Optimal Humanized Scg3-Neutralizing Antibodies for Anti-Angiogenic Therapy of Diabetic Retinopathy
Abstract
:1. Introduction
2. Results
2.1. Binding Activity
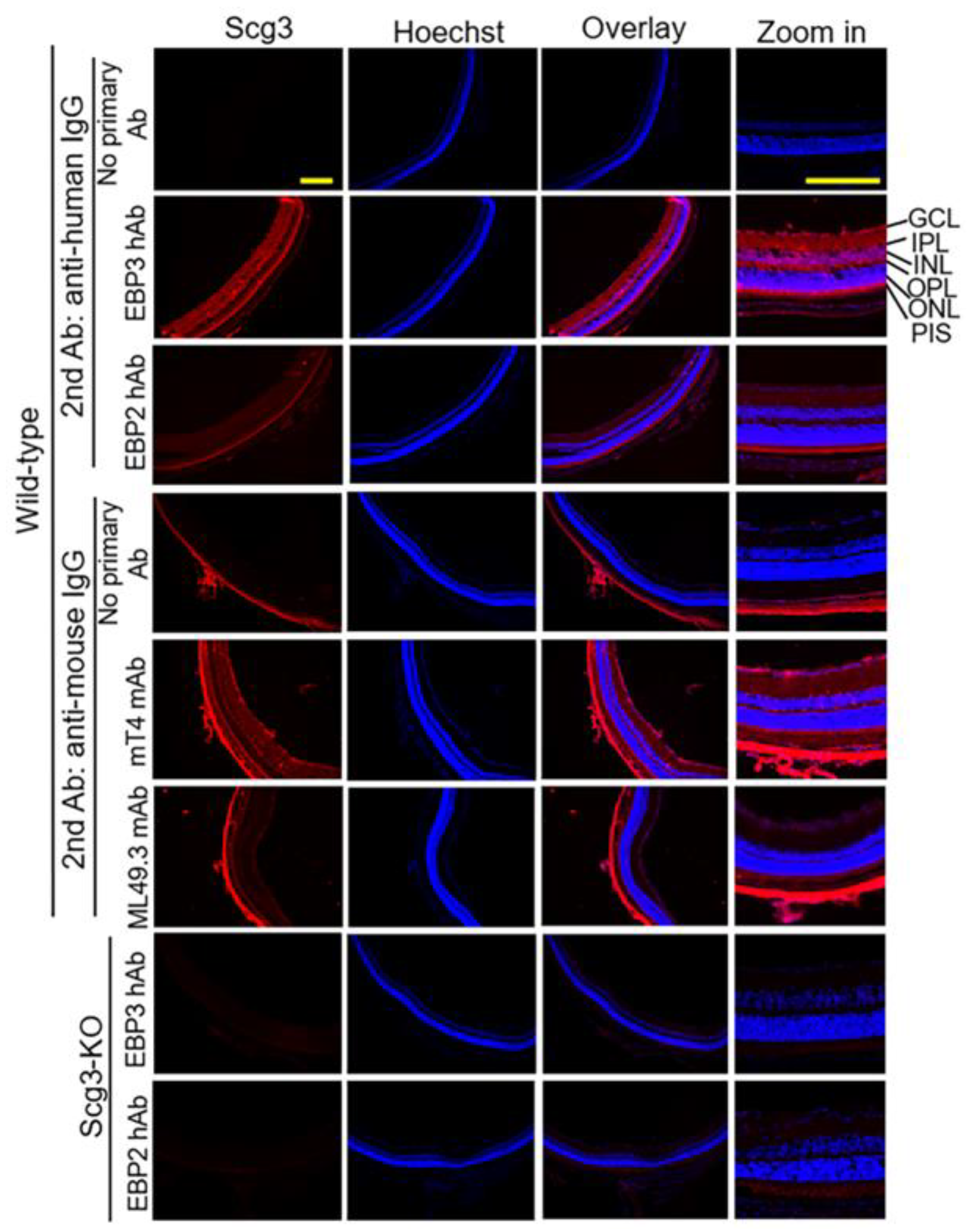
2.2. Detection of Scg3 by Immunohistochemistry
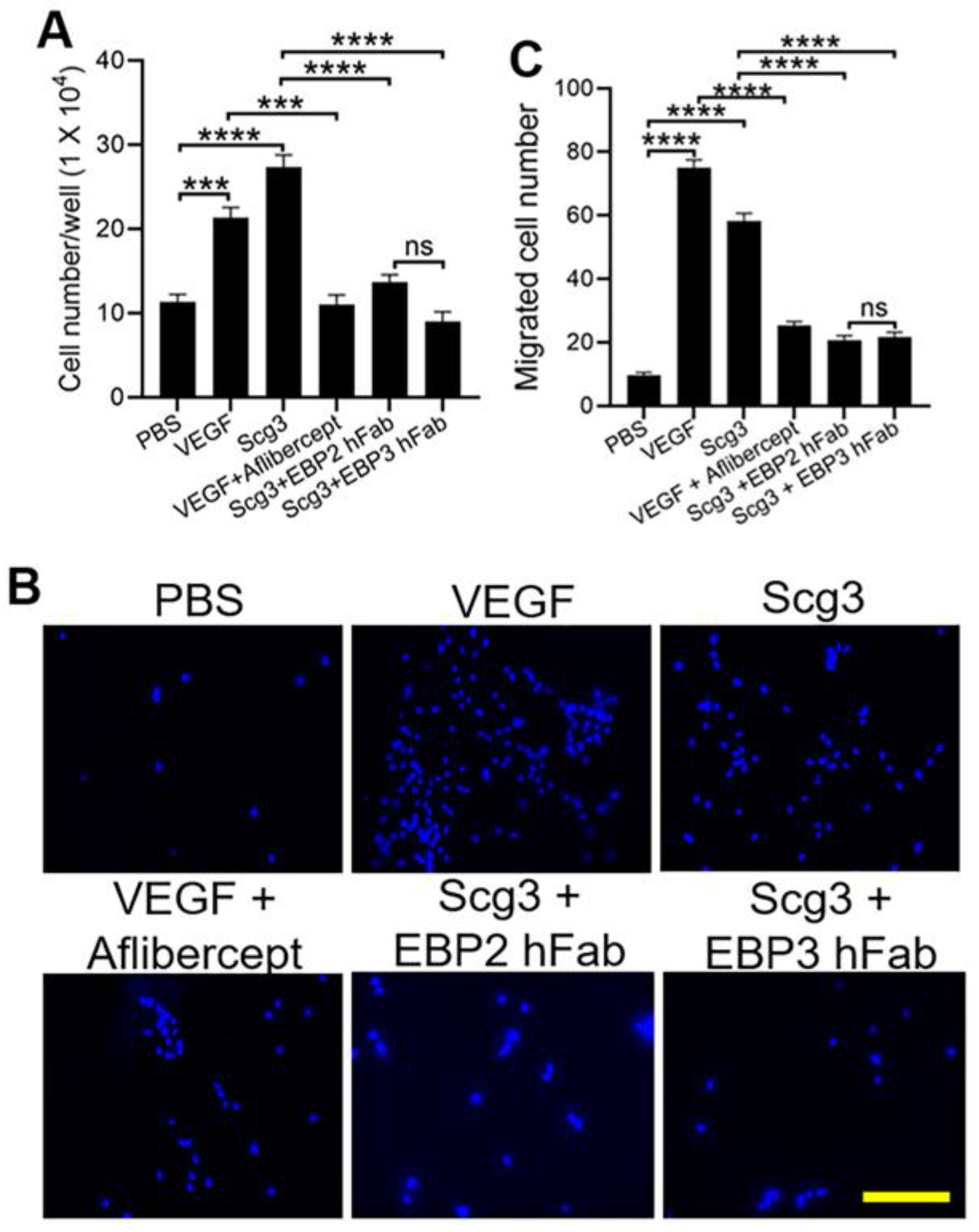
2.3. Neutralizing Activity
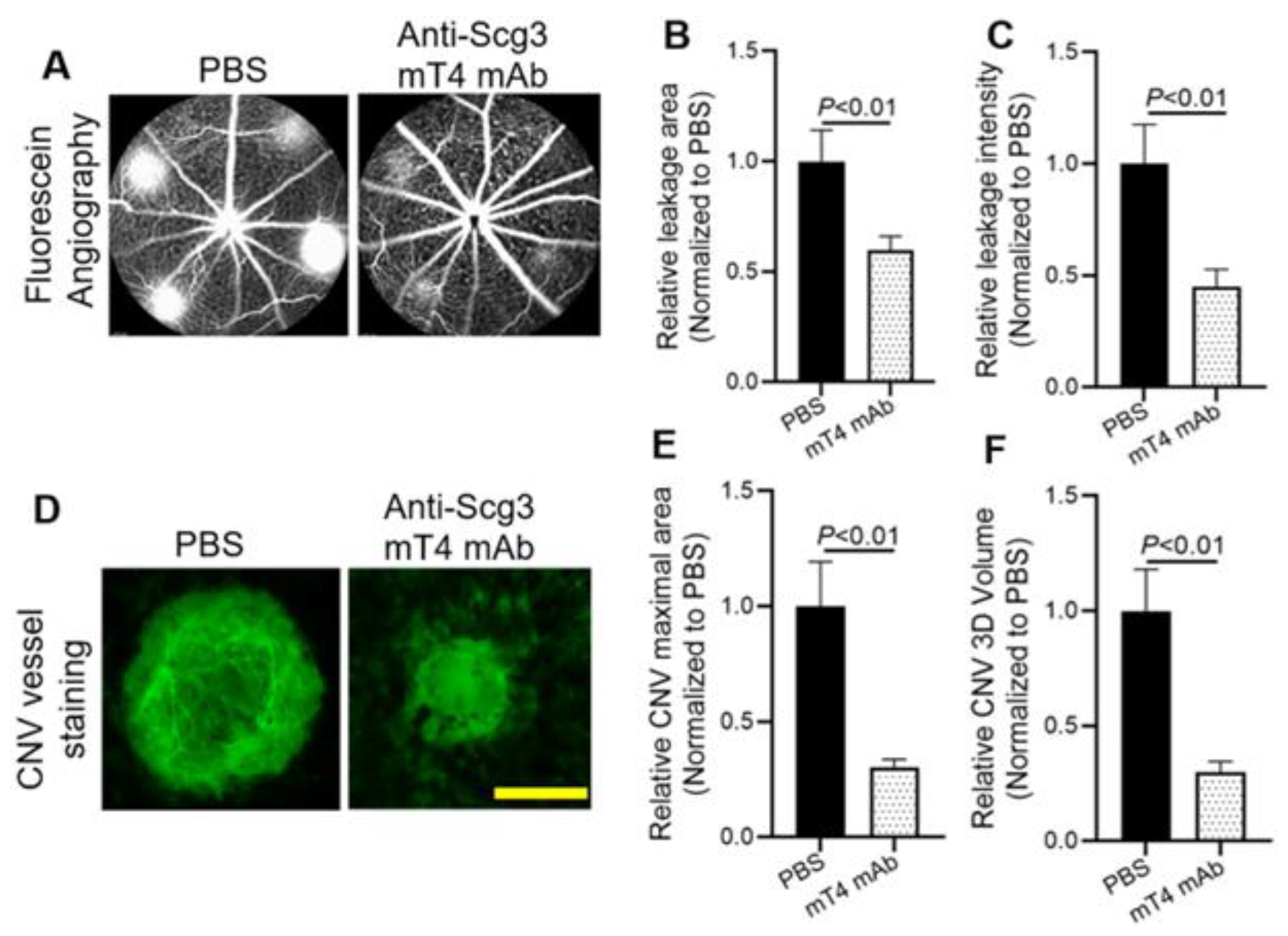
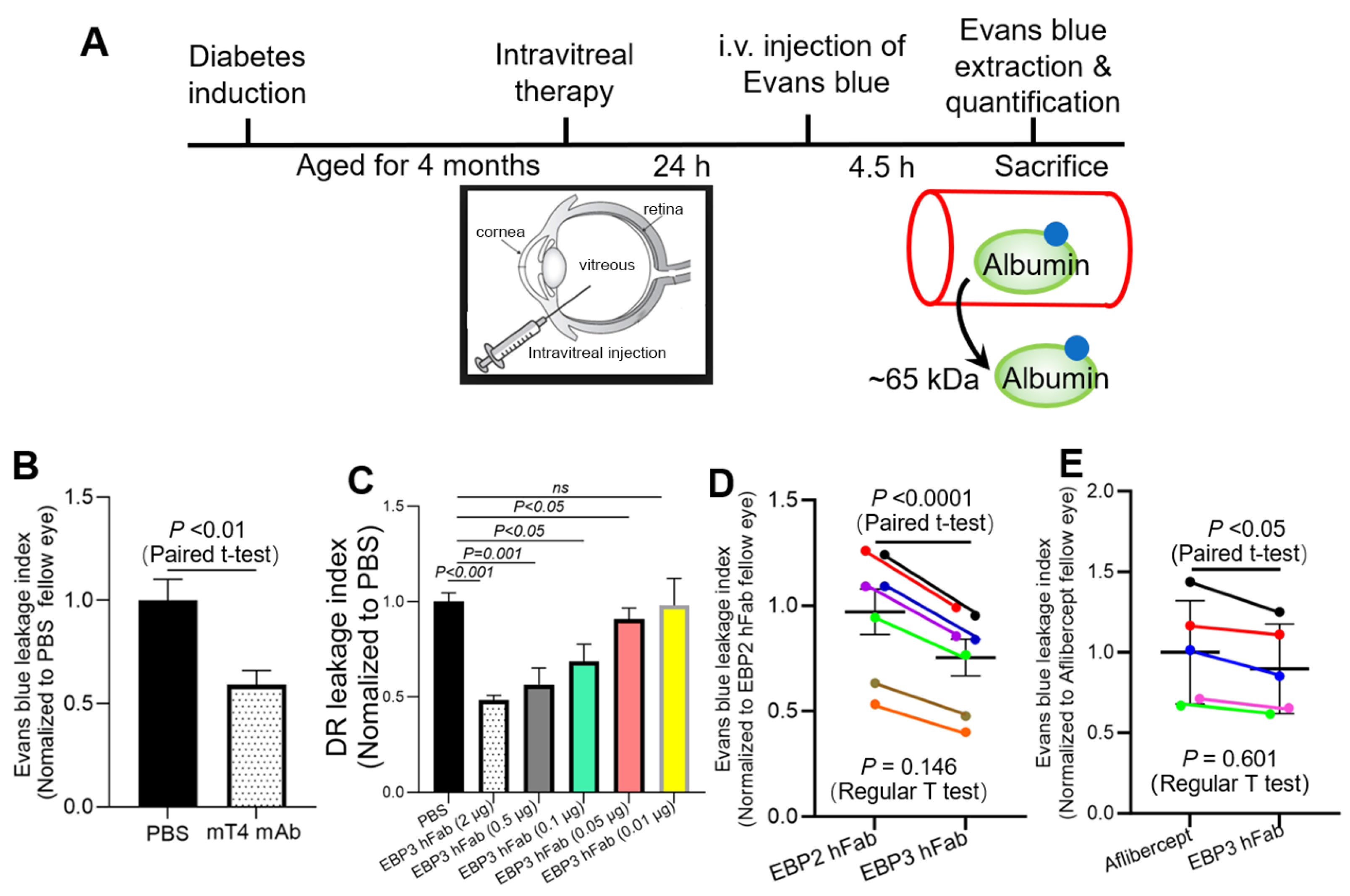
2.4. Alleviation of CNV
2.5. Amelioration of DR
2.6. Efficacy Comparison
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. ELISA
4.3. Binding Kinetics Measurements
4.4. Endothelial Proliferation Assay
4.5. Endothelial Transwell Migration Assay
4.6. Immunohistochemistry
4.7. DR Mice
4.8. Evans Blue Leakage Assay
4.9. Paired Assay for Efficacy Comparison
4.10. Laser-Induced CNV Mouse Model
4.11. Fluorescein Angiography
4.12. CNV Vessel Staining
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uludag, G.; Hassan, M.; Matsumiya, W.; Pham, B.H.; Chea, S.; Trong Tuong Than, N.; Doan, H.L.; Akhavanrezayat, A.; Halim, M.S.; Do, D.V.; et al. Efficacy and Safety of Intravitreal Anti-VEGF Therapy in Diabetic Retinopathy: What We Have Learned and What Should We Learn Further? Expert Opin. Biol. Ther. 2022, 22, 1275–1291. [Google Scholar] [CrossRef]
