Functional Study on the Key Gene LaLBD37 Related to the Lily Bulblets Formation
Abstract
:1. Introduction
2. Results
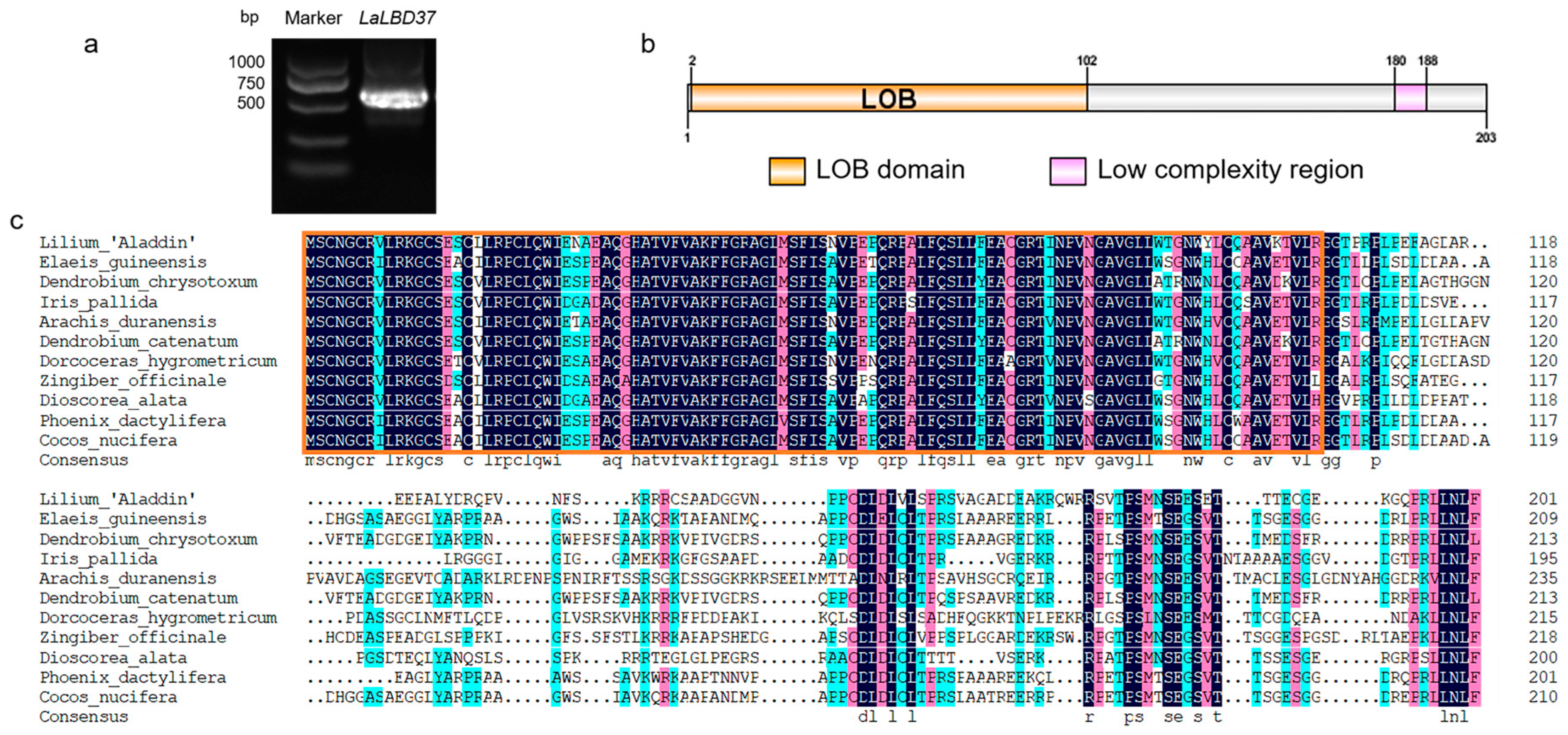
2.1. Full-Length Cloning and Sequence Analysis of LaLBD37
2.2. Subcellular Localization and Transcriptional Activation of LaLBD37
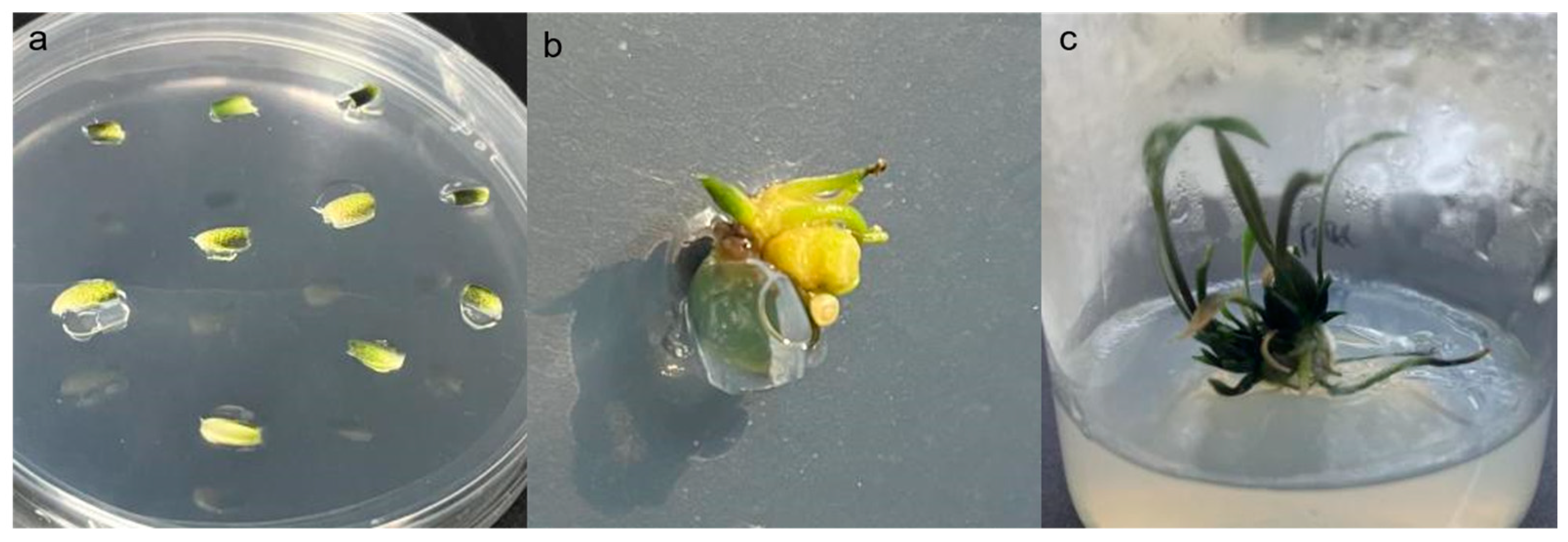
2.3. Acquisition of Resistant Plants
2.4. Identification of Genetically Modified Plants
2.4.1. GUS Staining Identification
2.4.2. DNA Level Identification
2.4.3. RNA Level Identification
2.5. Morphological Changes in Transgenic Lilies
2.6. Changes in Soluble Sugar, Starch Content, and Sucrose Synthase Activity in Transgenic Lines
2.7. Determination of Endogenous Hormone Content in Transgenic Lines
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Gene Cloning and Analysis of LaLBD37
4.3. Subcellular Localization of LaLBD37
4.4. Detection of Transcriptional Autoactivation Activity in LaLBD37
4.5. Agrobacterium Mediated Transformation of Small Scales from Sterile Seedlings of ‘Sorbonne’
4.6. Identification of Genetically Modified Plants
4.6.1. Detection of GUS Activity in Transformed Plants
4.6.2. PCR Detection of Transformed Plants
4.6.3. Real-Time Fluorescence Quantitative Detection of Transformed Plants
4.7. Phenotypic Observation of Transgenic ‘Sorbonne’ Lines
4.8. Metabolite Analysis
4.9. Determination of Endogenous Hormone Content in Transgenic Lines
4.10. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shahin, A. Development of Genomic Resources for Ornamental Lilies (Lilium L.); Wageningen University and Research: Wageningen, The Netherlands, 2012. [Google Scholar]
- Zhang, Y.; Yong, Y.B.; Wang, Q.; Lu, Y.M. Physiological and Molecular Changes during Lily Underground Stem Axillary Bulbils Formation. Russ. J. Plant Physiol. 2018, 65, 372–383. [Google Scholar] [CrossRef]
- Chen, Y.; Hou, X.; Zheng, Y.; Lyu, Y. The Establishment of a Genetic Transformation System and the Acquisition of Transgenic Plants of Oriental Hybrid Lily (Lilium L.). Int. J. Mol. Sci. 2023, 24, 782. [Google Scholar] [CrossRef]
- Sun, B.; Liu, J.; Ge, Y.; Sheng, L.; Chen, L.; Hu, X.; Yang, Z.; Huang, H.; Xu, L. Recent progress on plant regeneration. Chin. Sci. Bull. 2016, 61, 3887–3902. [Google Scholar] [CrossRef]
- Mao, J.L.; Miao, Z.Q.; Wang, Z.; Yu, L.H.; Cai, X.T.; Xiang, C.B. Arabidopsis ERF1 Mediates Cross-Talk between Ethylene and Auxin Biosynthesis during Primary Root Elongation by Regulating ASA1 Expression. PLoS Genet. 2016, 12, e1005760. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.T.; Xu, P.; Zhao, P.X.; Liu, R.; Yu, L.H.; Xiang, C.B. Arabidopsis ERF109 mediates cross-talk between jasmonic acid and auxin biosynthesis during lateral root formation. Nat. Commun. 2014, 5, 5833. [Google Scholar] [CrossRef] [PubMed]
