Thanatin: A Promising Antimicrobial Peptide Targeting the Achilles’ Heel of Multidrug-Resistant Bacteria
Abstract
:1. Introduction
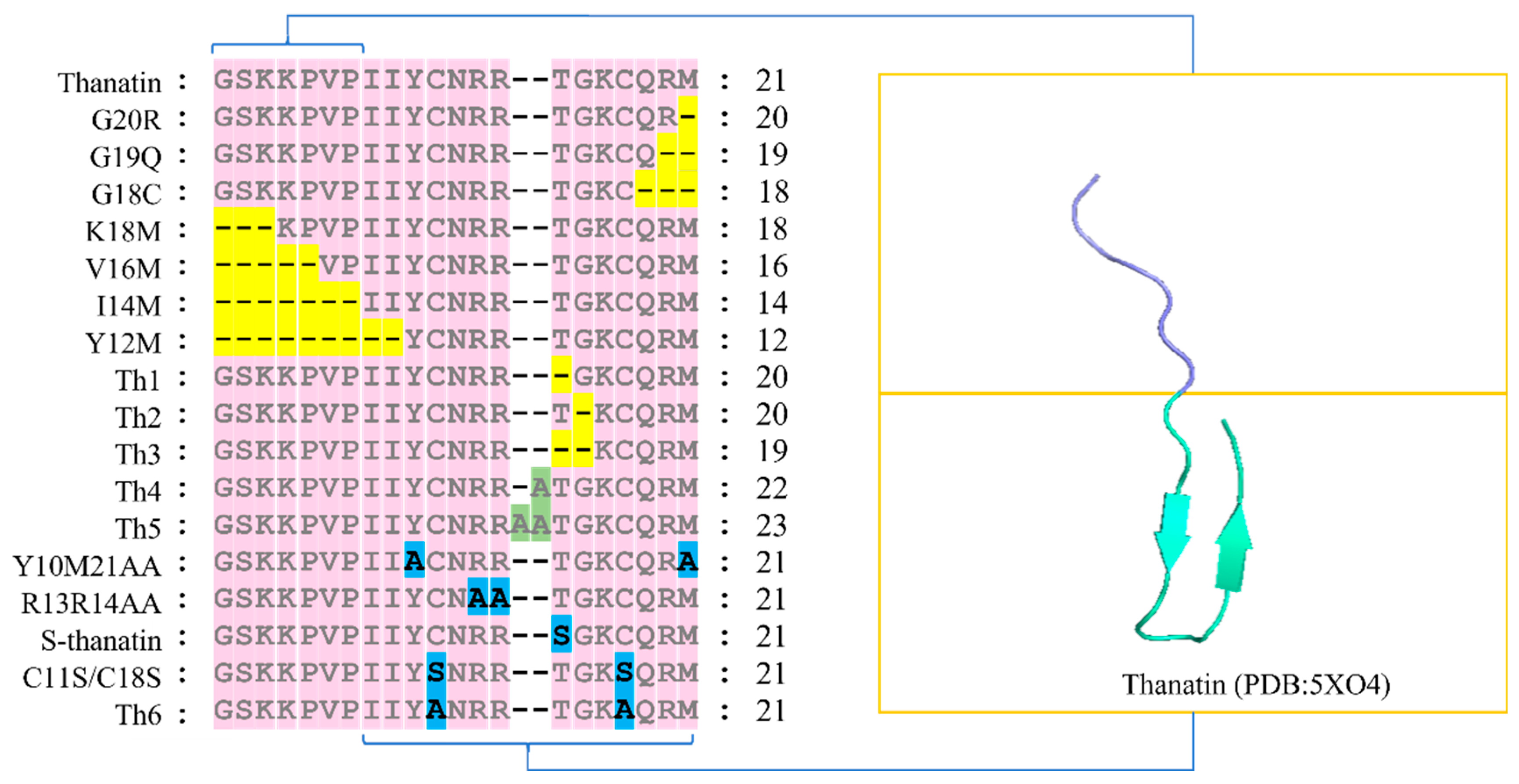
2. Structure–Activity Analysis of Thanatin
2.1. Relationship between Residues G1-P7 and the Antimicrobial Activity of Thanatin
2.2. Relationship between Residues I8-M21 and the Antimicrobial Activity of Thanatin
3. Synthetic Method of Thanatin
3.1. Recombinant Protein Expression
3.2. Solid-Phase Synthesis
4. Antimicrobial Activity of Thanatin
4.1. Gram-Negative Bacteria
4.2. Gram-Positive Bacteria
4.3. Antimicrobial Activity of Thanatin Combined with Other Antimicrobials
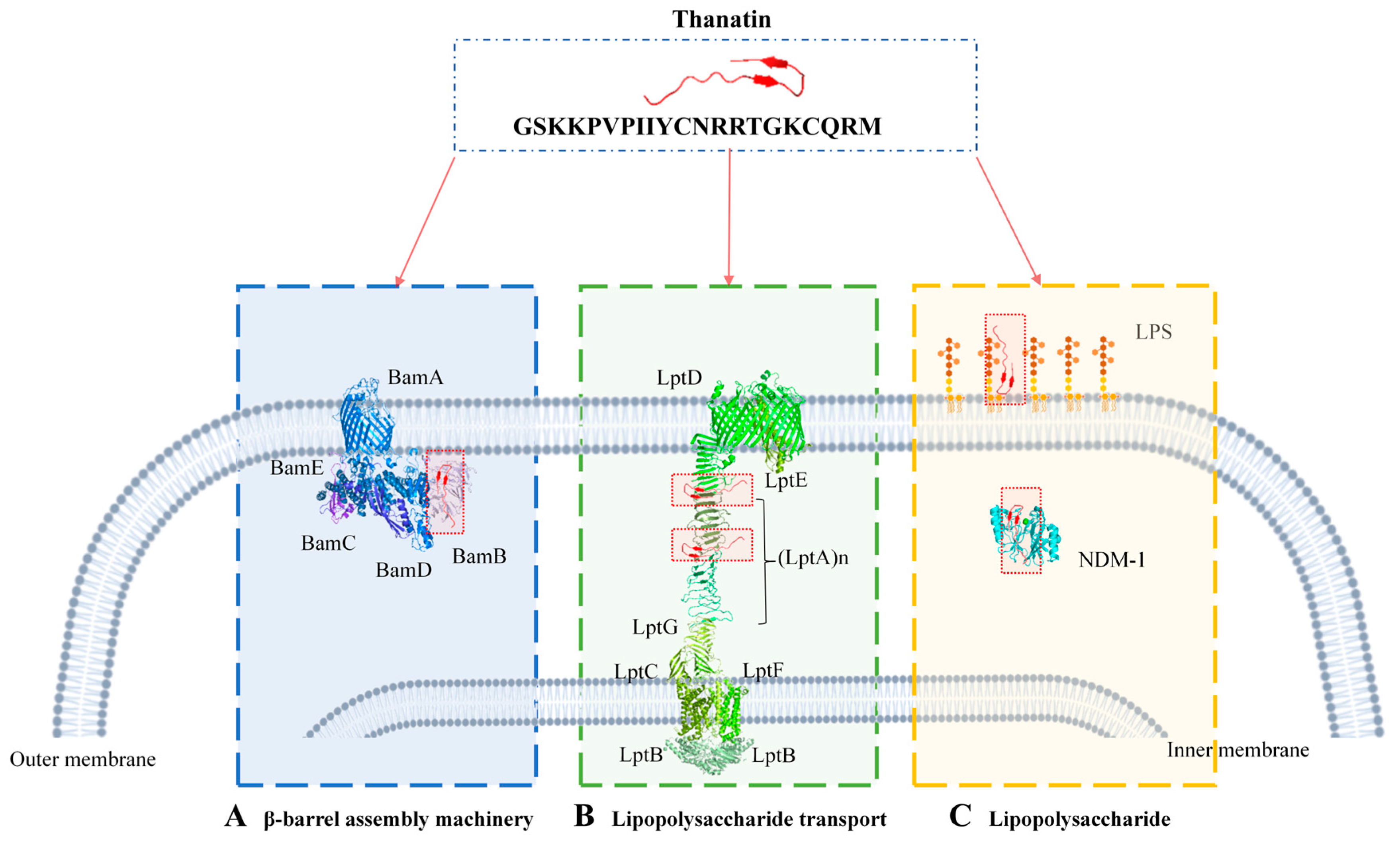
5. Multiple Bactericidal Mechanisms of Thanatin
5.1. Targeting Lipopolysaccharide Transport
5.1.1. Targeting LptA
5.1.2. Targeting LptD
5.2. Targeting the β-Barrel Assembly Machinery
5.3. Targeting LPS
6. Conclusions and Prospects
6.1. Clinical Applications
6.2. Environmental Applications
6.3. Applications in Food and Agriculture
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, R.; Fan, X.; Jiang, X.; Zou, M.; Xiao, H.; Wu, G. Multiple Mechanisms of the Synthesized Antimicrobial Peptide TS against Gram-Negative Bacteria for High Efficacy Antibacterial Action In Vivo. Molecules 2021, 26, 60. [Google Scholar] [CrossRef]
- World Health Organization. Ten Threats to Global Health in 2019. 2019. Available online: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 (accessed on 30 March 2024).
- De Kraker, M.E.A.; Stewardson, A.J.; Harbarth, S. Will 10 Million People Die a Year Due to Antimicrobial Resistance by 2050? PLoS Med. 2016, 13, e1002184. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- World Health Organization. WHO Outlines 40 Research Priorities on Antimicrobial Resistance. 2023. Available online: https://www.who.int/news/item/22-06-2023-who-outlines-40-research-priorities-on-antimicrobial-resistance (accessed on 30 March 2024).
