The Application of Green Solvents in the Synthesis of S-Heterocyclic Compounds—A Review
Abstract
:1. Introduction
2. Solvents in Organic Synthesis
3. Synthesis of S-Heterocycles in Water as a Green Solvent
3.1. Synthesis of S-Heterocycles in Water Using Conventional Methods
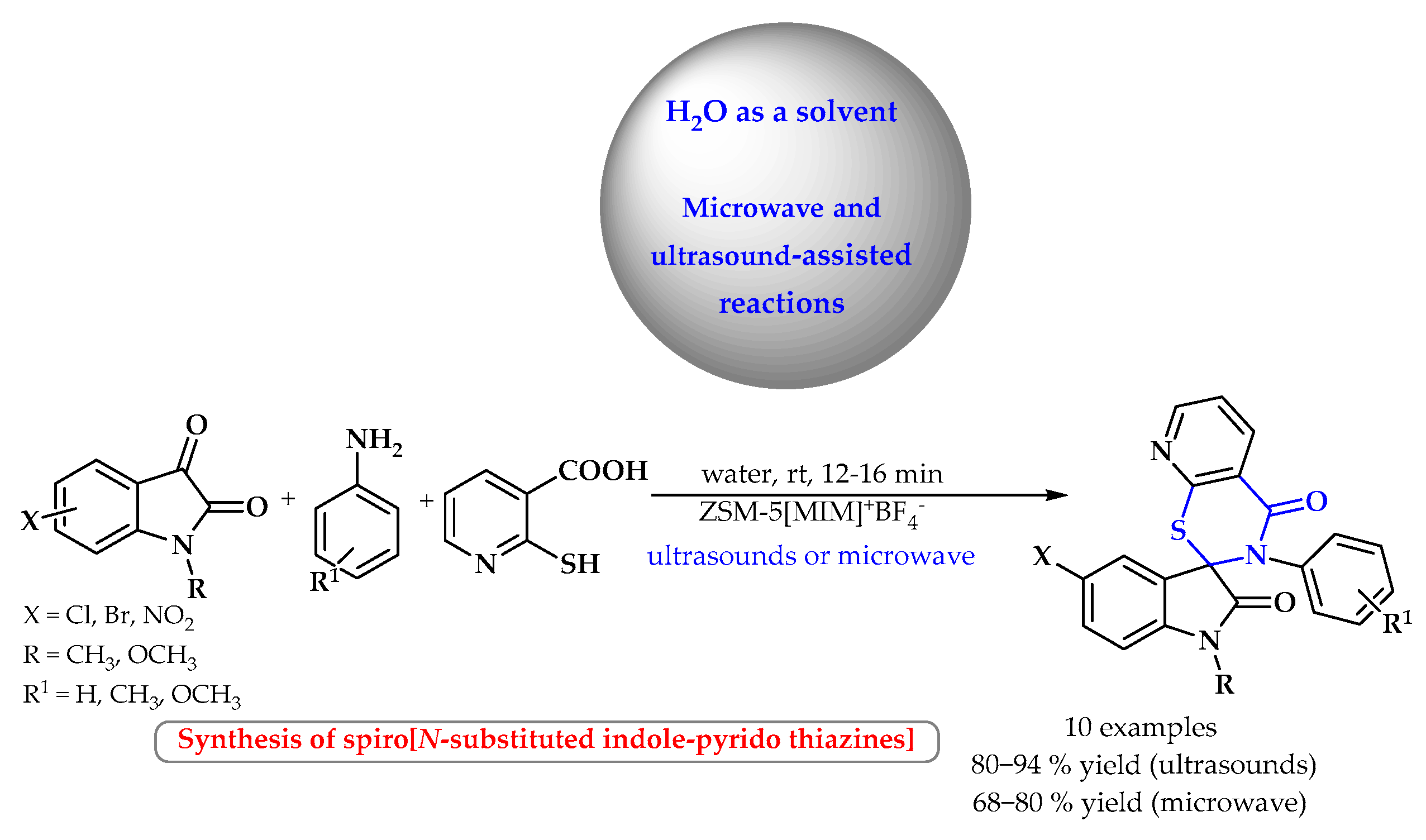
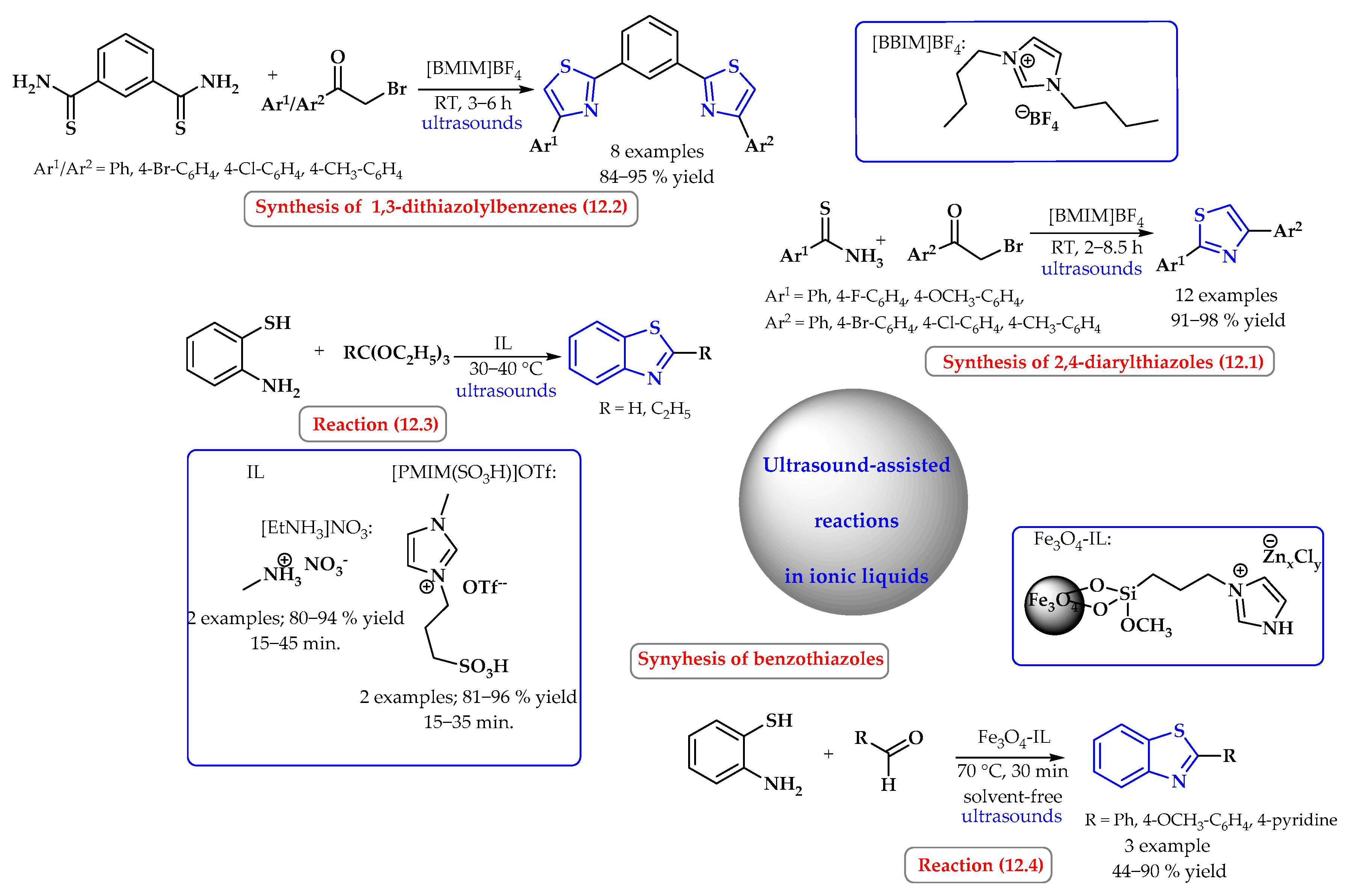
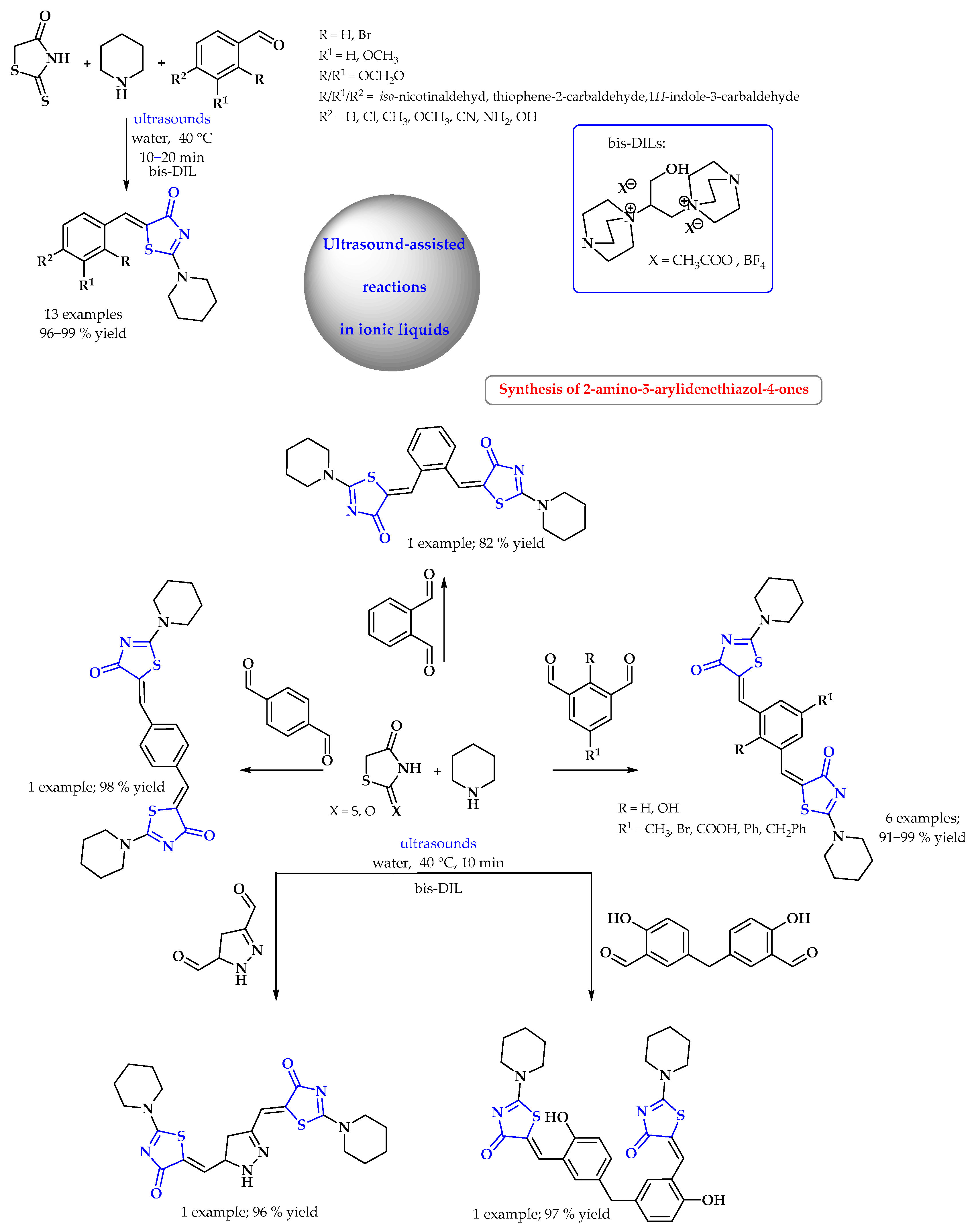
3.2. Synthesis of S-Heterocycles in Water Using Microwave or Ultrasound Radiation
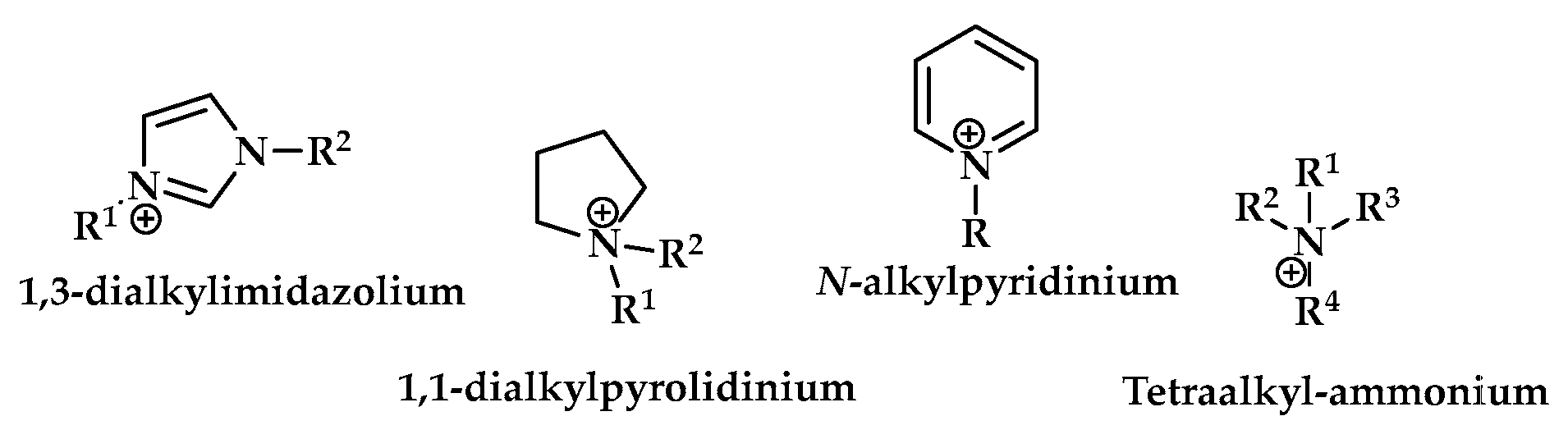
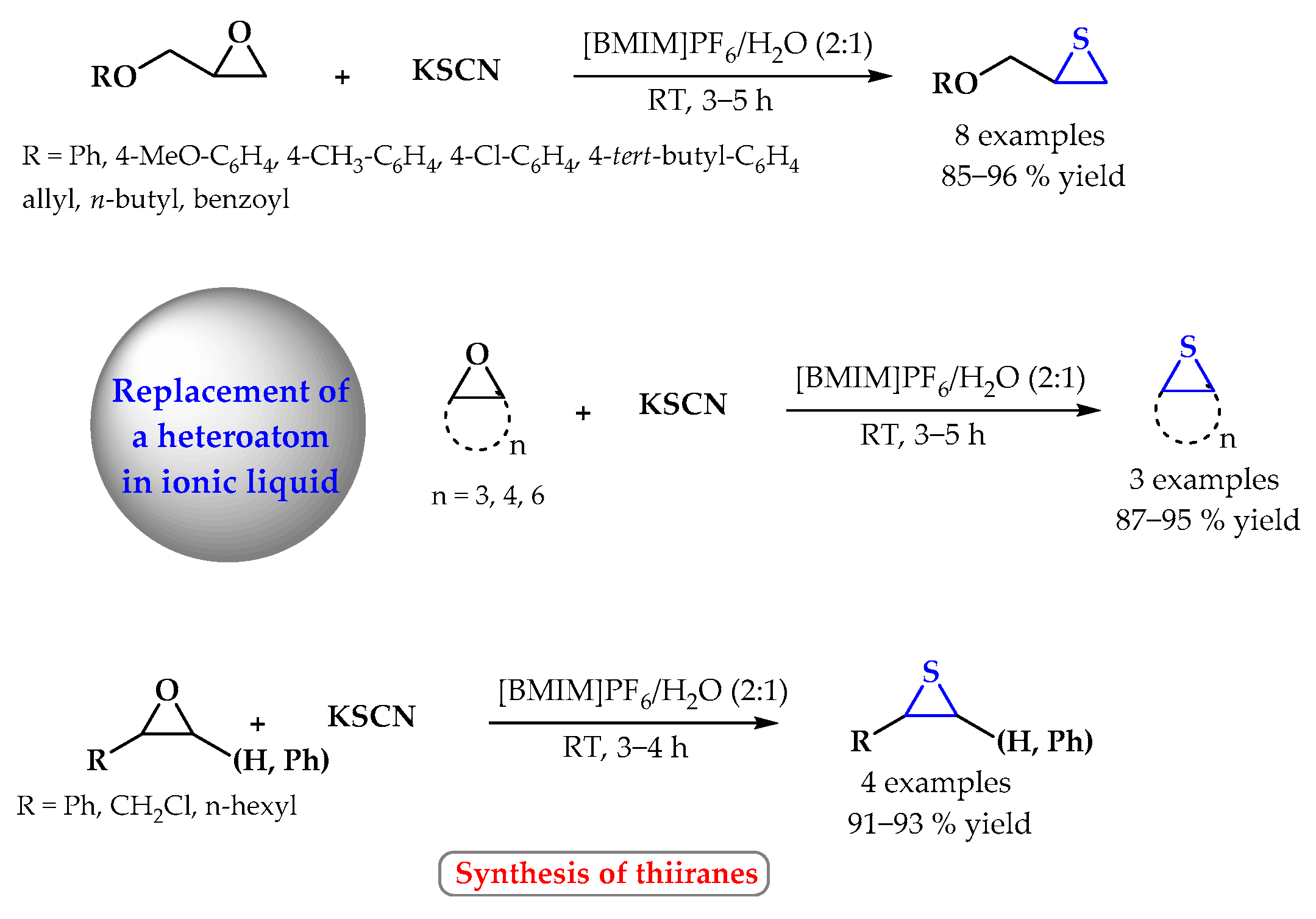
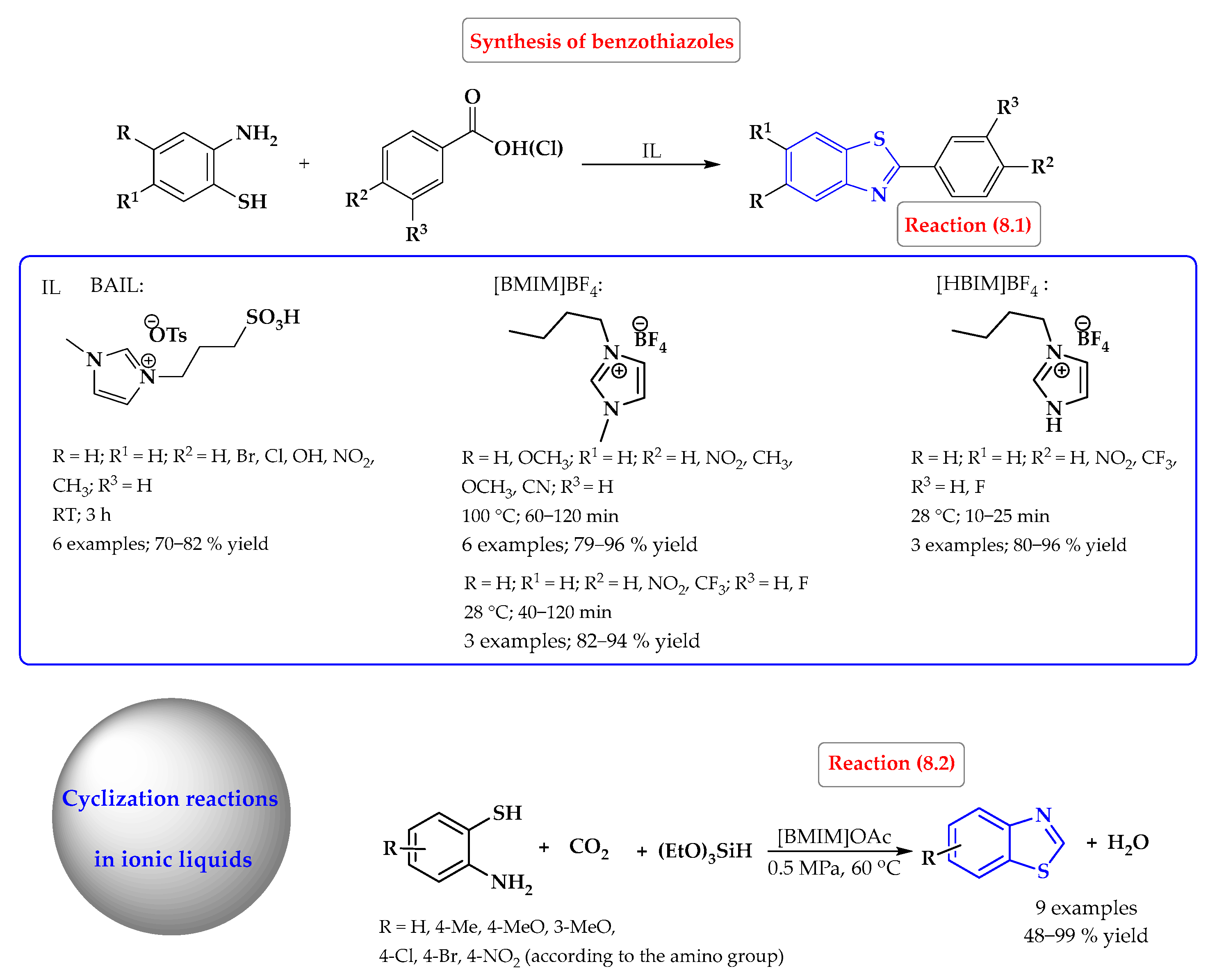
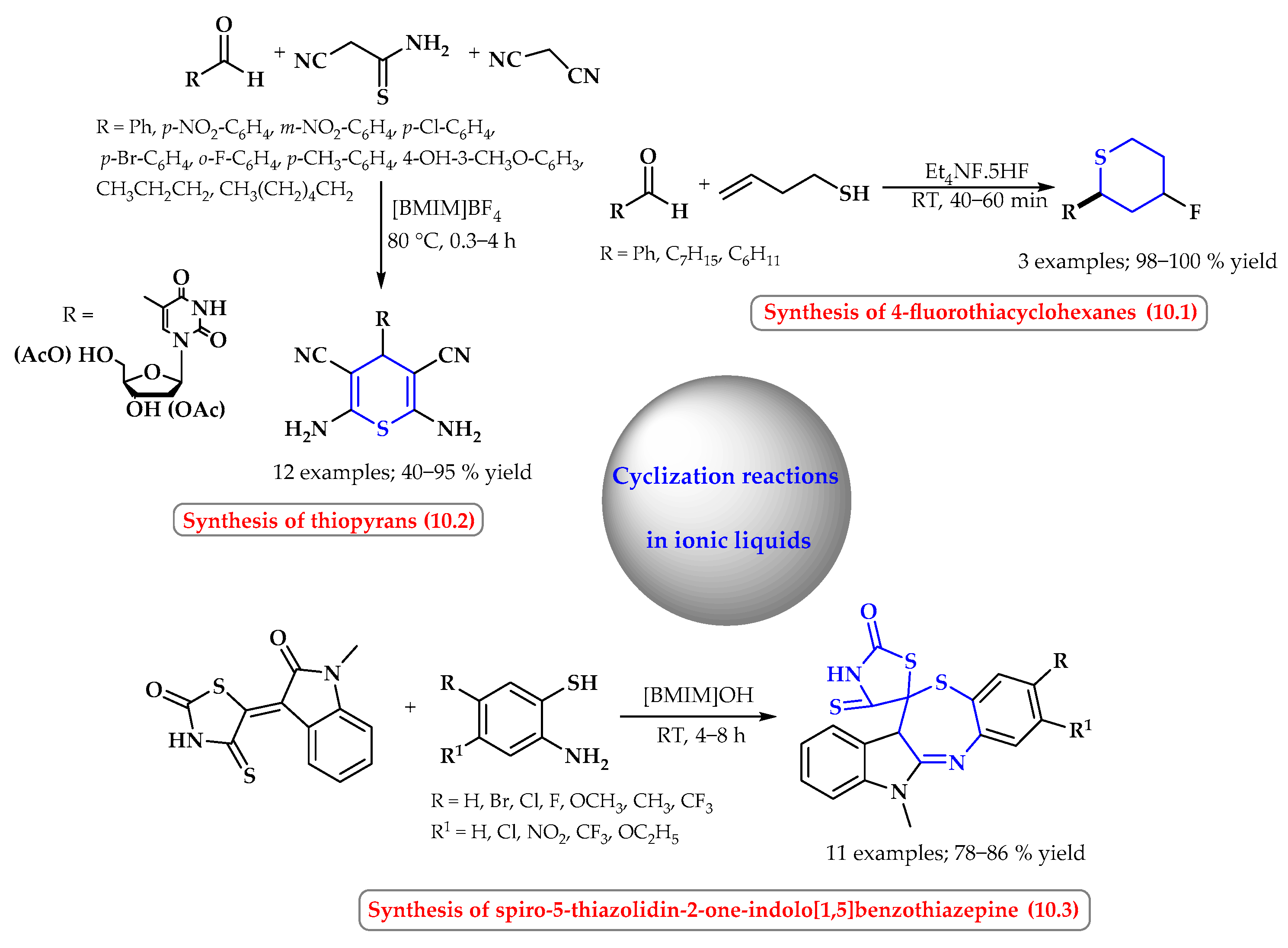
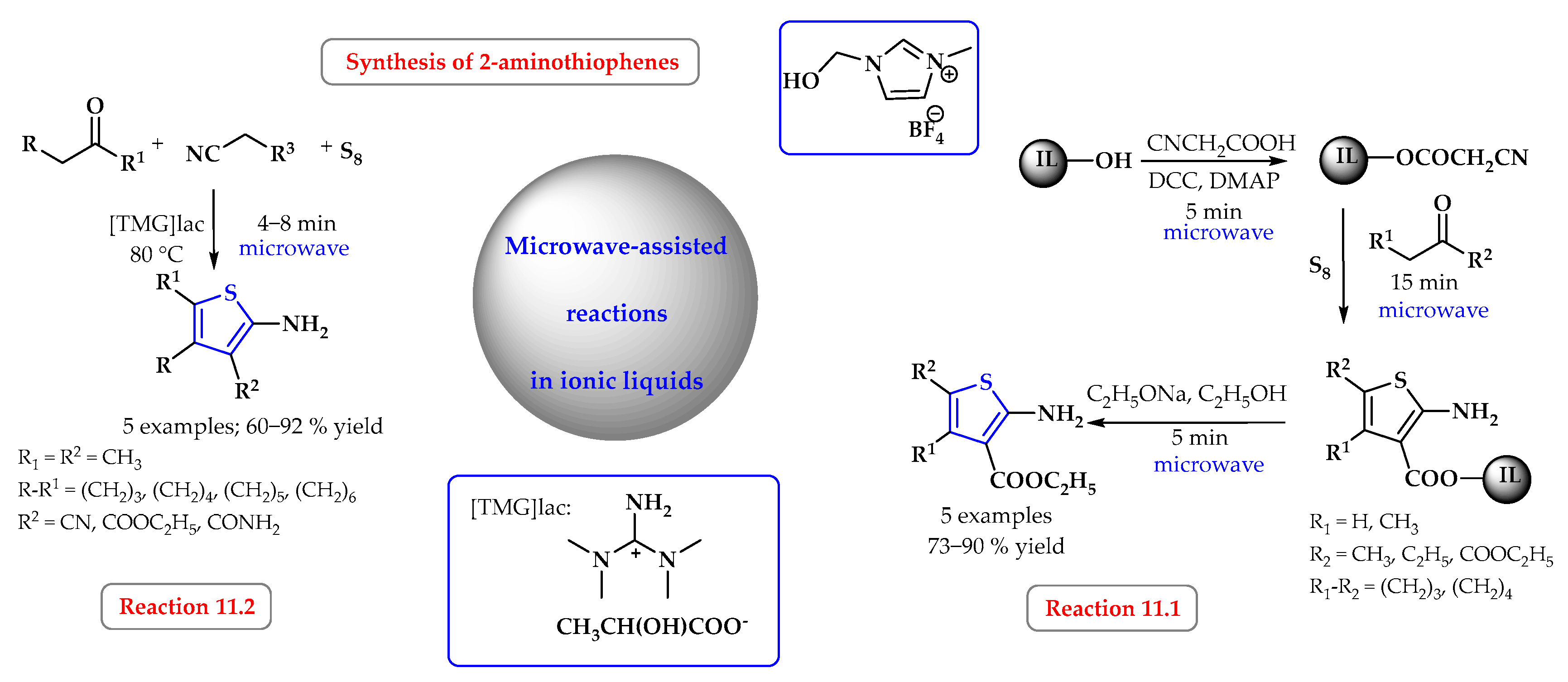
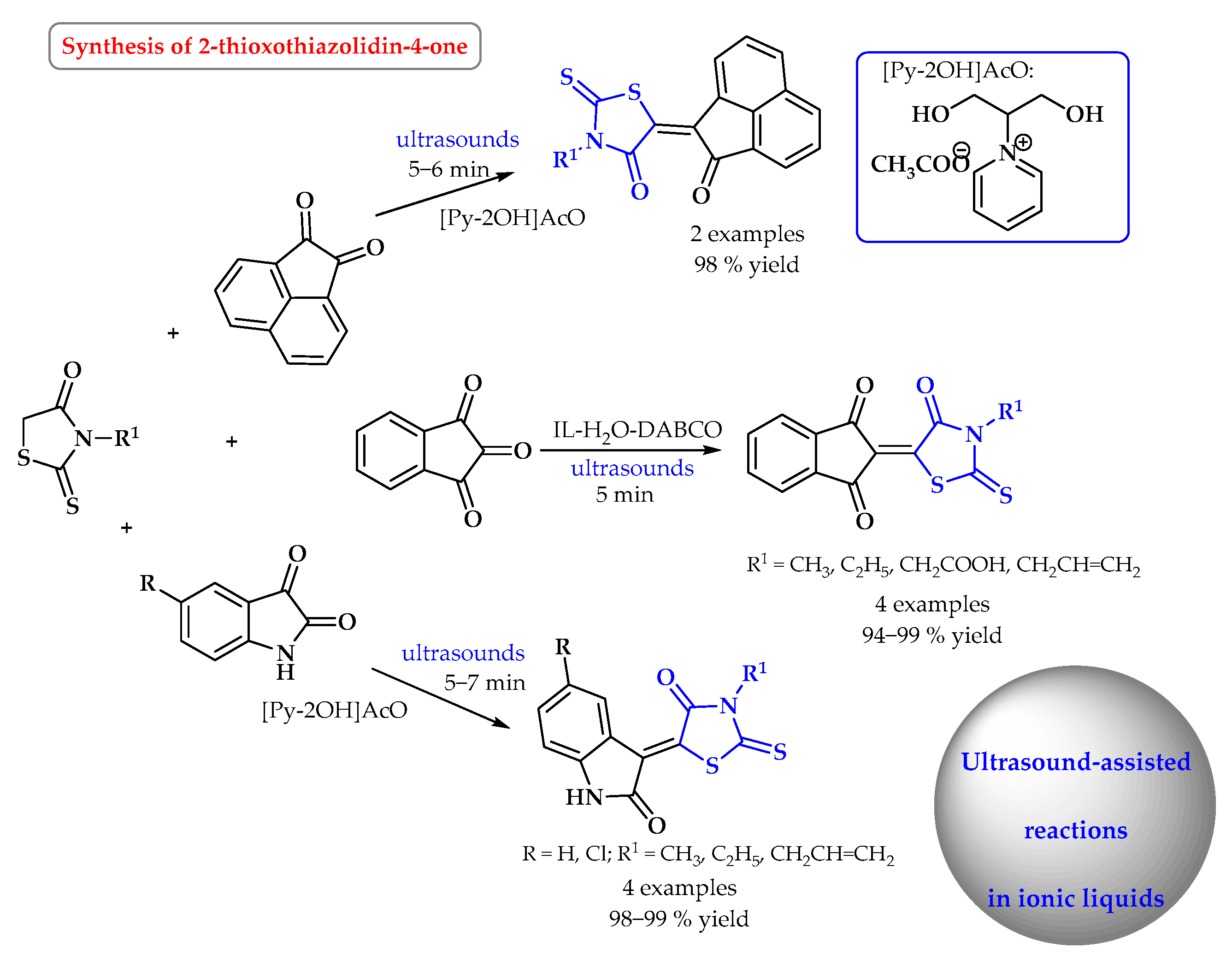
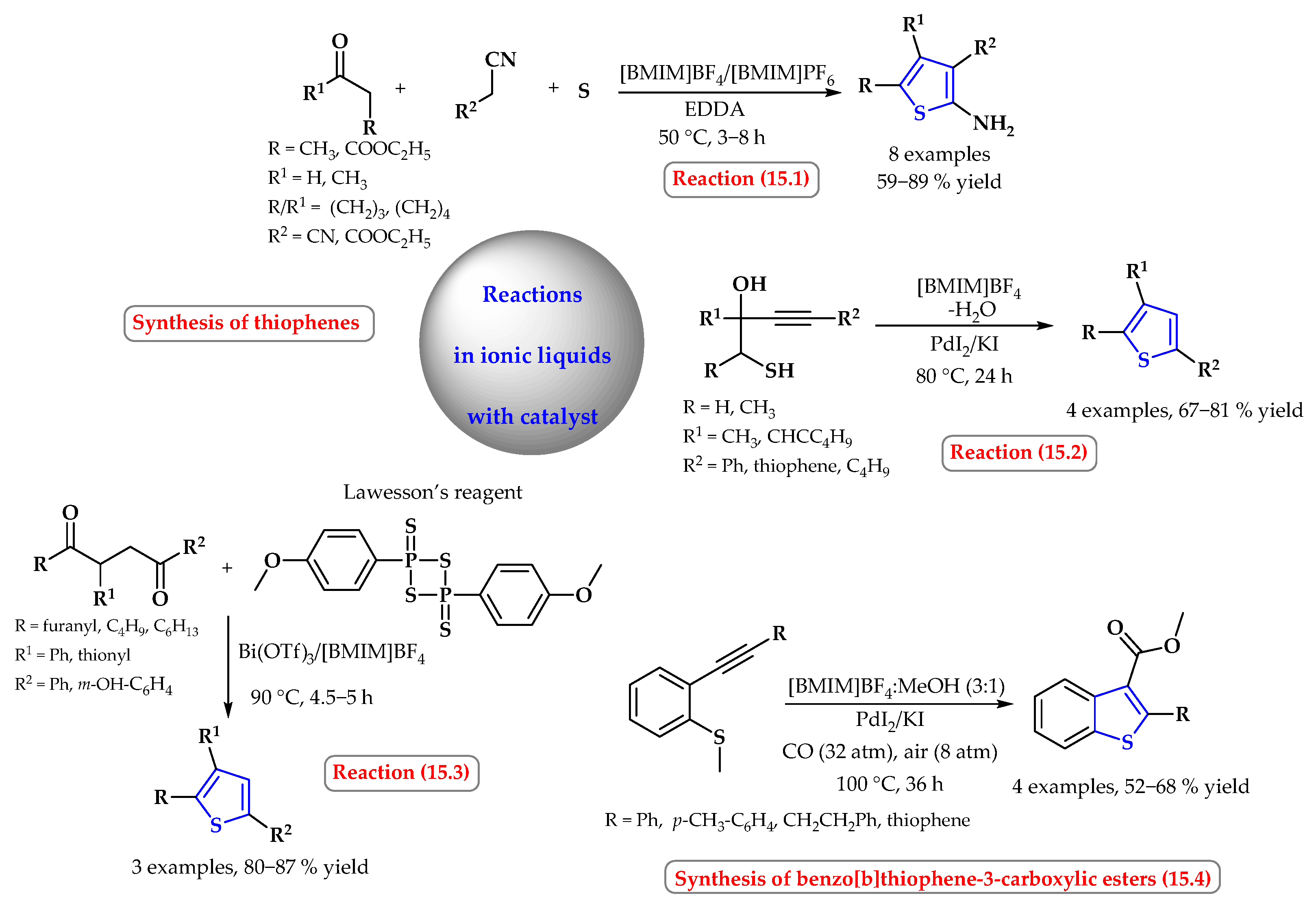
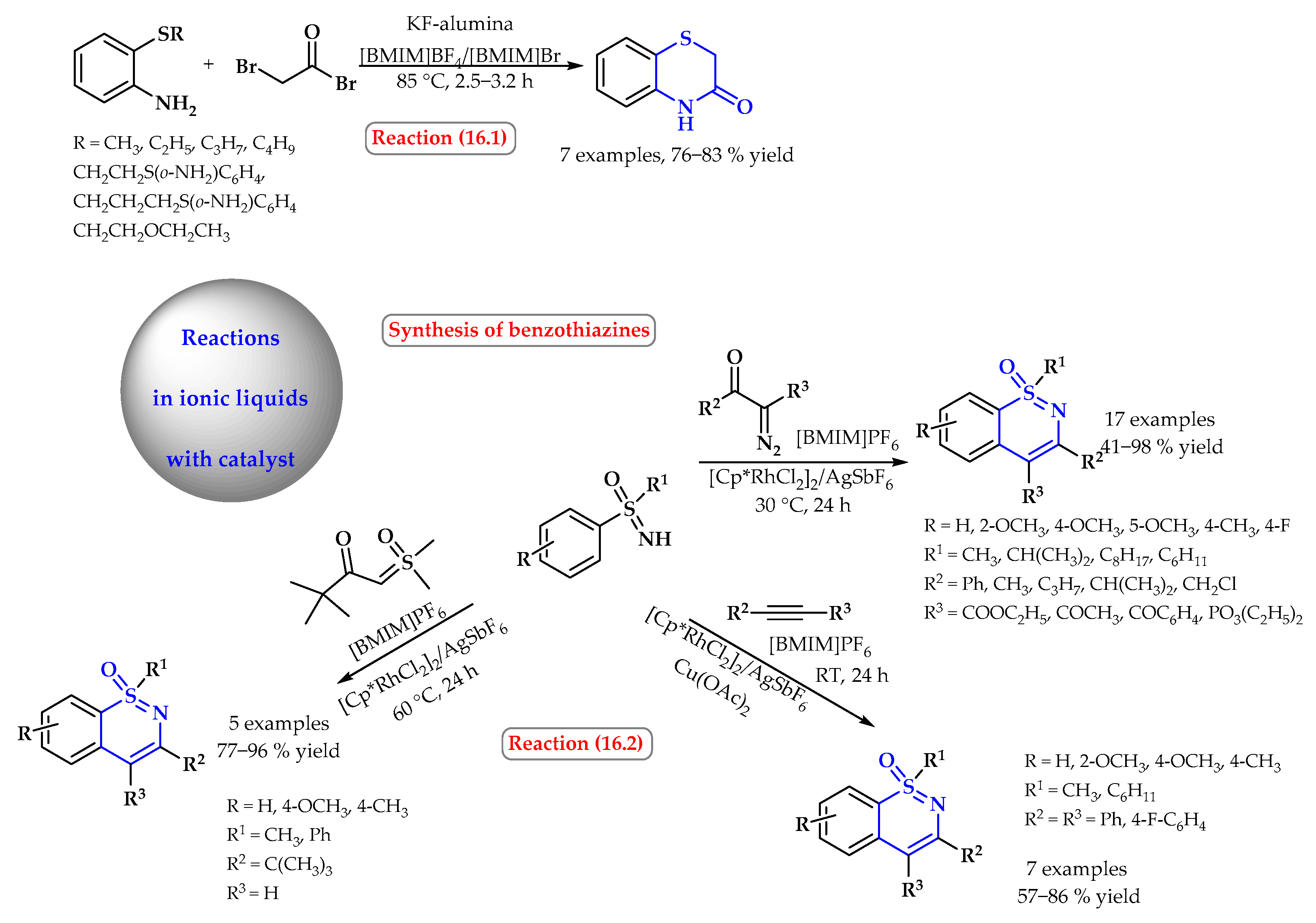
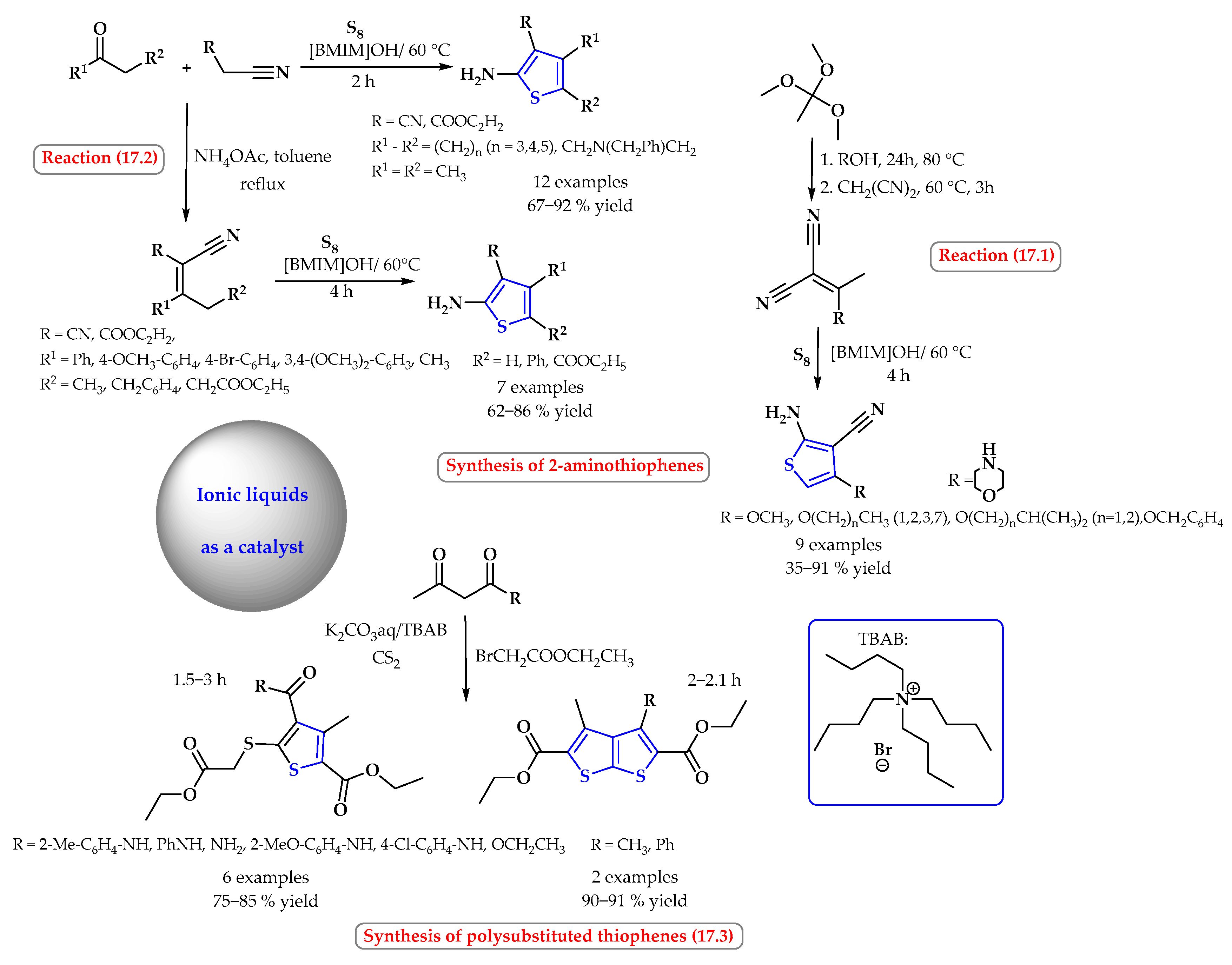
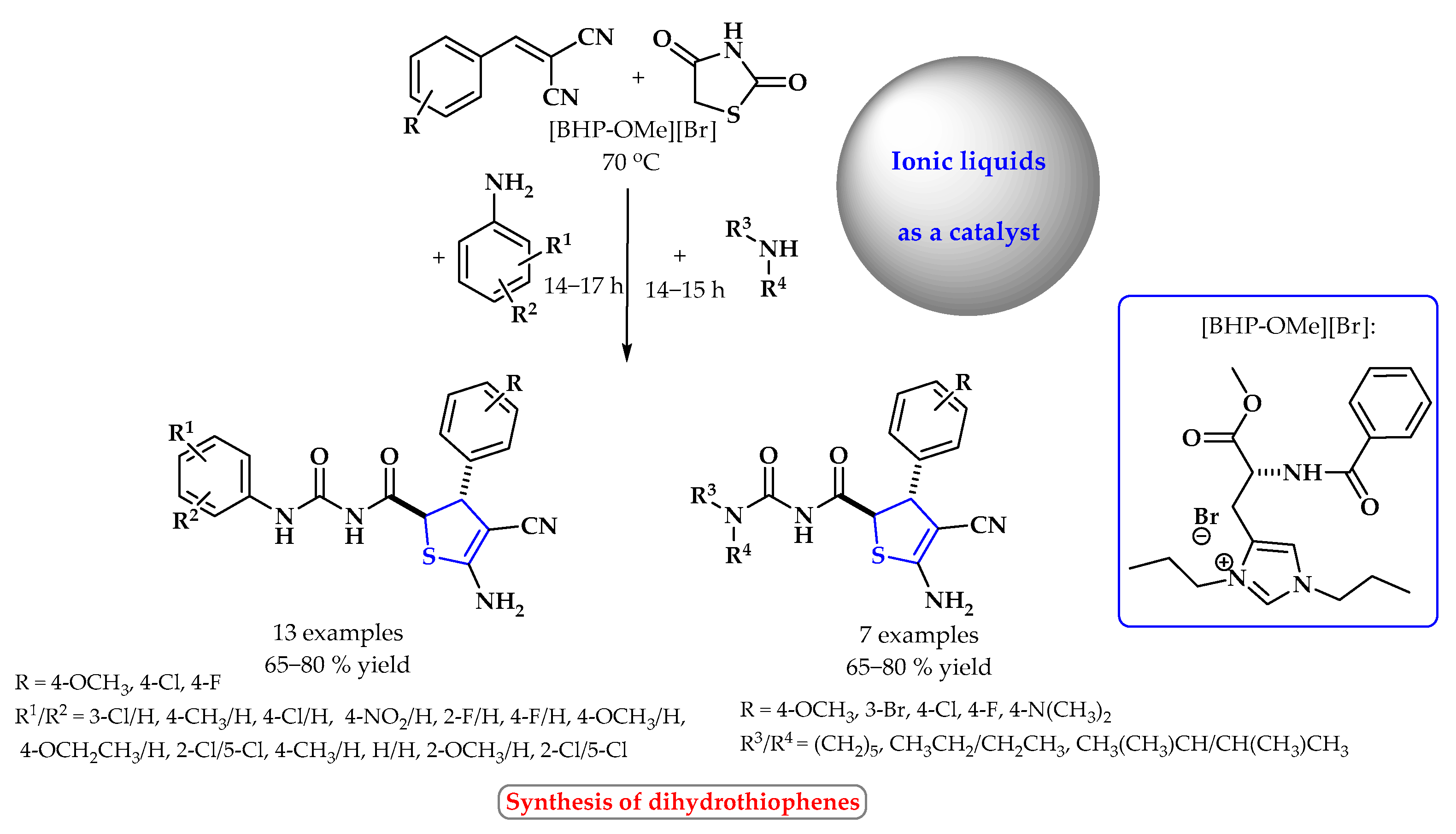
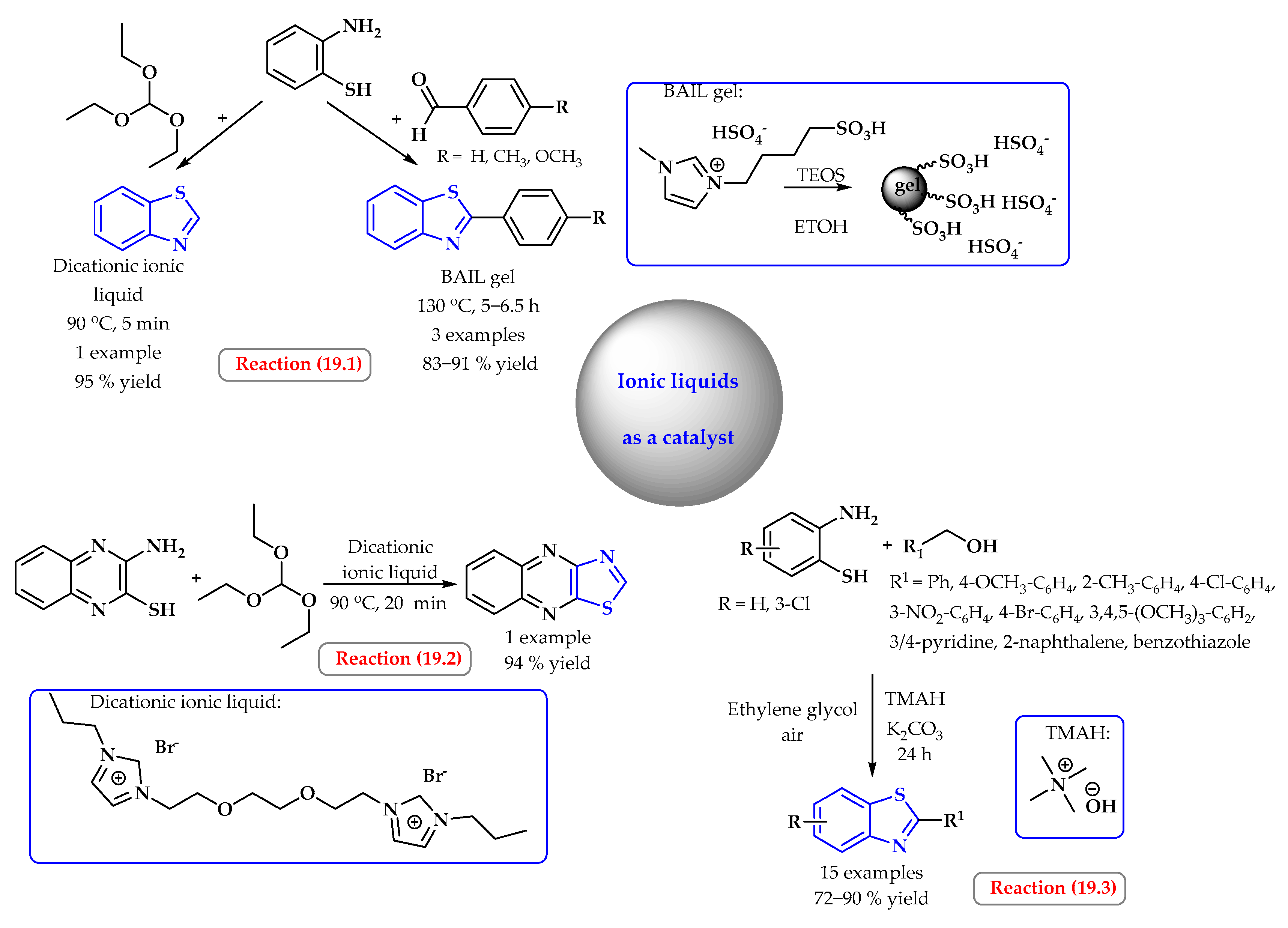
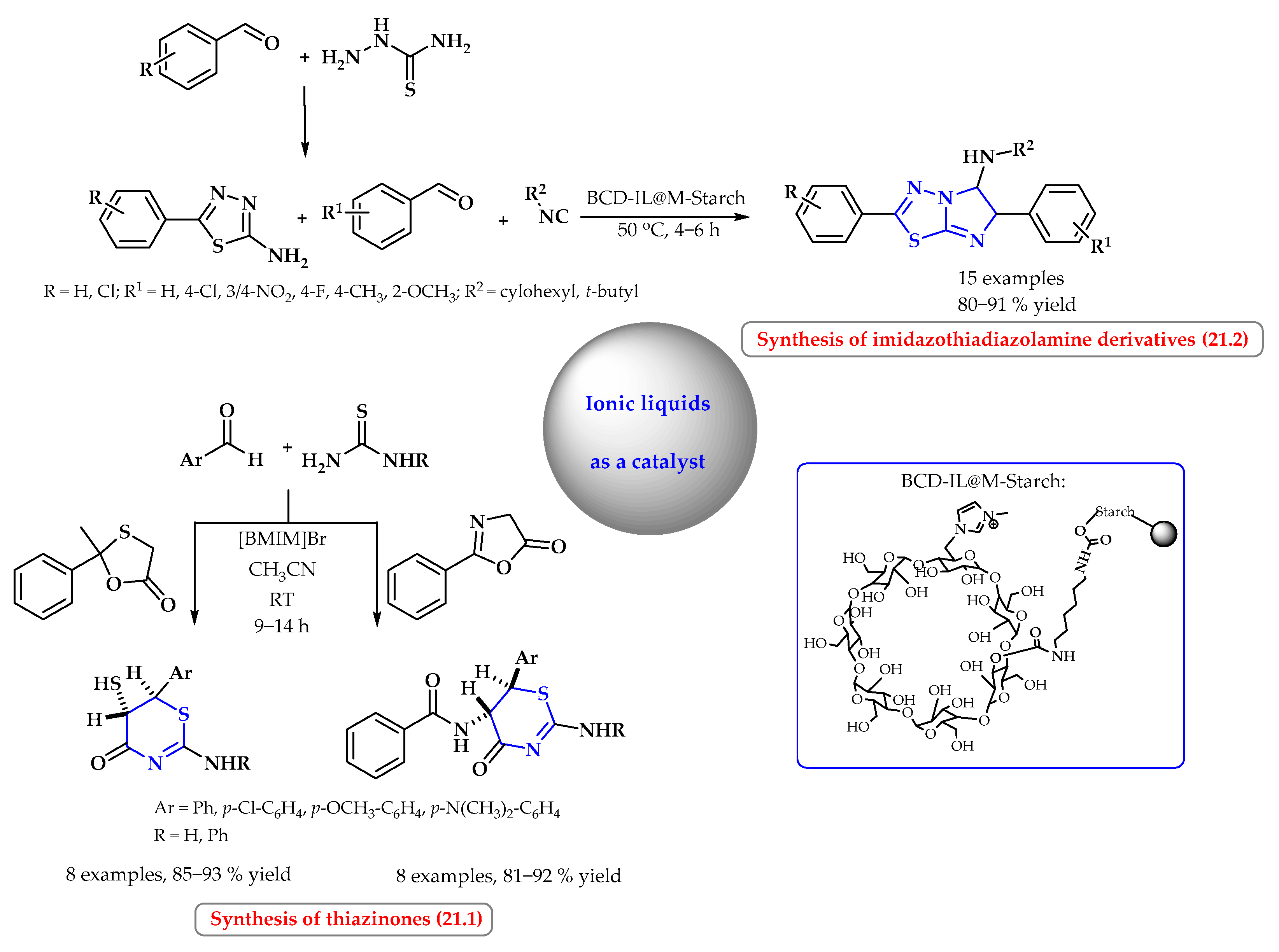
4. Synthesis of S-Heterocycles in Ionic Liquids as Green Solvents
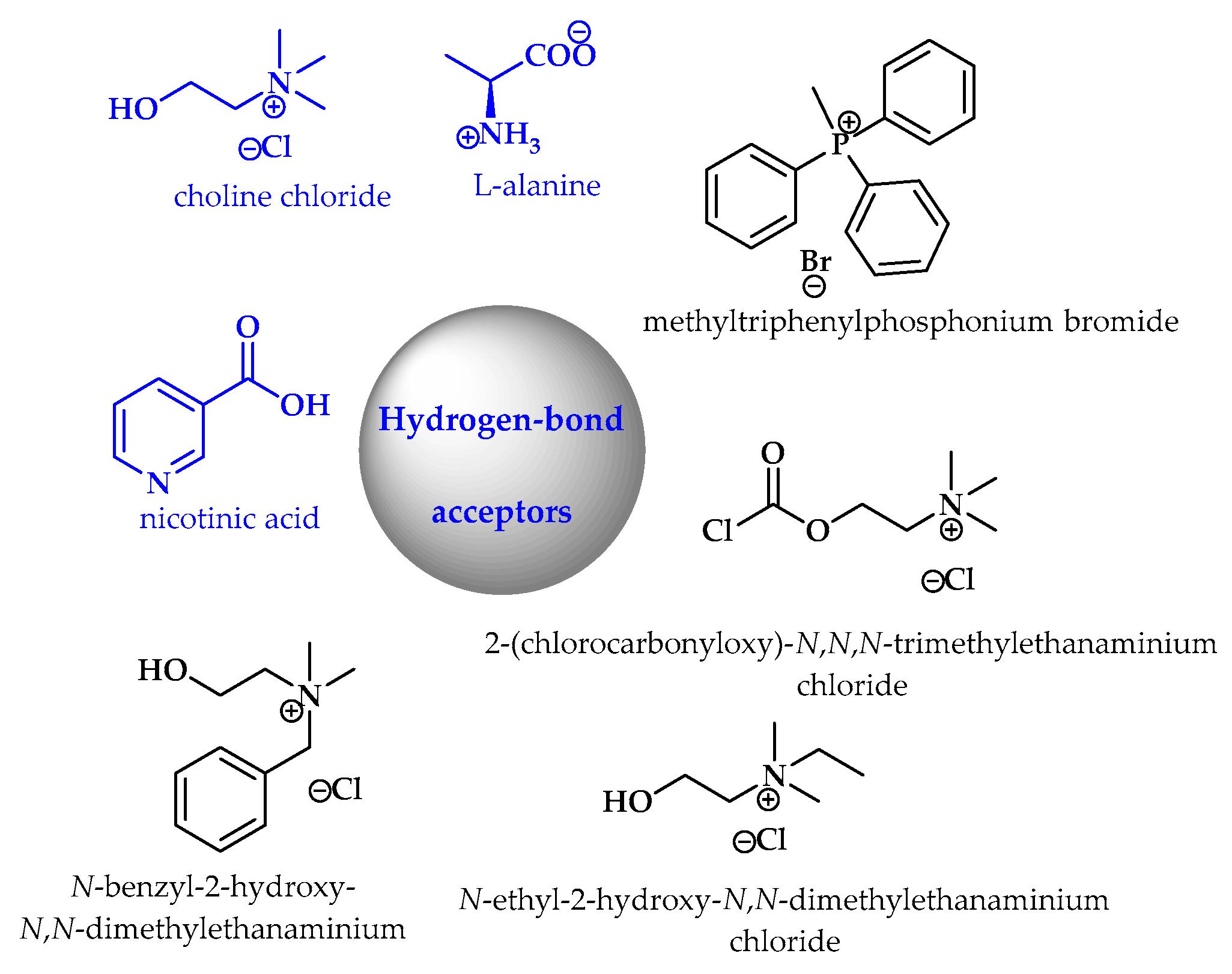
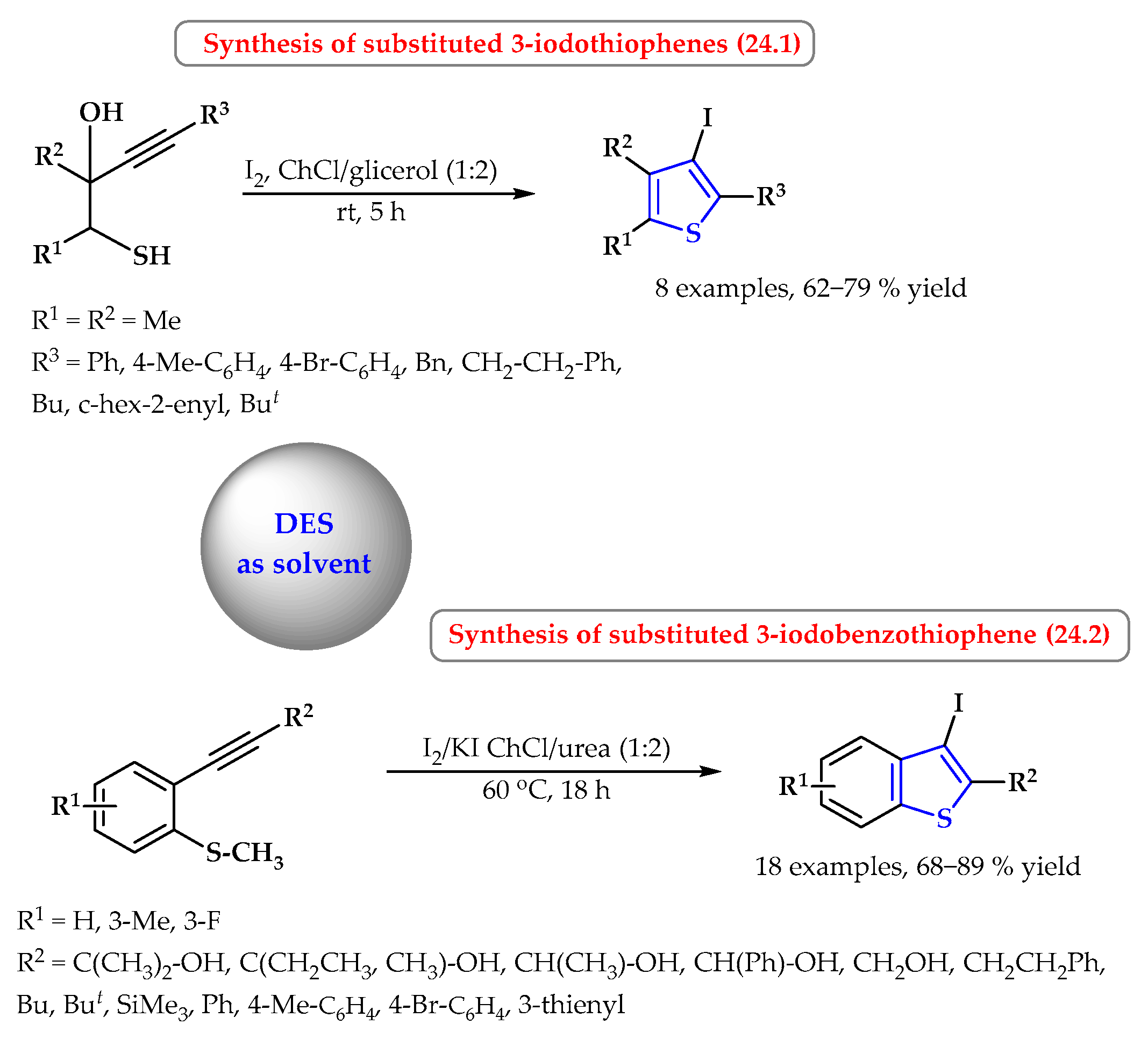
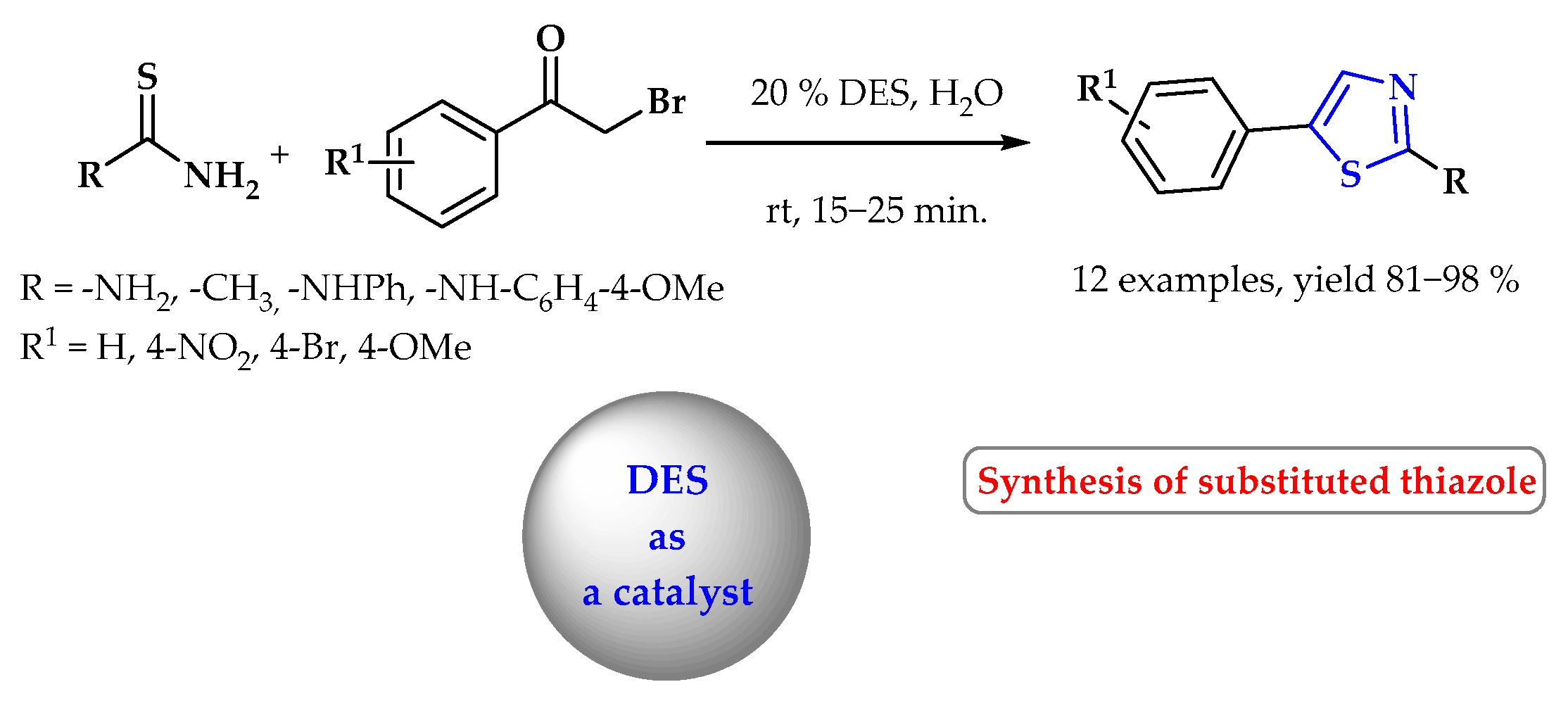
5. Synthesis of S-Heterocycles in Deep Eutectic Solvents as Green Solvents
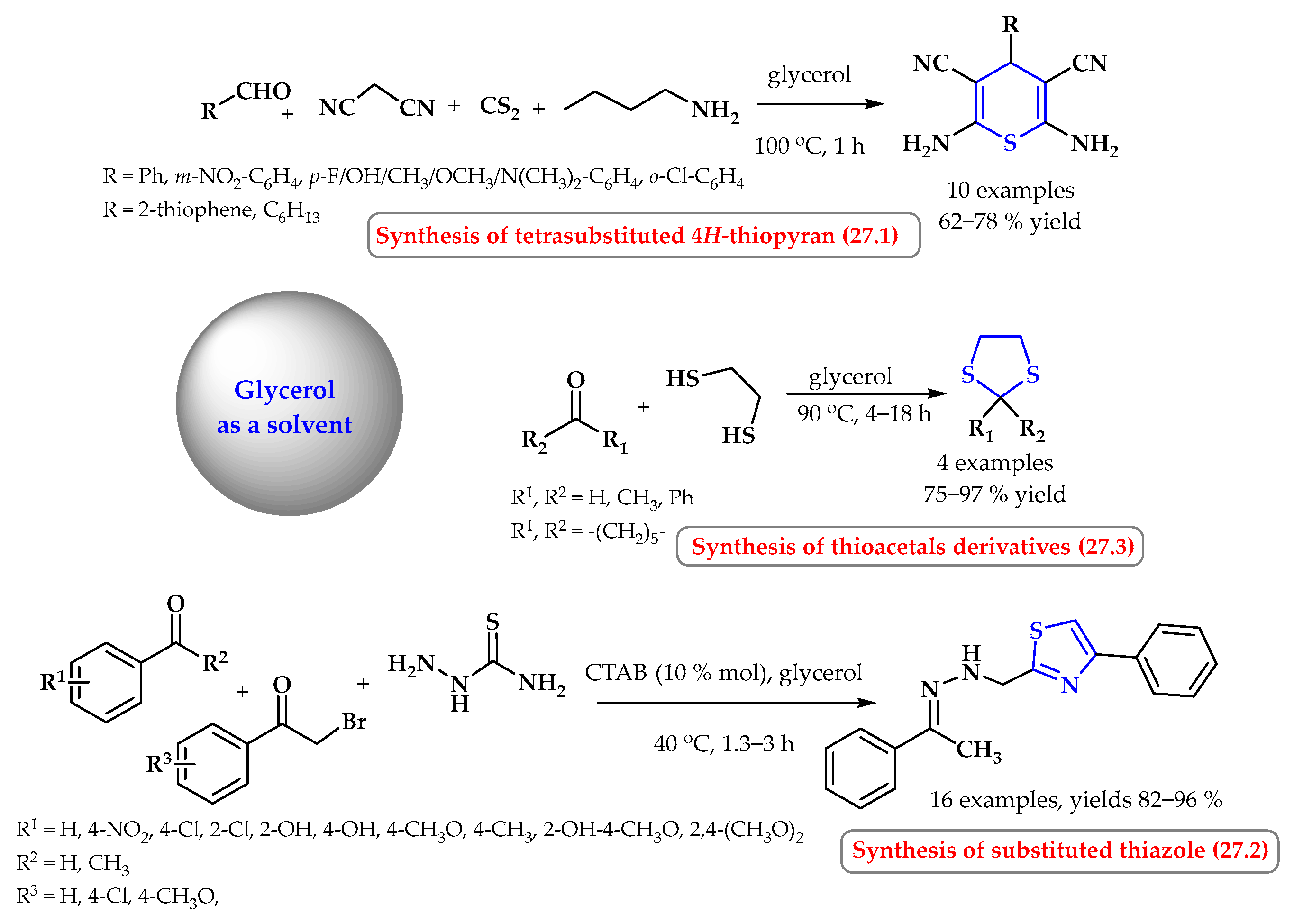
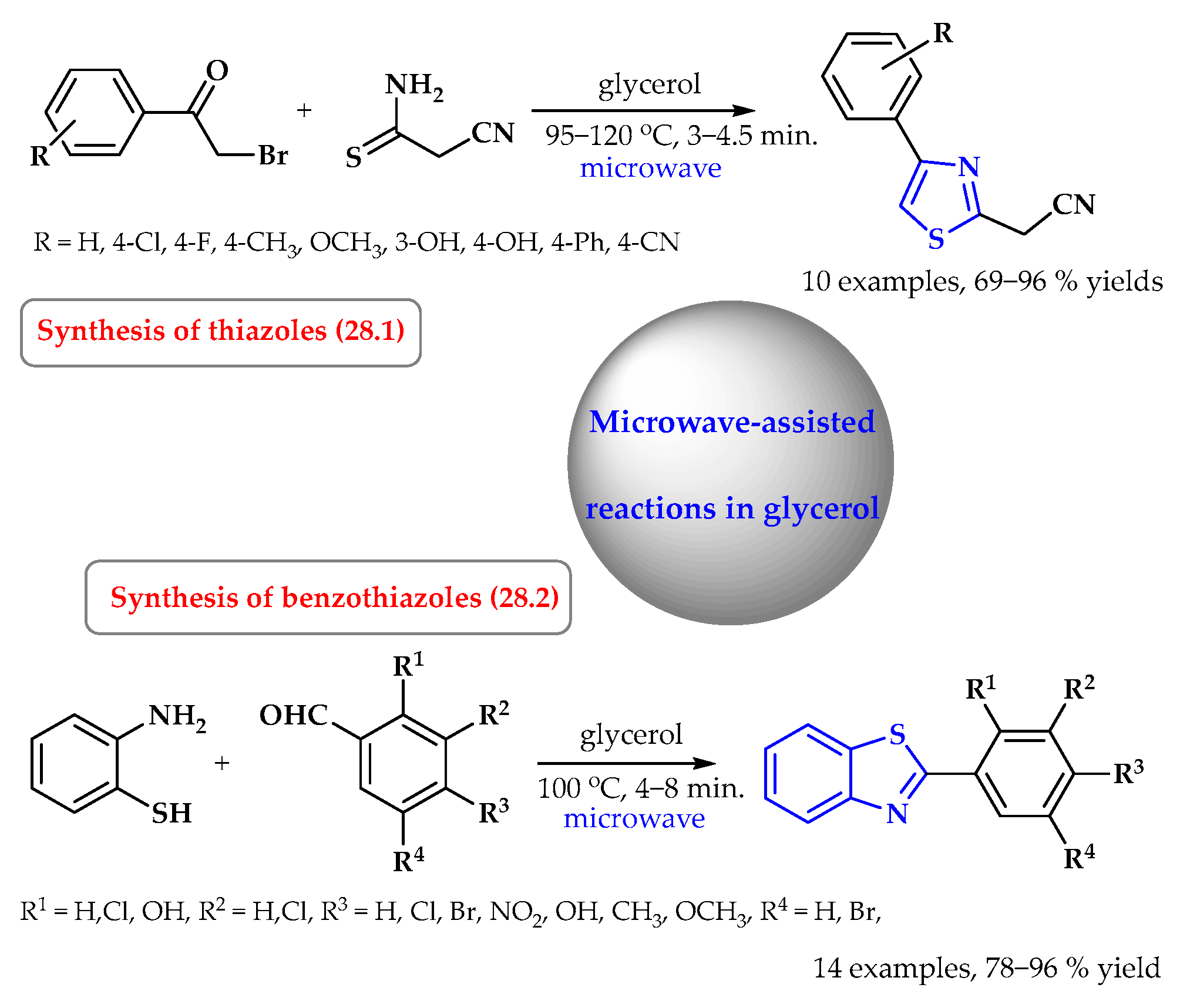
6. Synthesis of S-Heterocycles in Glycerol as a Green Solvent
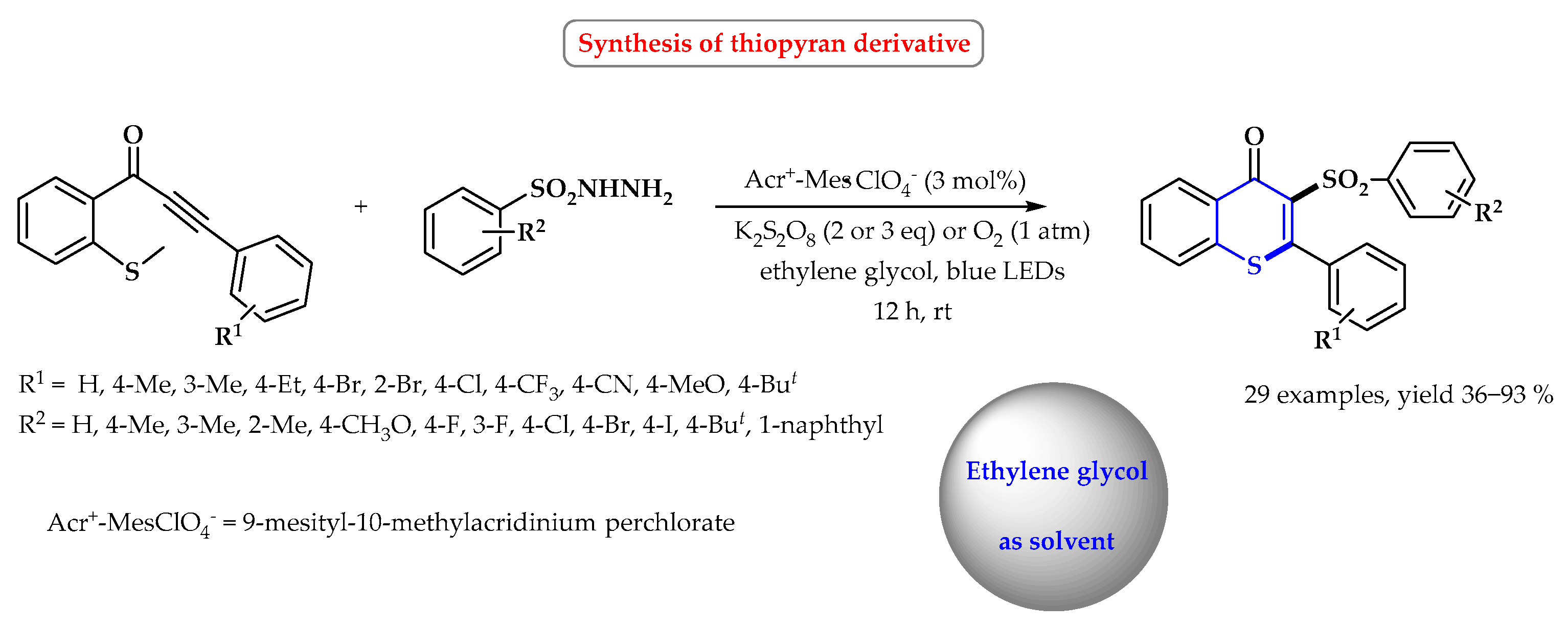
7. Synthesis of S-Heterocycles in Ethylene Glycol as a Green Solvent
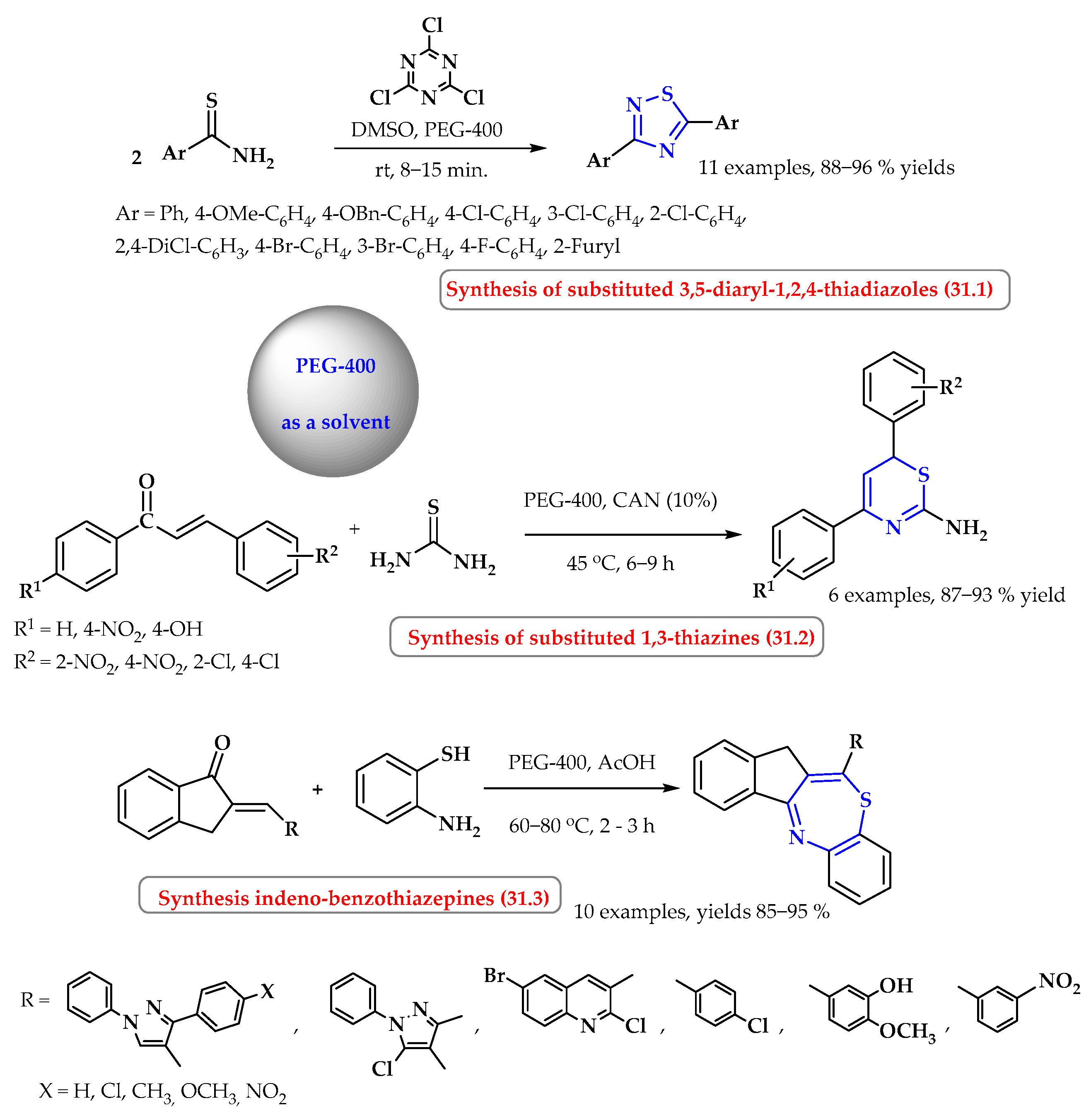
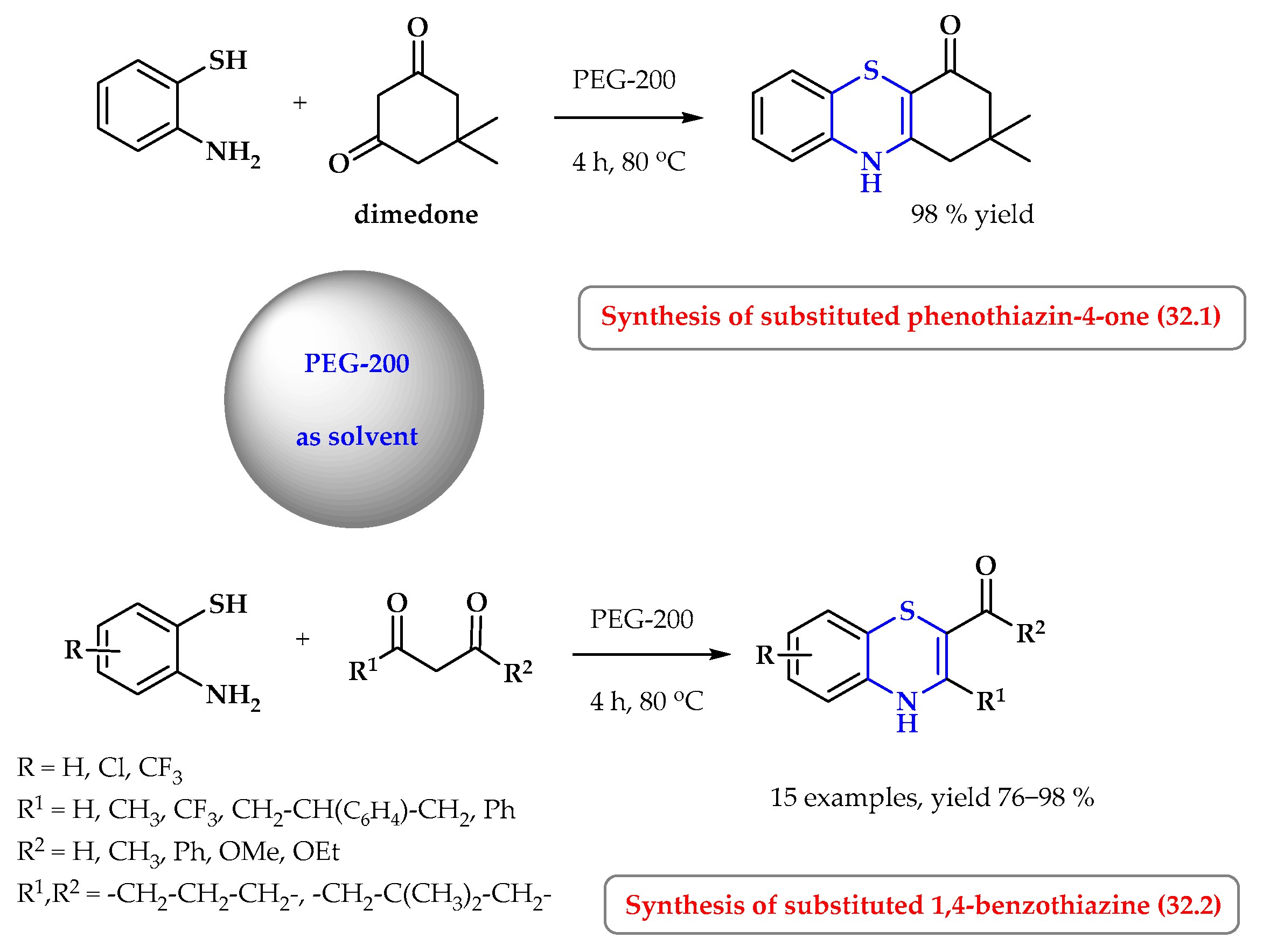
8. Synthesis of S-Heterocycles in Polyethylene Glycol (PEG) as a Green Solvent
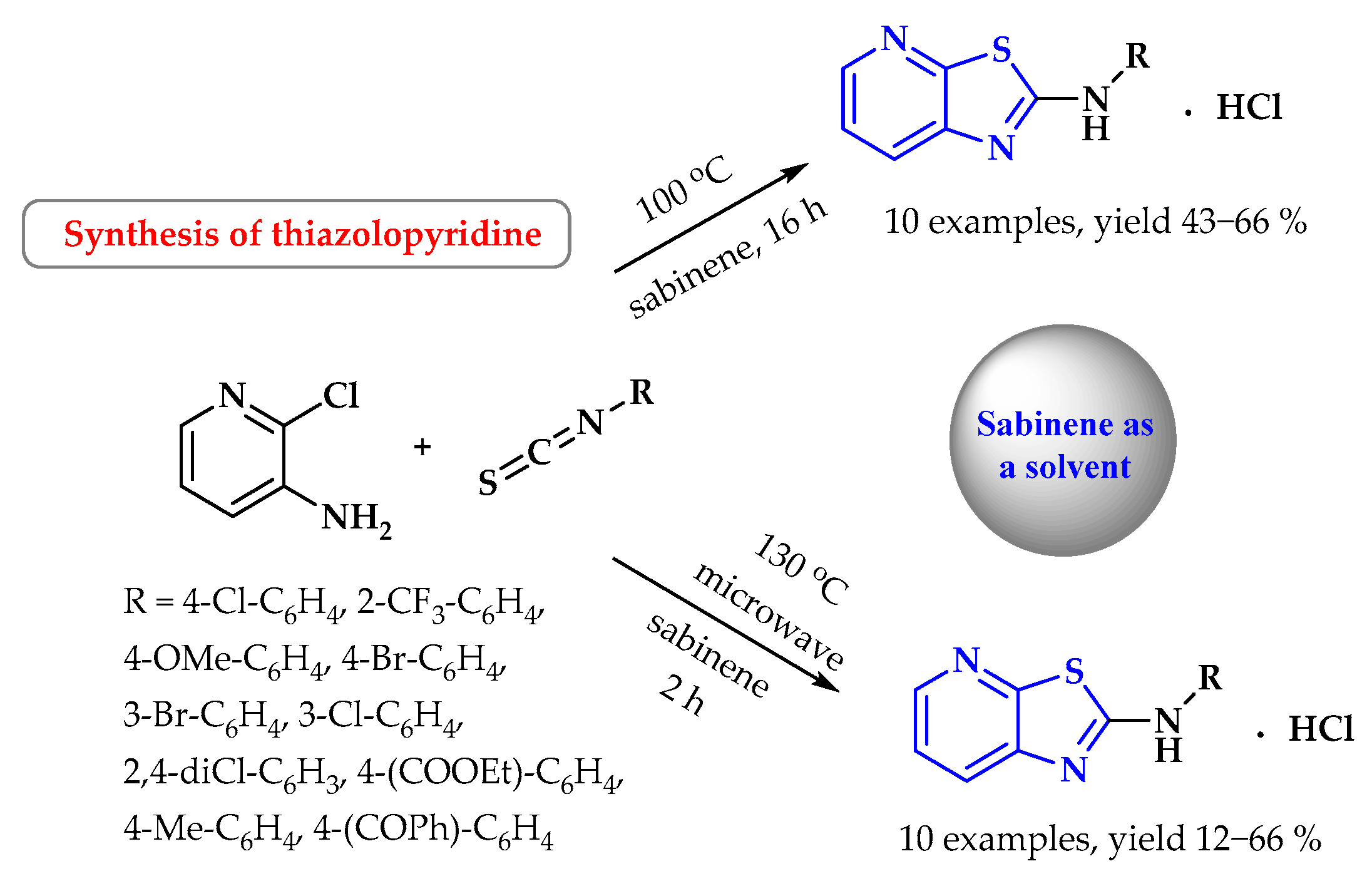
9. Synthesis of S-Heterocycles in Sabinene as a Green Solvent
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Katritzky, A.R.; Ramsden, C.A.; Scriven, E.F.V.; Taylor, R.J.K. Introduction. In Comprehensive Heterocyclic Chemistry III; Elsevier: Amsterdam, The Netherlands, 2008; pp. 21–29. [Google Scholar] [CrossRef]
- Lancaster, S.G.; Gonzalez, J.P. Dothiepin. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in depressive illness. Drugs 1989, 38, 123–147. [Google Scholar] [CrossRef] [PubMed]
- da Cruz, R.M.D.; Mendonça-Junior, F.J.B.; de Mélo, N.B.; Scotti, L.; de Araújo, R.S.A.; de Almeida, R.N.; de Moura, R.O. Thiophene-based compounds with potential anti-inflammatory activity. Pharmaceuticals 2021, 14, 692. [Google Scholar] [CrossRef]
- Al-Goul, S.T.; AlSalem, H.S.; Binkadem, M.S.; El Hamd, M.A.; Alsaggaf, W.T.; Saleh, S.F.; Sallam, S.; Abdel-Lateef, M.A. Synthesis of photoluminescence carbon dots from red beetroot and utilizing its extract as blue-emitted fluorescence probes for spectrofluorimetric determination of tenoxicam in varied pharmaceutical samples. J. Photochem. Photobiol. 2023, 445, 115028. [Google Scholar] [CrossRef]
- Aberg, A.K.G.; Arulnesan, N.; Bolger, G.T.; Ciofalo, V.B.; Pucaj, K.; Walle, K.; Walle, T. Ketotifen is a prodrug. norketotifen is the active metabolite. Drug Dev. Res. 2021, 83, 3623–3667. [Google Scholar] [CrossRef]
- Xu, R.; Huang, Y.; Lu, C.; Lv, W.; Hong, S.; Zeng, S.; Xia, W.; Guo, L.; Lu, H.; Chen, Y. Ticlopidine induces cardiotoxicity in zebrafish embryos through AHR-mediated oxidative stress signaling pathway. Ecotoxicol. Environ. Saf. 2022, 230, 11313. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Ren, Y.; Sha, J.; Cao, Z.; Ma, T.; Chong, Y.; Niu, H.; Li, Y.; Li, T.; Ren, B. Solubility, molecular simulation, Hansen solubility parameter and thermodynamic properties of citiolone in thirteen organic pure solvents at different temperatures. J. Chem. Thermodyn. 2022, 164, 106624. [Google Scholar] [CrossRef]