- Heyman, J.; Cools, T.; Canher, B.; Shavialenka, S.; Traas, J.; Vercauteren, I.; Van den Daele, H.; Persiau, G.; De Jaeger, G.; Sugimoto, K.; et al. The heterodimeric transcription factor complex ERF115-PAT1 grants regeneration competence. Nat. Plants 2016, 2, 16165. [Google Scholar] [CrossRef]
- Licausi, F.; Ohme-Takagi, M.; Perata, P. APETALA2/Ethylene Responsive Factor (AP2/ERF) transcription factors: Mediators of stress responses and developmental programs. New Phytologist 2013, 199, 639–649. [Google Scholar] [CrossRef]
- Moreno-Pachon, N.M. Mechanisms of Vegetative Propagation in Bulbs: A Molecular Approach. Ph.D. Thesis, Wageningen University & Research, Wageningen, The Netherlands, 2017. [Google Scholar]
- He, G.; Cao, Y.; Wang, J.; Song, M.; Bi, M.; Tang, Y.; Xu, L.; Ming, J.; Yang, P. WUSCHEL-Related Homeobox Genes Cooperate with Cytokinin to Promote Bulbil Formation in Lilium lancifolium. Plant Physiol. 2022, 190, 387–402. [Google Scholar] [CrossRef]
- Yang, P.X.H.; Xu, L.; Tang, Y.; He, G.; Cao, Y.; Yuan, S.; Ren, J.; Ming, J. Cloning and expression analysis of LlAGO1 in Lilium lancifolium. Acta Hortic. Sin. 2018, 45, 784–794. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, Z.; Yong, Y.B.; Lyu, Y.M. Hormonal Regulatory Patterns of LaKNOXs and LaBEL1 Transcription Factors Reveal Their Potential Role in Stem Bulblet Formation in LA Hybrid Lily. Int. J. Mol. Sci. 2021, 22, 13502. [Google Scholar] [CrossRef]
- Li, X.Y.; Wang, C.X.; Cheng, J.Y.; Zhang, J.; da Silva, J.A.T.; Liu, X.Y.; Duan, X.; Li, T.L.; Sun, H.M. Transcriptome analysis of carbohydrate metabolism during bulblet formation and development in Lilium davidii var. unicolor. BMC Plant Biol. 2014, 14, 358. [Google Scholar] [CrossRef]
- Wu, Y.; Ren, Z.; Gao, C.; Sun, M.; Li, S.; Min, R.; Wu, J.; Li, D.; Wang, X.; Wei, Y.; et al. Change in Sucrose Cleavage Pattern and Rapid Starch Accumulation Govern Lily Shoot-to-Bulblet Transition in vitro. Front. Plant Sci. 2020, 11, 564713. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.M.; Zhang, D.; Jiao, C.; Li, D.Q.; Wu, Y.; Wang, X.Y.; Gao, C.; Lin, Y.F.; Ruan, Y.L.; Xia, Y.P. Comparative transcriptome and metabolome analyses identified the mode of sucrose degradation as a metabolic marker for early vegetative propagation in bulbs of Lycoris. Plant J. 2022, 112, 115–134. [Google Scholar] [CrossRef]
- Ren, Z.; Xu, Y.; Lvy, X.; Zhang, D.; Gao, C.; Lin, Y.; Liu, Y.; Wu, Y.; Xia, Y. Early Sucrose Degradation and the Dominant Sucrose Cleavage Pattern Influence Lycoris sprengeri Bulblet Regeneration in Vitro. Int. J. Mol. Sci. 2021, 22, 1890. [Google Scholar] [CrossRef]
