Large Extracellular Vesicles Derived from Natural Killer Cells Affect the Functions of Monocytes
Abstract
1. Introduction
2. Results
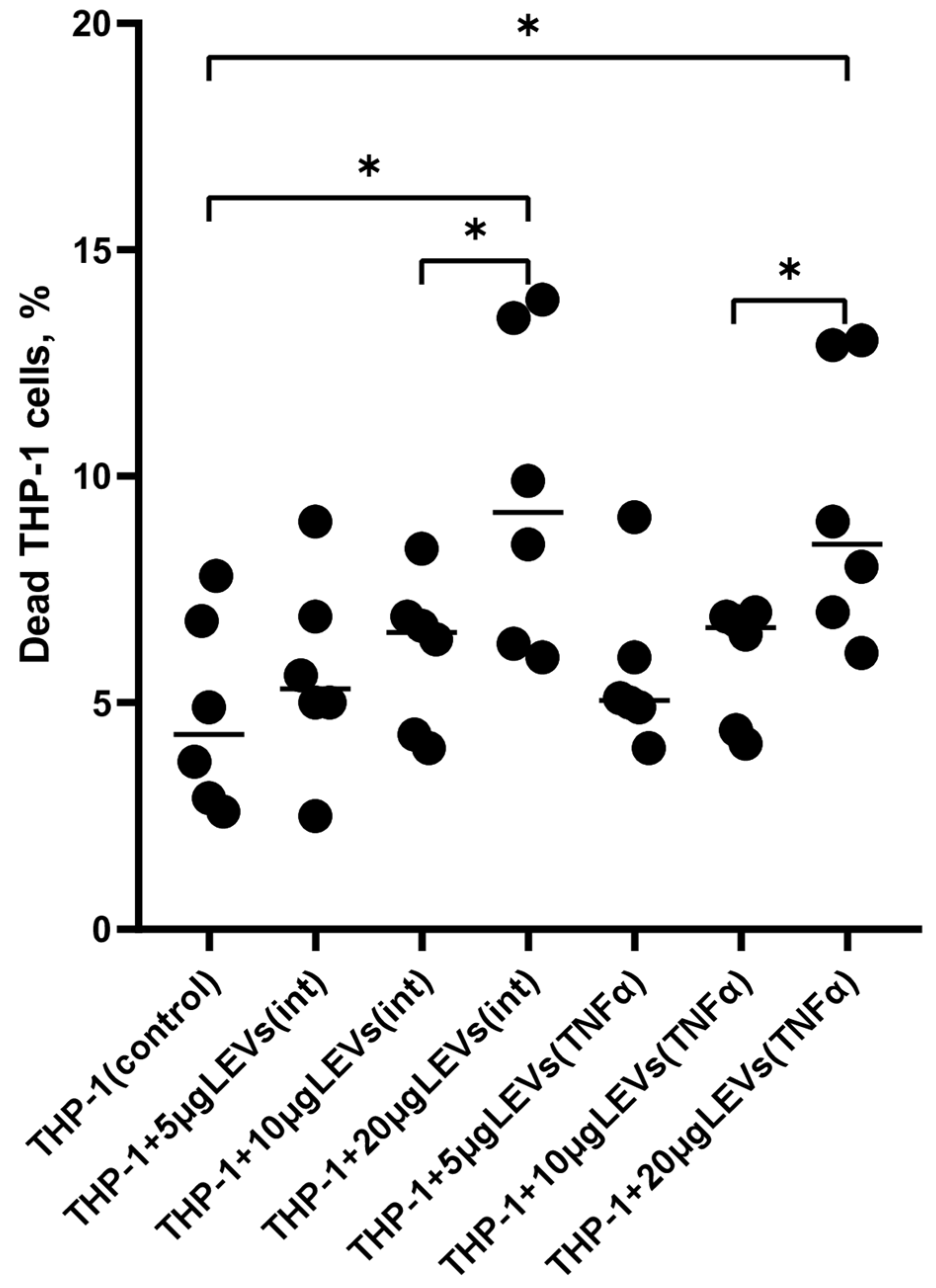
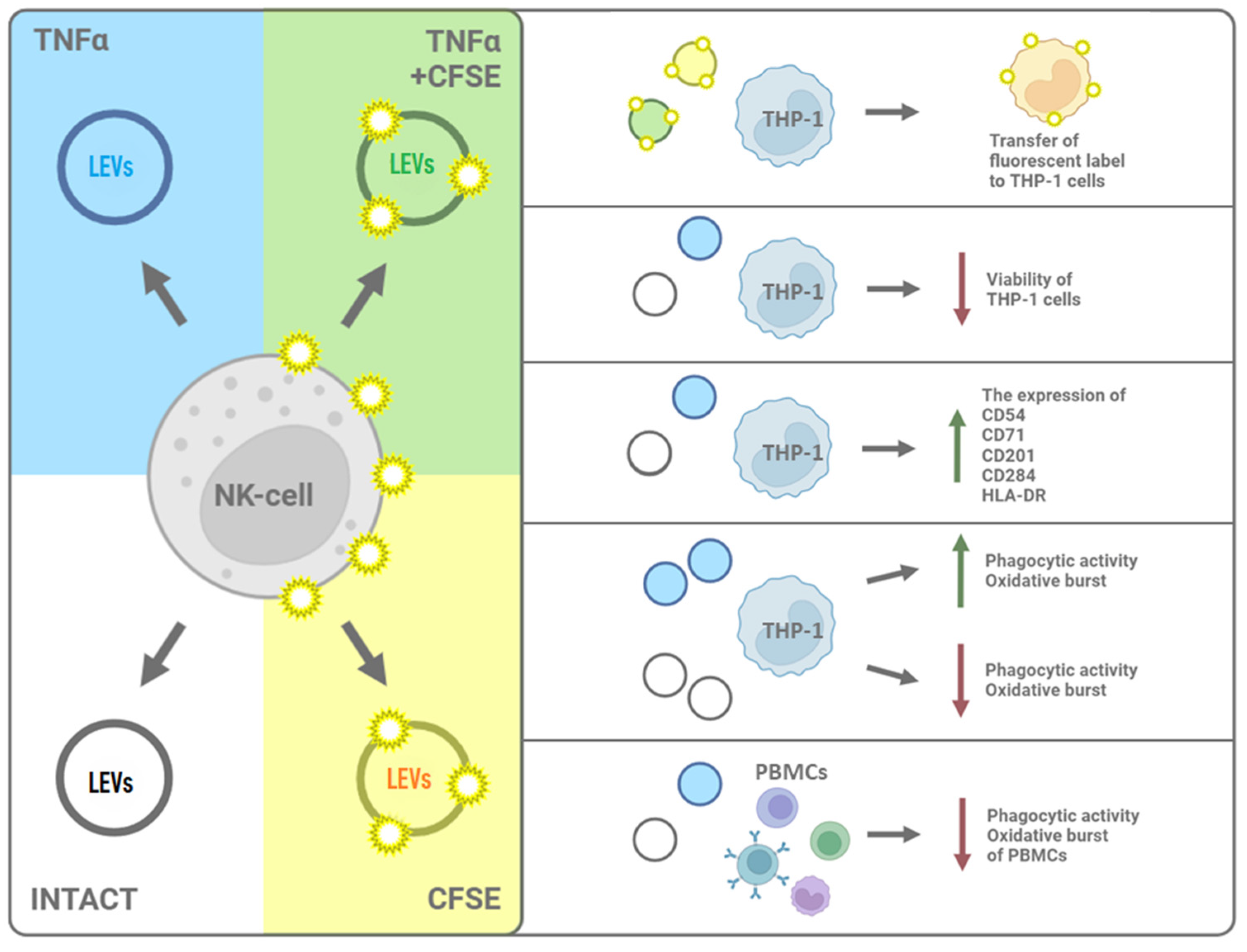
2.1. A High Concentration of LEVs Derived from NK Cells Causes a Decrease in the Viability of THP-1 Cells
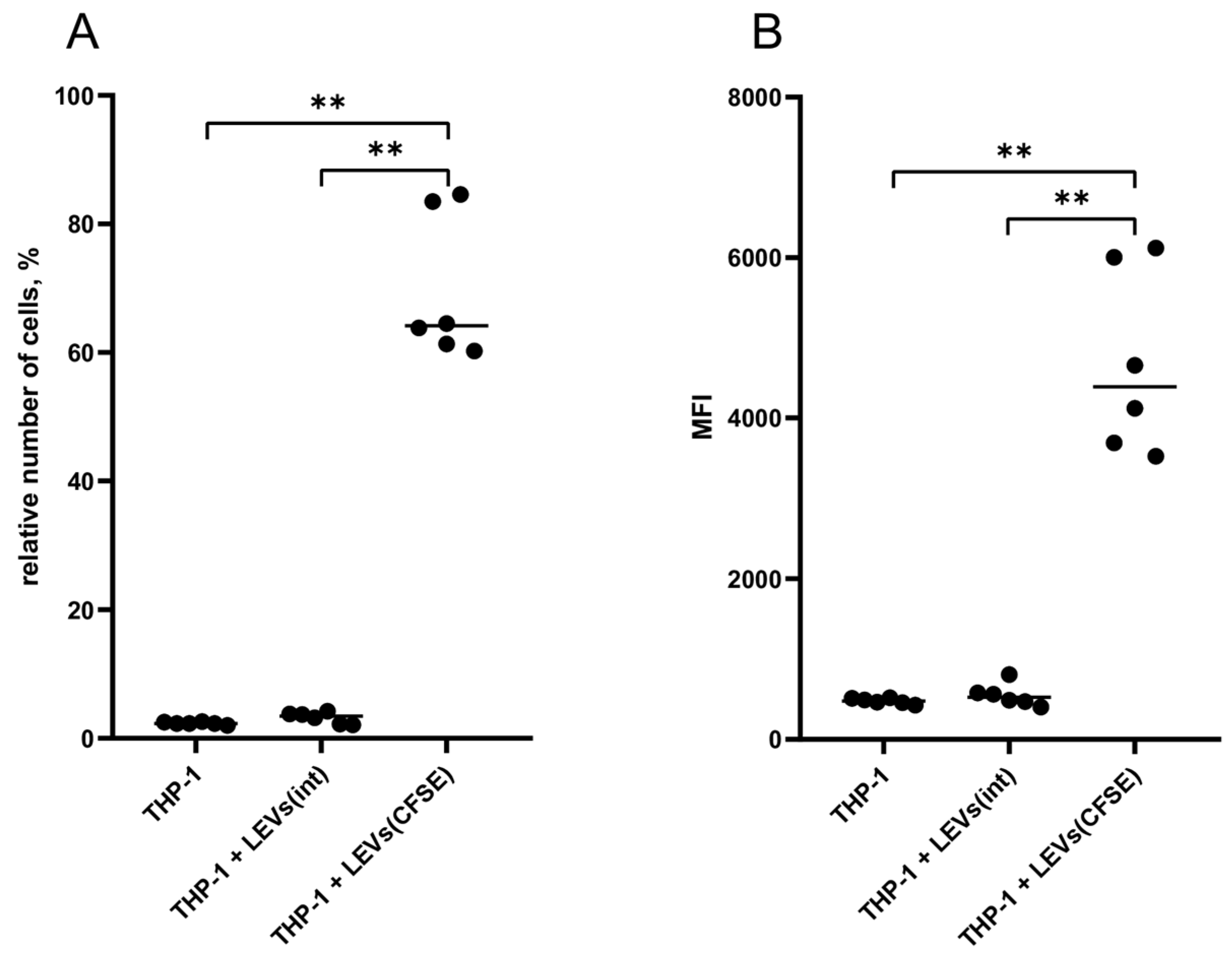
2.2. LEVs Derived from CFSE-Stained NK Cells Transfer a Fluorescent Label to THP-1 Cells
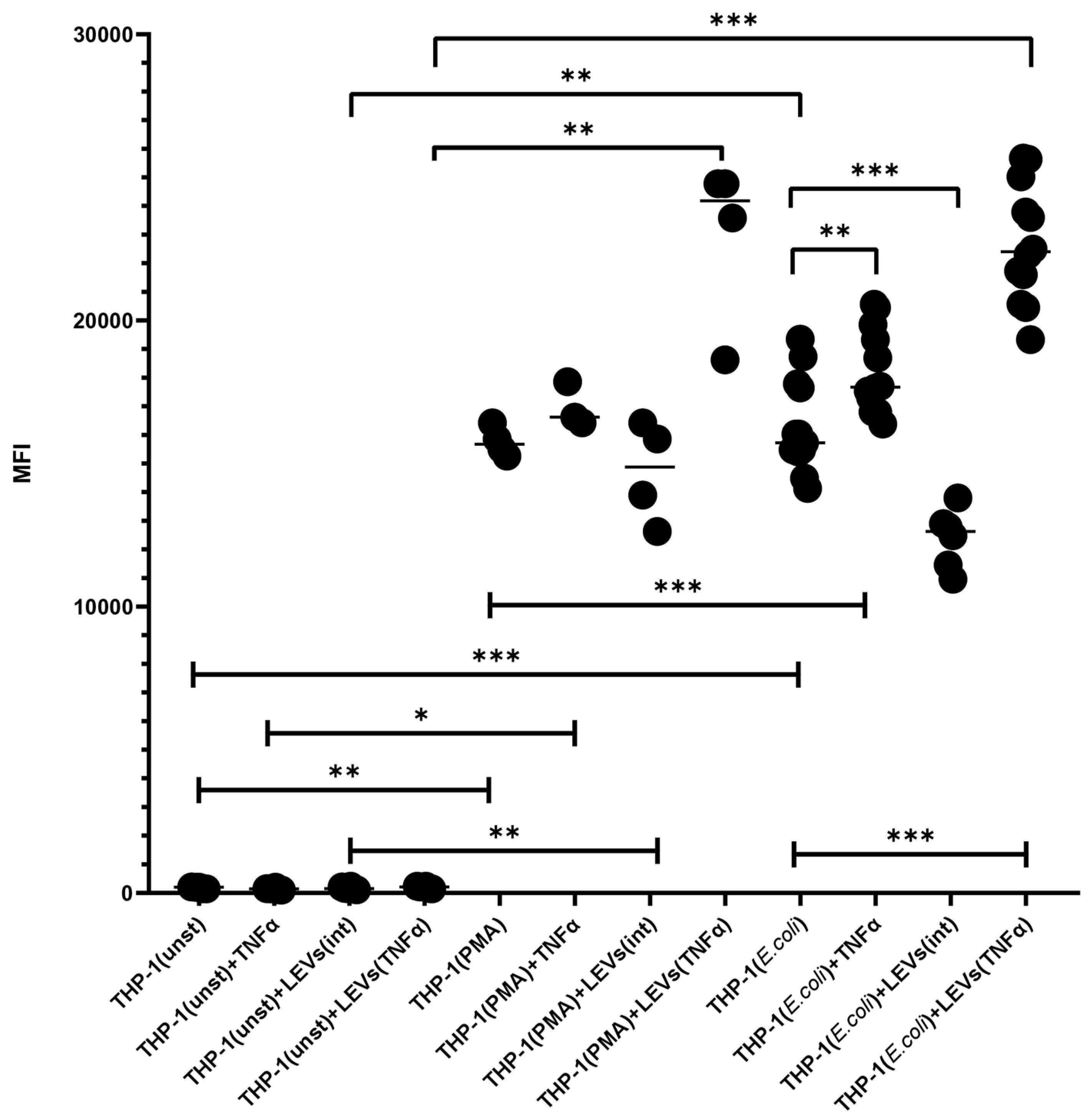
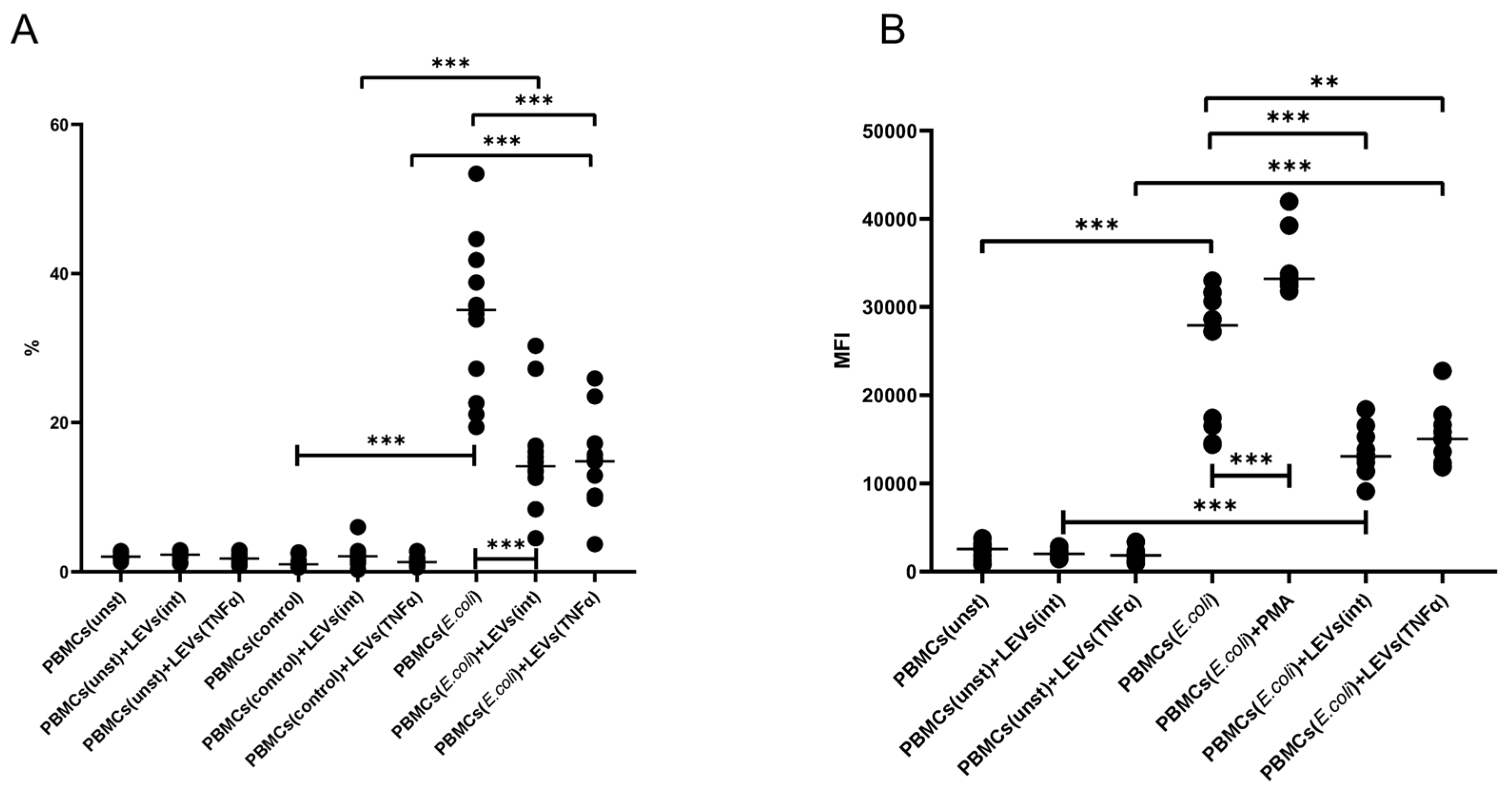
2.3. TNFα and LEVs Derived from TNFα-Activated NK Cells Increase the Phagocytic Activity and the Oxidative Burst of THP-1 Cells
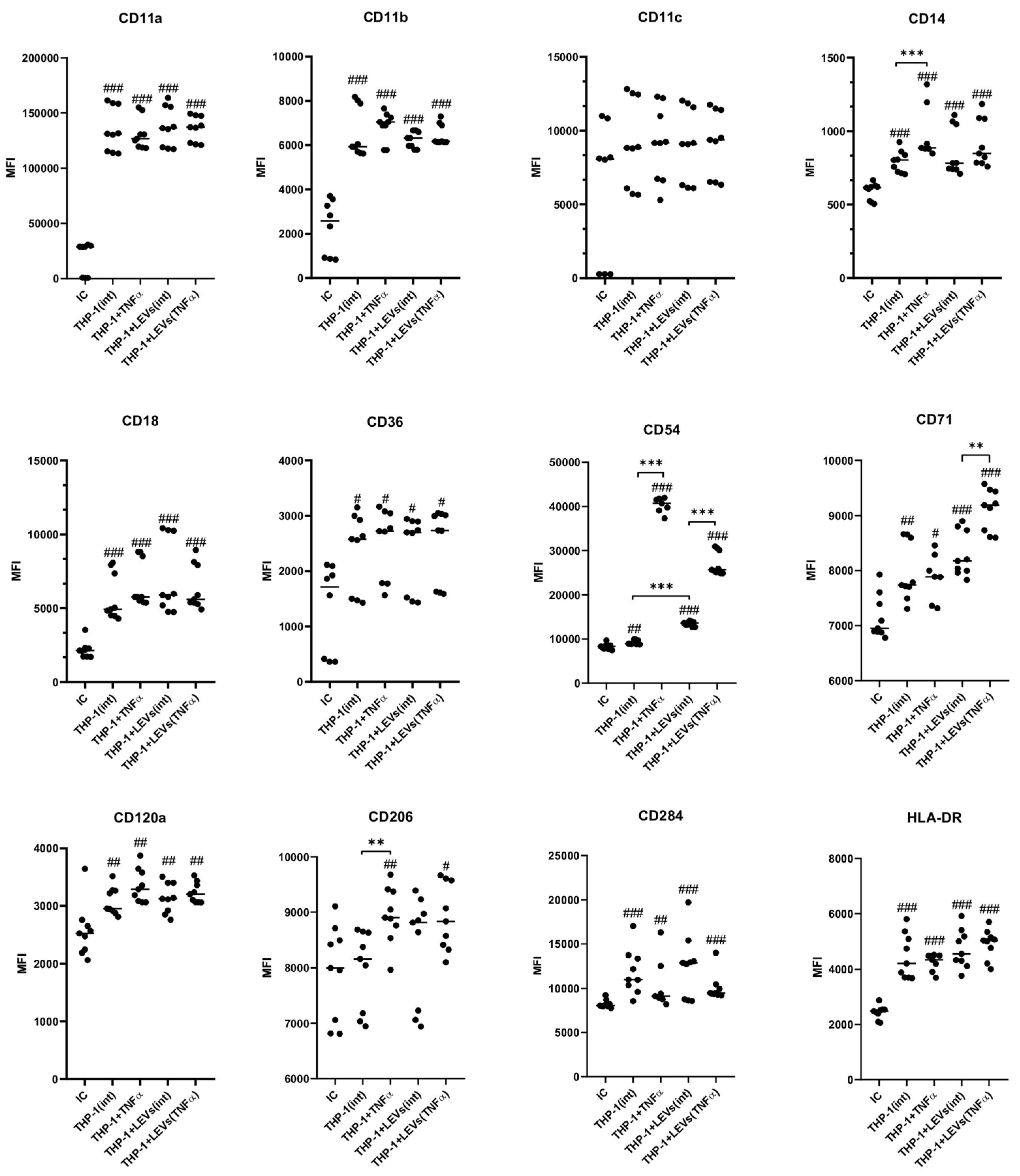
2.4. LEVs Derived from NK-92 Cells Alter the Phenotype of THP-1 Cells
2.5. LEVs Derived from NK-92 Cells Decrease the Phagocytic Activity and the Oxidative Burst of PBMCs
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. LEVs Isolation
4.3. Laser Correlation Analysis
4.4. Evaluation of the Transfer of a Fluorescent Label from LEVs Derived from NK-92 Cells to THP-1 Cells
4.5. Evaluation of the Effect of LEVs of NK-92 Cells on the THP-1 Cells Viability
4.6. Evaluation of the Effect of LEVs Derived from NK-92 Cells on Phagocytic Activity and Oxidative Burst in THP-1 Cells
4.7. Evaluation of the Effect of LEVs Derived from NK-92 Cells on Phagocytic Activity and the Realization of Oxidative Explosion via the PBMCs of Healthy Donors
4.8. Evaluation of the Effect of LEVs Derived from NK-92 Cells on the Phenotype of THP-1 Cells
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Del Zotto, G.; Marcenaro, E.; Vacca, P.; Sivori, S.; Pende, D.; Della Chiesa, M.; Moretta, F.; Ingegnere, T.; Mingari, M.C.; Moretta, A.; et al. Markers and function of human NK cells in normal and pathological conditions. Cytom. Part B Clin. Cytom. 2017, 92, 100–114. [Google Scholar] [CrossRef]
- Mandal, A.; Viswanathan, C. Natural killer cells: In health and disease. Hematol./Oncol. Stem Cell Ther. 2015, 8, 47–55. [Google Scholar] [CrossRef]
- Auffray, C.; Sieweke, M.H.; Geissmann, F. Blood monocytes: Development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 2009, 27, 669–692. [Google Scholar] [CrossRef]
- Walajtys-Rode, E.; Dzik, J.M. Monocyte/Macrophage: NK Cell Cooperation-Old Tools for New Functions. Results Probl. Cell Differ. 2017, 62, 73–145. [Google Scholar] [CrossRef]
- Lapaque, N.; Walzer, T.; Meresse, S.; Vivier, E.; Trowsdale, J. Interactions between human NK cells and macrophages in response to Salmonella infection. J. Immunol. 2009, 182, 4339–4348. [Google Scholar] [CrossRef]
- Empson, V.G.; McQueen, F.M.; Dalbeth, N. The natural killer cell: A further innate mediator of gouty inflammation? Immunol. Cell Biol. 2010, 88, 24–31. [Google Scholar] [CrossRef]
- Söderström, K.; Stein, E.; Colmenero, P.; Purath, U.; Müller-Ladner, U.; de Matos, C.T.; Tarner, I.H.; Robinson, W.H.; Engleman, E.G. Natural killer cells trigger osteoclastogenesis and bone destruction in arthritis. Proc. Natl. Acad. Sci. USA 2010, 107, 13028–13033. [Google Scholar] [CrossRef]
- Zhang, A.L.; Colmenero, P.; Purath, U.; Teixeira de Matos, C.; Hueber, W.; Klareskog, L.; Tarner, I.H.; Engleman, E.G.; Söderström, K. Natural killer cells trigger differentiation of monocytes into dendritic cells. Blood 2007, 110, 2484–2493. [Google Scholar] [CrossRef]