- Mandard, N.; Sodano, P.; Labbe, H.; Bonmatin, J.M.; Bulet, P.; Hetru, C.; Ptak, M.; Vovelle, F. Solution Structure of Thanatin, a Potent Bactericidal and Fungicidal Insect Peptide, Determined from Proton Two-Dimensional Nuclear Magnetic Resonance Data. Eur. J. Biochem. 1998, 256, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Fehlbaum, P.; Bulet, P.; Chernysh, S.; Briand, J.P.; Roussel, J.P.; Letellier, L.; Hetru, C.; Hoffmann, J.A. Structure-Activity Analysis of Thanatin, a 21-Residue Inducible Insect Defense Peptide with Sequence Homology to Frog Skin Antimicrobial Peptides. Proc. Natl. Acad. Sci. USA 1996, 93, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Robert, E.; Lefevre, T.; Fillion, M.; Martial, B.; Dionne, J.; Auger, M. Mimicking and Understanding the Agglutination Effect of the Antimicrobial Peptide Thanatin Using Model Phospholipid Vesicles. Biochemistry 2015, 54, 3932–3941. [Google Scholar] [CrossRef]
- Sperandeo, P.; Dehò, G.; Polissi, A. The Lipopolysaccharide Transport System of Gram-Negative Bacteria. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2009, 1791, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Choi, U.; Lee, C.-R. Antimicrobial Agents That Inhibit the Outer Membrane Assembly Machines of Gram-Negative Bacteria. J. Microbiol. Biotechnol. 2019, 29, 1–10. [Google Scholar] [CrossRef]
- Das, C.; Shankaramma, S.C.; Balaram, P. Molecular Carpentry: Piecing Together Helices and Hairpins in Designed Peptides. Chem. Eur. J. 2001, 7, 840–847. [Google Scholar] [CrossRef]
- Maget-Dana, R.; Lelièvre, D.; Brack, A. Surface Active Properties of Amphiphilic Sequential Isopeptides: Comparison between a-Helical and β-Sheet Conformations. Biopolymers 1999, 49, 415–423. [Google Scholar] [CrossRef]
- Lee, M.K.; Cha, L.; Lee, S.H.; Hahm, K.S. Role of Amino Acid Residues within the Disulfide Loop of Thanatin, a Potent Antibiotic Peptide. J. Biochem. Mol. Biol. 2002, 35, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Ding, J.; Li, H.; Li, L.; Zhao, R.; Shen, Z.; Fan, X.; Xi, T. Effects of Cations and PH on Antimicrobial Activity of Thanatin and S-Thanatin Against Escherichia coli ATCC25922 and B. subtilis ATCC 21332. Curr. Microbiol. 2008, 57, 552–557. [Google Scholar] [CrossRef]
- Sinha, S.; Zheng, L.; Mu, Y.; Ng, W.J.; Bhattacharjya, S. Structure and Interactions of A Host Defense Antimicrobial Peptide Thanatin in Lipopolysaccharide Micelles Reveal Mechanism of Bacterial Cell Agglutination. Sci. Rep. 2017, 7, 17795. [Google Scholar] [CrossRef] [PubMed]
- Hongbiao, W.; Baolong, N.; Mengkui, X.; Lihua, H.; Weifeng, S.; Zhiqi, M. Biological Activities of Cecropin B-Thanatin Hybrid Peptides. J. Pept. Res. 2005, 66, 382–386. [Google Scholar] [CrossRef]
- Sinha, S.; Ng, W.J.; Bhattacharjya, S. NMR Structure and Localization of the Host Defense Antimicrobial Peptide Thanatin in Zwitterionic Dodecylphosphocholine Micelle: Implications in Antimicrobial Activity. Biochim. Biophys. Acta (BBA)—Biomembr. 2020, 1862, 183432. [Google Scholar] [CrossRef]
- Sinha, S.; Dhanabal, V.B.; Sperandeo, P.; Polissi, A.; Bhattacharjya, S. Linking Dual Mode of Action of Host Defense Antimicrobial Peptide Thanatin: Structures, Lipopolysaccharide and LptAm Binding of Designed Analogs. Biochim. Biophys. Acta-Biomembr. 2022, 1864, 183839. [Google Scholar] [CrossRef]
- Grieco, P.; Luca, V.; Auriemma, L.; Carotenuto, A.; Saviello, M.R.; Campiglia, P.; Barra, D.; Novellino, E.; Mangoni, M.L. Alanine Scanning Analysis and Structurefunction Relationships of the Frogskin Antimicrobial Peptide temporin1Ta. J. Pept. Sci. 2011, 17, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Hänchen, A.; Rausch, S.; Landmann, B.; Toti, L.; Nusser, A.; Süssmuth, R.D. Alanine Scan of the Peptide Antibiotic Feglymycin: Assessment of Amino Acid Side Chains Contributing to Antimicrobial Activity. Chembiochem 2013, 14, 625–632. [Google Scholar] [CrossRef]
- Wu, G.; Wu, H.; Li, L.; Fan, X.; Ding, J.; Li, X.; Xi, T.; Shen, Z. Membrane Aggregation and Perturbation Induced by Antimicrobial Peptide of S-Thanatin. Biochem. Biophys. Res. Commun. 2010, 395, 31–35. [Google Scholar] [CrossRef]
- Wu, G.; Li, X.; Fan, X.; Wu, H.; Wang, S.; Shen, Z.; Xi, T. The Activity of Antimicrobial Peptide S-Thanatin Is Independent on Multidrug-Resistant Spectrum of Bacteria. Peptides 2011, 32, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Wu, P.; Xue, X.; Yan, X.; Liu, S.; Zhang, C.; Shen, Z.; Xi, T. Application of S-Thanatin, an Antimicrobial Peptide Derived from Thanatin, in Mouse Model of Klebsiella pneumoniae Infection. Peptides 2013, 45, 73–77. [Google Scholar] [CrossRef]
