Interactions at the Oviposition Scar: Molecular and Metabolic Insights into Elaeagnus angustifolia’s Resistance Response to Anoplophora glabripennis
Abstract
:1. Introduction
2. Results
2.1. ALBs’ OS Quantity on the Russian Olive
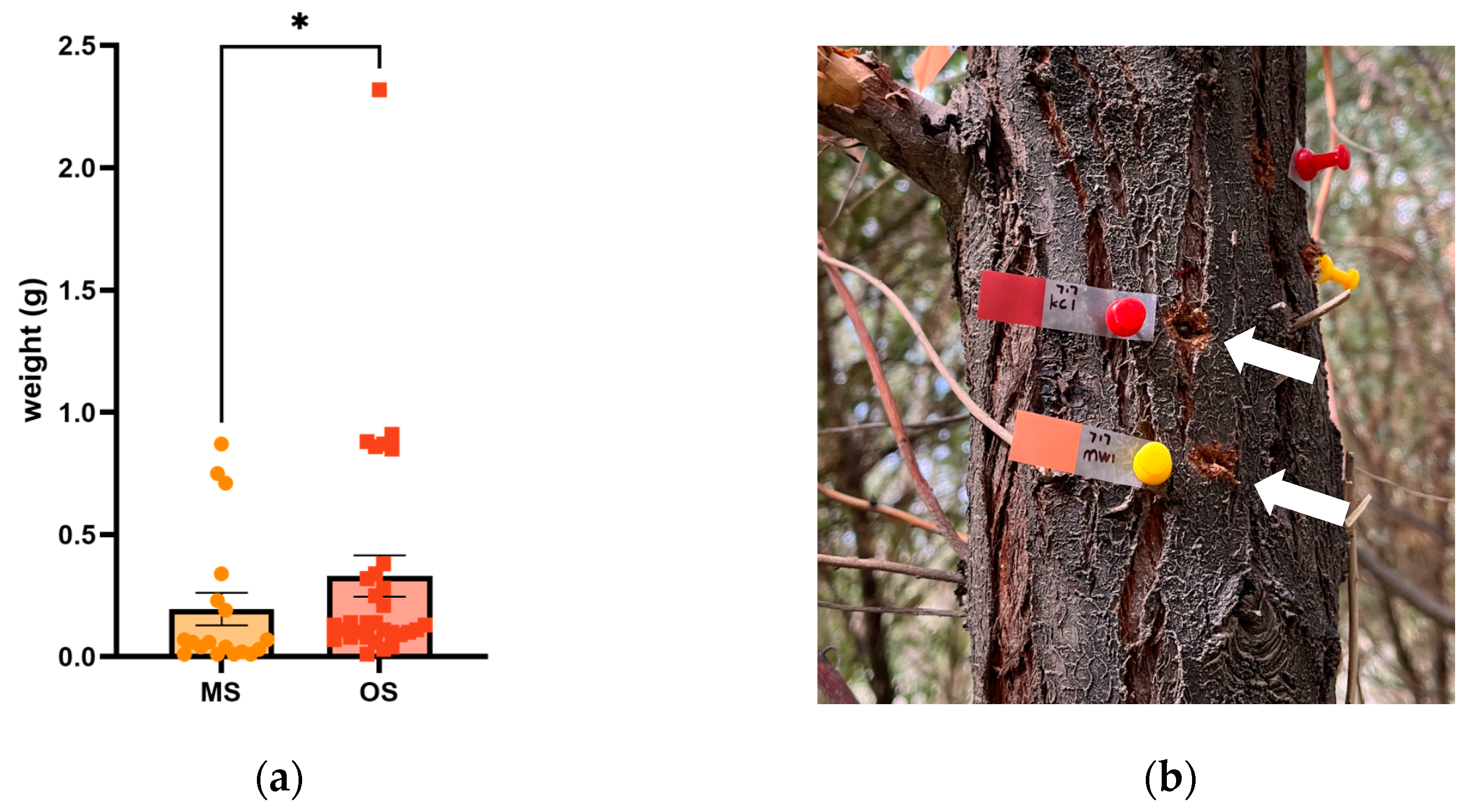
2.2. Differences in the Gum Rate and Weight between OSs and MSs in Russian Olive Trees
2.3. Transcriptome Analysis of OSs, MSs, and HBs
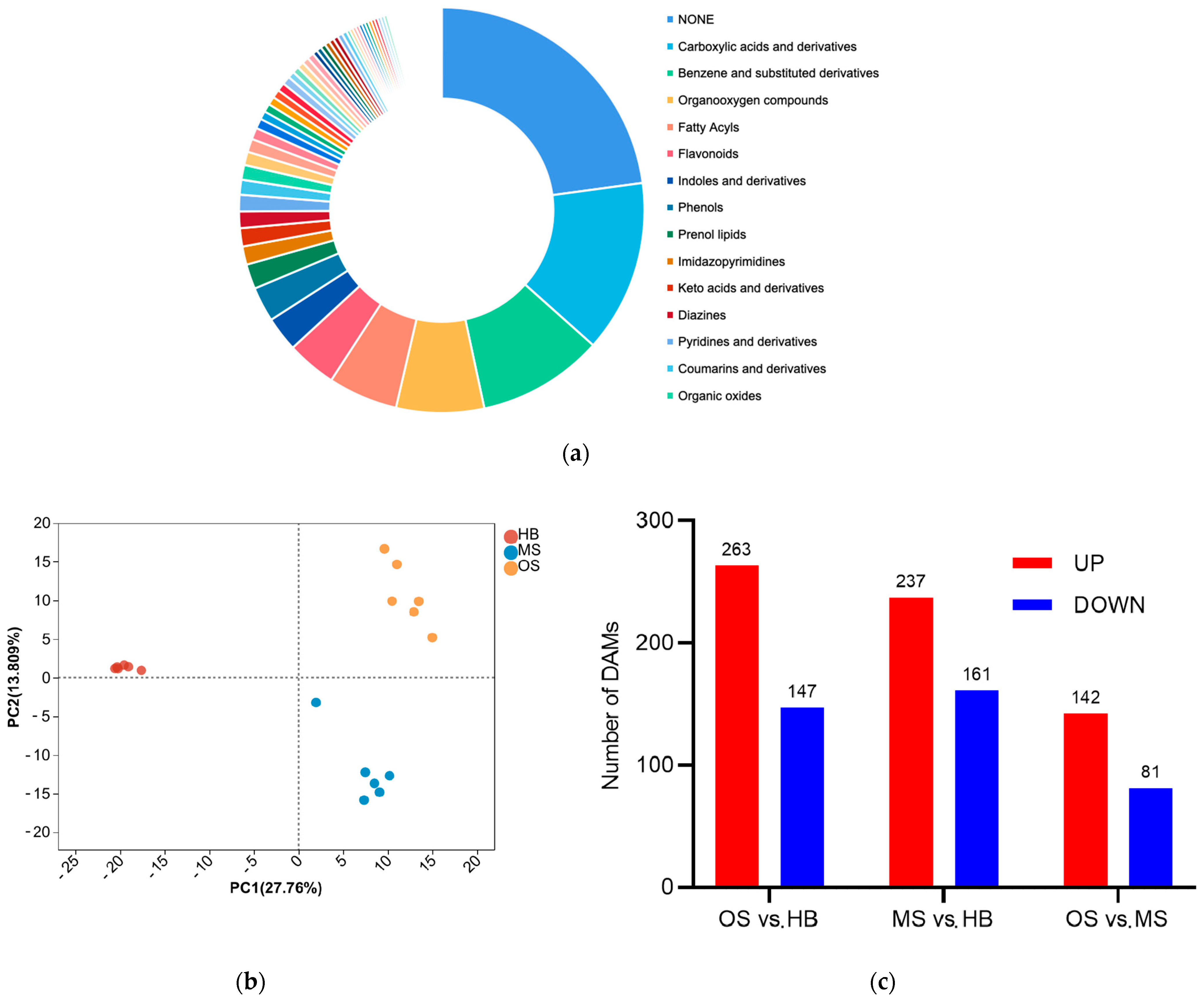
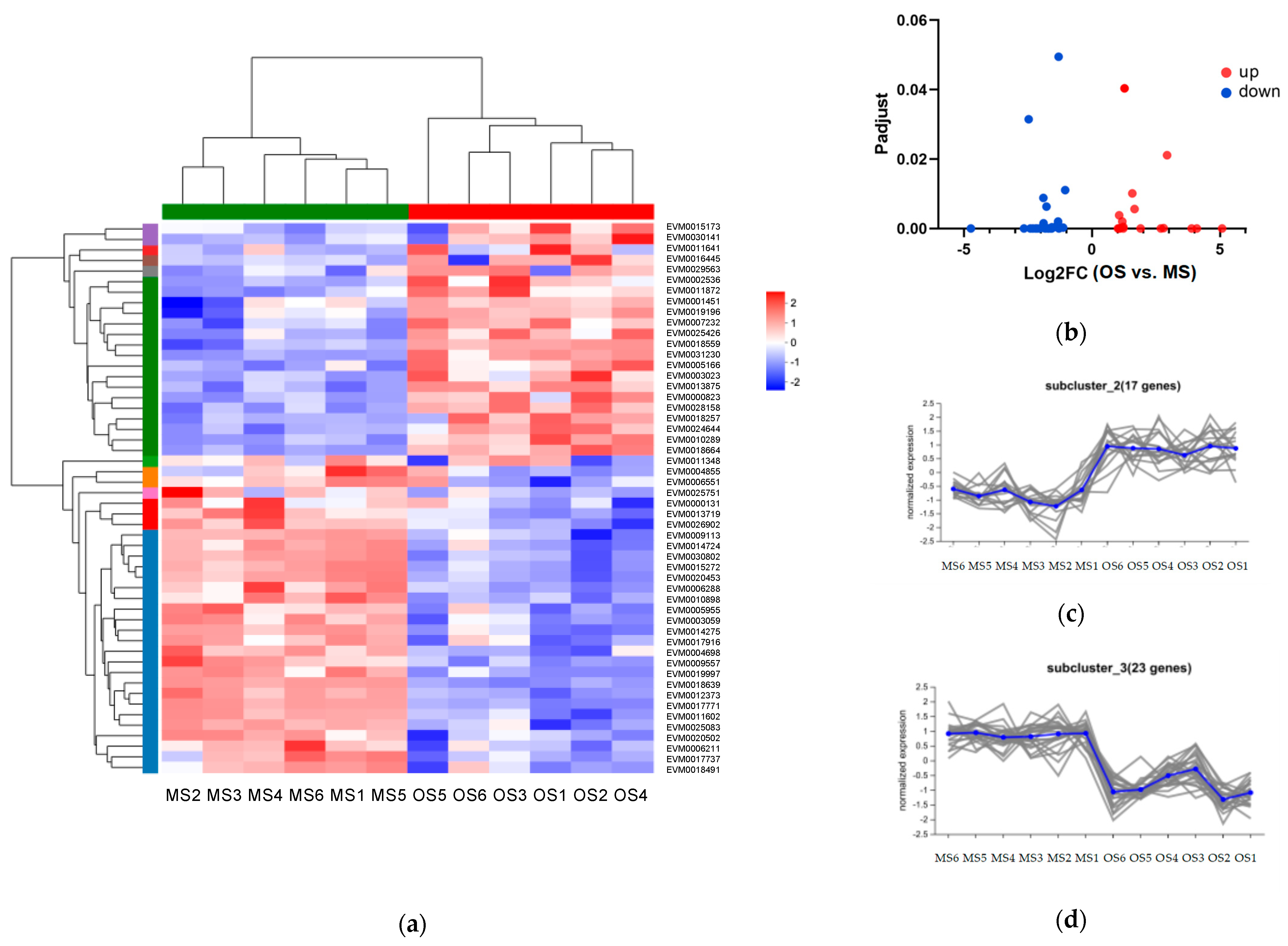
2.3.1. DEGs among OSs, MSs, and HBs
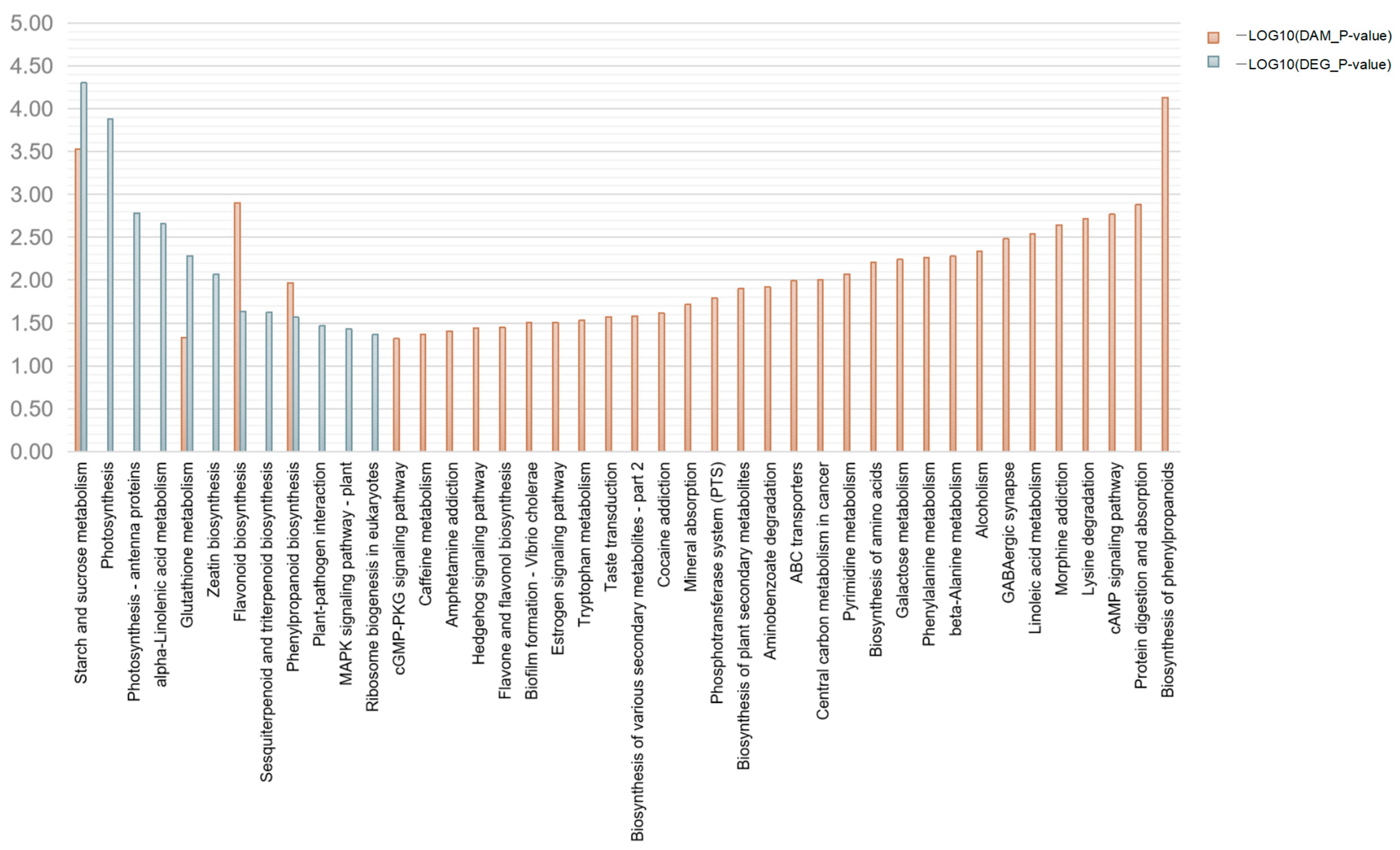
2.3.2. KEGG Pathway Annotations of DEGs
2.4. Differences in Metabolic Components among OSs, MSs, and HBs
2.4.1. Differentially Accumulated Metabolites among OSs, MSs, and HBs
2.4.2. KEGG Pathway Annotations of DAMs
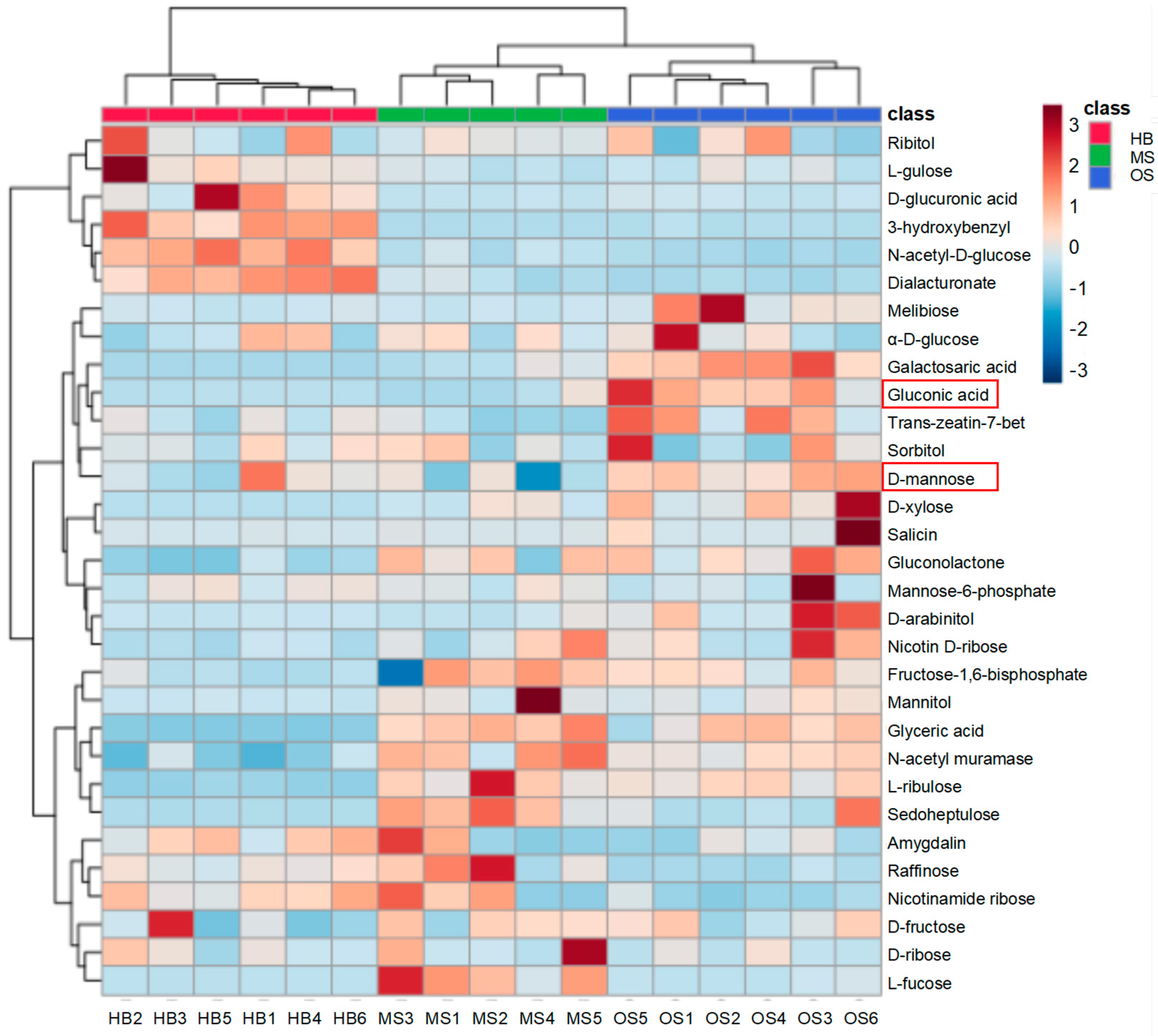
2.5. Differences in the Carbohydrates and Carbohydrate Conjugates among OSs, MSs, and HBs
2.6. Differential Co-Expression of Genes and Metabolites of Russian Olive Induced by ALBs
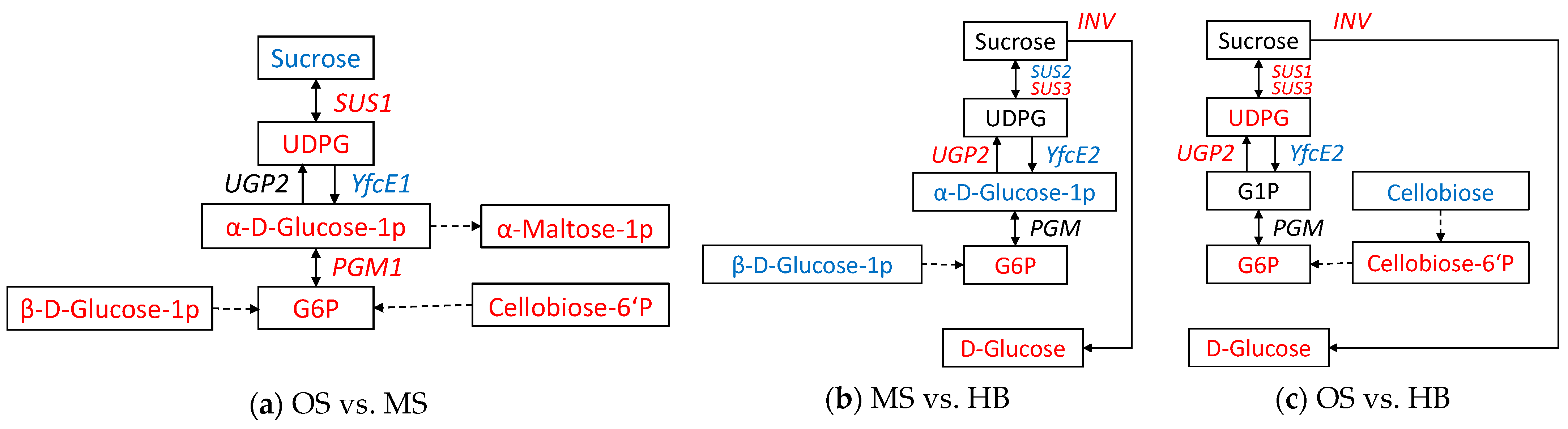
2.7. Potential Candidate DEGs and DAMs in the Starch and Sucrose Metabolism Pathway
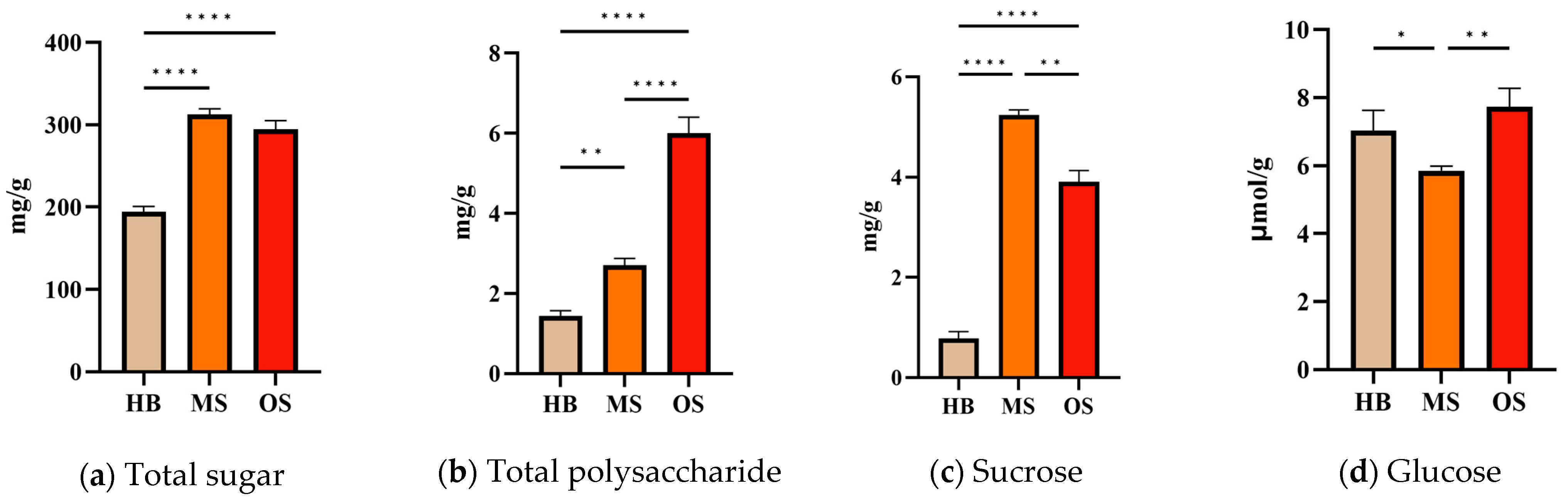
2.8. Total Sugar, Total Polysaccharide, Sucrose, and Glucose Content of OSs, MSs, and HBs
3. Discussion
4. Materials and Methods
4.1. Sampling Site and Sample Preparation
4.2. High-Throughput Transcriptomics Analysis
4.3. Metabolomics Profiling Analysis
4.4. Correlation Network Analysis of Transcriptomics and Metabolomics Data
4.5. Total Sugar, Total Polysaccharide, Sucrose, and Glucose Content