- Podwyszyńska, M. The Mechanisms of In Vitro Storage Organ Formation in Ornamental Geophytes. Floric. Ornam. Biotechnol. 2015, 6, 9. [Google Scholar]
- Chang, L.; Xiao, Y.M.; She, L.F.; Xia, Y.P. Analysis of gene expression and enzyme activities related to starch metabolism in ‘Lycoris sprengeri’ bulbs of different sizes. Sci. Hortic. 2013, 161, 118–124. [Google Scholar] [CrossRef]
- Xu, J.X.; Li, Q.Z.; Yang, L.Y.; Li, X.; Wang, Z.; Zhang, Y.C. Changes in carbohydrate metabolism and endogenous hormone regulation during bulblet initiation and development in Lycoris radiata. BMC Plant Biol. 2020, 20, 180. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y. Functional Studies on the Sucrose Synthase Gene during Lily Bulb Development; Shenyang Agricultural University: Shenyang, China, 2020. [Google Scholar]
- Tang, N.; Ju, X.; Hu, Y.; Jia, R.; Tang, D. Effects of Temperature and Plant Growth Regulators on the Scale Propagation of Lilium davidii var. unicolor. Hortscience 2020, 55, 870–875. [Google Scholar] [CrossRef]
- Woodward, A.W.; Bartel, B. Auxin: Regulation, action, and interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef]
- Azadi, P.; Khosh-Khui, M. Micropropagation of Lilium ledebourii (Baker) Boiss as affected by plant growth regulator, sucrose concentration, harvesting season and cold treatments. Electron. J. Biotechnol. 2008, 10, 582–591. [Google Scholar] [CrossRef]
- Kapoora, R.; Kumara, S.; Kanwara, J.K. Bulblet production from node explant grown in vitro in hybrid lilies. Int. J. Plant Prod. 2009, 3, 1–6. [Google Scholar]
- Maesato, K.; Sharada, K.; Fukui, H.; Hara, T.; Sarma, K.S. In vitro bulblet regeneration from bulbscale explants of Lilium japonicum Thunb. Effect of plant growth regulators and culture environment. J. Pomol. Hortic. Sci. 2015, 69, 289–297. [Google Scholar] [CrossRef]
- Macmillan, J.; Takahashi, N. Proposed Procedure for the Allocation of Trivial Names to the Gibberellins. Nature 1968, 217, 170–171. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, Y.; Liu, Y.; Zhang, Q.; Gao, H.; Zhang, F. Comparative proteomics illustrates the molecular mechanism of potato (Solanum tuberosum L.) tuberization inhibited by exogenous gibberellins in vitro. Physiol. Plant 2018, 163, 103–123. [Google Scholar] [CrossRef] [PubMed]
- Marković, M.; Trifunović Momčilov, M.; Uzelac, B.; Cingel, A.; Milošević, S.; Jevremović, S.; Subotić, A. Breaking the Dormancy of Snake’s Head Fritillary (Fritillaria meleagris L.) In Vitro Bulbs—Part 1: Effect of GA3, GA Inhibitors and Temperature on Fresh Weight, Sprouting and Sugar Content. Plants 2020, 9, 1449. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Seng, S.; Li, D.; Zhang, F.; Liu, Y.; Yao, T.; Liang, J.; Yi, M.; Wu, J. Antagonism between abscisic acid and gibberellin regulates starch synthesis and corm development in Gladiolus hybridus. Hortic. Res. 2021, 8, 155. [Google Scholar] [CrossRef]