- Imamura, T.; Yamamoto, N.; Tamura, A.; Murabayashi, S.; Hashimoto, S.; Shimada, H.; Taguchi, S. NMR Based Structure-Activity Relationship Analysis of an Antimicrobial Peptide, Thanatin, Engineered by Site-Specific Chemical Modification: Activity Improvement and Spectrum Alteration. Biochem. Biophys. Res. Commun. 2008, 369, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Karbalaei, M.; Rezaee, S.A.; Farsiani, H. Pichia Pastoris: A Highly Successful Expression System for Optimal Synthesis of Heterologous Proteins. J. Cell. Physiol. 2020, 235, 5867–5881. [Google Scholar] [CrossRef] [PubMed]
- Sabokkhiz, M.A.; Tanhaeian, A.; Mamarabadi, M. Study on Antiviral Activity of Two Recombinant Antimicrobial Peptides Against Tobacco Mosaic Virus. Probiotics Antimicrob. Proteins 2019, 11, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Smart, T.G. HEK293 Cell Line: A Vehicle for the Expression of Recombinant Proteins. J. Pharmacol. Toxicol. Methods 2005, 51, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Han, J.; Li, H.; Zhang, X.; Liu, L.L.; Chen, F.; Zeng, B. Human Embryonic Kidney 293 Cells: A Vehicle for Biopharmaceutical Manufacturing, Structural Biology, and Electrophysiology. Cells Tissues Organs 2018, 205, 1–8. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, M.; Chen, X.; Yang, G.; Yang, T.; Yu, L.; Hui, L.; Wang, X. Expression and Antibacterial Activity of Hybrid Antimicrobial Peptide cecropinA-Thanatin in Pichia Pastoris. Front. Lab. Med. 2018, 2, 23–29. [Google Scholar] [CrossRef]
- Tanhaeian, A.; Azghandi, M.; Mousavi, Z.; Javadmanesh, A. Expression of Thanatin in HEK293 Cells and Investigation of Its Antibacterial Effects on Some Human Pathogens. Protein Pept. Lett. 2020, 27, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Mamarabadi, M.; Tanhaeian, A.; Ramezany, Y. Antifungal Activity of Recombinant Thanatin in Comparison with Two Plant Extracts and a Chemical Mixture to Control Fungal Plant Pathogens. AMB Express 2018, 8, 180. [Google Scholar] [CrossRef]
- Wurm, D.J.; Veiter, L.; Ulonska, S.; Eggenreich, B.; Herwig, C.; Spadiut, O. The E. coli pET Expression System Revisited—Mechanistic Correlation between Glucose and Lactose Uptake. Appl. Microbiol. Biotechnol. 2016, 100, 8721–8729. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Chen, Y.; Zhao, Y.; Che, L.; Ma, Y.; Li, J.; Wang, Y.; Tao, H.; Ma, J.; Pan, B.; et al. Sortase A-Aided Escherichia Coli Expression System for Functional Osteoprotegerin Cysteine-Rich Domain. Appl. Microbiol. Biotechnol. 2017, 101, 4923–4933. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Deng, X.; Li, X.; Wang, X.; Wang, S.; Xu, H. Application of Immobilized Thrombin for Production of S-Thanatin Expressed in Escherichia coli. Appl. Microbiol. Biotechnol. 2011, 92, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Merrifield, R.B. Solid-Phase Peptide Synthesis. Adv. Enzymol. Relat. Areas Mol. Biol. 1969, 32, 221–296. [Google Scholar] [PubMed]
- Behrendt, R.; White, P.; Offer, J. Advances in Fmoc Solid-phase Peptide Synthesis. J. Pept. Sci. 2016, 22, 4–27. [Google Scholar] [CrossRef]
- Pires, D.A.T.; Bemquerer, M.P.; Do Nascimento, C.J. Some Mechanistic Aspects on Fmoc Solid Phase Peptide Synthesis. Int. J. Pept. Res. Ther. 2014, 20, 53–69. [Google Scholar] [CrossRef]
- Moura, E.C.C.M.; Baeta, T.; Romanelli, A.; Laguri, C.; Martorana, A.M.; Erba, E.; Simorre, J.-P.; Sperandeo, P.; Polissi, A. Thanatin Impairs Lipopolysaccharide Transport Complex Assembly by Targeting LptC-LptA Interaction and Decreasing LptA Stability. Front. Microbiol. 2020, 11, 909. [Google Scholar] [CrossRef]
- Vetterli, S.U.; Zerbe, K.; Mueller, M.; Urfer, M.; Mondal, M.; Wang, S.-Y.; Moehle, K.; Zerbe, O.; Vitale, A.; Pessi, G.; et al. Thanatin Targets the Intermembrane Protein Complex Required for Lipopolysaccharide Transport in Escherichia coli. Sci. Adv. 2018, 4, eaau2634. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, W.; Deng, L.; Ming, J.; Yao, S.; Zeng, K. Control of Sour Rot in Citrus Fruit by Three Insect Antimicrobial Peptides. Postharvest Biol. Technol. 2019, 149, 200–208. [Google Scholar] [CrossRef]