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhigang, W. Study on the Occurrence Dynamics of Anoplophora glabripennis (Coleoptera: Cerambycidae) and Its Control Measures. Ph.D. Thesis, Northeast Forestry University, Harrbin, China, April 2004. [Google Scholar]
- Chen, S. Cerambycidae. In List of Chinese Insects; Huayu Nature Book Trade Co., Ltd.: Beijing, China, 1959; Volume 1. (In Chinese) [Google Scholar]
- Hua, L. Chelisochidae. In List of Chinese Insects; Huayu Nature Book Trade Co., Ltd.: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Wang, Y. Risk Analysis of Anoplophora glabripennis in Western China. Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2006. [Google Scholar]
- Cavey, J.F.; Hoebeke, E.R.; Passoa, S.; Lingafelter, S.W. A new exotic threat to North American hardwood forests: An Asian longhorned beetle, Anoplophora glabripennis (motschulsky) (Coleoptera: Cerambycidae). I. Larval description and diagnosis. Proc. Entomol. Soc. Wash 1998, 100, 373–381. [Google Scholar]
- Hérard, F.; Ciampitti, M.; Maspero, M.; Krehan, H.; Bialooki, P. Anoplophora species in Europe: Infestations and management processes. Bull. OEPP/EPPO Bull. 2006, 36, 470–474. [Google Scholar] [CrossRef]
- Cocquempot, C.; Prost, M.; Carmignac, D. Interceptions and introductions in France of Asian longhorn beetles: Case of Anoplophora glabripennis (Motschulsky) and A. chinensis (Forster) (Coleoptera Cerambycidae). Bull. Mens. De La Société Linnéenne De Lyon. 2003, 72, 273–278. [Google Scholar]
- Wubier, A.; Li, Y.; Ailie, W.; Dong, X.; Su, W.; Maimaiti, A.; Tuerxun, A. Study on exudation of Elaeagnus angustifolia and its influencing factors. Arid. Land Geogr. 2005, 28, 521–525. (In Chinese) [Google Scholar] [CrossRef]
