Correlation with Apoptosis Process through RNA-Seq Data Analysis of Hep3B Hepatocellular Carcinoma Cells Treated with Glehnia littoralis Extract (GLE)
Abstract
:1. Introduction
2. Results
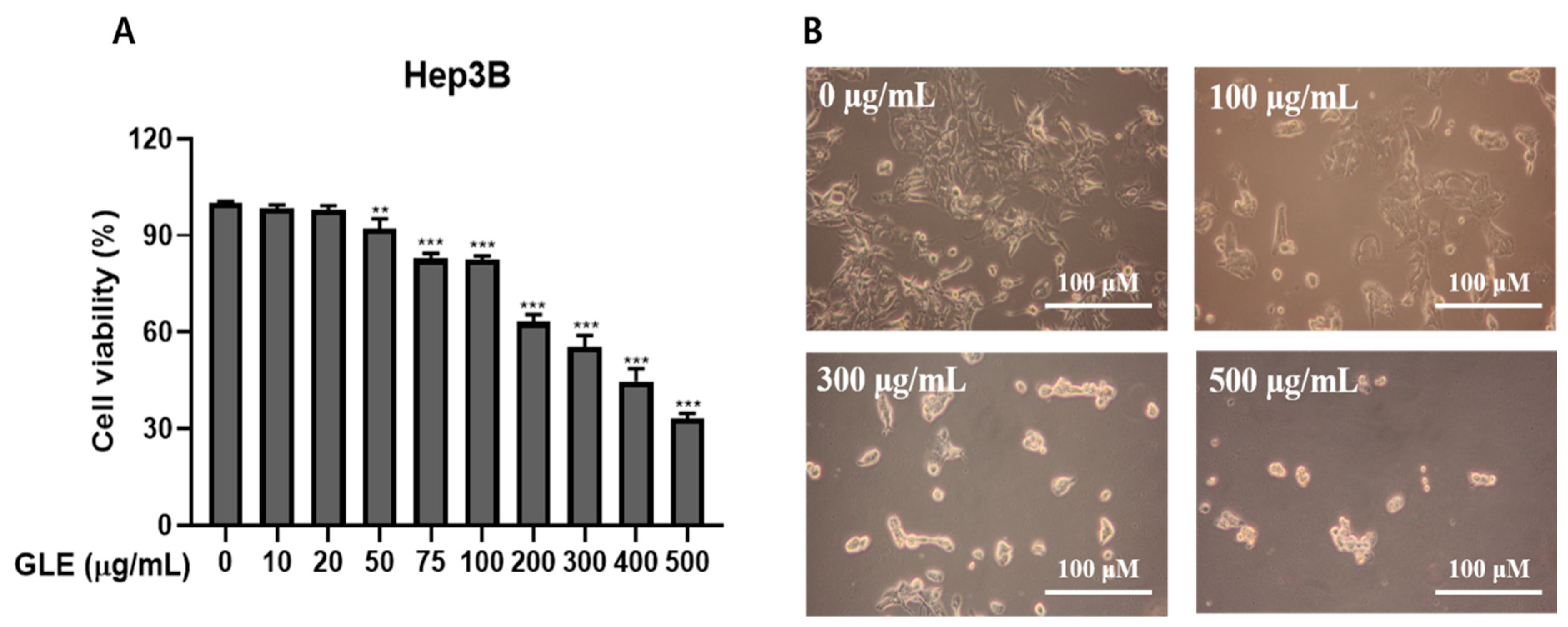
2.1. Glehnia littoralis Extract (GLE) Induces Cell Death of Hep3B Cells
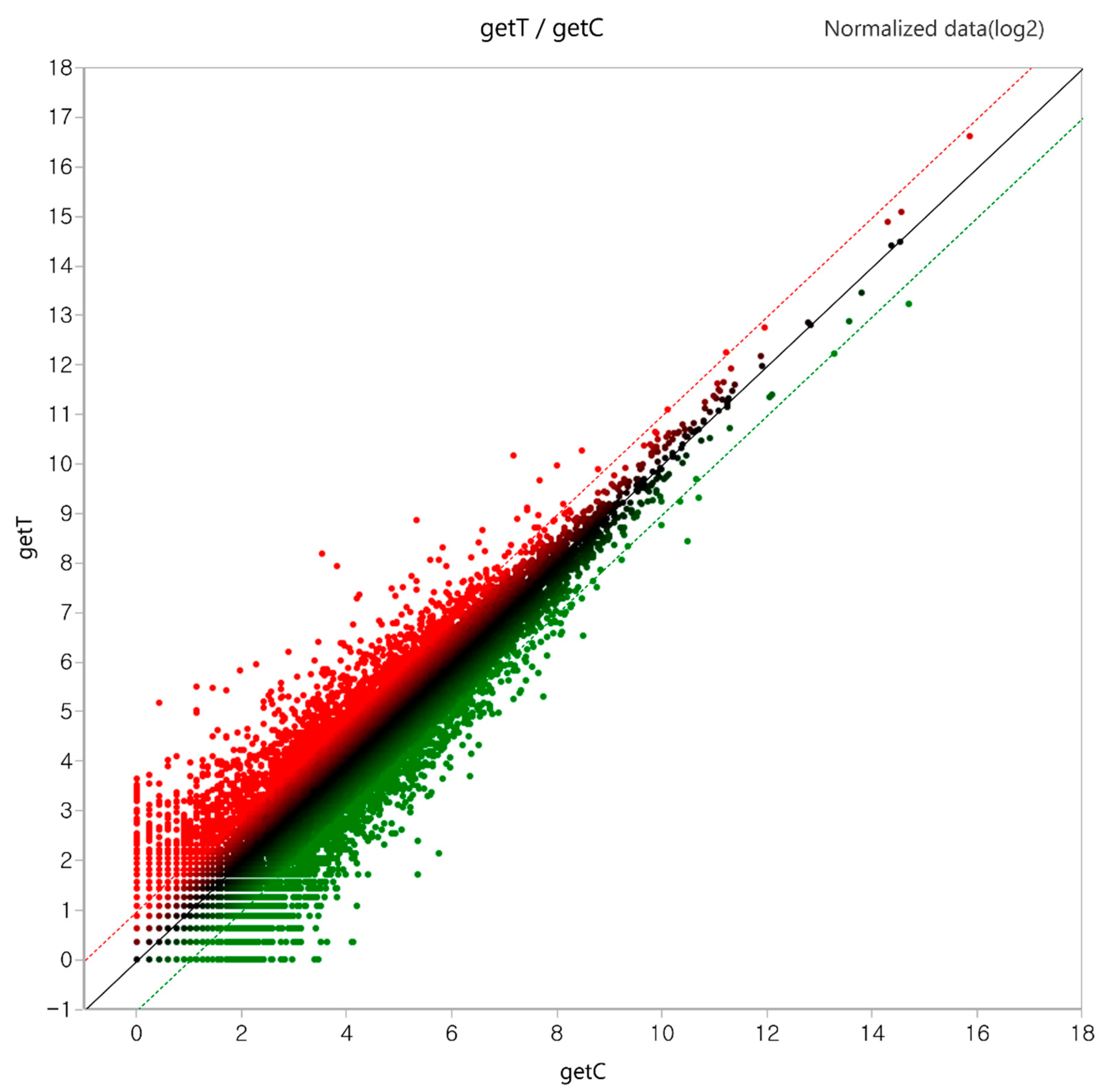
2.2. Identification of Genes and Differentially Expressed Genes (DEGs)
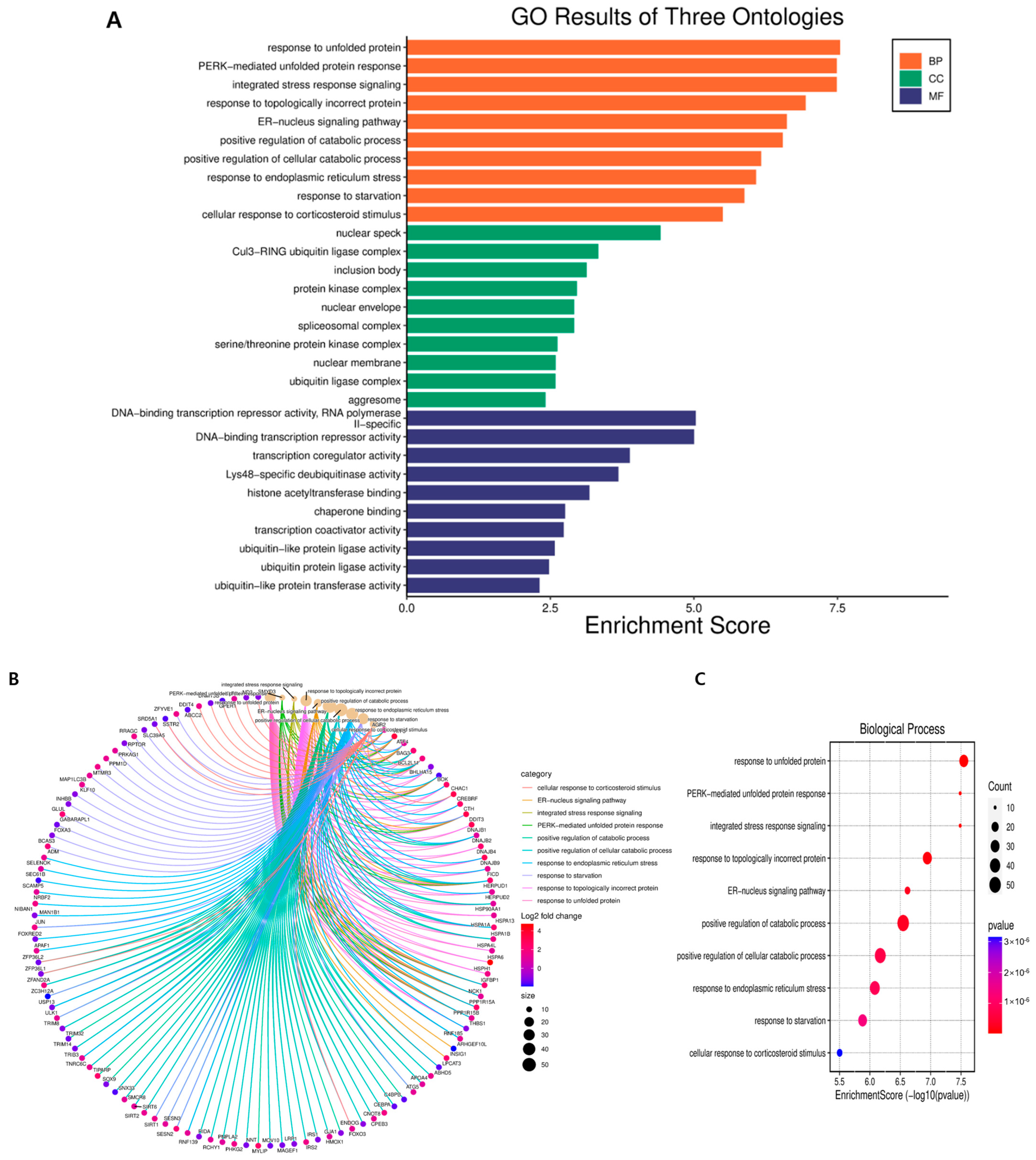
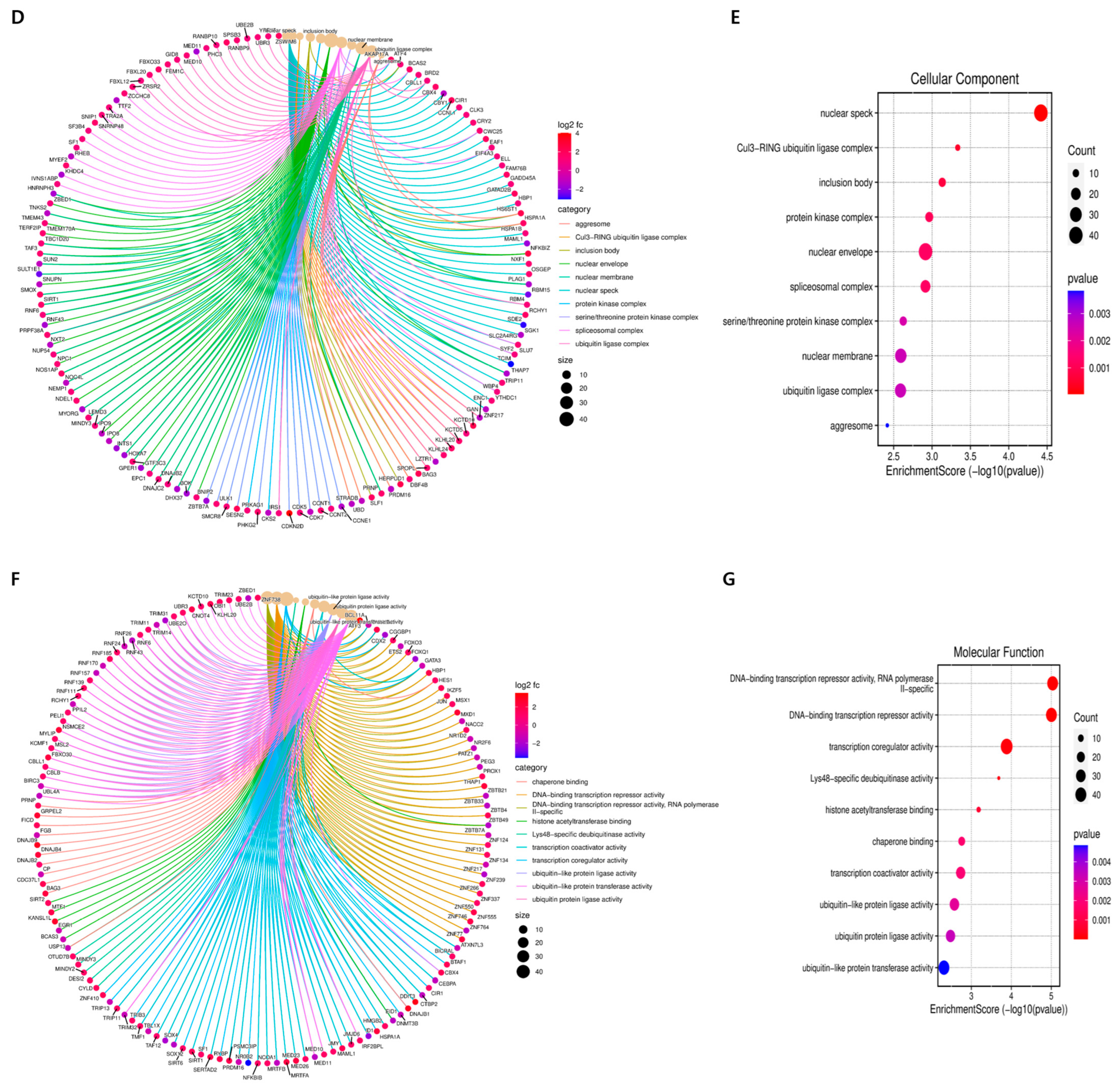
2.3. Gene Ontology Term Enrichment Analysis of DEGs in GLE-Treated Hep3B Cells
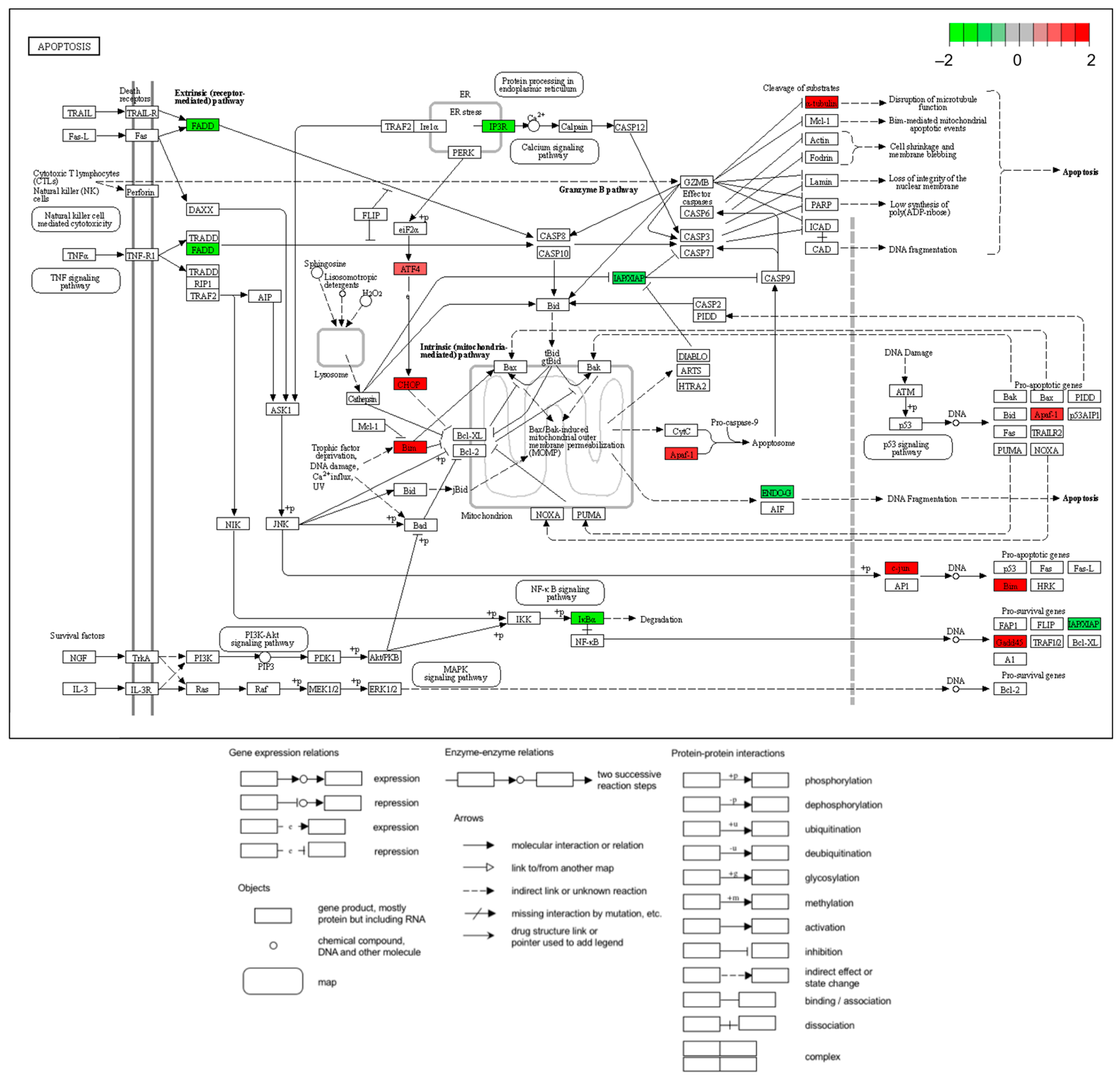
2.4. KEGG (Kyoto Encyclopedia of Genes and Genomes) Enrichment Pathway and Protein–Protein Interaction (PPI)
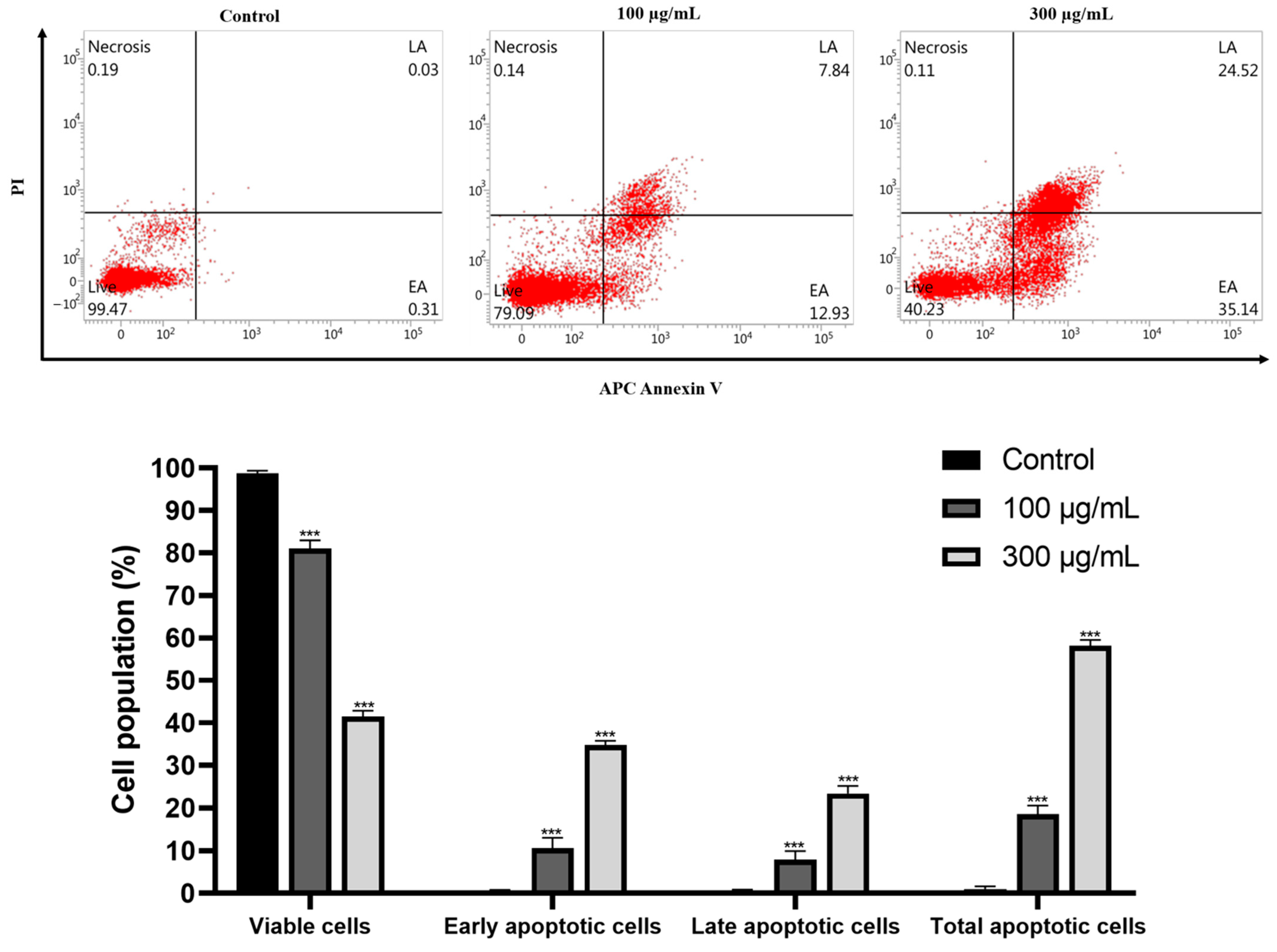
2.5. GLE Induces Apoptotic Cell Death in Hep3B Cells
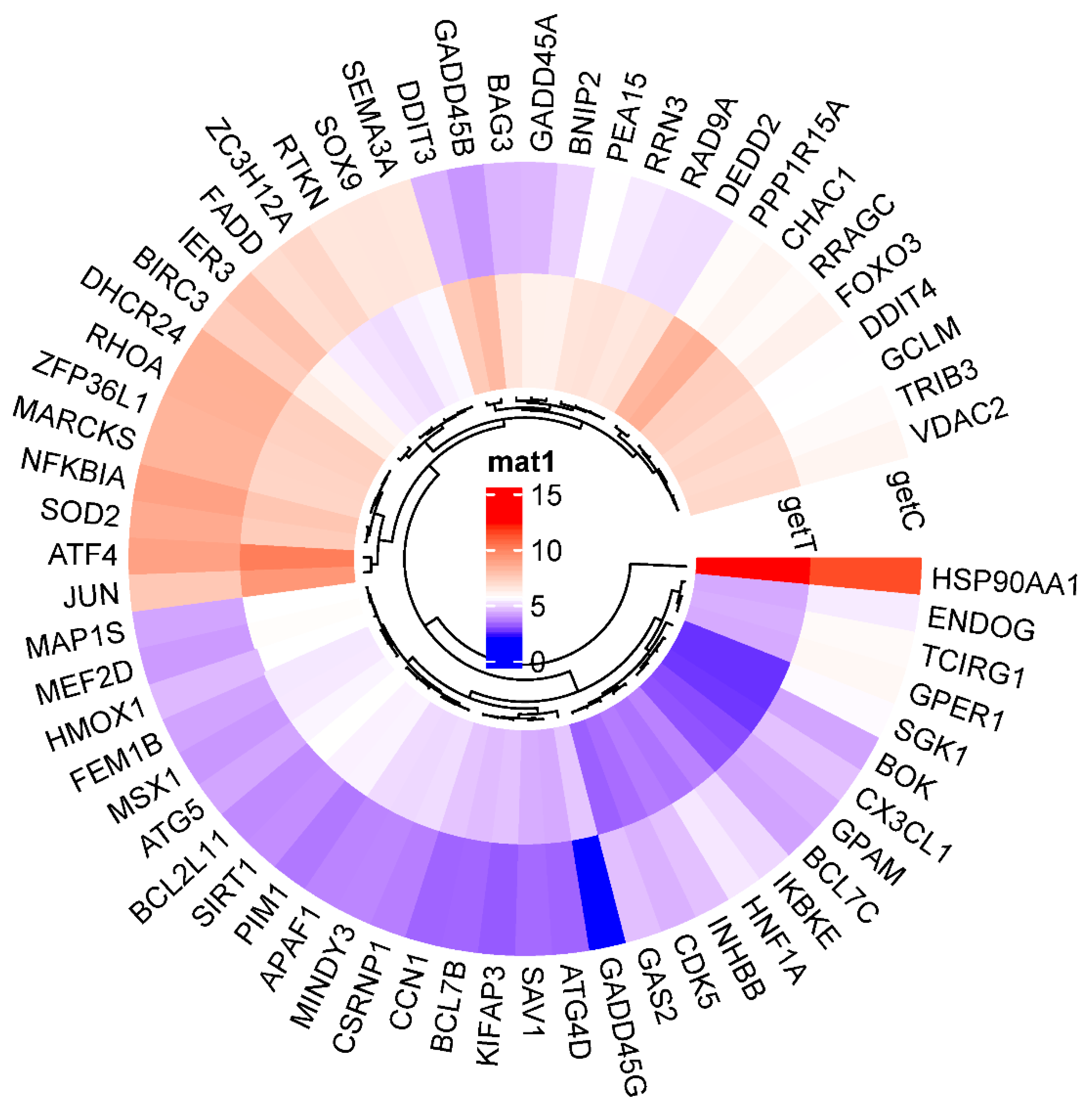
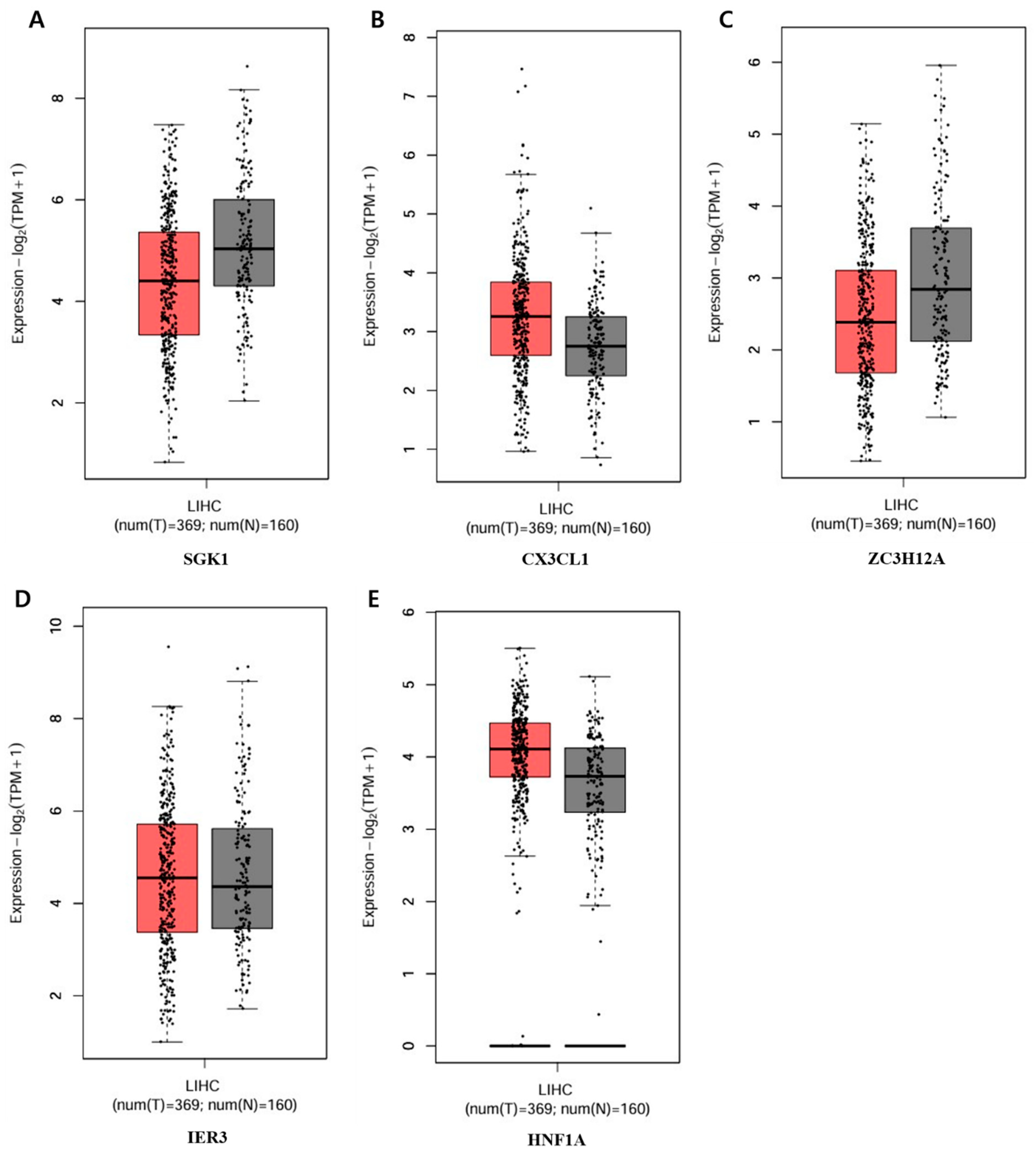
2.6. Increase or Decrease in Genes Related to the Apoptotic Process
3. Discussion
4. Material and Methods
4.1. Reagents and Chemicals
4.2. Cell Culture and Viability Assay
4.3. Annexin V/Propidium Iodide Apoptosis Detection
4.4. RNA Isolation
4.5. Library Preparation and Sequencing
4.6. Data Analysis
4.7. Source Code
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GLE | Glehnia littoralis extract |
| HCC | Hepatocellular carcinoma |
| DEG | Differentially expressed gene |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| PPI | Protein–protein interaction |
| BP | Biological process |
| MF | Molecular function |
| CC | Cellular component |
References
- Liu, G.; Luo, Y.; Liu, J.; Yang, T.; Ma, Z.; Sun, J.; Zhou, W.; Li, H.; Wen, J.; Zeng, X. Identification of a Novel Circadian Rhythm-Related Signature for Predicting Prognosis and Therapies in Hepatocellular Carcinoma Based on Bulk and Single-Cell RNA Sequencing. Eur. J. Cancer Care 2024, 2024, 1834636. [Google Scholar] [CrossRef]
- Choi, S.; Kim, B.K.; Yon, D.K.; Lee, S.W.; Lee, H.G.; Chang, H.H.; Park, S.; Koyanagi, A.; Jacob, L.; Dragioti, E.; et al. Global burden of primary liver cancer and its association with underlying aetiologies, sociodemographic status, and sex differences from 1990–2019: A DALY-based analysis of the Global Burden of Disease 2019 study. Clin. Mol. Hepatol. 2023, 29, 433. [Google Scholar] [CrossRef]
