Development and Optimization of a Bromothymol Blue-Based PLA2 Assay Involving POPC-Based Self-Assemblies
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization of POPC Liposomes and Mixed Micelles
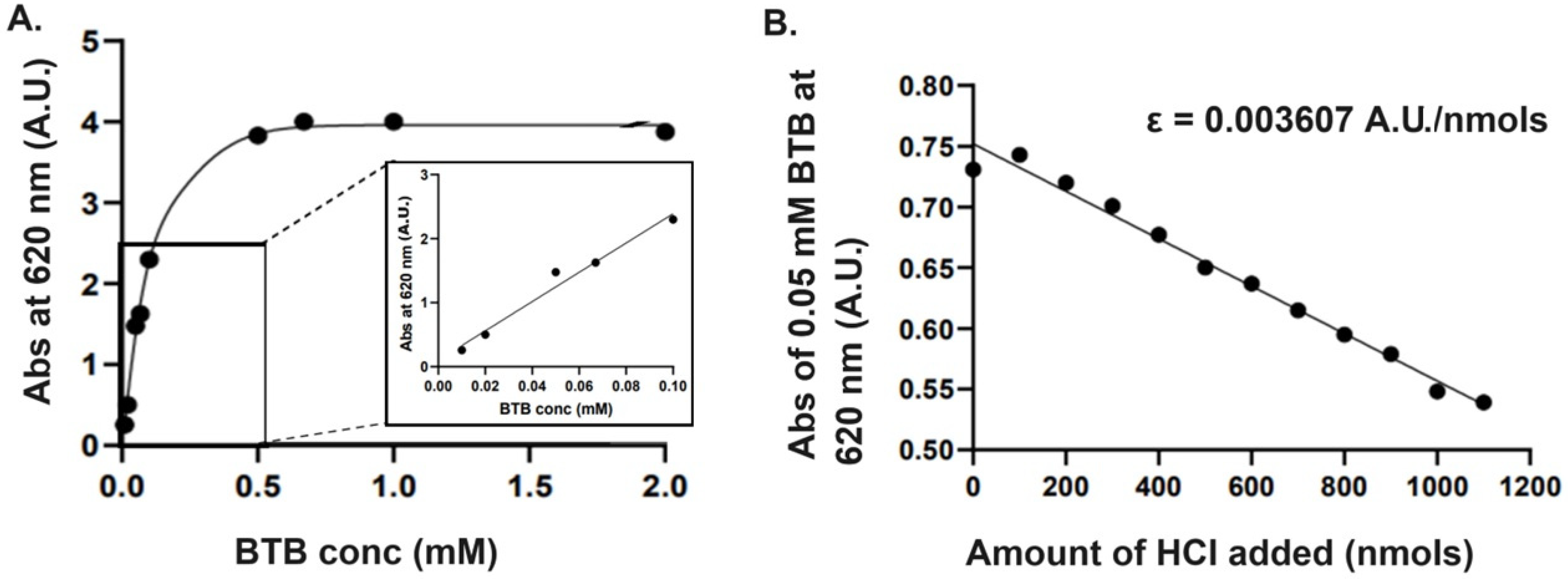
2.2. Preparation of Standard Absorption Curve of Bromothymol Blue (BTB)
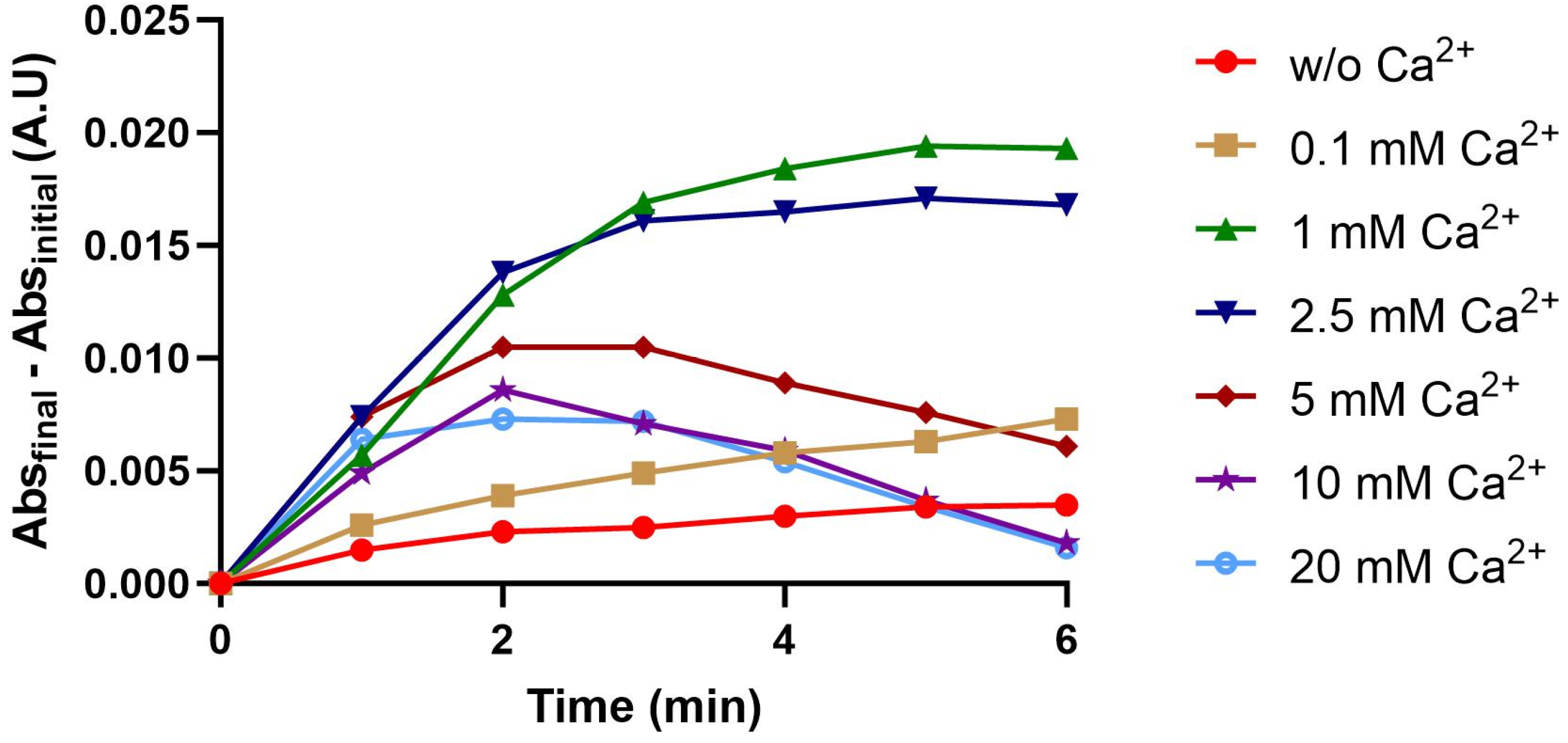
2.3. Effect of CaCl2 on sPLA2 Activity
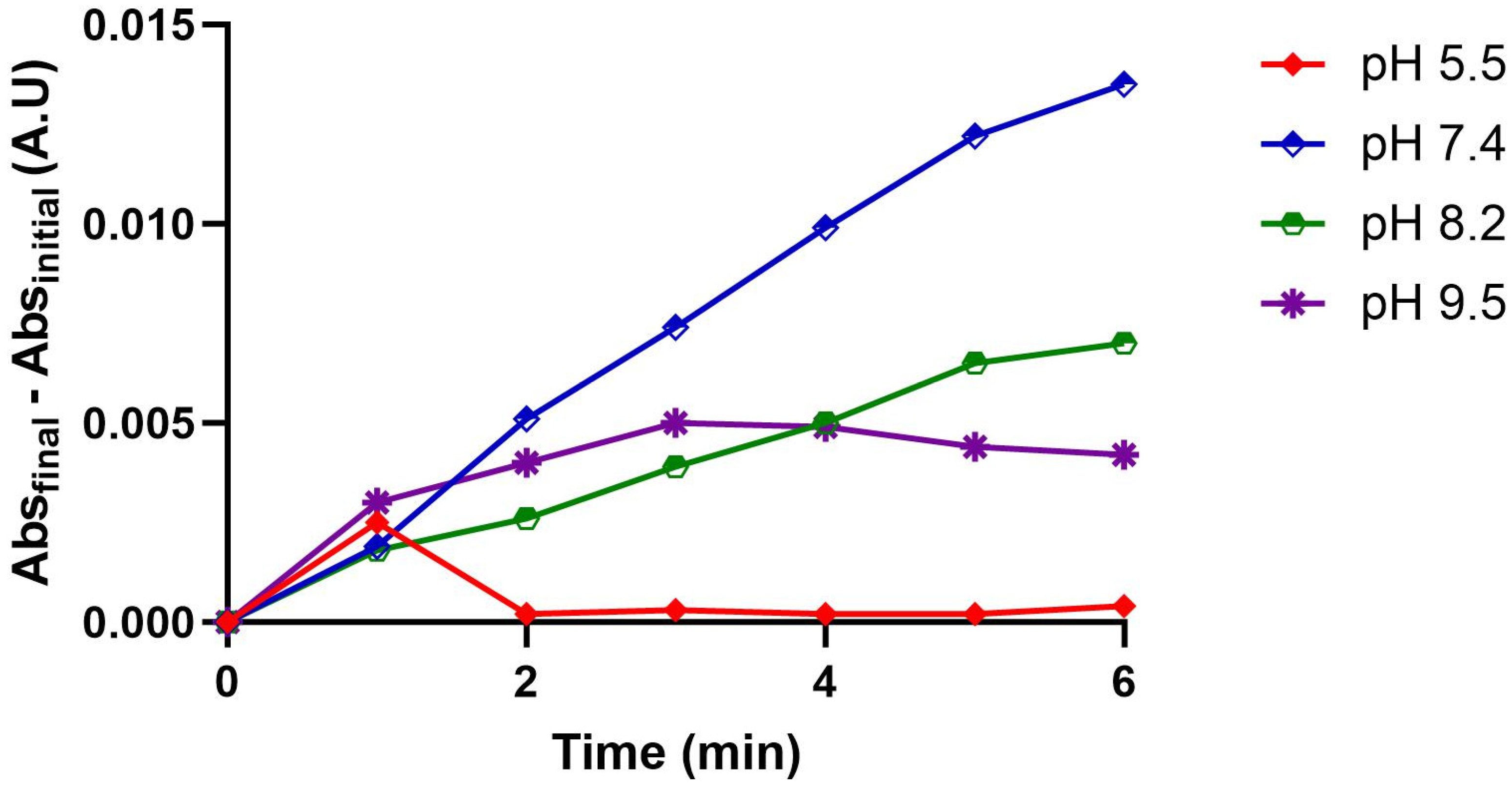
2.4. Effect of pH on PLA2 Activity
2.5. Comparison of PLA2 IIA Kinetics on POPC-Based Self-Assemblies (Liposomes vs. Mixed Micelles)
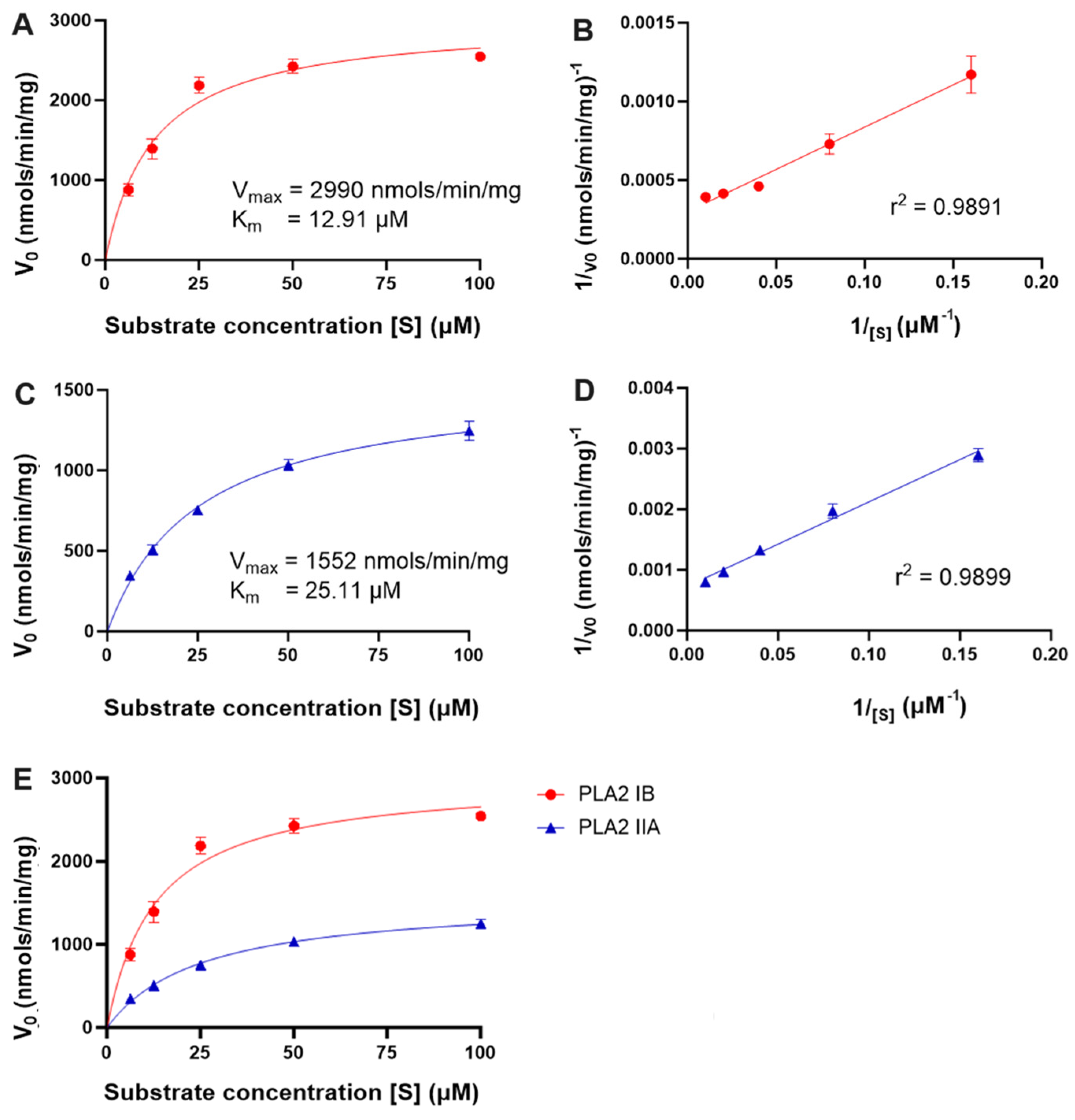
2.6. Comparison of PLA2 IIA and IB Assay Kinetics with POPC-Based Liposomal Vesicles and Mixed Micelles