- Mao, Y.; Cui, X.; Wang, H.; Qin, X.; Liu, Y.; Yin, Y.; Su, X.; Tang, J.; Wang, F.; Ma, F.; et al. De novo assembly provides new insights into the evolution of Elaeagnus angustifolia L. Plant Methods 2022, 18, 84. [Google Scholar] [CrossRef]
- Katz, G.L.; Shafroth, P.B. Biology, ecology and management of Elaeagnus angustifolia L. (Russian olive) in western North America. Wetlands 2003, 23, 763–777. [Google Scholar] [CrossRef]
- Peter Lesica, S.M. Russian olive invasion into cottonwood forests along a regulated river in north-central Montana. Can. J. Bot. 1999, 77, 1077–1083. [Google Scholar] [CrossRef]
- He, P.; Huang, J. Behavior of Anoplophora glabripennis adults. Acta Entomol. Sin. 1993, 36, 51–55. (In Chinese) [Google Scholar] [CrossRef]
- Tian, R.; Yu, J.; Zhao, W. Preliminary study on the trap function of Elaeagnus angustifolia on Anoplophora glabripennis population. J. Inn. Mong. For. Sci. Tech. 2003, 4, 23–25. (In Chinese) [Google Scholar] [CrossRef]
- Li, F.; Liu, R.; Baoshan, W.; Wu, T. Selection of Trap Trees for Controlling Anoplophora glabripennis and A. nobilis. J. Beijing For. Univ. 1999, 4, 85–89. (In Chinese) [Google Scholar]
- Wang, L.; Li, C.; Luo, Y.; Wang, G.; Dou, Z.; Haq, I.U.; Shang, S.; Cui, M. Current and future control of the wood-boring pest Anoplophora glabripennis. Insect Sci. 2023, 30, 1534–1551. [Google Scholar] [CrossRef]
- Shelton, A.M.; Nault, B.A. Dead-end trap cropping: A technique to improve management of the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Crop Prot. 2004, 23, 497–503. [Google Scholar] [CrossRef]