- Xu, C.; Luo, F.; Hochholdinger, F. LOB Domain Proteins: Beyond Lateral Organ Boundaries. Trends Plant Sci. 2016, 21, 159–167. [Google Scholar] [CrossRef]
- Albinsky, D.; Kusano, M.; Higuchi, M.; Hayashi, N.; Kobayashi, M.; Fukushima, A.; Mori, M.; Ichikawa, T.; Matsui, K.; Kuroda, H. Metabolomic Screening Applied to Rice FOX Arabidopsis Lines Leads to the Identification of a Gene-Changing Nitrogen Metabolism. Mol. Plant 2010, 18, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.M.; Lin, W.C.; Husbands, A.Y.; Yu, L.; Jaganatha, V.; Jablonska, B.; Mangeon, A.; Neff, M.M.; Girke, T.; Springer, P.S. Arabidopsis lateral organ boundaries negatively regulates brassinosteroid accumulation to limit growth in organ boundaries. Proc. Natl. Acad. Sci. USA 2012, 109, 21146–21151. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, M.; Lee, M.R.; Park, S.K.; Kim, J. LATERAL ORGAN BOUNDARIES DOMAIN (LBD)10 interacts with SIDECAR POLLEN/LBD27 to control pollen development in Arabidopsis. Plant J. 2015, 81, 794–809. [Google Scholar] [CrossRef]
- Matsumura, Y.; Iwakawa, H.; Machida, Y.; Machida, C. Characterization of genes in the ASYMMETRIC LEAVES2/LATERAL ORGAN BOUNDARIES (AS2/LOB) family in Arabidopsis thaliana, and functional and molecular comparisons between AS2 and other family members. Plant J. 2009, 58, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Rubin, G.; Tohge, T.; Matsuda, F.; Saito, K.; Scheible, W.-R. Members of the LBD Family of Transcription Factors Repress Anthocyanin Synthesis and Affect Additional Nitrogen Responses in Arabidopsis. Plant Cell 2009, 21, 3567–3584. [Google Scholar] [CrossRef] [PubMed]
- Semiarti, E.; Ueno, Y.; Tsukaya, H.; Iwakawa, H.; Machida, C.; Machida, Y. The ASYMMETRIC LEAVES2 gene of Arabidopsis thaliana regulates formation of a symmetric lamina, establishment of venation and repression of meristem-related homeobox genes in leaves. Development 2001, 128, 1771–1783. [Google Scholar] [CrossRef]
- Taramino, G.; Sauer, M.; Stauffer, J.L., Jr.; Multani, D.; Niu, X.; Sakai, H.; Hochholdinger, F. The maize (Zea mays L.) RTCS gene encodes a LOB domain protein that is a key regulator of embryonic seminal and post-embryonic shoot-borne root initiation. Plant J. 2007, 50, 649–659. [Google Scholar] [CrossRef]
- Thatcher, L.F.; Powell, J.J.; Aitken, E.A.B.; Kazan, K.; Manners, J.M. The lateral organ boundaries domain transcription factor lbd20 functions in fusarium wilt susceptibility and jasmonate signaling in arabidopsis 1[w]. Plant Physiol. 2018, 160, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Rast, M.I.; Simon, R. Arabidopsis JAGGED LATERAL ORGANS acts with ASYMMETRIC LEAVES2 to coordinate KNOX and PIN expression in shoot and root meristems. Plant Cell 2012, 24, 2917–2933. [Google Scholar] [CrossRef]