- Parravano, M.; Costanzo, E.; Scondotto, G.; Trifirò, G.; Virgili, G. Anti-VEGF and Other Novel Therapies for Neovascular Age-Related Macular Degeneration: An Update. BioDrugs 2021, 35, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.M.; Pastor, A.M.; de la Cruz, R.R. Vascular Endothelial Growth Factor: An Essential Neurotrophic Factor for Motoneurons? Neural Regen. Res. 2018, 13, 1181–1182. [Google Scholar] [PubMed]
- Sondell, M.; Sundler, F.; Kanje, M. Vascular Endothelial Growth Factor Is a Neurotrophic Factor Which Stimulates Axonal Outgrowth through the Flk-1 Receptor. Eur. J. Neurosci. 2000, 12, 4243–4254. [Google Scholar] [CrossRef] [PubMed]
- Dedania, V.S.; Bakri, S.J. Current Perspectives on Ranibizumab. Clin. Ophthalmol. 2015, 9, 533–542. [Google Scholar]
- Chung, A.S.; Ferrara, N. Developmental and Pathological Angiogenesis. Annu. Rev. Cell Dev. Biol. 2011, 27, 563–584. [Google Scholar] [CrossRef] [PubMed]
- Panos, G.D.; Lakshmanan, A.; Dadoukis, P.; Ripa, M.; Motta, L.; Amoaku, W.M. Faricimab: Transforming the Future of Macular Diseases Treatment—A Comprehensive Review of Clinical Studies. Drug Des. Dev. Ther. 2023, 17, 2861–2873. [Google Scholar] [CrossRef]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef]