- Welte, S.; Kuttruff, S.; Waldhauer, I.; Steinle, A. Mutual activation of natural killer cells and monocytes mediated by NKp80-AICL interaction. Nat. Immunol. 2006, 7, 1334–1342. [Google Scholar] [CrossRef]
- Nedvetzki, S.; Sowinski, S.; Eagle, R.A.; Harris, J.; Vely, F.; Pende, D.; Trowsdale, J.; Vivier, E.; Gordon, S.; Davis, D.M. Reciprocal regulation of human natural killer cells and macrophages associated with distinct immune synapses. Blood 2007, 109, 3776–3785. [Google Scholar] [CrossRef]
- Schulz, U.; Kreutz, M.; Multhoff, G.; Stoelcker, B.; Köhler, M.; Andreesen, R.; Holler, E. Interleukin-10 promotes NK cell killing of autologous macrophages by stimulating expression of NKG2D ligands. Scand. J. Immunol. 2010, 72, 319–331. [Google Scholar] [CrossRef]
- Cichocki, F.; Sitnicka, E.; Bryceson, Y.T. NK cell development and function--plasticity and redundancy unleashed. Semin. Immunol. 2014, 26, 114–126. [Google Scholar] [CrossRef]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef]
- Michel, T.; Hentges, F.; Zimmer, J. Consequences of the crosstalk between monocytes/macrophages and natural killer cells. Front. Immunol. 2013, 3, 403. [Google Scholar] [CrossRef]
- Wu, M.; Zhou, C.; Li, M.; Yu, H.; Zhao, D.; Xue, W.; Qin, L.; Peng, A. Depletion of NK cells attenuates paraquat-induced acute lung injury by manipulating macrophage polarization. Int. Immunopharmacol. 2020, 86, 106698. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Borges, F.T.; Reis, L.A.; Schor, N. Extracellular vesicles: Structure, function, and potential clinical uses in renal diseases. Braz. J. Med. Biol. Res. 2013, 46, 824–830. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.p.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Muralidharan-Chari, V.; Clancy, J.; Plou, C.; Romao, M.; Chavrier, P.; Raposo, G.; D’Souza-Schorey, C. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr. Biol. CB 2009, 19, 1875–1885. [Google Scholar] [CrossRef]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef]
- Simak, J.; Gelderman, M.P. Cell Membrane Microparticles in Blood and Blood Products: Potentially Pathogenic Agents and Diagnostic Markers. Transfus. Med. Rev. 2006, 20, 1–26. [Google Scholar] [CrossRef]
- Simak, J.; Gelderman, M.P.; Yu, H.; Wright, V.; Baird, A.E. Circulating endothelial microparticles in acute ischemic stroke: A link to severity, lesion volume and outcome. J. Thromb. Haemost. 2006, 4, 1296–1302. [Google Scholar] [CrossRef]
- Stahl, P.D.; Raposo, G. Extracellular Vesicles: Exosomes and Microvesicles, Integrators of Homeostasis. Physiology 2019, 34, 169–177. [Google Scholar] [CrossRef]
- Camussi, G.; Deregibus, M.C.; Bruno, S.; Grange, C.; Fonsato, V.; Tetta, C. Exosome/microvesicle-mediated epigenetic reprogramming of cells. Am. J. Cancer Res. 2011, 1, 98–110. [Google Scholar]
- György, B.; Szabó, T.G.; Pásztói, M.; Pál, Z.; Misják, P.; Aradi, B.; László, V.; Pállinger, É.; Pap, E.; Kittel, Á.; et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell. Mol. Life Sci. CMLS 2011, 68, 2667–2688. [Google Scholar] [CrossRef]
- Burbano, C.; Rojas, M.; Vasquez, G.; Castano, D. Microparticles That Form Immune Complexes as Modulatory Structures in Autoimmune Responses. Mediat. Inflamm. 2015, 2015, 267590. [Google Scholar] [CrossRef]
- Sokolov, D.I.; Markova, K.L.; Mikhailova, V.A.; Vyazmina, L.P.; Milyutina, Y.P.; Kozyreva, A.R.; Zhdanova, A.A.; Malygina, D.A.; Onokhin, K.V.; Ivanova, A.N.; et al. Phenotypic and Functional Characteristics of Microvesicles Produced by Natural Killer Cells. Med. Immunol. 2019, 21, 669–688. [Google Scholar] [CrossRef]