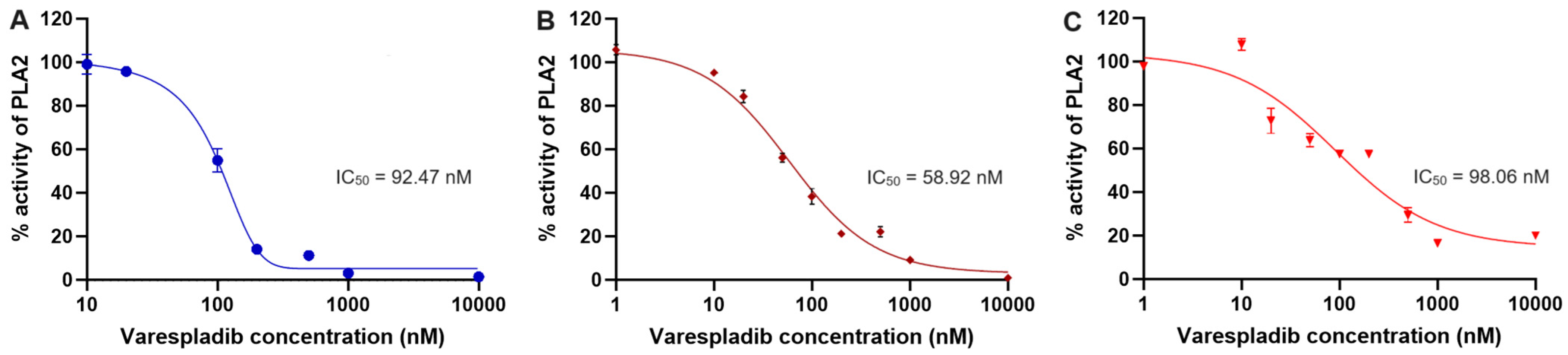
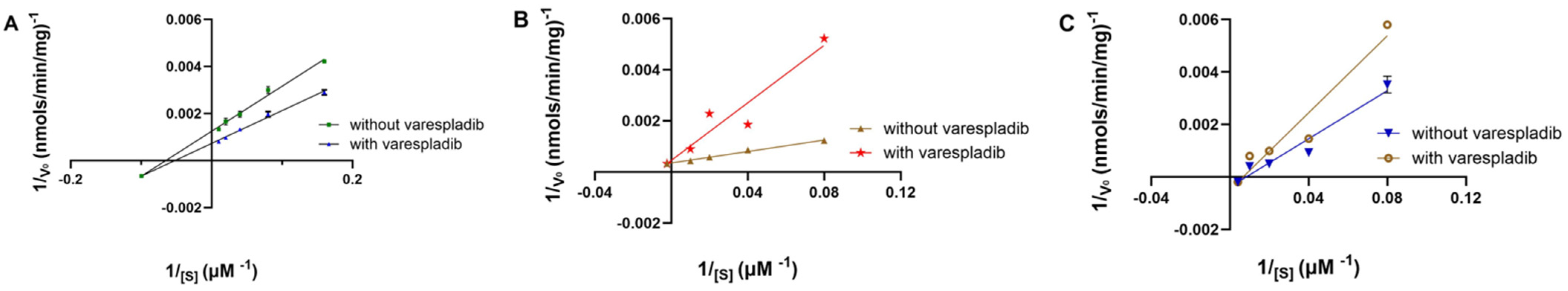
2.7. Inhibition Study of PLA2 IIA Using Varespladib
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of Buffers and Stock Solutions
3.2.2. Preparation of 1.0 mM POPC Liposomes via Extrusion Method
3.2.3. Preparation of POPC Mixed Micelles with Triton X-100 via Sonication Method
3.2.4. Preparation of Standard Curve Using Bromothymol Blue (BTB)
3.2.5. Effect of Ca2+ Ion on PLA2 Activity
3.2.6. Effect of pH on PLA2 Activity
3.2.7. Assay Method for PLA2 Kinetic Study Using POPC-Based Liposomes and Mixed Micelles
3.2.8. PLA2 Inhibition Study with the Developed Assay Using a Standard Inhibitor Varespladib