- Safri, F.; Nguyen, R.; Zerehpooshnesfchi, S.; George, J.; Qiao, L. Heterogeneity of hepatocellular carcinoma: From mechanisms to clinical implications. Cancer Gene Therapy 2024, 31, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Chen, Q.; Liu, Z.; Chen, X.; Zhu, P. Adjuvant therapy following curative treatments for hepatocellular carcinoma: Current dilemmas and prospects. Front. Oncol. 2023, 13, 1098958. [Google Scholar] [CrossRef]
- Lohitesh, K.; Chowdhury, R.; Mukherjee, S. Resistance a major hindrance to chemotherapy in hepatocellular carcinoma: An insight. Cancer Cell Int. 2018, 18, 44. [Google Scholar] [CrossRef]
- Omeroglu Ulu, Z.; Bolat, Z.B.; Sahin, F. Integrated transcriptome and in vitro analysis revealed anti-proliferative effect of sodium perborate on hepatocellular carcinoma cells. J. Trace Elem. Med. Biol. 2022, 73, 127011. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, B.J.C.; Reviews, M. Hepatocellular carcinoma: Molecular mechanism, targeted therapy, and biomarkers. Cancer Metastasis Rev. 2023, 42, 629–652. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, X.; Zhang, L.; Wang, C.; Ji, M.; Xu, J.; Zhang, K.; Liu, J.; Zhang, C.; Li, M.; et al. Ethnopharmacology, phytochemistry, and pharmacology of the genus glehnia: A systematic review. Evid.-Based Complement. Altern. Med. 2019, 2019, 1253493. [Google Scholar] [CrossRef]
- Kim, C.J.; Ghimire, B.K.; Choi, S.K.; Yu, C.Y.; Lee, J.G. Sustainable Bioactive Composite of Glehnia littoralis Extracts for Osteoblast Differentiation and Bone Formation. Processes 2023, 11, 1491. [Google Scholar] [CrossRef]
- Shao, C.; Wang, G.; Ding, X.; Yang, C.; Yan, M. Physiological and biochemical characteristics of cold stratification to overcome morphophysiological dormancy in Glehnia littoralis seed. Seed Sci. Technol. 2021, 49, 19–24. [Google Scholar] [CrossRef]