- Vajen, T.; Mause, S.F.; Koenen, R.R. Microvesicles from platelets: Novel drivers of vascular inflammation. Thromb. Haemost. 2015, 114, 228–236. [Google Scholar] [CrossRef]
- Lugini, L.; Cecchetti, S.; Huber, V.; Luciani, F.; Macchia, G.; Spadaro, F.; Paris, L.; Abalsamo, L.; Colone, M.; Molinari, A.; et al. Immune surveillance properties of human NK cell-derived exosomes. J. Immunol. 2012, 189, 2833–2842. [Google Scholar] [CrossRef]
- Burnett, L.A.; Nowak, R.A. Exosomes mediate embryo and maternal interactions at implantation and during pregnancy. Front. Biosci. (Sch. Ed) 2016, 8, 79–96. [Google Scholar] [CrossRef]
- Tong, M.; Chamley, L.W. Placental extracellular vesicles and feto-maternal communication. Cold Spring Harb. Perspect. Med. 2015, 5, a023028. [Google Scholar] [CrossRef]
- Federici, C.; Shahaj, E.; Cecchetti, S.; Camerini, S.; Casella, M.; Iessi, E.; Camisaschi, C.; Paolino, G.; Calvieri, S.; Ferro, S.; et al. Natural-Killer-Derived Extracellular Vesicles: Immune Sensors and Interactors. Front. Immunol. 2020, 11, 262. [Google Scholar] [CrossRef]
- Mikhailova, V.A.; Belyakova, K.L.; Vyazmina, L.P.; Sheveleva, A.R.; Selkov, S.A.; Sokolov, D.I. Evaluation of Microvesicles Formed by Natural Killer (Nk) Cells Using Flow Cytometry. Med. Immunol. 2018, 20, 251–254. [Google Scholar] [CrossRef]
- Sedgwick, A.E.; D’Souza-Schorey, C. The biology of extracellular microvesicles. Traffic 2018, 19, 319–327. [Google Scholar] [CrossRef]
- Gong, J.H.; Maki, G.; Klingemann, H.G. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leuk. Off. J. Leuk. Soc. Am. Leuk. Res. Fund UK 1994, 8, 652–658. [Google Scholar]
- Tannetta, D.; Dragovic, R.; Alyahyaei, Z.; Southcombe, J. Extracellular vesicles and reproduction-promotion of successful pregnancy. Cell Mol. Immunol. 2014, 11, 548–563. [Google Scholar] [CrossRef]
- Burger, D.; Schock, S.; Thompson, C.S.; Montezano, A.C.; Hakim, A.M.; Touyz, R.M. Microparticles: Biomarkers and beyond. Clin. Sci. 2013, 124, 423–441. [Google Scholar] [CrossRef]
- Tricarico, C.; Clancy, J.; D’Souza-Schorey, C. Biology and biogenesis of shed microvesicles. Small GTPases 2017, 8, 220–232. [Google Scholar] [CrossRef]
- Fuhrmann, G.; Serio, A.; Mazo, M.; Nair, R.; Stevens, M.M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Control Release 2015, 205, 35–44. [Google Scholar] [CrossRef]
- Deng, S.Z.; Lai, M.F.; Li, Y.P.; Xu, C.H.; Zhang, H.R.; Kuang, J.G. Human marrow stromal cells secrete microRNA-375-containing exosomes to regulate glioma progression. Cancer Gene Ther. 2020, 27, 203–215. [Google Scholar] [CrossRef]
- Markova, K.L.; Mikhailova, V.A.; Korenevsky, A.V.; Milyutina, Y.P.; Rodygina, V.V.; Aleksandrova, E.P.; Markov, A.S.; Balabas, O.A.; Selkov, S.A.; Sokolov, D.I. Microvesicles produced by natural killer cells of the NK-92 cell line affect the phenotype and functions of endothelial cells of the EA.Hy926 cell line. Med. Immunol. 2020, 22, 249–268. [Google Scholar] [CrossRef]
- Korenevskii, A.V.; Milyutina, Y.P.; Zhdanova, A.A.; Pyatygina, K.M.; Sokolov, D.I.; Sel’kov, S.A. Mass-Spectrometric Analysis of Proteome of Microvesicles Produced by NK-92 Natural Killer Cells. Bull. Exp. Biol. Med. 2018, 165, 564–571. [Google Scholar] [CrossRef]