- Brogden, R.N.; Heel, R.C.; Speight, T.M.; Avery, G.S. Cefoxitin: A review of its antibacterial activity, pharmacological properties and therapeutic use. Drugs 1979, 17, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, A.; Dlugy, C.; Shotland, Y. Glycerol as a green solvent for high product yields and selectivities. Environ. Chem. Lett. 2007, 5, 67–71. [Google Scholar] [CrossRef]
- de Marco, B.A.; Rechelo, B.S.; Tótoli, E.G.; Kogawa, A.C.; Salgado, H.R.N. Evolution of green chemistry and its multidimensional impacts: A review. Saudi Pharm. J. 2019, 27, 1–8. [Google Scholar] [CrossRef]
- Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Sherwood, J. Opportunities for bio-based solvents created as petrochemical and fuel products transition towards renewable resources. Int. J. Mol. Sci. 2015, 16, 17101–17159. [Google Scholar] [CrossRef]
- Clarke, C.J.; Tu, W.C.; Levers, O.; Brohl, A.; Hallett, J.P. Green and sustainable solvents in chemical processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry, 4th ed.; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar] [CrossRef]
- Abou-Shehada, S.; Clark, J.H.; Paggiola, G.; Sherwood, J. Tunable solvents: Shades of green. Chem. Eng. Process. 2016, 99, 88–96. [Google Scholar] [CrossRef]
- Jimenez-Gonzalez, C.; Ponder, C.S.; Broxterman, Q.B.; Manley, J.B. Using the right green yardstick: Why process mass intensity is used in the pharmaceutical industry to drive more sustainable processes. Org. Process Res. Dev. 2011, 15, 912–917. [Google Scholar] [CrossRef]
- Ashcroft, C.P.; Dunn, P.J.; Hayler, J.D.; Wells, A.S. Survey of solvent usage in papers published in organic process research & development 1997–2012. Org. Process Res. Dev. 2015, 19, 740–747. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Nakashima, K.; Kamiyaa, N.; Goto, M. Recent advances of enzymatic reactions in ionic liquids. Biochem. Eng. J. 2010, 48, 295–314. [Google Scholar] [CrossRef]
- Menges, N. The role of green solvents and catalysts at the future of drug design and of synthesis. In Green Chemistry; Saleh, H.E.-D.M., Kolle, M., Eds.; IntechOpen: London, UK, 2018; Volume 5, pp. 73–100. [Google Scholar] [CrossRef]
- Narayan, S.; Muldoon, J.; Finn, M.G.; Fokin, V.V.; Kolb, H.C.; Sharpless, K.B. “On Water”: Unique reactivity of organic compounds in aqueous suspension. Angew. Chem. Int. Ed. 2005, 44, 3275–3279. [Google Scholar] [CrossRef]
- Li, C.J.; Trost, B.M. Green chemistry for chemical synthesis. Proc. Natl. Acad. Sci. USA 2008, 105, 13197–13202. [Google Scholar] [CrossRef]
- Butler, R.N.; Coyne, A.G. Organic synthesis reactions on-water at the organic–liquid water interface. Org. Biomol. Chem. 2016, 14, 9945–9960. [Google Scholar] [CrossRef]
- Weissler, A. Sonochemistry: The production of chemical changes with sound waves. J. Acoust. Soc. Am. 1953, 25, 651–657. [Google Scholar] [CrossRef]
- Abaee, M.S.; Cheraghi, S. Efficient three-component Gewald reactions under Et3N/H2O conditions. J. Sulfur Chem. 2014, 35, 261–269. [Google Scholar] [CrossRef]
- Aryanasab, F.; Halimehjani, A.Z.; Saidi, M.R. Dithiocarbamate as an efficient intermediate for the synthesis of 2-amino-1,3,4-thiadiazoles in water. Tetrahedron Lett. 2010, 51, 790–792. [Google Scholar] [CrossRef]
- Zhang, S.B.; Liu, X.; Gao, M.Y.; Dong, Z.B. One-pot synthesis of 2-benzyl/2-allyl-substituted thiobenzoazoles using transition-metal-free conditions in water. J Org. Chem. 2018, 83, 14933–14941. [Google Scholar] [CrossRef] [PubMed]