- Dunn, E.N.; Hariprasad, S.M.; Sheth, V.S. An Overview of the Fovista and Rinucumab Trials and the Fate of Anti-PDGF Medications. Ophthalmic Surg Lasers Imaging Retin. 2017, 48, 100–104. [Google Scholar] [CrossRef]
- Wang, D.; Huang, H.J.; Kazlauskas, A.; Cavenee, W.K. Induction of Vascular Endothelial Growth Factor Expression in Endothelial Cells by Platelet-Derived Growth Factor through the Activation of Phosphatidylinositol 3-Kinase. Cancer Res. 1999, 59, 1464–1472. [Google Scholar]
- Lobov, I.B.; Brooks, P.C.; Lang, R.A. Angiopoietin-2 Displays VEGF-Dependent Modulation of Capillary Structure and Endothelial Cell Survival in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 11205–11210. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Zhang, H.; Hui, R. Single Chain Fv Antibody against Angiopoietin-2 Inhibits VEGF-Induced Endothelial Cell Proliferation and Migration in vivo. Biochem. Biophys. Res. Commun. 2003, 309, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Heier, J.S.; Khanani, A.M.; Quezada Ruiz, C.; Basu, K.; Ferrone, P.J.; Brittain, C.; Figueroa, M.S.; Lin, H.; Holz, F.G.; Patel, V.; et al. Efficacy, Durability, and Safety of Intravitreal Faricimab up to Every 16 Weeks for Neovascular Age-Related Macular Degeneration (TENAYA and LUCERNE): Two Randomised, Double-Masked, Phase 