- Krzewski, K.; Coligan, J.E. Human NK cell lytic granules and regulation of their exocytosis. Front. Immunol. 2012, 3, 335. [Google Scholar] [CrossRef]
- Daigneault, M.; Preston, J.A.; Marriott, H.M.; Whyte, M.K.; Dockrell, D.H. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS ONE 2010, 5, e8668. [Google Scholar] [CrossRef]
- Mangan, D.F.; Welch, G.R.; Wahl, S.M. Lipopolysaccharide, tumor necrosis factor-alpha, and IL-1 beta prevent programmed cell death (apoptosis) in human peripheral blood monocytes. J. Immunol. 1991, 146, 1541–1546. [Google Scholar] [CrossRef]
- Titov, L.P. Monocytes, Macrophages, Dendritic and Myeloid Suppressor Cells: Genesis, Classification, Immunobiological Properties. Proc. Natl. Acad. Sci. Belarus Med. Ser. 2018, 15, 363–382. [Google Scholar] [CrossRef]
- Navegantes, K.C.; de Souza Gomes, R.; Pereira, P.A.T.; Czaikoski, P.G.; Azevedo, C.H.M.; Monteiro, M.C. Immune modulation of some autoimmune diseases: The critical role of macrophages and neutrophils in the innate and adaptive immunity. J. Transl. Med. 2017, 15, 36. [Google Scholar] [CrossRef]
- Goldszmid, R.S.; Caspar, P.; Rivollier, A.; White, S.; Dzutsev, A.; Hieny, S.; Kelsall, B.; Trinchieri, G.; Sher, A. NK cell-derived interferon-gamma orchestrates cellular dynamics and the differentiation of monocytes into dendritic cells at the site of infection. Immunity 2012, 36, 1047–1059. [Google Scholar] [CrossRef]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef]
- Tsuchiya, S.; Yamabe, M.; Yamaguchi, Y.; Kobayashi, Y.; Konno, T.; Tada, K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 1980, 26, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005, 5, 953–964. [Google Scholar] [CrossRef]
- Moh, M.C.; Lorenzini, P.A.; Gullo, C.; Schwarz, H. Tumor necrosis factor receptor 1 associates with CD137 ligand and mediates its reverse signaling. FASEB J. 2013, 27, 2957–2966. [Google Scholar] [CrossRef] [PubMed]
- Baqui, A.A.; Meiller, T.F.; Kelley, J.I.; Turng, B.F.; Falkler, W.A. Antigen activation of THP-1 human monocytic cells after stimulation with lipopolysaccharide from oral microorganisms and granulocyte-macrophage colony-stimulating factor. J. Periodontal Res. 1999, 34, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Mitachi, T.; Kouzui, M.; Maruyama, R.; Yamashita, K.; Ogata, S.; Kojima, H.; Itagaki, H. Some non-sensitizers upregulate CD54 expression by activation of the NLRP3 inflammasome in THP-1 cells. J. Toxicol. Sci. 2019, 44, 213–224. [Google Scholar] [CrossRef]
- Gonzalez, N.; Quintana, J.A.; García-Silva, S.; Mazariegos, M.; de la Aleja, A.G.; Nicolás-Ávila, J.A.; Walter, W.; Adrover, J.M.; Crainiciuc, G.; Kuchroo, V.K.; et al. Phagocytosis imprints heterogeneity in tissue-resident macrophages. J. Exp. Med. 2017, 214, 1281–1296. [Google Scholar] [CrossRef]
- Popēna, I.; Ābols, A.; Saulīte, L.; Pleiko, K.; Zandberga, E.; Jēkabsons, K.; Endzeliņš, E.; Llorente, A.; Linē, A.; Riekstiņa, U. Effect of colorectal cancer-derived extracellular vesicles on the immunophenotype and cytokine secretion profile of monocytes and macrophages. Cell Commun. Signal 2018, 16, 17. [Google Scholar] [CrossRef]
- Portillo, G.; Turner, M.; Chantry, D.; Feldmann, M. Effect of cytokines on HLA-DR and IL-1 production by a monocytic tumour, THP-1. Immunology 1989, 66, 170–175. [Google Scholar]
- Ubanako, P.; Xelwa, N.; Ntwasa, M. LPS induces inflammatory chemokines via TLR-4 signalling and enhances the Warburg Effect in THP-1 cells. PLoS ONE 2019, 14, e0222614. [Google Scholar] [CrossRef]