- Taterao, M.P.; Ingale, S.A.; Srinivasan, K.V. Catalyst–free efficient synthesis of 2-aminothiazoles in water at ambient temperature. Tetrahedron 2008, 64, 5019–5022. [Google Scholar] [CrossRef]
- Moghaddam, F.M.; Kiamehr, M.; Khodabakhshi, M.R.; Mirjafary, Z.; Fathi, S.; Saeidian, H.A. New domino Knoevenagel–hetero–Diels–Alder reaction: An efficient catalyst–free synthesis of novel thiochromone–annulated thiopyranocoumarin derivatives in aqueous medium. Tetrahedron 2010, 66, 8615–8622. [Google Scholar] [CrossRef]
- Li, P.; Du, J.; Xie, Y.; Tao, M.; Zhang, W.Q. Highly efficient polyacrylonitrile fiber catalysts functionalized by aminopyridines for the synthesis of 3-substituted 2-aminothiophenes in water. ACS Sustain. Chem. Eng. 2015, 4, 1139–1147. [Google Scholar] [CrossRef]
- Tizhoosh, N.Y.; Khataee, A.; Hassandoost, R.; Soltani, R.D.C.; Doustkhah, E. Ultrasound-engineered synthesis of WS2@CeO2 heterostructure for sonocatalytic degradation of tylosin. Ultrason. Sonochem. 2020, 67, 105114. [Google Scholar] [CrossRef] [PubMed]
- Rad, T.S.; Ansarian, Z.; Khataee, A.; Vahid, B.; Doustkhah, E. N-doped graphitic carbon as a nanoporous MOF-derived nanoarchitecture for the efficient sonocatalytic degradation process. Sep. Purif. Technol. 2021, 256, 117811. [Google Scholar] [CrossRef]
- Oskouia, S.A.; Khataee, A.; Safarpour, M.; Orooji, Y.; Vatanpoure, V. A review on the applications of ultrasonic technology in membrane bioreactors. Ultrason. Sonochem. 2019, 58, 104633. [Google Scholar] [CrossRef]
- Pagadala, P.; Kasi, V.; Shabalala, N.S.; Jonnalagadda, S.B. Ultrasound-assisted multicomponent synthesis of heterocycles in water—A review. Arab. J. Chem. 2022, 15, 103544. [Google Scholar] [CrossRef]
- Arya, K.; Rawat, D.S.; Sasai, H. Zeolite supported brønsted acid ionic liquids: An eco approach for synthesis of spiro[indolepyrido[3,2-e]thiazine] in water under ultrasonication. Green Chem. 2012, 14, 1956–1963. [Google Scholar] [CrossRef]
- Dandia, A.; Singh, R.; Joshi, J.; Maheshwari, S.; Soni, P. Ultrasound promoted catalyst-free and selective synthesis of spiro[indole-3,40-pyrazolo[3,4-e][1,4]thiazepines] in aqueous media and evaluation of their anti-hyperglycemic activity. RSC Adv. 2013, 3, 18992–19001. [Google Scholar] [CrossRef]
- Singh, R.; Ahmad Ganaie, S.; Singh, A. PEG-OSO 3 H catalyzed synthesis of spiro [acenaphthylene-thiazine] diones under sonica- tion in aqueous medium. Chem. Biol. Interface. 2017, 7, 286–292. [Google Scholar] [CrossRef]
- Zhang, D.N.; Li, J.T.; Song, Y.L.; Liu, H.M.; Li, H.Y. Efficient one-pot three-component synthesis of N-(4-arylthiazol-2-yl) hydrazones in water under ultrasound irradiation. Ultrason. Sonochem. 2012, 19, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Shaabani, A.; Hooshmand, S.E. Diversity-oriented catalyst free synthesis of pseudopeptides containing rhodanine scaffolds via a one-pot sequential isocyanide-based six-component reactions in water using ultrasound irradiation. Ultrason. Sonochem. 2018, 40, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Lei, D.; Wang, X.; Zhang, Q.; Yao, Q. First Gewald reaction ignited by sodium polysulfde: Greener ultrasound-promoted synthesis of substituted 2-aminothiophenes in the absence of catalyst. J. Sulfur Chem. 2013, 34, 458–463. [Google Scholar] [CrossRef]
- Mukku, N.; Maiti, B. On water catalyst-free synthesis of benzo[d]imidazo[2,1-b] thiazoles and novel N-alkylated 2-aminobenzo[d]oxazoles under microwave irradiation. RSC Adv. 2020, 10, 770–778. [Google Scholar] [CrossRef]