- Pages, J.M.; Dimarcq, J.L.; Quenin, S.; Hetru, C. Thanatin Activity on Multidrug Resistant Clinical Isolates of Enterobacter aerogenes and Klebsiella pneumoniae. Int. J. Antimicrob. Agents 2003, 22, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Fan, X.; Li, L.; Wang, H.; Ding, J.; Hongbin, W.; Zhao, R.; Gou, L.; Shen, Z.; Xi, T. Interaction of Antimicrobial Peptide S-Thanatin with Lipopolysaccharide in Vitro and in an Experimental Mouse Model of Septic Shock Caused by a Multidrug-Resistant Clinical Isolate of Escherichia coli. Int. J. Antimicrob. Agents 2010, 35, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Lu, J.; Fang, C.; Zhou, Y.; Bai, H.; Zhang, X.; Xue, X.; Chen, Y.; Luo, X. Underlying Mechanism of In Vivo and In Vitro Activity of C-Terminal-Amidated Thanatin Against Clinical Isolates of Extended-Spectrum Beta-Lactamase-Producing Escherichia coli. J. Infect. Dis. 2011, 203, 273–282. [Google Scholar] [CrossRef]
- Ma, B.; Niu, C.; Zhou, Y.; Xue, X.; Meng, J.; Luo, X.; Hou, Z. The Disulfide Bond of the Peptide Thanatin Is Dispensible for Its Antimicrobial Activity In Vivo and In Vitro. Antimicrob. Agents Chemother. 2016, 60, 4283–4289. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Da, F.; Liu, B.; Xue, X.; Xu, X.; Zhou, Y.; Li, M.; Li, Z.; Ma, X.; Meng, J.; et al. R-Thanatin Inhibits Growth and Biofilm Formation of Methicillin-Resistant Staphylococcus epidermidis In Vivo and In Vitro. Antimicrob. Agents Chemother. 2013, 57, 5045–5052. [Google Scholar] [CrossRef] [PubMed]
- Javadmanesh, A.; Mohammadi, E.; Mousavi, Z.; Azghandi, M.; Tanhaiean, A. Antibacterial Effects Assessment on Some Livestock Pathogens, Thermal Stability and Proposing a Probable Reason for Different Levels of Activity of Thanatin. Sci. Rep. 2021, 11, 10890. [Google Scholar] [CrossRef]
- Ma, B.; Fang, C.; Lu, L.; Wang, M.; Xue, X.; Zhou, Y.; Li, M.; Hu, Y.; Luo, X.; Hou, Z. The Antimicrobial Peptide Thanatin Disrupts the Bacterial Outer Membrane and Inactivates the NDM-1 Metallo-β-Lactamase. Nat. Commun. 2019, 10, 3517. [Google Scholar] [CrossRef]
- Akhlaghi, M.; Tarighi, S.; Taheri, P. Effect of Antimicrobial Peptides and Monoterpenes on Control of Fire Blight. Span. J. Agric. Res. 2020, 18, e1002. [Google Scholar] [CrossRef]
- Cirioni, O.; Wu, G.; Li, L.; Orlando, F.; Silvestri, C.; Ghiselli, R.; Shen, Z.; Gabrielli, E.; Brescini, L.; Lezoche, G.; et al. S-Thanatin in Vitro Prevents Colistin Resistance and Improves Its Efficacy in an Animal Model of Pseudomonas aeruginosa Sepsis. Peptides 2011, 32, 697–701. [Google Scholar] [CrossRef]
- Wu, G.-Q.; Ding, J.-X.; Li, L.-X.; Wang, H.; Zhao, R.; Shen, Z.-L. Activity of the Antimicrobial Peptide and Thanatin Analog S-Thanatin on Clinical Isolates of Klebsiella pneumoniae Resistant to Conventional Antibiotics with Different Structures. Curr. Microbiol. 2009, 59, 147–153. [Google Scholar] [CrossRef]
- Xia, X.; Song, S.; Zhang, S.; Wang, W.; Zhou, J.; Fan, B.; Li, L.; Dong, H.; Luo, C.; Li, B.; et al. The Synergy of Thanatin and Cathelicidin-BF-15a3 Combats Escherichia coli O157:H7. Int. J. Food Microbiol. 2023, 386, 110018. [Google Scholar] [CrossRef]
- Raghuraman, H.; Chattopadhyay, A. Melittin: A Membrane-Active Peptide with Diverse Functions. Biosci. Rep. 2007, 27, 189–223. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Qian, K.; Liu, G.; Sun, L.; Zhou, G.; Li, J.; Fang, X.; Ge, H.; Lv, Z. Design and Activity Study of a Melittin-Thanatin Hybrid Peptide. AMB Express 2019, 9, 14. [Google Scholar] [CrossRef]
- Suits, M.D.L.; Sperandeo, P.; Deho, G.; Polissi, A.; Jia, Z. Novel Structure of the Conserved Gram-Negative Lipopolysaccharide Transport Protein A and Mutagenesis Analysis. J. Mol. Biol. 2008, 380, 476–488. [Google Scholar] [CrossRef]
- Qiao, S.; Luo, Q.; Zhao, Y.; Zhang, X.C.; Huang, Y. Structural Basis for Lipopolysaccharide Insertion in the Bacterial Outer Membrane. Nature 2014, 511, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Iadanza, M.G.; Higgins, A.J.; Schiffrin, B.; Calabrese, A.N.; Brockwell, D.J.; Ashcroft, A.E.; Radford, S.E.; Ranson, N.A. Lateral Opening in the Intact β-Barrel Assembly Machinery Captured by Cryo-EM. Nat. Commun. 2016, 7, 12865. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, J.; Niu, G.; Shui, W.; Sun, Y.; Zhou, H.; Zhang, Y.; Yang, C.; Lou, Z.; Rao, Z. A Structural View of the Antibiotic Degradation Enzyme NDM-1 from a Superbug. Protein Cell 2011, 2, 384–394. [Google Scholar] [CrossRef]