- Nie, X. Research status and prospect of Elaeagnus angustifolia. North. Hortic. 2007, 4, 67–69. (In Chinese) [Google Scholar] [CrossRef]
- Wang, G.; Nuermaimaiti, A.; Ajiaikebair, A. Analysis of factors influencing exudation of medicinal resource Elaeagnus angustifolia. Xinjiang Agric. Sci. 2018, 55, 697–702. [Google Scholar] [CrossRef]
- Li, Y.; Wubier, A.; Ailie, W.; Dong, X.; Su, W.; Aihaiti, Y.; Aibi, T.; Tan, X. Effects of different soil moisture conditions and temperatures on gum exudation of Elaeagnus angustifolia. J. Desert Res. 2006, 26, 155–158. (In Chinese) [Google Scholar] [CrossRef]
- Li, Y.; Adili, W.; Wumanjiang, A.; Dong, X.; Su, W.; Yakup, A.; Tuerxun, A.; Tan, X. The effect of irrigation on gum exudation of Elaeagnus Angustifolia. For. Pest. Dis. Control 2006, 5–7. (In Chinese) [Google Scholar] [CrossRef]
- Li, Y.; Pan, B.; Gulnur, S.; Adili, W.; Liu, L. Gum flow and geographical variations of two Elaeagnus angustifolia species. For. Sci. Tech. Dev. 2012, 26, 28–30. [Google Scholar] [CrossRef]
- Basu, S.; Varsani, S.; Louis, J. Altering Plant Defenses: Herbivore-Associated Molecular Patterns and Effector Arsenal of Chewing Herbivores. Mol. Plant Microbe Interact. 2018, 31, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Rse, U.S.R.; Tumlinson, J.H. Systemic induction of volatile release in cotton: How specific is the signal to herbivory? Planta 2005, 222, 327–335. [Google Scholar] [CrossRef]