- Park, J.H.; Shin, B.N.; Ahn, J.H.; Cho, J.H.; Lee, T.-K.; Lee, J.-C.; Jeon, Y.H.; Kang, I.J.; Yoo, K.-Y.; Hwang, I.K. Glehnia littoralis extract promotes neurogenesis in the hippocampal dentate gyrus of the adult mouse through increasing expressions of brain-derived neurotrophic factor and tropomyosin-related kinase B. Chin. Med. J. 2018, 131, 689–695. [Google Scholar] [CrossRef]
- Zhou, C.; An, K.; Zhang, X.; Tong, B.; Liu, D.; Kong, D.; Bian, F.J.B.P.B. Sporogenesis, gametophyte development and embryogenesis in Glehnia littoralis. BMC Plant Biol. 2023, 23, 114. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, R.; Kim, Y.-S.; Kim, G.-W.; Kim, W.-J.; Hong, S.-M.; Kim, C.-G.; Choi, D.-K. Standardized extract of Glehnia littoralis abrogates memory impairment and neuroinflammation by regulation of CREB/BDNF and NF-κB/MAPK signaling in scopolamine-induced amnesic mice model. Biomed. Pharmacother. 2023, 165, 115106. [Google Scholar] [CrossRef]
- Li, S.; Xu, N.; Fang, Q.; Cheng, X.; Chen, J.; Liu, P.; Li, L.; Wang, C.; Liu, W. Glehnia littoralis Fr. Schmidtex Miq.: A systematic review on ethnopharmacology, chemical composition, pharmacology and quality control. J. Ethnopharmacol. 2023, 317, 116831. [Google Scholar] [CrossRef]
- Choe, S.-Y.; Hong, J.-H.; Gu, Y.-R.; Kim, I.-D.; Dhungana, S.; Moon, K.-D. Hot water extract of Glehnia littoralis leaf showed skin-whitening and anti-wrinkle properties. S. Afr. J. Bot. 2019, 127, 104–109. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Ketkar, S.; Burrage, L.C.; Lee, B. RNA sequencing as a diagnostic tool. JAMA 2023, 329, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Xu, S. Transcriptome profiling in systems vascular medicine. Front. Pharmacol. 2017, 8, 294149. [Google Scholar] [CrossRef]
- Xu, F.; Jiang, L.; Zhao, Q.; Zhang, Z.; Liu, Y.; Yang, S.; Yu, M.; Chen, H.; Zhang, J.; Zhang, J. Whole-transcriptome and proteome analyses identify key differentially expressed mRNAs, miRNAs, lncRNAs and circRNAs associated with HCC. Oncogene 2021, 40, 4820–4831. [Google Scholar] [CrossRef]