- Wu, F.; Xie, M.; Hun, M.; She, Z.; Li, C.; Luo, S.; Chen, X.; Wan, W.; Wen, C.; Tian, J. Natural Killer Cell-Derived Extracellular Vesicles: Novel Players in Cancer Immunotherapy. Front. Immunol. 2021, 12, 658698. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Quan, J. Exosomal miR-223 derived from natural killer cells inhibits hepatic stellate cell activation by suppressing autophagy. Mol. Med. 2020, 26, 81. [Google Scholar] [CrossRef]
- Ma, W.T.; Gao, F.; Gu, K.; Chen, D.K. The Role of Monocytes and Macrophages in Autoimmune Diseases: A Comprehensive Review. Front. Immunol. 2019, 10, 1140. [Google Scholar] [CrossRef]
- Kerrigan, A.M.; Brown, G.D. C-type lectins and phagocytosis. Immunobiology 2009, 214, 562–575. [Google Scholar] [CrossRef]
- Uribe-Querol, E.; Rosales, C. Phagocytosis: Our Current Understanding of a Universal Biological Process. Front. Immunol. 2020, 11, 1066. [Google Scholar] [CrossRef]
- Babior, B.M. NADPH oxidase. Curr. Opin. Immunol. 2004, 16, 42–47. [Google Scholar] [CrossRef]
- Hannan, N.J.; Paiva, P.; Dimitriadis, E.; Salamonsen, L.A. Models for study of human embryo implantation: Choice of cell lines? Biol. Reprod. 2010, 82, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, F.; Kajiwara, M. Relation of natural killer cell line NK-92-mediated cytolysis (NK-92-lysis) with the surface markers of major histocompatibility complex class I antigens, adhesion molecules, and Fas of target cells. Oncol. Res. 1998, 10, 483–489. [Google Scholar]
- Crowley, L.C.; Scott, A.P.; Marfell, B.J.; Boughaba, J.A.; Chojnowski, G.; Waterhouse, N.J. Measuring Cell Death by Propidium Iodide Uptake and Flow Cytometry. Cold Spring Harb. Protoc. 2016, 2016, pdb-prot087163. [Google Scholar] [CrossRef] [PubMed]
- Van Der Pol, E.; Coumans, F.A.; Grootemaat, A.E.; Gardiner, C.; Sargent, I.L.; Harrison, P.; Sturk, A.; van Leeuwen, T.G.; Nieuwland, R. Particle size distribution of exosomes and microvesicles determined by transmission electron microscopy, flow cytometry, nanoparticle tracking analysis, and resistive pulse sensing. J. Thromb. Haemost. 2014, 12, 1182–1192. [Google Scholar] [CrossRef] [PubMed]
- Gelderman, M.P.; Simak, J. Flow Cytometric Analysis of Cell Membrane Microparticles. In Functional Proteomics; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2008; pp. 79–93. [Google Scholar]
- Kornilov, R.; Puhka, M.; Mannerström, B.; Hiidenmaa, H.; Peltoniemi, H.; Siljander, P.; Seppänen-Kaijansinkko, R.; Kaur, S. Efficient ultrafiltration-based protocol to deplete extracellular vesicles from fetal bovine serum. J. Extracell. Vesicles 2018, 7, 1422674. [Google Scholar] [CrossRef]
- Eitan, E.; Zhang, S.; Witwer, K.W.; Mattson, M.P. Extracellular vesicle-depleted fetal bovine and human sera have reduced capacity to support cell growth. J. Extracell. Vesicles 2015, 4, 26373. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Muth, D.C.; Eitan, E.; Travers, M.; Learman, L.N.; Lehrmann, E.; Witwer, K.W. Serum extracellular vesicle depletion processes affect release and infectivity of HIV-1 in culture. Sci. Rep. 2017, 7, 2558. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, D.I.; Ovchinnikova, O.M.; Korenkov, D.A.; Viknyanschuk, A.N.; Benken, K.A.; Onokhin, K.V.; Selkov, S.A. Influence of peripheral blood microparticles of pregnant women with preeclampsia on the phenotype of monocytes. Transl. Res. J. Lab. Clin. Med. 2016, 170, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Dragovic, R.A.; Collett, G.P.; Hole, P.; Ferguson, D.J.; Redman, C.W.; Sargent, I.L.; Tannetta, D.S. Isolation of syncytiotrophoblast microvesicles and exosomes and their characterisation by multicolour flow cytometry and fluorescence Nanoparticle Tracking Analysis. Methods 2015, 87, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Korenevsky, A.V.; Shcherbitskaia, A.D.; Berezkina, M.E.; Markova, K.L.; Alexandrova, E.P.; Balabas, O.A.; Selkov, S.A.; Sokolov, D.I. MALDI-TOF mass spectrometric protein profiling of microvesicles produced by the NK-92 natural killer cell line. Med. Immunol. 2020, 22, 633–646. [Google Scholar] [CrossRef]
- Sokolov, D.; Gorshkova, A.; Markova, K.; Milyutina, Y.; Pyatygina, K.; Zementova, M.; Korenevsky, A.; Mikhailova, V.; Selkov, S. Natural Killer Cell Derived Microvesicles Affect the Function of Trophoblast Cells. Membranes 2023, 13, 213. [Google Scholar] [CrossRef]
- Evans-Osses, I.; Reichembach, L.H.; Ramirez, M.I. Exosomes or microvesicles? Two kinds of extracellular vesicles with different routes to modify protozoan-host cell interaction. Parasitol. Res. 2015, 114, 3567–3575. [Google Scholar] [CrossRef]
- Markova, K.L.; Kozyreva, A.R.; Gorshkova, A.A.; Aleksandrova, E.p.; Berezkina, M.E.; Mikhailova, V.A.; Ivanova, A.N.; Kaputkina, S.Y.; Onokhin, K.V.; Benken, K.A.; et al. Methodological Approaches to Assessing the Size and Morphology of Microvesicles of Cell Lines. Bull. Exp. Biol. Med. 2020, 169, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Al-Numani, D.; Segura, M.; Doré, M.; Gottschalk, M. Up-regulation of ICAM-1, CD11a/CD18 and CD11c/CD18 on human THP-1 monocytes stimulated by Streptococcus suis serotype 2. Clin. Exp. Immunol. 2003, 133, 67–77. [Google Scholar] [CrossRef]
- Tobias, P.S.; Soldau, K.; Kline, L.; Lee, J.D.; Kato, K.; Martin, T.P.; Ulevitch, R.J. Cross-linking of lipopolysaccharide (LPS) to CD14 on THP-1 cells mediated by LPS-binding protein. J. Immunol. 1993, 150, 3011–3021. [Google Scholar] [CrossRef] [PubMed]
- Zingg, J.M.; Azzi, A.; Meydani, M. α-Tocopheryl Phosphate Induces VEGF Expression via CD36/PI3Kgamma in THP-1 Monocytes. J. Cell Biochem. 2017, 118, 1855–1867. [Google Scholar] [CrossRef] [PubMed]
- Schierloh, P.; Alemán, M.; Yokobori, N.; Alves, L.; Roldan, N.; Abbate, E.; María del, C.S.; de la Barrera, S. NK cell activity in tuberculosis is associated with impaired CD11a and ICAM-1 expression: A regulatory role of monocytes in NK activation. Immunology 2005, 116, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.; Almeidab, J.; Teixeira, M.d.A.; Queirós, M.L.; Justiçaa, B.; Orfãob, A. The “ex vivo” patterns of CD2/CD7, CD57/CD11c, CD38/CD11b, CD45RA/CD45RO, and CD11a/HLA-DR expression identify acute/early and chronic/late NK-cell activation states. Blood Cells Mol. Dis. 2002, 28, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Ma, J.; Chen, Y.; Zhang, J.; Zhao, W.; Zhang, J.; Wei, H.; Ling, B.; Sun, R.; Tian, Z. Establishment, characterization, and successful adaptive therapy against human tumors of NKG cell, a new human NK cell line. Cell Transplant. 2011, 20, 1731–1746. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, Z.; Lei, Z.; Lei, P. CD14: Biology and role in the pathogenesis of disease. Cytokine Growth Factor Rev. 2019, 48, 24–31. [Google Scholar] [CrossRef]