3, Non-Inferiority Trials. Lancet 2022, 399, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, N.; Parachuri, N.; Karanam, D.; Kuppermann, B.D.; Bandello, F.; Regillo, C.D. Faricimab Phase 3 DME Trial Significance of Personalized Treatment Intervals (PTI) Regime for Future DME Trials. Eye 2022, 36, 679–680. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, M.E.; Wang, W.; Chen, X.; Caberoy, N.B.; Guo, F.; Shen, C.; Ji, Y.; Tian, H.; Wang, H.; Chen, R.; et al. Secretogranin III as a Disease-Associated Ligand for Antiangiogenic Therapy of Diabetic Retinopathy. J. Exp. Med. 2017, 214, 1029–1047. [Google Scholar] [CrossRef]
- Ji, L.; Waduge, P.; Wan, W.; Tian, H.; Li, J.; Zhang, J.; Chen, R.; Li, W. Comparative Ligandomics Implicates Secretogranin III as a Disease-Restricted Angiogenic Factor in Laser-Induced Choroidal Neovascularization. FEBS J. 2022, 289, 3521–3534. [Google Scholar] [CrossRef]
- Ji, L.; Waduge, P.; Hao, L.; Kaur, A.; Wan, W.; Wu, Y.; Tian, H.; Zhang, J.; Webster, K.A.; Li, W. Selectively Targeting Disease-Restricted Secretogranin III to Alleviate Choroidal Neovascularization. FASEB J. 2022, 36, e22106. [Google Scholar] [CrossRef]