- Guo, M.; Thomas, J.; Collins, G.; Timmermans, M.C. Direct repression of KNOX loci by the ASYMMETRIC LEAVES1 complex of Arabidopsis. Plant Cell 2008, 20, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Shuai, B.; Springer, P.S. The Arabidopsis LATERAL ORGAN BOUNDARIES-domain gene ASYMMETRIC LEAVES2 functions in the repression of KNOX gene expression and in adaxial-abaxial patterning. Plant Cell 2003, 15, 2241–2252. [Google Scholar] [CrossRef]
- Chalfun-Junior, A.; Franken, J.; Mes, J.J.; Marsch-Martinez, N.; Pereira, A.; Angenent, G.C. ASYMMETRIC LEAVES2-LIKE1gene a member of the AS2/LOB family, controls proximal-distal patterning in Arabidopsis petals. Plant Mol. Biol. 2005, 57, 559–575. [Google Scholar] [CrossRef]
- Nakazawa, M. Activation tagging, a novel tool to dissect the functions of a gene family. Plant J. 2010, 34, 741–750. [Google Scholar] [CrossRef]
- Berckmans, B.; Vassileva, V.; Schmid, S.P.C.; Maes, S.; Parizot, B.; Naramoto, S.; Magyar, Z.; Kamei, C.L.A.; Koncz, C.; Bgre, L. Auxin-dependent cell cycle reactivation through transcriptional regulation of Arabidopsis E2Fa by lateral organ boundary proteins. Plant Cell 2011, 23, 3671–3683. [Google Scholar] [CrossRef]
- Laurent, L.; Boris, P.; Andrew, B.; Lilian, R.; Alexandre, M.; Florence, A.; Claudine, F.; Laurent, N.; Didier, B.; Jim, H. GAL4-GFP enhancer trap lines for genetic manipulation of lateral root development in Arabidopsis thaliana. J. Exp. Bot. 2005, 56, 2433–2442. [Google Scholar]
- Okushima, Y. Functional Genomic Analysis of the AUXIN RESPONSE FACTOR Gene Family Members in Arabidopsis thaliana: Unique and Overlapping Functions of ARF7 and ARF19. Plant Cell 2005, 17, 444–463. [Google Scholar] [CrossRef]
- Ma, X.J.; Tao, Y.; Zhao, X.Y.; Zhang, X.S. Wheat TaAS2, a member of LOB family, affects the adaxial–abaxial polarity of leaves in transgenic Arabidopsis. Plant Sci. 2007, 172, 181–188. [Google Scholar] [CrossRef]
- Li, C.; Zhu, S.; Zhang, H.; Chen, L.; Cai, M.; Wang, J.; Chai, J.; Wu, F.; Cheng, Z.; Guo, X. OsLBD37 and OsLBD38, two class II type LBD proteins, are involved in the regulation of heading date by controlling the expression of Ehd1 in rice. Biochem. Biophys. Res. Commun. 2017, 486, 720. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Zhang, Y.; Wu, X.; Tang, W.; Wu, R.; Dai, Z.; Liu, G.; Zhang, H.; Wu, C.; Chen, G. DH1, a LOB domain-like protein required for glume formation in rice. Plant Mol. Biol. 2008, 66, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Jingrong, Z.; Wei, T.; Yulan, H.; Xiangli, N.; Yu, Z.; Yi, H.; Yongsheng, L. Down-regulation of a LBD-like gene, OsIG1, leads to occurrence of unusual double ovules and developmental abnormalities of various floral organs and megagametophyte in rice. J. Exp. Bot. 2015, 66, 99–112. [Google Scholar]