- Fan, L.; Li, H.; Chen, Q. Applications and mechanisms of ionic liquids in whole-cell biotransformation. Int. J. Mol. Sci. 2014, 15, 12196–12216. [Google Scholar] [CrossRef]
- Quijano, G.; Couvert, A.; Amrane, A. Ionic liquids: Applications and future trends in bioreactor technology. Bioresour. Technol. 2010, 101, 8923–8930. [Google Scholar] [CrossRef]
- Bourbigou, O.H.; Magna, L.; Morvan, D. Ionic liquids and catalysis: Recent progress from knowledge to applications. App. Catal. A-Gen. 2010, 373, 1–56. [Google Scholar] [CrossRef]
- Wilkes, J. Properties of ionic liquid solvents for catalysis. J. Mol. Catal. A Chem. 2004, 214, 11–17. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, N.; He, X.; Lu, X.; Zhang, X. Physical properties of ionic liquids: Database and evaluation. J. Phys. Chem. Ref. Data 2006, 35, 1475–1517. [Google Scholar] [CrossRef]
- Zhao, H. Review: Current studies on some physical properties of ionic liquids. Phys. Chem. Liq. 2003, 41, 545–557. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Srinivas, C.; Rajasekhar, K. [Bmim]PF6: A novel and recyclable ionic liquid for conversion of oxiranes to thiiranes in aqueous media. J. Org. Chem. 2003, 68, 2525–2527. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.S.; Wang, H.M.; Tsai, H.H.; Chen, L.C.J. Synthesis of 2-phenylthiazoles from α-tosyloxyketones and thiobenzamide in [Bmim][PF6] ionic liquid at ambient temperature. Chin. Chem. Soc. 2006, 53, 863. [Google Scholar] [CrossRef]
- Izumisawa, Y.; Togo, H. Preparation of α-bromoketones and thiazoles from ketones with NBS and thioamides in ionic liquids. Green Sustain. Chem. 2011, 1, 54–62. [Google Scholar] [CrossRef]
- Potewar, T.M.; Ingale, S.A.; Srinivasan, K.V. Efficient synthesis of 2,4-disubstituted thiazoles using ionic liquid under ambient conditions: A practical approach towards the synthesis of fanetizole. Tetrahedron 2007, 63, 11066–11069. [Google Scholar] [CrossRef]
- Nadaf, R.N.; Siddiqui, S.A.; Daniel, T.; Lahoti, R.J.; Srinivasan, K.V. Room temperature ionic liquid promoted regioselective synthesis of 2-aryl benzimidazoles, benzoxazoles and benzthiazoles under ambient conditions. J. Mol. Catal. A Chem. 2004, 214, 155–160. [Google Scholar] [CrossRef]
- Maradolla, M.B.; Allam, S.K.; Mandha, A.; Chandramouli, G.V.P. One pot synthesis of benzoxazoles, benzthiazoles and benzimidazoles from carboxylic acids using ionic liquids. Arkivoc 2008, 15, 42–46. [Google Scholar] [CrossRef]
- Pal, S.; Chatterjee, R.; Santra, S.; Zyryanov, G.V.; Majee, A. Ionic liquid catalyzed simple and green synthesis of benzothiazole under neat condition. AIP Conf. Proc. 2020, 2280, 050037. [Google Scholar] [CrossRef]
- Gao, X.; Yu, B.; Yang, Z.; Zhao, Y.; Zhang, H.; Hao, L.; Han, B.; Liu, Z. Ionic liquid-catalyzed C-S bond construction using CO2 as a C1 building block under mild conditions: A metal-free route to synthesis of benzothiazoles. ACS Catal. 2015, 5, 6648–6652. [Google Scholar] [CrossRef]
- Yadav, A.K.; Kumar, M.; Yadav, T.; Jain, R. An ionic liquid mediated one-pot synthesis of substituted thiazolidinones and benzimidazoles. Tetrahedron Lett. 2009, 50, 5031–5034. [Google Scholar] [CrossRef]
- Lingampalle, D.; Jawale, D.; Waghmare, R.; Mane, R. Ionic liquid–mediated, one-pot synthesis for 4-thiazolidinones. Synth. Commun. 2010, 40, 2397–2401. [Google Scholar] [CrossRef]