- Rashid, S.; Wilson, S.G.; Zhu, K.; Walsh, J.P.; Xu, J.; Mullin, B.H. Identification of differentially expressed genes and molecular pathways involved in osteoclastogenesis using RNA-seq. Genes 2023, 14, 916. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Fu, M.; Li, J.; Chen, L.; Feng, K.; Huang, T.; Cai, Y.-D. Analysis and prediction of protein stability based on interaction network, gene ontology, and KEGG pathway enrichment scores. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2023, 1871, 140889. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Zhu, J.; Yu, H.; Bazhin, A.V.; Westphalen, C.B.; Renz, B.W.; Jacob, S.N.; Lampert, C.; Werner, J.; Angele, M.K. Angiogenesis-related gene expression signatures predicting prognosis in gastric cancer patients. Cancers 2020, 12, 3685. [Google Scholar] [CrossRef] [PubMed]
- Byron, S.A.; Van Keuren-Jensen, K.R.; Engelthaler, D.M.; Carpten, J.D.; Craig, D.W. Translating RNA sequencing into clinical diagnostics: Opportunities and challenges. Nat. Rev. Genet. 2016, 17, 257–271. [Google Scholar] [CrossRef]
- Van de Sande, B.; Lee, J.S.; Mutasa-Gottgens, E.; Naughton, B.; Bacon, W.; Manning, J.; Wang, Y.; Pollard, J.; Mendez, M.; Hill, J. Applications of single-cell RNA sequencing in drug discovery and development. Nat. Rev. Drug Discov. 2023, 22, 496–520. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Wu, H.; Yang, C.; Zhu, W.; Shi, B.; Zheng, B.; Wang, S.; Zhou, K.; Gao, A. RNA-Seq reveals the key pathways and genes involved in the light-regulated flavonoids biosynthesis in mango (Mangifera indica L.) peel. Front. Plant Sci. 2023, 13, 1119384. [Google Scholar] [CrossRef]
- Sakle, N.S.; More, S.A.; Mokale, S.N. A network pharmacology-based approach to explore potential targets of Caesalpinia pulcherima: An updated prototype in drug discovery. Sci. Rep. 2020, 10, 17217. [Google Scholar] [CrossRef]
- Khatoon, Z.; Figler, B.; Zhang, H.; Cheng, F. Introduction to RNA-Seq and its applications to drug discovery and development. Drug Dev. Res. 2014, 75, 324–330. [Google Scholar] [CrossRef]
- Bertoldo, J.B.; Müller, S.; Hüttelmaier, S. RNA-binding proteins in cancer drug discovery. Drug Discov. Today 2023, 28, 103580. [Google Scholar] [CrossRef]