- Fang, S.; Yang, C.; Ali, M.M.; Lin, M.; Tian, S.; Zhang, L.; Chen, F.; Lin, Z. Transcriptome Analysis Reveals the Molecular Regularity Mechanism Underlying Stem Bulblet Formation in Oriental Lily ‘Siberia’; Functional Characterization of the LoLOB18 Gene. Int. J. Mol. Sci. 2022, 23, 15246. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.Y.; He, G.R.; Bi, M.M.; Tang, Y.C.; Hao, C.L.; Qu, Y.X.; Ming, J. Cloning and Functional Analysis of LlLBD18 in Lilium lancifolium. Acta Hortic. Sin. 2023, 50, 2117–2127. [Google Scholar]
- Majer, C.; Hochholdinger, F. Defining the boundaries: Structure and function of LOB domain proteins. Trends Plant Sci. 2011, 16, 47–52. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, N.Y.; Lee, D.J.; Kim, J. LBD18/ASL20 Regulates Lateral Root Formation in Combination with LBD16/ASL18 Downstream of ARF7 and ARF19 in Arabidopsis. Plant Physiol. 2009, 151, 1377–1389. [Google Scholar] [CrossRef]
- Ji, X.; Wang, J.; Hou, X.; Xu, J.; Shao, J.; Hu, C. Functional analysis of transcription factor bclbd37 in non-heading Chinese cabbage. J. Nucl. Agric. Sci. 2022, 36, 2338–2348. [Google Scholar]
- Kim, M.; Kim, M.J.; Pandey, S.; Kim, J. Expression and Protein Interaction Analyses Reveal Combinatorial Interactions of LBD Transcription Factors During Arabidopsis Pollen Development. Plant Cell Physiol. 2016, 57, 2291. [Google Scholar] [CrossRef] [PubMed]
- Marinangeli, P.A.; Hernández, L.F.; Pellegrini, C.P.; Curvetto, N.R. Bulblet Differentiation after Scale Propagation of Lilium longiflorum. J. Am. Soc. Hortic. Sci. 2003, 128, 324–329. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, F.; Guo, J.; Zhang, X.S. Rice OsAS2 Gene, a Member of LOB Domain Family, Functions in the Regulation of Shoot Differentiation and Leaf Development. J. Plant Biol. 2009, 52, 374–381. [Google Scholar] [CrossRef]
- Meng, L.S.; Liu, H.L.; Cui, X.; Sun, X.D.; Zhu, J. ASYMMETRIC LEAVES2-LIKE38 gene, a Member of AS2/LOB family of Arabidopsis, causes leaf dorsoventral alternation in transgenic cockscomb plants. Acta Physiol. Plant. 2009, 31, 1301. [Google Scholar] [CrossRef]
- Hong-Mei, S.; Tian-Lai, L.I.; Yun-Fei, L.I. Physiology Mechanism of Metabolisms in the Middle Scales of Lilium davidii var. unicolor Bulbs Stored at Low Temperature for Dormancy-Release. Agric. Sci. China 2005, 4, 521–527. [Google Scholar]
- Wang, X.; Zhang, Y.; Niu, L. Changes in carbohydrate and protein content of bulbs during tulip bud differentiation. J. Plant Physiol. 2011, 47, 379–384. [Google Scholar]