- Ji, L.; Waduge, P.; Wu, Y.; Huang, C.; Kaur, A.; Oliveira, P.; Tian, H.; Zhang, J.; Stout, J.T.; Weng, C.Y.; et al. Secretogranin III Selectively Promotes Vascular Leakage in the Deep Vascular Plexus of Diabetic Retinopathy. Int. J. Mol. Sci. 2023, 24, 10531. [Google Scholar] [CrossRef]
- Huang, C.; Ji, L.; Kaur, A.; Tian, H.; Waduge, P.; Webster, K.A.; Li, W. Anti-Scg3 Gene Therapy to Treat Choroidal Neovascularization in Mice. Biomedicines 2023, 11, 1910. [Google Scholar] [CrossRef]
- Li, W.; Webster, K.A.; LeBlanc, M.E.; Tian, H. Secretogranin III: A Diabetic Retinopathy-Selective Angiogenic Factor. Cell. Mol. Life Sci. 2018, 75, 635–647. [Google Scholar] [CrossRef]
- Dai, C.; Waduge, P.; Ji, L.; Huang, C.; He, Y.; Tian, H.; Zuniga-Sanchez, E.; Bhatt, A.; Pang, I.-H.; Su, G.; et al. Secretogranin III Stringently Regulates Pathological but Not Physiological Angiogenesis in Oxygen-Induced Retinopathy. Cell. Mol. Life Sci. 2022, 79, 63. [Google Scholar] [CrossRef]
- Rong, X.; Tian, H.; Yang, L.; Li, W. Function-First Ligandomics for Ocular Vascular Research and Drug Target Discovery. Exp. Eye Res. 2019, 182, 57–64. [Google Scholar] [CrossRef]
- Tang, F.; LeBlanc, M.E.; Wang, W.; Liang, D.; Chen, P.; Chou, T.-H.; Tian, H.; Li, W. Anti-Secretogranin III Therapy of Oxygen-Induced Retinopathy with Optimal Safety. Angiogenesis 2019, 22, 369–382. [Google Scholar] [CrossRef]