- Jaudan, A.; Sharma, S.; Malek, S.N.A.; Dixit, A. Induction of apoptosis by pinostrobin in human cervical cancer cells: Possible mechanism of action. PLoS ONE 2018, 13, e0191523. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603. [Google Scholar] [CrossRef]
- Humayun, A.; Fornace, A.J., Jr. GADD45 in stress signaling, cell cycle control, and apoptosis. In Gadd45 Stress Sensor Genes; Springer: Cham, Switzerland, 2022; pp. 1–22. [Google Scholar]
- Zaidi, M.R.; Liebermann, D.A. Gadd45 in senescence. In Gadd45 Stress Sensor Genes; Springer: Cham, Switzerland, 2022; pp. 109–116. [Google Scholar]
- Ou, D.-L.; Shyue, S.-K.; Lin, L.-I.; Feng, Z.-R.; Liou, J.-Y.; Fan, H.-H.; Lee, B.-S.; Hsu, C.; Cheng, A.-L. Growth arrest DNA damage-inducible gene 45 gamma expression as a prognostic and predictive biomarker in hepatocellular carcinoma. Oncotarget 2015, 6, 27953. [Google Scholar] [CrossRef] [PubMed]
- de la Cruz, J.F.; Vergara, E.; Cho, Y.; Hong, H.O.; Oyungerel, B.; Hwang, S.G. Glehnia littoralis root extract induces G0/G1 phase cell cycle arrest in the MCF-7 human breast cancer cell line. Asian Pac. J. Cancer Prev. 2015, 16, 8113–8117. [Google Scholar] [CrossRef]
- Wang, L.; Yuan, Z. Research progress in polysaccharide compositions of Glehnia littoralis. Asian J. Tradit. Med. 2020, 15, 136–143. [Google Scholar]
- Kong, C.-S.; Um, Y.R.; Im Lee, J.; Kim, Y.A.; Yea, S.S.; Seo, Y. Constituents isolated from Glehnia littoralis suppress proliferations of human cancer cells and MMP expression in HT1080 cells. Food Chem. 2010, 120, 385–394. [Google Scholar] [CrossRef]
- Kim, H.H.; Jeong, S.H.; Park, M.Y.; Bhosale, P.B.; Abusaliya, A.; Kim, H.W.; Seong, J.K.; Kim, D.I.; Lee, S.J.; Park, K.I. Potential Antioxidant and Anti-Inflammatory Properties of Polyphenolic Compounds from Cirsium japonicum Extract. Int. J. Mol. Sci. 2024, 25, 785. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef]


| Name | Type | Reads | Bases | Bases (Gb) | GC | N | Q30 |
|---|---|---|---|---|---|---|---|