- Jacob, V.; Tibère, R.; Nibouche, S. Few Sensory Cues Differentiate Host and Dead-End Trap Plant for the Sugarcane Spotted Borer Chilo sacchariphagus (Lepidoptera: Crambidae). J. Chem. Ecol. 2021, 47, 153–166. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.; Zhang, M.; Ye, Y.; Wang, L.; Zhu, J.; Chen, Z.; Zheng, X.; Wang, Y.; Wang, Y. Microbe-derived non-necrotic glycoside hydrolase family 12 proteins act as immunogenic signatures triggering plant defenses. J. Integr. Plant Biol. 2022, 64, 1966–1978. [Google Scholar] [CrossRef]
- Samara, R. Evaluation of 11 potential trap crops for root-knot nematode (RKN) control under glasshouse conditions. Open Agric. 2022, 7, 61–68. [Google Scholar] [CrossRef]
- Tian, R.; Lu, Q.; Zhang, Y.; Sang, H. Induce and control of Anoplophora glabripennis. J. Inn. Mong. For. Sci. Tech. 2009, 35, 42–46. [Google Scholar] [CrossRef]
- Wallace, J.W.; Mansell, R.L. Biochemical interaction between plants and insects || toward a general theory of plant antiherbivore chemistry. In Biochemical Interaction Between Plants and Insects; Springer: Berlin/Heidelberg, Germany, 1976. [Google Scholar]
- Hilker, M.; Fatouros, N.E. Resisting the onset of herbivore attack: Plants perceive and respond to insect eggs. Curr. Opin. Plant Biol. 2016, 32, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Seino, Y.; Suzuki, Y.; Sogawa, K. An Ovicidal Substance Produced by Rice Plants in Response to Oviposition by the Whitebacked Planthopper, Sogatella furcifera (HORVATH) (Homoptera: Delphacidae). Appl. Entomol. Zool. 1996, 31, 467–473. [Google Scholar] [CrossRef]
- Petzold-Maxwell, J.; Wong, S.; Arellano, C.; Gould, F. Host plant direct defence against eggs of its specialist herbivore, Heliothis subflexa. Ecol. Entomol. 2011, 36, 700–708. [Google Scholar] [CrossRef]