- Henderson, J.C.; Zimmerman, S.M.; Crofts, A.A.; Boll, J.M.; Kuhns, L.G.; Herrera, C.M.; Trent, M.S. The Power of Asymmetry: Architecture and Assembly of the Gram-Negative Outer Membrane Lipid Bilayer. Annu. Rev. Microbiol. 2016, 70, 255–278. [Google Scholar] [CrossRef]
- May, K.L.; Silhavy, T.J. Making a Membrane on the Other Side of the Wall. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2017, 1862, 1386–1393. [Google Scholar] [CrossRef]
- Martorana, A.M.; Moura, E.C.C.M.; Sperandeo, P.; Di Vincenzo, F.; Liang, X.; Toone, E.; Zhou, P.; Polissi, A. Degradation of Components of the Lpt Transenvelope Machinery Reveals LPS-Dependent Lpt Complex Stability in Escherichia coli. Front. Mol. Biosci. 2021, 8, 758228. [Google Scholar] [CrossRef]
- Sherman, D.J.; Xie, R.; Taylor, R.J.; George, A.H.; Okuda, S.; Foster, P.J.; Needleman, D.J.; Kahne, D. Lipopolysaccharide Is Transported to the Cell Surface by a Membrane-to-Membrane Protein Bridge. Science 2018, 359, 798–801. [Google Scholar] [CrossRef]
- Ruiz, N.; Gronenberg, L.S.; Kahne, D.; Silhavy, T.J. Identification of Two Inner-Membrane Proteins Required for the Transport of Lipopolysaccharide to the Outer Membrane of Escherichia coli. Proc. Natl. Acad. Sci. USA 2008, 105, 5537–5542. [Google Scholar] [CrossRef]
- Freinkman, E.; Okuda, S.; Ruiz, N.; Kahne, D. Regulated Assembly of the Transenvelope Protein Complex Required for Lipopolysaccharide Export. Biochemistry 2012, 51, 4800–4806. [Google Scholar] [CrossRef] [PubMed]
- Villa, R.; Martorana, A.M.; Okuda, S.; Gourlay, L.J.; Nardini, M.; Sperandeo, P.; Deho, G.; Bolognesi, M.; Kahne, D.; Polissi, A. The Escherichia coli Lpt Transenvelope Protein Complex for Lipopolysaccharide Export Is Assembled via Conserved Structurally Homologous Domains. J. Bacteriol. 2013, 195, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Merten, J.A.; Schultz, K.M.; Klug, C.S. Concentration-dependent Oligomerization and Oligomeric Arrangement of LptA. Protein Sci. 2012, 21, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Narita, S.; Tokuda, H. Biochemical Characterization of an ABC Transporter LptBFGC Complex Required for the Outer Membrane Sorting of Lipopolysaccharides. FEBS Lett. 2009, 583, 2160–2164. [Google Scholar] [CrossRef] [PubMed]
- Sperandeo, P.; Martorana, A.M.; Polissi, A. The Lpt ABC Transporter for Lipopolysaccharide Export to the Cell Surface. Res. Microbiol. 2019, 170, 366–373. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, Z.; Tang, X.; Paterson, N.G.; Dong, C. Structural and Functional Insights into the Lipopolysaccharide ABC Transporter LptB2FG. Nat. Commun. 2017, 8, 222. [Google Scholar] [CrossRef]
- Schultz, K.M.; Feix, J.B.; Klug, C.S. Disruption of LptA Oligomerization and Affinity of the LptA–LptC Interaction. Protein Sci. 2013, 22, 1639–1645. [Google Scholar] [CrossRef]
- Robinson, J.A. Folded Synthetic Peptides and Other Molecules Targeting Outer Membrane Protein Complexes in Gram-Negative Bacteria. Front. Chem. 2019, 7, 45. [Google Scholar] [CrossRef]
- Zha, Z.; Li, C.; Li, W.; Ye, Z.; Pan, J. LptD Is a Promising Vaccine Antigen and Potential Immunotherapeutic Target for Protection against Vibrio Species Infection. Sci. Rep. 2016, 6, 38577. [Google Scholar] [CrossRef]
- Lundquist, K.P.; Gumbart, J.C. Presence of Substrate Aids Lateral Gate Separation in LptD. Biochim. Biophys. Acta (BBA)—Biomembr. 2020, 1862, 183025. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, F.; Sauer, J.B.; Qiu, X.; Corey, R.A.; Cassidy, C.K.; Mynors-Wallis, B.; Mehmood, S.; Bolla, J.R.; Stansfeld, P.J.; Robinson, C.V. Dynamics of an LPS Translocon Induced by Substrate and an Antimicrobial Peptide. Nat. Chem. Biol. 2021, 17, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Heuck, A.; Schleiffer, A.; Clausen, T. Augmenting β-Augmentation: Structural Basis of How BamB Binds BamA and May Support Folding of Outer Membrane Proteins. J. Mol. Biol. 2011, 406, 659–666. [Google Scholar] [CrossRef]
- Mikheyeva, I.V.; Sun, J.; Huang, K.C.; Silhavy, T.J. Mechanism of Outer Membrane Destabilization by Global Reduction of Protein Content. Nat. Commun. 2023, 14, 5715. [Google Scholar] [CrossRef] [PubMed]
- Bakelar, J.; Buchanan, S.K.; Noinaj, N. Structural Snapshots of the Β-barrel Assembly Machinery. FEBS J. 2017, 284, 1778–1786. [Google Scholar] [CrossRef]
- Dong, C.; Hou, H.-F.; Yang, X.; Shen, Y.-Q.; Dong, Y.-H. Structure of Escherichia coli BamD and Its Functional Implications in Outer Membrane Protein Assembly. Acta Crystallogr. D Biol. Crystallogr. 2012, 68, 95–101. [Google Scholar] [CrossRef]