- LeBlanc, M.E.; Wang, W.; Ji, Y.; Tian, H.; Liu, D.; Zhang, X.; Li, W. Secretogranin III as a Novel Target for the Therapy of Choroidal Neovascularization. Exp. Eye Res. 2019, 181, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Fava, R.A.; Olsen, N.J.; Spencer-Green, G.; Yeo, K.T.; Yeo, T.K.; Berse, B.; Jackman, R.W.; Senger, D.R.; Dvorak, H.F.; Brown, L.F. Vascular Permeability Factor/Endothelial Growth Factor (VPF/VEGF): Accumulation and Expression in Human Synovial Fluids and Rheumatoid Synovial Tissue. J. Exp. Med. 1994, 180, 341–346. [Google Scholar] [CrossRef]
- Yu, H.; Huang, X.; Ma, Y.; Gao, M.; Wang, O.; Gao, T.; Shen, Y.; Liu, X. Interleukin-8 Regulates Endothelial Permeability by down-Regulation of Tight Junction but Not Dependent on Integrins Induced Focal Adhesions. Int. J. Biol. Sci. 2013, 9, 966–979. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Dubey, S.; Varney, M.L.; Dave, B.J.; Singh, R.K. IL-8 Directly Enhanced Endothelial Cell Survival, Proliferation, and Matrix Metalloproteinases Production and Regulated Angiogenesis. J. Immunol. 2003, 170, 3369–3376. [Google Scholar] [CrossRef]