- Fatouros, N.E.; Pineda, A.; Huigens, M.E.; Broekgaarden, C.; Shimwela, M.M.; Figueroa Candia, I.A.; Verbaarschot, P.; Bukovinszky, T. Synergistic effects of direct and indirect defences on herbivore egg survival in a wild crucifer. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141254. [Google Scholar] [CrossRef] [PubMed]
- Broekaert, I.; Lee, H.I.; Kush, A.; Chua, N.H.; Raikhel, N. Wound-induced accumulation of mRNA containing a hevein sequence in laticifers of rubber tree (Hevea brasiliensis). Proc. Natl. Acad. Sci. USA 1990, 87, 7633–7637. [Google Scholar] [CrossRef]
- Chien, S.; Xiao, J.; Tseng, Y.; Kuo, Y.; Wang, S. Composition and antifungal activity of balsam from Liquidambar formosana Hance. Holzforschung 2013, 67, 345–351. [Google Scholar] [CrossRef]
- Gong, L.J.; Guo, S.X. Endophytic fungi from Dracaena cambodiana and Aquilaria sinensis and their antimicrobial activity. Afr. J. Biotechnol. 2009, 8, 731–736. [Google Scholar] [CrossRef]
- Amiri Tehranizadeh, Z.; Baratian, A.; Hosseinzadeh, H. Russian olive (Elaeagnus angustifolia) as a herbal healer. Bioimpacts 2016, 6, 155–167. [Google Scholar] [CrossRef]
- Chen, H.; Yang, Y.; Xue, J.; Wei, J.; Zhang, Z.; Chen, H. Comparison of Compositions and Antimicrobial Activities of Essential Oils from Chemically Stimulated Agarwood, Wild Agarwood and Healthy Aquilaria sinensis (Lour.) Gilg Trees. Molecules 2011, 16, 4884. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hou, Y. Chemical composition analysis of four types of domestic tree gums. Sci. Silv. Sin. 1988, 2, 253–254. CNKI:SUN:LYKE.0.1988-02-019. (In Chinese) [Google Scholar]