- Swaim, C.D.; Scott, A.F.; Canadeo, L.A.; Huibregtse, J.M. Extracellular ISG15 Signals Cytokine Secretion through the LFA-1 Integrin Receptor. Mol. Cell 2017, 68, 581–590.e5. [Google Scholar] [CrossRef]
- Bosch, N.C.; Voll, R.E.; Voskens, C.J.; Gross, S.; Seliger, B.; Schuler, G.; Schaft, N.; Dorrie, J. NF-kappaB activation triggers NK-cell stimulation by monocyte-derived dendritic cells. Ther. Adv. Med Oncol. 2019, 11, 1758835919891622. [Google Scholar] [CrossRef]
- Komatsu, F.; Yoshida, S. Characteristics of human T-lymphotropic virus type-1 (HTLV-1)-infected cell line MT-2, which is not killed by a natural killer cell line NK-92 but is killed by lymphokine-activated killer cells. Oncol. Res. 1999, 11, 213–218. [Google Scholar]
- Sakaguchi, H.; Ashikaga, T.; Miyazawa, M.; Kosaka, N.; Ito, Y.; Yoneyama, K.; Sono, S.; Itagaki, H.; Toyoda, H.; Suzuki, H. The relationship between CD86/CD54 expression and THP-1 cell viability in an in vitro skin sensitization test--human cell line activation test (h-CLAT). Cell Biol. Toxicol. 2009, 25, 109–126. [Google Scholar] [CrossRef]
- Salzberger, W.; Martrus, G.; Bachmann, K.; Goebels, H.; Hess, L.; Koch, M.; Langeneckert, A.; Lunemann, S.; Oldhafer, K.J.; Pfeifer, C.; et al. Tissue-resident NK cells differ in their expression profile of the nutrient transporters Glut1, CD98 and CD71. PLoS ONE 2018, 13, e0201170. [Google Scholar] [CrossRef]
- Ma, H.; Shu, Q.; Li, D.; Wang, T.; Li, L.; Song, X.; Lou, K.; Xu, H. Accumulation of Intracellular Ferrous Iron in Inflammatory-Activated Macrophages. Biol. Trace Elem Res. 2023, 201, 2303–2310. [Google Scholar] [CrossRef] [PubMed]
- Mason, A.T.; McVicar, D.W.; Smith, C.A.; Young, H.A.; Ware, C.F.; Ortaldo, J.R. Regulation of NK cells through the 80-kDa TNFR (CD120b). J. Leukoc. Biol. 1995, 58, 249–255. [Google Scholar] [CrossRef]
- Azad, A.K.; Rajaram, M.V.; Schlesinger, L.S. Exploitation of the Macrophage Mannose Receptor (CD206) in Infectious Disease Diagnostics and Therapeutics. J. Cytol. Mol. Biol. 2014, 1, 1000003. [Google Scholar] [CrossRef]
- Bahrami, M.; Haji Molla Hoseini, M.; Rezaei, M.; Ziai, S.A. Umbelliprenin Increases the M1/M2 Ratio of Macrophage Polarization and Improves the M1 Macrophage Activity in THP-1 Cells Cocultured with AGS Cells. Evid. Based Complement. Altern. Med. 2021, 2021, 9927747. [Google Scholar] [CrossRef] [PubMed]
- Duriez, M.; Quillay, H.; Madec, Y.; El Costa, H.; Cannou, C.; Marlin, R.; de Truchis, C.; Rahmati, M.; Barre-Sinoussi, F.; Nugeyre, M.T.; et al. Human decidual macrophages and NK cells differentially express Toll-like receptors and display distinct cytokine profiles upon TLR stimulation. Front. Microbiol. 2014, 5, 316. [Google Scholar] [CrossRef] [PubMed]
- Souza-Fonseca-Guimaraes, F.; Parlato, M.; Philippart, F.; Misset, B.; Cavaillon, J.-M.; Adib-Conquy, M.; the Captain study group. Toll-like receptors expression and interferon-gamma production by NK cells in human sepsis. Crit. Care 2012, 16, R206. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Ma, L.; Zhou, Y.; Shen, W.; Xu, D.; Dou, J.; Shen, B.; Zhou, C. Polysaccharide enhanced NK cell cytotoxicity against pancreatic cancer via TLR4/MAPKs/NF-κB pathway in vitro/vivo. Carbohydr. Polym. 2019, 225, 115223. [Google Scholar] [CrossRef]
- Kim, Y.K.; Hwang, J.H.; Lee, H.T. Differential susceptibility to lipopolysaccharide affects the activation of toll-like-receptor 4 signaling in THP-1 cells and PMA-differentiated THP-1 cells. Innate Immun. 2022, 28, 122–129. [Google Scholar] [CrossRef]