- Zhao, Y. Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef]
- Azizi, P.; Rafii, M.Y.; Maziah, M.; Abdullah, S.N.A.; Hanafi, M.M.; Latif, M.A.; Rashid, A.A.; Sahebi, M. Understanding the shoot apical meristem regulation: A study of the phytohormones, auxin and cytokinin, in rice. Mech. Dev. 2015, 135, 1–15. [Google Scholar] [CrossRef]
- Tang, Y. The Mechanism of Auxin Involving in Bulbil Formation of Lilium Lancifolium; Chinese Academy of Agricultural Sciences: Beijing, China, 2019. [Google Scholar]
- Xu, J.; Li, Q.; Li, Y.; Yang, L.; Zhang, Y.; Cai, Y. Effect of Exogenous Gibberellin, Paclobutrazol, Abscisic Acid, and Ethrel Application on Bulblet Development in Lycoris radiata. Front. Plant Sci. 2020, 11, 615547. [Google Scholar] [CrossRef]
- Aklade, S.A.; Bardhan, K.; Singh, P.; Kakade, D.K.; Pathan, A.B. Effect of PGR’s on growth, flowering and flower yield of chrysanthemum (Chrysanthemum indicum L.) cv. ‘LOCAL WHITE’. Asian J. Hortic. 2009, 491–493. [Google Scholar]
- Askari, N. Effect of gibberellin on in vitro bulblet induction of lily (Lilium orientalis L. cv. Santander). J. Hortic. Postharvest Res. 2023, 6, 421–432. [Google Scholar]
- Kim, K.S.; Davelaar, E.; De Klerk, G.J. Abscisic acid controls dormancy development and bulb formation in lily plantlets regenerated in vitro. Physiol. Plant. 1994, 90, 59–64. [Google Scholar] [CrossRef]
- Askari, N.; Visser, R.G.; De Klerk, G.-J. Growth of lily bulblets in vitro, a review. Int. J. Hortic. Sci. Technol. 2018, 5, 133–143. [Google Scholar]
- Jeong, E.-J.; Imran, M.; Kang, S.-M.; Khan, M.A.; Lee, I.-J. The application of diniconazole and prohydrojasmon as plant growth regulators to induce growth and tuberization of potato. J. Appl. Bot. Food Qual. 2021, 94, 39–46. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, B.; Liu, X.; Shan, N.; Sun, J.; Zhang, H.; Huang, Y.; Xiao, Y.; Zhou, Q. Uncovering the mechanism preliminarily of formation and development of taro corm in vitro by morphological physiology and transcriptomic analysis. Sci. Hortic. 2022, 291, 110575. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, X.; Zhang, K.; Lyu, Y. Functional Study on the Key Gene LaLBD37 Related to the Lily Bulblets Formation. Int. J. Mol. Sci. 2024, 25, 9456. https://doi.org/10.3390/ijms25179456
Hou X, Zhang K, Lyu Y. Functional Study on the Key Gene LaLBD37 Related to the Lily Bulblets Formation. International Journal of Molecular Sciences. 2024; 25(17):9456. https://doi.org/10.3390/ijms25179456
Chicago/Turabian StyleHou, Xinru, Kewen Zhang, and Yingmin Lyu. 2024. "Functional Study on the Key Gene LaLBD37 Related to the Lily Bulblets Formation" International Journal of Molecular Sciences 25, no. 17: 9456. https://doi.org/10.3390/ijms25179456