- Song, Z.; Qin, J.; Yao, Y.; Lai, X.; Li, X. A transcriptomic analysis unravels key factors in the regulation of stay-green disorder in peel of banana fruit (Fenjiao) caused by treatment with 1-MCP. Postharvest Biol. Tec. 2020, 168, 111290. [Google Scholar] [CrossRef]
- Blancoherrera, F.; Moreno, A.A.; Tapia, R.; Reyes, F.; Araya, M.; Alessio, C.D.; Parodi, A.; Orellana, A. The UDP-glucose: Glycoprotein glucosyltransferase (UGGT), a key enzyme in ER quality control, plays a significant role in plant growth as well as biotic and abiotic stress in Arab. Thaliana. BMC Plant Biol. 2015, 15, 127. [Google Scholar] [CrossRef]
- BHringer, J.; Fischer, D.; Mosler, G.; Hengge-Aronis, R. UDP-glucose is a potential intracellular signal molecule in the control of expression of sigma S and sigma S-dependent genes in Escherichia coli. J. Bacteriol. 1995, 177, 413–422. [Google Scholar] [CrossRef]
- He, M.J.; Liu, L.F.; Mu, G.J.; Hou, M.Y.; Cui, S.L. Molecular Cloning of Sucrose Synthase Gene and Expression Analysis under Drought Stress in Peanut (Arachis hypogaea L.). Acta Agron. Sin. 2012, 38, 2139–2146. [Google Scholar] [CrossRef]
- Stein, O.; Granot, D. An Overview of Sucrose Synthases in Plants. Front. Plant Sci. 2019, 10, 95. [Google Scholar] [CrossRef] [PubMed]
- Fernie, A.R.; Tauberger, E.; Lytovchenko, A.; Roessner, U.; Willmitzer, L.; Trethewey, R.N. Antisense repression of cytosolic phosphoglucomutase in potato (Solanum tuberosum ) results in severe growth retardation, reduction in tuber number and altered carbon metabolism. Planta 2002, 214, 510–520. [Google Scholar] [CrossRef]