- Kishi, Y.; Nagura, H.; Inagi, S.; Fuchigami, T. Facile and highly efficient synthesis of fluorinated heterocycles via Prins cyclization in ionic liquid hydrogen fluoride salts. Chem. Commun. 2008, 33, 3876–3878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.; Fan, X.; Wang, X.; Li, D.; Qu, G.; Wang, J. Ionic liquid promoted preparation of 4H-thiopyran and pyrimidine nucleoside-thiopyran hybrids through one-pot multi-component reaction of thioamide. Mol. Divers. 2009, 13, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.R.; Shamim, S.; Abumhdi, A.A.; Waseem, M.A.; Srivastava, A.; Srivastava, A. Ionic liquid promoted spiroannulation via hetero-Michael addition and intramolecular heterocyclisation. New J. Chem. 2013, 37, 1258–1263. [Google Scholar] [CrossRef]
- Hu, Y.; Wei, P.; Huang, H.; Han, S.Q.; Ouyang, P.K. Microwave-assisted Gewald synthesis of 2-aminothiophenes using functional ionic liquid as soluble support. Heterocycles 2006, 68, 375–380. [Google Scholar] [CrossRef]
- Chavan, S.S.; Pedgaonkar, Y.Y.; Jadhav, A.J.; Degani, M.S. Microwave accelerated synthesis of 2-aminothiophenes in ionic liquid via three component Gewald reaction. Indian J. Chem. 2012, 43, 657. [Google Scholar] [CrossRef]
- Noei, J.; Khosropour, A.R. Ultrasound-promoted a green protocol for the synthesis of 2,4-diarylthiazoles under ambient temperature in [bmim]BF4. Ultrason. Sonochem. 2009, 16, 711–717. [Google Scholar] [CrossRef]
- Aridoss, G.; Laali, K.K. Building heterocyclic systems with RC(OR)2 þ carbocations in recyclable Brønsted acidic ionic liquids: Facile synthesis of 1-substituted 1H-1,2,3,4-tetrazoles, benzazoles and other ring systems with CH(OEt)3 and EtC(OEt)3 in [EtNH3][NO3] and [PMIM(SO3H)][OTf]. Eur. J. Org. Chem. 2011, 15, 2827–2835. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Nguyen, T.H.; Pham, D.D.; Nguyen, C.T.; Tran, P.H. A green approach for the synthesis of 2-substituted benzoxazoles and benzothiazoles via coupling/cyclization reactions. Heliyon 2021, 7, e08309. [Google Scholar] [CrossRef]
- Arafa, W.A.A.; Mourad, A.K. New dicationic DABCO-based ionic liquids: A scalable metal-free one-pot synthesis of bis-2-amino-5-arylidenethiazol-4-ones. R. Soc. Open. Sci. 2019, 6, 190997. [Google Scholar] [CrossRef] [PubMed]
- Nayl, A.A.; Arafa, W.A.A.; Ahmed, I.M.; Abd-Elhamid, A.I.; El-Fakharany, E.M.; Abdelgawad, M.A.; Gomha, S.M.; Ibrahim, H.M.; Aly, A.A.; Bräse, S.; et al. Novel pyridinium based ionic liquid promoter for aqueous Knoevenagel condensation: Green and efficient synthesis of new derivatives with their anticancer evaluation. Molecules 2022, 27, 2940. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, S.; Wu, W.; Jiang, H. Recent advances in Pd-catalyzed cross-coupling reaction in ionic liquids. Eur. J. Org. Chem. 2018, 11, 1248–1306. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, Z.C.; Le, Z.G.; Zheng, Q.G. Organic reactions in ionic liquids: Gewald synthesis of 2-aminothiophenes catalyzed by ethylenediammonium diacetate. Synth. Commun. 2004, 34, 3801–3806. [Google Scholar] [CrossRef]
- Gabriele, B.; Mancuso, R.; Veltri, L.; Maltese, V.; Salerno, G. Synthesis of substituted thiophenes by palladium-catalyzed heterocyclodehydration of 1-mercapto-3-yn-2-ols in conventional and nonconventional solvents. J. Org. Chem. 2012, 77, 9905–9909. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Eeshwaraiah, B.; Gupta, M.K. Bi(OTf)3/[Bmim]BF4 as novel and reusable catalytic system for the synthesis of furan, pyrrole and thiophene derivatives. Tetrahedron Lett. 2004, 45, 5873–5876. [Google Scholar] [CrossRef]