| getC | Raw | 22,236,783 (100%) | 1,689,995,508 (100%) | 1.69 | 722,506,852 (42.75%) | 122,257 (0.0%) | 1,546,777,236 (91.53%) |
| Clean | 21,935,961 (98.65%) | 1,563,188,663 (92.50%) | 1.56 | 685,071,168 (43.83%) | 17,858 (0.0%) | 1,471,970,080 (94.16%) | |
| getT | Raw | 16,504,954 (100%) | 1,254,376,504 (100%) | 1.25 | 525,542,567 (41.90%) | 91,010 (0.0%) | 1,138,775,855 (90.78%) |
| Clean | 16,279,626 (98.63%) | 1,148,658,184 (91.57%) | 1.15 | 496,909,263 (43.26%) | 13,046 (0.0%) | 1,075,923,116 (93.67%) |
| Biological Process | ||||
| ID | Description | Gene Ratio | BgRatio | p Value |
| GO:0042594 | response to starvation | 29/994 | 206/18,866 | 1.31859 × 10−6 |
| GO:0001558 | regulation of cell growth | 38/994 | 420/18,866 | 0.000847655 |
| GO:0006984 | ER-nucleus signaling pathway | 14/994 | 51/18,866 | 2.40565 × 10−7 |
| Molecular Function | ||||
| ID | Description | Gene Ratio | BgRatio | p Value |
| GO:0001217 | DNA-binding transcription repressor activity | 39/1015 | 336/18,352 | 9.92372 × 10−6 |
| GO:0003712 | transcription coregulator activity | 48/1015 | 498/18,352 | 0.000130717 |
| GO:0004402 | histone acetyltransferase activity | 8/1015 | 55/18,352 | 0.010272488 |
| Cellular Component | ||||
| ID | Description | Gene Ratio | BgRatio | p Value |
| GO:0016607 | nuclear speck | 41/1029 | 401/19,559 | 3.81569 × 10−5 |
| GO:0005635 | nuclear envelope | 41/1029 | 473/19,559 | 0.001216517 |
| GO:0019908 | nuclear cyclin-dependent protein kinase holoenzyme complex | 3/1029 | 12/19,559 | 0.022350144 |
| ID | Description | Gene Ratio | Bg Ratio | p Value | p.Adjust | q Value | GeneID | Count |
|---|---|---|---|---|---|---|---|---|
| hsa04722 | Neurotrophin signaling pathway | 14/480 | 119/8223 | 0.00923833 | 0.22409163 | 0.21320458 | ATF4/FOXO3/FRS2/IRAK2/IRS1/JUN/MAP3K3/ MAPK12/NFKBIA/NFKBIB/NFKBIE/RHOA/RPS6KA5/SOS2 | 14 |
| hsa04152 | AMPK signaling pathway | 12/480 | 121/8223 | 0.04947161 | 0.50476492 | 0.48024193 | ADIPOR2/FOXO3/HNF4A/IRS1/IRS2/PRKAG1/ RHEB/RPTOR/SIRT1/STRADB/TBC1D1/ULK1 | 12 |
| hsa04110 | Cell cycle | 13/480 | 127/8223 | 0.03374595 | 0.48461782 | 0.46107364 | CCNE1/CDC6/CDK7/CDKN1C/CDKN2D/DBF4B/GADD45A/ GADD45B/GADD45G/ORC1/ORC3/ORC6/TTK | 13 |