- Brindle, N.P.J.; Saharinen, P.; Alitalo, K. Signaling and Functions of Angiopoietin-1 in Vascular Protection. Circ. Res. 2006, 98, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, L.M.; Barillas, S.; Weis, S.M.; Göthert, J.R.; Cheresh, D.A. Semaphorin 3A Suppresses VEGF-Mediated Angiogenesis yet Acts as a Vascular Permeability Factor. Blood 2008, 111, 2674–2680. [Google Scholar] [CrossRef]
- Yau, J.W.Y.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.-J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global Prevalence and Major Risk Factors of Diabetic Retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef]
- Caberoy, N.B.; Zhou, Y.; Jiang, X.; Alvarado, G.; Li, W. Efficient Identification of Tubby-Binding Proteins by an Improved System of T7 Phage Display. J. Mol. Recognit. 2010, 23, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Kamat, V.; Rqfique, A. Designing Binding Kinetic Assay on the Bio-Layer Interferometry (BLI) Biosensor to Characterize Antibody-Antigen Interactions. Anal. Biochem. 2017, 536, 16–31. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, M.E.; Wang, W.; Caberoy, N.B.; Chen, X.; Guo, F.; Alvarado, G.; Shen, C.; Wang, F.; Wang, H.; Chen, R.; et al. Hepatoma-Derived Growth Factor-Related Protein-3 Is a Novel Angiogenic Factor. PLoS ONE 2015, 10, e0127904. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Pacheco, M.T.F.; Chen, P.; Liang, D.; Li, W. Secretogranin III Promotes Angiogenesis through MEK/ERK Signaling Pathway. Biochem. Biophys. Res. Commun. 2018, 495, 781–786. [Google Scholar] [CrossRef]