- Mancuso, R.; Cuglietta, S.; Strangis, R.; Gabriele, B. Synthesis of benzothiophene-3-carboxylic esters by palladium iodide-catalyzed oxidative cyclization−deprotection−alkoxycarbonylation sequence under aerobic conditions. J. Org. Chem. 2023, 88, 5180–5186. [Google Scholar] [CrossRef]
- Chhikara, B.S.; Mishra, A.K.; Tandon, V.K. F-alumina immobilized in ionic liquids: A novel heterogeneous base for heterocyclization of alkylsulfanylphenylamines into 1,4-benzothiazine. Heterocycles 2004, 63, 1057–1066. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Y.; Lv, G.; Nie, R.; Peng, Y.; Zhang, C.; Lv, S.; Hai, L.; Wang, H.; Wu, Y. Synthesis of 1,2-benzothiazines via C-H activation/cyclization in a recyclable, mild system. ACS Sustainable Chem. Eng. 2019, 326, 13–139. [Google Scholar] [CrossRef]
- Kaki, V.R.; Akkinepalli, R.R.; Deb, P.K.; Pichika, M.R. Basic ionic liquid [bmIm]OH mediated Gewald reaction as green protocol for the synthesis of 2-aminothiophenes. Synth Commun. 2015, 45, 119–126. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, D.; Yang, Y.; Huang, J.; Ouyang, Y.; Liu, Q. A facile and convenient one-pot synthesis of polysubstituted thiophenes from 1,3-dicarbonyl compounds in water. Tetrahedron 2007, 63, 2724–2728. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, G.; Srivastava, S. Functional ionic liquid mediated synthesis (FILMS) of dihydrothiophenes and tacrine derivatives. Green Chem. 2011, 13, 2459–2463. [Google Scholar] [CrossRef]
- Nguyen, T.; Nguyen, X.T.T.; Nguyen, T.L.H.; Tran, P.H. Synthesis of benzoxazoles, benzimidazoles, and benzothiazoles using a Brønsted acidic ionic liquid gel as an efficient heterogeneous catalyst under a aolvent-free condition. ACS Omega 2019, 4, 368–373. [Google Scholar] [CrossRef]
- Hasanpour, M.; Eshghi, H.; Bakavoli, M.; Mirzaei, M. A novel ionic liquid based on imidazolium cation as an efficient and reusable catalyst for the one-pot synthesis of benzoxazoles, benzthiazoles, benzimidazoles and 2-arylsubstituted benzimidazoles. J. Chin. Chem. Soc. 2015, 62, 412–419. [Google Scholar] [CrossRef]
- Badhani, G.; Joshi, A.; Adimurthy, S. Ionic-liquid-catalyzed synthesis of imines, benzimidazoles, benzothiazoles, quinoxalines and quinolines through C−N, C−S, and C−C bond formation. Eur. J. Org. 2021, 2021, 6705–6716. [Google Scholar] [CrossRef]
- Deshmukh, A.R.; Dhumal, S.T.; Nawale, L.U.; Khedkar, V.M.; Sarkar, D.; Mane, R.A. Dicationic liquid mediated synthesis of tetrazoloquinolinyl methoxy phenyl 4-thiazolidinones and their antibacterial and antitubercular evaluation. Synth. Commun. 2019, 69, 587–601. [Google Scholar] [CrossRef]
- Mirakmahaleh, M.S.; Rad-Moghadam, K.; Kefayati, H.; Falakro, S. Expedient synthesis of novel antibacterial hydrazono-4-thiazolidinones under catalysis of a natural-based binary ionic liquid. Mol. Divers. 2021, 25, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Fekri, L.Z.; Hamidian, H.; Chekosaran, M.A. Urazolium diacetate as a new, efficient and reusable Brønsted acid ionic liquid for the synthesis of novel derivatives of thiazolidine-4-ones. RSC Adv. 2020, 10, 556. [Google Scholar] [CrossRef]
- Waseem, M.A.; Srivastava, A.; Srivastava, A.; Siddiqui, I.R. Water and ionic liquid synergy: A novel approach for the synthesis of benzothiazole-2(3H)-one. J. Saudi Chem. Soc. 2015, 19, 334–339. [Google Scholar] [CrossRef]
- Yadav, L.D.S.; Rai, V.K.; Yadav, B.S. The first ionic liquid-promoted one-pot diastereoselective synthesis of 2,5-diamino-/2-amino-5-mercapto-1,3-thiazin-4-ones using masked amino/mercapto acids. Tetrahedron 2009, 65, 1306–1315. [Google Scholar] [CrossRef]