| hsa04068 | FoxO signaling pathway | 16/480 | 131/8223 | 0.00387417 | 0.13927125 | 0.13250504 | BCL2L11/CDKN2D/FOXO3/GABARAPL1/GADD45A/GADD45B/ GADD45G/IRS1/IRS2/MAPK12/PRKAG1/SGK1/SGK2/SIRT1/SOD2/SOS2 | 16 |
| hsa04210 | Apoptosis | 14/480 | 136/8223 | 0.02708404 | 0.47755381 | 0.45435282 | APAF1/ATF4/BCL2L11/BIRC3/DDIT3/ENDOG/FADD/GADD45A/ GADD45B/GADD45G/ITPR2/JUN/NFKBIA/TUBA4A | 14 |
| Number of nodes: 64 | Avg. local clustering coefficient: 0.678 |
| Number of edges: 197 | Expected number of edges: 47 |
| Average node degree: 6.16 | PPI enrichment p-value: <1.0 × 10−16 |
| Biological Process | ||||
| GO-Term | Description | Count in Network | Strength | False Discovery Rate |
| GO:0006915 | Apoptotic process | 64 of 1041 | 1.28 | 1.87 × 10−77 |
| GO:0050794 | Regulation of cellular process | 61 of 11,025 | 0.23 | 5.23 × 10−10 |
| GO:0050896 | Response to stimulus | 54 of 7835 | 0.33 | 9.81 × 10−11 |
| GO:0051716 | Cellular response to stimulus | 52 of 6357 | 0.4 | 6.42 × 10−13 |
| GO:0048518 | Positive regulation of the biological process | 51 of 6207 | 0.4 | 1.61 × 10−12 |
| Molecular Function | ||||
| GO-Term | Description | Count in Network | Strength | False Discovery Rate |
| GO:0005515 | Protein binding | 47 of 7242 | 0.3 | 7.04 × 10−6 |
| GO:0019899 | Enzyme binding | 28 of 2084 | 0.62 | 6.33 × 10−8 |
| GO:0042802 | Identical protein binding | 20 of 2144 | 0.46 | 0.0093 |
| GO:0098772 | Molecular function regulator activity | 19 of 1960 | 0.47 | 0.0093 |
| GO:0044877 | Protein-containing complex binding | 15 of 1261 | 0.56 | 0.0093 |
| KEGG Pathways | ||||
| Pathway | Description | Count in Network | Strength | False Discovery Rate |
| hsa05200 | Pathways in cancer | 13 of 515 | 0.89 | 1.29 × 10−6 |
| hsa04210 | Apoptosis | 12 of 131 | 1.45 | 1.03 × 10−11 |
| hsa04621 | NOD-like receptor signaling pathway | 9 of 173 | 1.2 | 1.17 × 10−6 |
| hsa05169 | Epstein–Barr virus infection | 9 of 192 | 1.16 | 1.29 × 10−6 |
| hsa04068 | FoxO signaling pathway | 8 of 126 | 1.29 | 1.29 × 10−6 |
| Reactome Pathways | ||||