- Jansen, K.B.; Baker, S.L.; Sousa, M.C. Crystal Structure of BamB Bound to a Periplasmic Domain Fragment of BamA, the Central Component of the Beta-Barrel Assembly Machine. J. Biol. Chem. 2015, 290, 2126–2136. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhan, L.-H.; Hou, H.-F.; Gao, Z.-Q.; Xu, J.-H.; Dong, C.; Dong, Y.-H. Structural Basis for the Interaction of BamB with the POTRA3–4 Domains of BamA. Acta Crystallogr. D Struct. Biol. 2016, 72, 236–244. [Google Scholar] [CrossRef]
- Sutterlin, H.A.; Zhang, S.; Silhavy, T.J. Accumulation of Phosphatidic Acid Increases Vancomycin Resistance in Escherichia coli. J. Bacteriol. 2014, 196, 3214–3220. [Google Scholar] [CrossRef]
- Snyder, D.S.; McIntosh, T.J. The Lipopolysaccharide Barrier: Correlation of Antibiotic Susceptibility with Antibiotic Permeability and Fluorescent Probe Binding Kinetics. Biochemistry 2000, 39, 11777–11787. [Google Scholar] [CrossRef]
- Sperandeo, P.; Martorana, A.M.; Polissi, A. The Lipopolysaccharide Transport (Lpt) Machinery: A Nonconventional Transporter for Lipopolysaccharide Assembly at the Outer Membrane of Gram-Negative Bacteria. J. Biol. Chem. 2017, 292, 17981–17990. [Google Scholar] [CrossRef]
- Edwards, I.A.; Elliott, A.G.; Kavanagh, A.M.; Zuegg, J.; Blaskovich, M.A.T.; Cooper, M.A. Contribution of Amphipathicity and Hydrophobicity to the Antimicrobial Activity and Cytotoxicity of Beta-Hairpin Peptides. ACS Infect. Dis. 2016, 2, 442–450. [Google Scholar] [CrossRef]
- Dash, R.; Bhattacharjya, S. Thanatin: An Emerging Host Defense Antimicrobial Peptide with Multiple Modes of Action. Int. J. Mol. Sci. 2021, 22, 1522. [Google Scholar] [CrossRef]
- Ofosu-Appiah, F.; Acquah, E.E.; Mohammed, J.; Sakyi Addo, C.; Agbodzi, B.; Ofosu, D.A.S.; Myers, C.J.; Mohktar, Q.; Ampomah, O.-W.; Ablordey, A.; et al. Klebsiella pneumoniae ST147 Harboring blaNDM-1, Multidrug Resistance and Hypervirulence Plasmids. Microbiol. Spectr. 2024, 12, e03017-23. [Google Scholar] [CrossRef]
- Salari-jazi, A.; Mahnam, K.; Sadeghi, P.; Damavandi, M.S.; Faghri, J. Discovery of Potential Inhibitors against New Delhi Metallo-β-Lactamase-1 from Natural Compounds: In Silico-Based Methods. Sci. Rep. 2021, 11, 2390. [Google Scholar] [CrossRef]
- Yong, D.; Toleman, M.A.; Giske, C.G.; Cho, H.S.; Sundman, K.; Lee, K.; Walsh, T.R. Characterization of a New Metallo-β-Lactamase Gene, blaNDM-1, and a Novel Erythromycin Esterase Gene Carried on a Unique Genetic Structure in Klebsiella pneumoniae Sequence Type 14 from India. Antimicrob. Agents Chemother. 2009, 53, 5046–5054. [Google Scholar] [CrossRef]
- Johnson, A.P.; Woodford, N. Global Spread of Antibiotic Resistance: The Example of New Delhi Metallo-β-Lactamase (NDM)-Mediated Carbapenem Resistance. J. Med. Microbiol. 2013, 62, 499–513. [Google Scholar] [CrossRef]
- Zhang, H.; Hao, Q. Crystal Structure of NDM-1 Reveals a Common Β-lactam Hydrolysis Mechanism. FASEB J. 2011, 25, 2574–2582. [Google Scholar] [CrossRef]
- Rehman, S.U.; De Castro, F.; Marini, P.; Aprile, A.; Benedetti, M.; Fanizzi, F.P. Vermibiochar: A Novel Approach for Reducing the Environmental Impact of Heavy Metals Contamination in Agricultural Land. Sustainability 2023, 15, 9380. [Google Scholar] [CrossRef]
- Kokilaramani, S.; Al-Ansari, M.M.; Rajasekar, A.; Al-Khattaf, F.S.; Hussain, A.; Govarthanan, M. Microbial Influenced Corrosion of Processing Industry by Re-Circulating Waste Water and Its Control Measures—A Review. Chemosphere 2021, 265, 129075. [Google Scholar] [CrossRef]
- Li, C.; Li, C.; Yu, H.; Cheng, Y.; Xie, Y.; Yao, W.; Guo, Y.; Qian, H. Chemical Food Contaminants during Food Processing: Sources and Control. Crit. Rev. Food Sci. Nutr. 2021, 61, 1545–1555. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.P.; Erickson, M.C. Opportunities for Mitigating Pathogen Contamination during On-Farm Food Production. Int. J. Food Microbiol. 2012, 152, 54–74. [Google Scholar] [CrossRef] [PubMed]

| Microorganism | MIC | Reference | ||
|---|---|---|---|---|
| Thanatin | A-Thanatin | S-Thanatin | ||
| Gram-negative bacteria | ||||
| Escherichia coli D22 | 0.6 µM | 1.2 µM | - | [8] |
| E. coli 1106 | 1.2 µM | 1.2 µM | - | [8] |
| E. coli ATCC 25922 | 1.6 µM | 6.4 µM | 1.6 µM | [24,44,48] |
| E. coli ATCC 35218 | 0.4 µM | - | - | [48] |
| E. coli BL21 | 0.5 µM | - | - | [19] |
| E. coli E79466 | - | - | 5 µM | [43] |
| E. coli O157:H7 | 2.5 µM | - | - | [47] |
| E. coli k99 | 5 µM | - | - | [47] |