- Bahadorikhalili, S.; Ansari, S.; Hamedifar, H.; Mahdavi, M. The use of magnetic starch as a support for an ionic liquid-β-cyclodextrin based catalyst for the synthesis of imidazothiadiazolamine derivatives. Int. J. Biol. Macromol. 2019, 135, 453–461. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Perna, F.M.; Vitale, P.; Capriati, V. Deep eutectic solvents and their applications as green solvents. Curr. Opin. Green Sustain. Chem. 2020, 21, 27–33. [Google Scholar] [CrossRef]
- Alonso, D.A.; Baeza, A.; Chinchilla, R.; Guillena, G.; Pastor, I.M.; Ramon, D.J. Deep eutectic solvents: The organic reaction medium of the century. Eur. J. Org. Chem. 2016, 2016, 612–632. [Google Scholar] [CrossRef]
- Mancuso, R.; Maner, A.; Cicco, L.; Perna, F.M.; Capriati, V.; Gabriele, B. Synthesis of thiophenes in a deep eutectic solvent: Heterocyclodehydration and iodocyclization of 1-mercapto-3-yn-2-ols in a choline chloride/glycerol medium. Tetrahedron 2016, 72, 4239–4244. [Google Scholar] [CrossRef]
- Mancuso, R.; Lettieri, M.; Strangis, R.; Russo, P.; Piccionello, A.P.; De Angelis, S.; Gabriele, B. Iodocyclization of 2-methylthiophenylacetylenes to 3-iodobenzothiophenes and their coupling reactions under more sustainable conditions. Asian J. Org. Chem. 2022, 11, e202200353. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, Y.; Li, Y.; Chen, Y. A green synthesis and antibacterial activity of ferrocene-based thiazole derivatives in cholinę chloride/glycerol eutectic solvent. RSC Adv. 2022, 12, 22054–22059. [Google Scholar] [CrossRef] [PubMed]
- Kaldareh, M.F.; Mokhtary, M.; Nikpassand, M. Deep eutectic solvent mediated one-pot synthesis of hydrazinyl-4-phenyl-1,3-thiazoles. Polycyclic Arom. Comp. 2019, 41, 1012–1019. [Google Scholar] [CrossRef]
- Lobo, H.R.; Singh, B.S.; Shankarling, G.S. Lipase and deep eutectic mixture catalyzed efficient synthesis of thiazoles in water at room temperature. Catal. Lett. 2012, 142, 1369–1375. [Google Scholar] [CrossRef]
- Mitra, B.; Pariyar, G.C.; Ghosh, P. Glycerol: A benign solvent-assisted metal-free one-pot multi-component synthesis of 4H-thiopyran and thioamides from easily accessible precursors. ChemistrySelect. 2019, 4, 5476–5483. [Google Scholar] [CrossRef]
- Tiwari, J.; Singh, S.; Tufail, F.; Jaiswal, D.; Singh, J.; Singh, J. Glycerol micellar catalysis: An efficient multicomponent-tandem green synthetic approach to biologically important 2,4-disubstituted thiazole derivatives. ChemistrySelect 2018, 3, 11634–11643. [Google Scholar] [CrossRef]
- Perin, G.; Mello, L.G.; Radatz, C.S.; Savegnago, L.; Alves, D.; Jacob, R.G.; Lenardăo, E.J. Green, catalyst-free thioacetalization of carbonyl compounds using glicerol as recyclable solvent. Tetrahedron Lett. 2010, 51, 4354–4356. [Google Scholar] [CrossRef]
- Deligeorgiev, T.; Kaloyanova, S.; Lesev, N.; Alajarín, R.; Vaquero, J.J.; Álvarez-Builla, J. An environmentally benign synthesis of 2-cyanomethyl-4-phenylthiazoles under focused microwave irradiation. Green Sustain. Chem. 2011, 1, 170–175. [Google Scholar] [CrossRef]
- Zhang, X.-Z.; Zhou, W.-J.; Yang, M.; Wang, J.-X.; Bai, L. Microwave-assisted synthesis of benzothiazole derivatives using glycerol as green solvent. J. Chem. Res. 2012, 36, 489–491. [Google Scholar] [CrossRef]
- Jiang, Y.-Q.; Li, J.; Feng, Z.-W.; Xu, G.-Q.; Shi, X.; Ding, Q.-J.; Li, W.; Ma, C.-H.; Yu, B. Ethylene glycol: A green solvent for visible light-promoted aerobic transition metal-free cascade sulfonation/cyclization reaction. Adv. Synth. Catal. 2020, 362, 2609–2614. [Google Scholar] [CrossRef]
