A.J. Identification of a novel gp130-responsive site in the vasoactive intestinal peptide cytokine response element. J. Biol. Chem. 2000, 275, 36013–36020. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, B.D.; Hu, Z.; Abad, C.; Yamamoto, M.; Rodriguez, W.I.; Cheng, J.; Tam, J.; Gomariz, R.P.; Patterson, P.H.; Waschek, J.A. Lymphocyte regulation of neuropeptide gene expression after neuronal injury. J. Neurosci. Res. 2003, 74, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Samal, B.; Hamelink, C.R.; Xiang, C.C.; Chen, Y.; Chen, M.; Vaudry, D.; Brownstein, M.J.; Hallenbeck, J.M.; Eiden, L.E. Neuroprotection by endogenous and exogenous PACAP following stroke. Regul. Pept. 2006, 137, 4–19. [Google Scholar] [CrossRef]
- Passemard, S.; Sokolowska, P.; Schwendimann, L.; Gressens, P. VIP-induced neuroprotection of the developing brain. Curr. Pharm. Des. 2011, 17, 1036–1039. [Google Scholar] [CrossRef] [PubMed]
- Leceta, J.; Gomariz, R.P.; Martinez, C.; Abad, C.; Ganea, D.; Delgado, M. Receptors and transcriptional factors involved in the anti-inflammatory activity of VIP and PACAP. Ann. N. Y. Acad. Sci. 2000, 921, 92–102. [Google Scholar] [CrossRef]
- Delgado, M.; Abad, C.; Martinez, C.; Juarranz, M.G.; Leceta, J.; Ganea, D.; Gomariz, R.P. PACAP in immunity and inflammation. Ann. N. Y. Acad. Sci. 2003, 992, 141–157. [Google Scholar] [CrossRef]
- Zeng, L.; Zhang, X.; Xia, M.; Ye, H.; Li, H.; Gao, Z. Heme and Cu(2+)-induced vasoactive intestinal peptide (VIP) tyrosine nitration: A possible molecular mechanism for the attenuated anti-inflammatory effect of VIP in inflammatory diseases. Biochimie 2023, 214, 176–187. [Google Scholar] [CrossRef]
- Abad, C.; Tan, Y.V. Immunomodulatory Roles of PACAP and VIP: Lessons from Knockout Mice. J. Mol. Neurosci. 2018, 66, 102–113. [Google Scholar] [CrossRef]
- Ganea, D.; Delgado, M. Neuropeptides as modulators of macrophage functions. Regulation of cytokine production and antigen presentation by VIP and PACAP. Arch. Immunol. Ther. Exp. 2001, 49, 101–110. [Google Scholar]
- Maino, B.; D’Agata, V.; Severini, C.; Ciotti, M.T.; Calissano, P.; Copani, A.; Chang, Y.C.; DeLisi, C.; Cavallaro, S. Igf1 and Pacap rescue cerebellar granule neurons from apoptosis via a common transcriptional program. Cell Death Discov. 2015, 1, 15029. [Google Scholar] [CrossRef] [PubMed]
- Vaudry, D.; Pamantung, T.F.; Basille, M.; Rousselle, C.; Fournier, A.; Vaudry, H.; Beauvillain, J.C.; Gonzalez, B.J. PACAP protects cerebellar granule neurons against oxidative stress-induced apoptosis. Eur. J. Neurosci. 2002, 15, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Shioda, S.; Ozawa, H.; Dohi, K.; Mizushima, H.; Matsumoto, K.; Nakajo, S.; Takaki, A.; Zhou, C.J.; Nakai, Y.; Arimura, A. PACAP protects hippocampal neurons against apoptosis: Involvement of JNK/SAPK signaling pathway. Ann. N. Y. Acad. Sci. 1998, 865, 111–117. [Google Scholar] [CrossRef]
- Chandrasekharan, B.; Nezami, B.G.; Srinivasan, S. Emerging neuropeptide targets in inflammation: NPY and VIP. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G949–G957. [Google Scholar] [CrossRef]
- Morell, M.; Souza-Moreira, L.; González-Rey, E. VIP in neurological diseases: More than a neuropeptide. Endocr. Metab. Immune Disord. Drug Target 2012, 12, 323–332. [Google Scholar] [CrossRef]