| Pathway | Description | Count in Network | Strength | False Discovery Rate |
| HSA-162582 | Signal transduction | 25 of 2540 | 0.48 | 4.14 × 10−5 |
| HSA-74160 | Gene expression (Transcription) | 17 of 1476 | 0.55 | 0.00080 |
| HSA-168256 | Immune system | 17 of 1979 | 0.42 | 0.0136 |
| HSA-2262752 | Cellular responses to stress | 15 of 747 | 0.79 | 1.69 × 10−5 |
| HSA-212436 | Generic transcription pathway | 15 of 1215 | 0.58 | 0.0014 |
| Wiki Pathways | ||||
| Pathway | Description | Count in Network | Strength | False Discovery Rate |
| WP1772 | Apoptosis modulation and signaling | 10 of 89 | 1.54 | 5.91 × 10−10 |
| WP3888 | VEGFA-VEGFR2 signaling | 10 of 428 | 0.86 | 0.00012 |
| WP254 | Apoptosis | 7 of 83 | 1.41 | 6.30 × 10−6 |
| WP4754 | IL-18 signaling pathway | 7 of 271 | 0.9 | 0.0015 |
| WP2882 | Nuclear receptors meta-pathway | 7 of 312 | 0.84 | 0.0024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, M.-Y.; Lee, S.; Kim, H.-H.; Jeong, S.-H.; Abusaliya, A.; Bhosale, P.B.; Seong, J.-K.; Park, K.-I.; Heo, J.-D.; Ahn, M.; et al. Correlation with Apoptosis Process through RNA-Seq Data Analysis of Hep3B Hepatocellular Carcinoma Cells Treated with Glehnia littoralis Extract (GLE). Int. J. Mol. Sci. 2024, 25, 9462. https://doi.org/10.3390/ijms25179462
Park M-Y, Lee S, Kim H-H, Jeong S-H, Abusaliya A, Bhosale PB, Seong J-K, Park K-I, Heo J-D, Ahn M, et al. Correlation with Apoptosis Process through RNA-Seq Data Analysis of Hep3B Hepatocellular Carcinoma Cells Treated with Glehnia littoralis Extract (GLE). International Journal of Molecular Sciences. 2024; 25(17):9462. https://doi.org/10.3390/ijms25179462
Chicago/Turabian StylePark, Min-Yeong, Sujin Lee, Hun-Hwan Kim, Se-Hyo Jeong, Abuyaseer Abusaliya, Pritam Bhangwan Bhosale, Je-Kyung Seong, Kwang-Il Park, Jeong-Doo Heo, Meejung Ahn, and et al. 2024. "Correlation with Apoptosis Process through RNA-Seq Data Analysis of Hep3B Hepatocellular Carcinoma Cells Treated with Glehnia littoralis Extract (GLE)" International Journal of Molecular Sciences 25, no. 17: 9462. https://doi.org/10.3390/ijms25179462