- Kauthale, S.S.; Tekale, S.U.; Jadhav, K.M.; Pawar, R.P. Ethylene glycol promoted catalyst-free pseudo three-component green synthesis of bis(coumarin)s and bis(3-methyl-1-phenyl-1H-pyrazol-5-ol)s. Mol Divers. 2016, 20, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Soni, J.; Sahiba, N.; Sethiya, A.; Agarwal, S. Polyethylene glycol: A promising approach for sustainable organic synthesis. J. Mol. Liq. 2020, 315, 113766. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Dekamin, M. A facile and environmentally benign polyethylene glycol 600-mediated method for the synthesis of densely functionalized 2-aminothiophene derivatives under ultrasonication. Green Chem. Lett. 2017, 10, 315–323. [Google Scholar] [CrossRef]
- Kavitha, K.; Srikrishna, D.; Dubey, P.K.; Aparna, P. An expedient one-pot tandem method for the synthesis of 3-(2-(phenylamino)thiazol-4-yl)-2H-chromen-2-ones under green conditions. J. Iran. Chem. Soc. 2019, 16, 1913–1921. [Google Scholar] [CrossRef]
- Khosropour, R.A.; Noei, J. A convenient strategy for the synthesis of 3,5-diaryl-1,2,4-thiadiazoles: Oxidative dimerization of arylthioamides using CC–DMSO in PEG-400. Monatsh. Chem. 2010, 141, 649–651. [Google Scholar] [CrossRef]
- Singh, U.P.; Bhat, H.R.; Singh, R.K. Ceric ammonium nitrate (CAN) catalysed expeditious one-pot synthesis of 1,3-thiazine as IspE kinase inhibitor of Gram-negative bacteria using polyethylene glycol (PEG-400) as an efficient recyclable reaction medium. C. R. Chim. 2013, 16, 462–468. [Google Scholar] [CrossRef]
- Badshah, S.L.; Naeem, A. Bioactive thiazine and benzothiazine derivatives: Green synthesis methods and their medicinal importance. Molecules 2016, 21, 1054. [Google Scholar] [CrossRef] [PubMed]
- Acharya, A.; Kamble, R.; Patil, S.; Hese, S.; Yemul, O.; Patil, S.; Halale, S.; Dawane, B. “Green synthesis” of benzothiazepine library of indeno analogues and their in vitro antimicrobial activity. Chem. Papers 2014, 68, 719–724. [Google Scholar] [CrossRef]
- Islam, A.; Singha, R.; Ghosh, P. Polyethylene glycol (PEG-200): An efficient, green and biocompatible reaction medium for the metal-free synthesis of functionalized 1,4-benzothiazines. ChemistrySelect 2023, 8, e202203780. [Google Scholar] [CrossRef]
- Messire, G.; Ferreira, V.; Caillet, E.; Bodin, L.; Auville, A.; Berteina-Raboin, S. Sabinene: A new green solvent used in the synthesis of thiazolo[5,4-b]pyridines by thermal or microwave activation. Molecules 2023, 28, 6924. [Google Scholar] [CrossRef] [PubMed]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosmalski, T.; Kołodziejska, R.; Przybysz, M.; Szeleszczuk, Ł.; Pawluk, H.; Mądra-Gackowska, K.; Studzińska, R. The Application of Green Solvents in the Synthesis of S-Heterocyclic Compounds—A Review. Int. J. Mol. Sci. 2024, 25, 9474. https://doi.org/10.3390/ijms25179474
Kosmalski T, Kołodziejska R, Przybysz M, Szeleszczuk Ł, Pawluk H, Mądra-Gackowska K, Studzińska R. The Application of Green Solvents in the Synthesis of S-Heterocyclic Compounds—A Review. International Journal of Molecular Sciences. 2024; 25(17):9474. https://doi.org/10.3390/ijms25179474
Chicago/Turabian StyleKosmalski, Tomasz, Renata Kołodziejska, Monika Przybysz, Łukasz Szeleszczuk, Hanna Pawluk, Katarzyna Mądra-Gackowska, and Renata Studzińska. 2024. "The Application of Green Solvents in the Synthesis of S-Heterocyclic Compounds—A Review" International Journal of Molecular Sciences 25, no. 17: 9474. https://doi.org/10.3390/ijms25179474