- Delgado, M.; Ganea, D. Vasoactive intestinal peptide: A neuropeptide with pleiotropic immune functions. Amino Acid 2013, 45, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Llobet, N.; Vidal-Sancho, L.; Masana, M.; Fournier, A.; Alberch, J.; Vaudry, D.; Xifró, X. Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) Enhances Hippocampal Synaptic Plasticity and Improves Memory Performance in Huntington’s Disease. Mol. Neurobiol. 2018, 55, 8263–8277. [Google Scholar] [CrossRef]
- Mercer, A.; Rönnholm, H.; Holmberg, J.; Lundh, H.; Heidrich, J.; Zachrisson, O.; Ossoinak, A.; Frisén, J.; Patrone, C. PACAP promotes neural stem cell proliferation in adult mouse brain. J. Neurosci. Res. 2004, 76, 205–215. [Google Scholar] [CrossRef]
- Masmoudi-Kouki, O.; Gandolfo, P.; Castel, H.; Leprince, J.; Fournier, A.; Dejda, A.; Vaudry, H.; Tonon, M.C. Role of PACAP and VIP in astroglial functions. Peptides 2007, 28, 1753–1760. [Google Scholar] [CrossRef]
- Dejda, A.; Jozwiak-Bebenista, M.; Nowak, J.Z. PACAP, VIP, and PHI: Effects on AC-, PLC-, and PLD-driven signaling systems in the primary glial cell cultures. Ann. N. Y. Acad. Sci. 2006, 1070, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, N.M.; Krueckl, S.L.; McRory, J.E. The origin and function of the pituitary adenylate cyclase-activating polypeptide (PACAP)/glucagon superfamily. Endocr. Rev. 2000, 21, 619–670. [Google Scholar] [CrossRef]
- Gębka-Kępińska, B.; Adamczyk, B.; Gębka, D.; Czuba, Z.; Szczygieł, J.; Adamczyk-Sowa, M. Cytokine Profiling in Cerebrospinal Fluid of Patients with Newly Diagnosed Relapsing-Remitting Multiple Sclerosis (RRMS): Associations between Inflammatory Biomarkers and Disease Activity. Int. J. Mol. Sci. 2024, 25, 7399. [Google Scholar] [CrossRef] [PubMed]
- Stampanoni Bassi, M.; Iezzi, E.; Mori, F.; Simonelli, I.; Gilio, L.; Buttari, F.; Sica, F.; De Paolis, N.; Mandolesi, G.; Musella, A.; et al. Interleukin-6 Disrupts Synaptic Plasticity and Impairs Tissue Damage Compensation in Multiple Sclerosis. Neurorehabilit. Neural Repair 2019, 33, 825–835. [Google Scholar] [CrossRef]
- Levraut, M.; Landes, C.; Mondot, L.; Cohen, M.; Bresch, S.; Brglez, V.; Seitz-Polski, B.; Lebrun-Frenay, C. Kappa Free Light Chains, Soluble Interleukin-2 Receptor, and Interleukin-6 Help Explore Patients Presenting with Brain White Matter Hyperintensities. Front. Immunol. 2022, 13, 864133. [Google Scholar] [CrossRef] [PubMed]
- Virupakshaiah, A.; Moseley, C.E.; Elicegui, S.; Gerwitz, L.M.; Spencer, C.M.; George, E.; Shah, M.; Cree, B.A.C.; Waubant, E.; Zamvil, S.S. Life-Threatening MOG Antibody-Associated Hemorrhagic ADEM With Elevated CSF IL-6. Neurol. Neuroimmunol. Neuroinflamm. 2024, 11, e200243. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castorina, A.; Scheller, J.; Keay, K.A.; Marzagalli, R.; Rose-John, S.; Campbell, I.L. Increased Expression of the Neuropeptides PACAP/VIP in the Brain of Mice with CNS Targeted Production of IL-6 Is Mediated in Part by Trans-Signalling. Int. J. Mol. Sci. 2024, 25, 9453. https://doi.org/10.3390/ijms25179453
Castorina A, Scheller J, Keay KA, Marzagalli R, Rose-John S, Campbell IL. Increased Expression of the Neuropeptides PACAP/VIP in the Brain of Mice with CNS Targeted Production of IL-6 Is Mediated in Part by Trans-Signalling. International Journal of Molecular Sciences. 2024; 25(17):9453. https://doi.org/10.3390/ijms25179453
Chicago/Turabian StyleCastorina, Alessandro, Jurgen Scheller, Kevin A. Keay, Rubina Marzagalli, Stefan Rose-John, and Iain L. Campbell. 2024. "Increased Expression of the Neuropeptides PACAP/VIP in the Brain of Mice with CNS Targeted Production of IL-6 Is Mediated in Part by Trans-Signalling" International Journal of Molecular Sciences 25, no. 17: 9453. https://doi.org/10.3390/ijms25179453