- Lertjuthaporn, S.; Somkird, J.; Lekmanee, K.; Atipimonpat, A.; Sukapirom, K.; Sawasdipokin, H.; Tiewcharoen, S.; Pattanapanyasat, K.; Khowawisetsut, L. Extracellular Vesicles from Naegleria fowleri Induce IL-8 Response in THP-1 Macrophage. Pathogens 2022, 11, 632. [Google Scholar] [CrossRef]
- Gordon, S.; Martinez-Pomares, L. Physiological roles of macrophages. Pflügers Arch. Eur. J. Physiol. 2017, 469, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Allden, S.J.; Ogger, P.P.; Ghai, P.; McErlean, P.; Hewitt, R.; Toshner, R.; Walker, S.A.; Saunders, P.; Kingston, S.; Molyneaux, P.L.; et al. The Transferrin Receptor CD71 Delineates Functionally Distinct Airway Macrophage Subsets during Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2019, 200, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Italiani, P.; Boraschi, D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front. Immunol. 2014, 5, 514. [Google Scholar] [CrossRef] [PubMed]


| Receptor | Surface Expression | Receptor’s Function | |||
|---|---|---|---|---|---|
| NK Cells | NK-92 Cells | LEVs | THP-1 | ||
| CD11a | Yes [84] | Yes [28,36] | Yes [28] | Yes [81] | Integrin |
| CD11b | Yes [85] | Yes [28,86] | Yes [28] | No [81] | Integrin |
| CD11c | Yes [85] | Yes [28,86] | Yes [28] | Yes [81] | Integrin |
| CD14 | No [87] | No [28,36] | No data | Yes [82] | LPS receptor, coreceptor for TLR4 |
| CD18 | Yes [85] | Yes [28,88] | Yes [28] | Yes [81] | Integrin |
| CD36 | No data | No data | No data | Yes [83] | Scavenger receptor |
| CD54 (ICAM-1) | Yes [89] | Yes [28,90] | Yes [28] | Yes [91] | Adhesion molecule, cell activation marker |
| CD71 | Yes [92] | No data | No data | Yes [93] | Transferrin receptor |
| CD120a (TNFR1) | Yes [94] | No data | No data | Yes [53] | TNFα receptor |
| CD206 | No [95] | No data | No data | Yes [96] | Mannose receptor |
| CD284 (TLR4) | Yes [97,98] | Yes [99] | No data | Yes, after activation [100] | TLR4 |
| HLA-DR | Yes [85], after activation | No [36] | No data | Yes, after activation [101] | MHC class II cell surface receptor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokolov, D.; Gorshkova, A.; Tyshchuk, E.; Grebenkina, P.; Zementova, M.; Kogan, I.; Totolian, A. Large Extracellular Vesicles Derived from Natural Killer Cells Affect the Functions of Monocytes. Int. J. Mol. Sci. 2024, 25, 9478. https://doi.org/10.3390/ijms25179478
Sokolov D, Gorshkova A, Tyshchuk E, Grebenkina P, Zementova M, Kogan I, Totolian A. Large Extracellular Vesicles Derived from Natural Killer Cells Affect the Functions of Monocytes. International Journal of Molecular Sciences. 2024; 25(17):9478. https://doi.org/10.3390/ijms25179478
Chicago/Turabian StyleSokolov, Dmitry, Alina Gorshkova, Elizaveta Tyshchuk, Polina Grebenkina, Maria Zementova, Igor Kogan, and Areg Totolian. 2024. "Large Extracellular Vesicles Derived from Natural Killer Cells Affect the Functions of Monocytes" International Journal of Molecular Sciences 25, no. 17: 9478. https://doi.org/10.3390/ijms25179478
APA StyleSokolov, D., Gorshkova, A., Tyshchuk, E., Grebenkina, P., Zementova, M., Kogan, I., & Totolian, A. (2024). Large Extracellular Vesicles Derived from Natural Killer Cells Affect the Functions of Monocytes. International Journal of Molecular Sciences, 25(17), 9478. https://doi.org/10.3390/ijms25179478