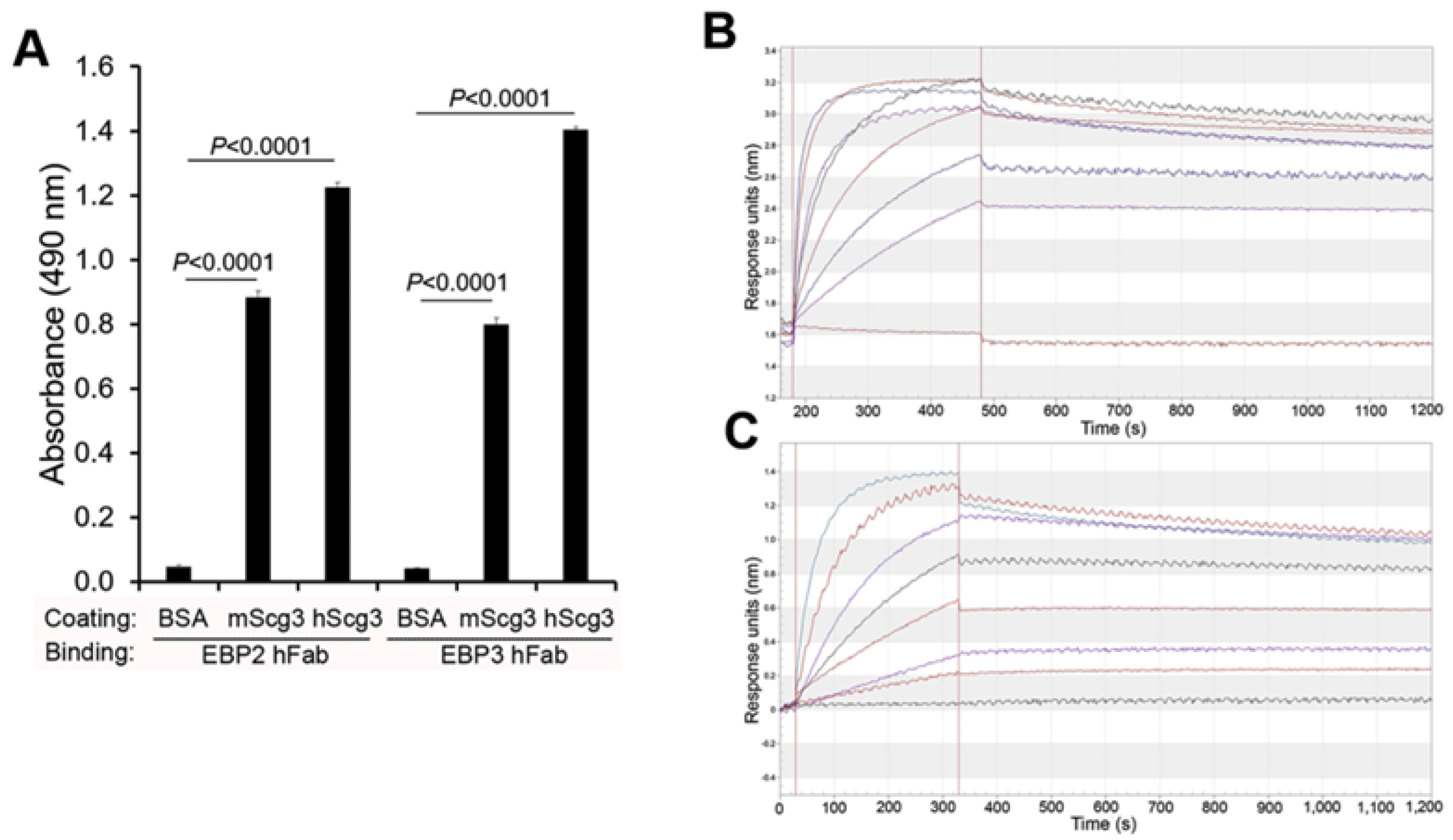

| Antibody | ML49.3 mAb | EBP2 hFab | mT4 mAb | EBP3 hFab |
|---|---|---|---|---|
| KD (nM) | 35 | 8.7 | 0.86 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Waduge, P.; Kaur, A.; Tian, H.; Weng, C.Y.; Stout, J.T.; Pang, I.-H.; Webster, K.A.; Li, W. Optimal Humanized Scg3-Neutralizing Antibodies for Anti-Angiogenic Therapy of Diabetic Retinopathy. Int. J. Mol. Sci. 2024, 25, 9507. https://doi.org/10.3390/ijms25179507
Huang C, Waduge P, Kaur A, Tian H, Weng CY, Stout JT, Pang I-H, Webster KA, Li W. Optimal Humanized Scg3-Neutralizing Antibodies for Anti-Angiogenic Therapy of Diabetic Retinopathy. International Journal of Molecular Sciences. 2024; 25(17):9507. https://doi.org/10.3390/ijms25179507
Chicago/Turabian StyleHuang, Chengchi, Prabuddha Waduge, Avinash Kaur, Hong Tian, Christina Y. Weng, John Timothy Stout, Iok-Hou Pang, Keith A. Webster, and Wei Li. 2024. "Optimal Humanized Scg3-Neutralizing Antibodies for Anti-Angiogenic Therapy of Diabetic Retinopathy" International Journal of Molecular Sciences 25, no. 17: 9507. https://doi.org/10.3390/ijms25179507