| MDR-EC XJ74283 | - | 3.2 µM | - | [44] |
| MDR-EC JT11092 | - | 0.8 µM | - | [44] |
| ESBL-EC ATCC35218 | - | 1.6 µM | - | [44] |
| ESBL-EC HN10318 | - | 0.8 µM | - | [44] |
| ESBL-EC JT11074 | - | 0.8 µM | - | [44] |
| ESBL-EC HN10322 | - | 0.8 µM | - | [44] |
| ESBL-EC SX49657 | - | 1.6 µM | - | [44] |
| ESBL-EC SX49660 | - | 1.6 µM | - | [44] |
| NDM-1 E. coli XJ141015 | 0.8 µM | - | - | [48] |
| NDM-1 E. coli XJ141026 | 0.8 µM | - | - | [48] |
| NDM-1 E. coli XJ141047 | 0.8 µM | - | - | [48] |
| Salmonella typhimurium | 1.2 µM | 1.2 µM | - | [8] |
| Salmonella enterica ATCC 14028 | 1 µM | - | - | [19] |
| Salmonella paratyphi C | 7.692 µM | - | - | [31] |
| Klebsiella pneumoniae | 1.2 µM | 1.2 µM | [8] | |
| K. pneumoniae ATCC 700603 | 3.2 µM | - | 1.6 µM | [24] |
| K. pneumoniae ATCC 13883 | 1 µM | - | 1.6 µM | [1,19] |
| NDM-1 K. pneumoniae XJ155017 | 3.2 µM | - | - | [48] |
| NDM-1 K. pneumoniae XJ155018 | 3.2 µM | - | - | [48] |
| NDM-1 K. pneumoniae XJ155019 | 3.2 µM | - | - | [48] |
| NDM-1 K. pneumoniae XJ155020 | 3.2 µM | - | - | [48] |
| ESBL-KP XJ75297 | - | 1.6 µM | - | [44] |
| ESBL-KP HN10349 | - | 0.8 µM | - | [44] |
| ESBL-KP HN10500 | - | 0.8 µM | - | [44] |
| Klebsiella ornithinolytica ATCC 31898 | - | - | 3.2 µM | [23] |
| Klebsiella oxytoca ATCC 43086 | - | - | 3.2 µM | [23] |
| Enterobacter cloacae | 2.5 µM | 2.5 µM | - | [8] |
| E. cloacae ATCC 13047 | - | - | 3.2 µM | [23] |
| Erwinia carotovora | 20 µM | 20 µM | - | [8] |
| Pseudomonas aeruginosa | 40 µM | 5 µM | - | [8] |
| P. aeruginosa ATCC 27853 | 1 µM | - | 12.8 µM | [1,19] |
| MDR-PA XJ75315 | - | 6.4 µM | - | [44] |
| Enterobacter aerogenes ATCC 49701 | - | - | 1.6 µM | [23] |
| Citrobacter freundii | 6 µM | - | - | [17] |
| Erwinia amylovora | 6.248 µM | - | - | [49] |
| Shigella dysenteriae | 15.384 µM | - | - | [31] |
| Microorganism | MIC | Reference | ||
|---|---|---|---|---|
| Thanatin | A-Thanatin | S-Thanatin | ||
| Gram-positive bacteria | ||||
| Aerococcus viridans | 1.2 µM | 1.2 µM | - | [8] |
| Micrococcus luteus | 2.5 µM | 2.5 µM | - | [8] |
| Bacillus megaterium | 5 µM | 1.2 µM | - | [8] |
| Bacillus subtilis | 2 µM | 5 µM | - | [8,19] |
| B. subtilis ATCC 21332 | 1.6 µM | - | 3.2 µM | [15] |
| Staphylococcus aureus | - | 40 µM | - | [8] |
| S. aureus ATCC 25923 | 1 µM | - | - | [19] |
| S. aureus ATCC 29213 | - | 102.4 µM | - | [44] |
| S. haemolyticus XJ31196 | 1.6 µM | - | - | [46] |
| S. haemolyticus XJ31245 | 0.8 µM | - | - | [46] |
| S. haemolyticus SX92421 | 3.2 µM | - | - | [46] |
| S. haemolyticus SX92464 | 1.6 µM | - | - | [46] |
| S.hominis XJ31287 | 0.8 µM | - | - | [46] |
| S.hominis XJ31303 | 3.2 µM | - | - | [46] |
| S.hominis SX92357 | 1.6 µM | - | - | [46] |
| S.hominis SX92433 | 0.8 µM | - | - | [46] |
| MRSA ATCC 33591 | 3.844 µM | - | - | [31] |
| MRSA XJ75302 | 25.6 µM | >102.4 µM | - | [44,46] |
| MRSE XJ75284 | 1.6 µM | 6.4 µM | - | [44,46] |
| MRSE XJ31106 | 0.8 µM | - | - | [46] |
| MRSE XJ31204 | 1.6 µM | - | - | [46] |
| MRSE XJ31276 | 1.6 µM | - | - | [46] |
| MRSE XJ31318 | 3.2 µM | - | - | [46] |
| MRSE SX70535 | 0.8 µM | - | - | [46] |
| MRSE SX70582 | 1.6 µM | - | - | [46] |
| MRSE SX70810 | 1.6 µM | - | - | [46] |
| MRSE SX70892 | 3.2 µM | - | - | [46] |
| MRSE SX70893 | 1.6 µM | - | - | [46] |
| Pediococcus acidolactici | 40 µM | 40 µM | - | [8] |
| Enterococcus faecalis ATCC 29212 | 0.5 µM | - | 25.6 µM | [1,19] |
| Streptococcus pyogenes ATCC 19615 | 0.5 µM | - | - | [19] |
| Listeria monocytegenes | 30.768 µM | - | - | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Wu, Q.; Xu, T.; Malakar, P.K.; Zhu, Y.; Liu, J.; Zhao, Y.; Zhang, Z. Thanatin: A Promising Antimicrobial Peptide Targeting the Achilles’ Heel of Multidrug-Resistant Bacteria. Int. J. Mol. Sci. 2024, 25, 9496. https://doi.org/10.3390/ijms25179496
Liu Q, Wu Q, Xu T, Malakar PK, Zhu Y, Liu J, Zhao Y, Zhang Z. Thanatin: A Promising Antimicrobial Peptide Targeting the Achilles’ Heel of Multidrug-Resistant Bacteria. International Journal of Molecular Sciences. 2024; 25(17):9496. https://doi.org/10.3390/ijms25179496
Chicago/Turabian StyleLiu, Qianhui, Qian Wu, Tianming Xu, Pradeep K. Malakar, Yongheng Zhu, Jing Liu, Yong Zhao, and Zhaohuan Zhang. 2024. "Thanatin: A Promising Antimicrobial Peptide Targeting the Achilles’ Heel of Multidrug-Resistant Bacteria" International Journal of Molecular Sciences 25, no. 17: 9496. https://doi.org/10.3390/ijms25179496