- Kofler, H.; Häusler, R.E.; Schulz, B.; Gröner, F.; Weber, A. Molecular characterisation of a new mutant allele of the plastid phosphoglucomutase in Arabidopsis, and complementation of the mutant with the wild-type cDNA. Mol. General. Genet. MGG 2000, 263, 978–986. [Google Scholar] [CrossRef]
- Huson, D.H.; Buchfink, B. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; Lopez, R.; Finn, R.D. Hmmer web server: 2018 update. Nucleic Acids Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Smyth, G.K.; Wei, S. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2013, 30, 923–930. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Pei, J.; Wang, L.; Tian, Y.; Ren, L.; Luo, Y. Interactions at the Oviposition Scar: Molecular and Metabolic Insights into Elaeagnus angustifolia’s Resistance Response to Anoplophora glabripennis. Int. J. Mol. Sci. 2024, 25, 9504. https://doi.org/10.3390/ijms25179504
Li C, Pei J, Wang L, Tian Y, Ren L, Luo Y. Interactions at the Oviposition Scar: Molecular and Metabolic Insights into Elaeagnus angustifolia’s Resistance Response to Anoplophora glabripennis. International Journal of Molecular Sciences. 2024; 25(17):9504. https://doi.org/10.3390/ijms25179504
Chicago/Turabian StyleLi, Chengcheng, Jiahe Pei, Lixiang Wang, Yi Tian, Lili Ren, and Youqing Luo. 2024. "Interactions at the Oviposition Scar: Molecular and Metabolic Insights into Elaeagnus angustifolia’s Resistance Response to Anoplophora glabripennis" International Journal of Molecular Sciences 25, no. 17: 9504. https://doi.org/10.3390/ijms25179504