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, S.A.; Ilies, M.A. The Phospholipase A2 Superfamily: Structure, Isozymes, Catalysis, Physiologic and Pathologic Roles. Int. J. Mol. Sci. 2023, 24, 1353. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.A.; Ilies, M.A. Chapter 2.8—Phospholipases A2. In Metalloenzymes; Supuran, C.T., Donald, W.A., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 101–136. [Google Scholar]
- Dennis, E.A.; Cao, J.; Hsu, Y.-H.; Magrioti, V.; Kokotos, G. Phospholipase A Enzymes: Physical Structure, Biological Function, Disease Implication, Chemical Inhibition, and Therapeutic Intervention. Chem. Rev. 2011, 111, 6130–6185. [Google Scholar] [CrossRef]
- Alekseeva, A.; Boldyrev, I. Phospholipase A2. Methods for Activity Monitoring. Biochem. (Mosc.) Suppl. Ser. A Membr. Cell Biol. 2020, 14, 267–278. [Google Scholar] [CrossRef]
- Cerminati, S.; Paoletti, L.; Aguirre, A.; Peirú, S.; Menzella, H.G.; Castelli, M.E. Industrial uses of phospholipases: Current state and future applications. Appl. Microbiol. Biotechnol. 2019, 103, 2571–2582. [Google Scholar] [CrossRef]
- Ali, S.; Khan, S.A.; Hamayun, M.; Lee, I.J. The Recent Advances in the Utility of Microbial Lipases: A Review. Microorganisms 2023, 11, 510. [Google Scholar] [CrossRef] [PubMed]
- Zappaterra, F.; Rodriguez, M.E.M.; Summa, D.; Semeraro, B.; Costa, S.; Tamburini, E. Biocatalytic Approach for Direct Esterification of Ibuprofen with Sorbitol in Biphasic Media. Int. J. Mol. Sci. 2021, 22, 3066. [Google Scholar] [CrossRef] [PubMed]
- Zappaterra, F.; Tupini, C.; Summa, D.; Cristofori, V.; Costa, S.; Trapella, C.; Lampronti, I.; Tamburini, E. Xylitol as a Hydrophilization Moiety for a Biocatalytically Synthesized Ibuprofen Prodrug. Int. J. Mol. Sci. 2022, 23, 2026. [Google Scholar] [CrossRef] [PubMed]
- Lam, L.; Ilies, M.A. Evaluation of the Impact of Esterases and Lipases from the Circulatory System against Substrates of Different Lipophilicity. Int. J. Mol. Sci. 2022, 23, 1262. [Google Scholar] [CrossRef]
- Volwerk, J.; Dedieu, A.; Verheij, H.; Dijkman, R.; De Haas, G. Hydrolysis of monomeric substrates by porcine pancreatic (pro) phospholipase A2. The use of a spectrophotometric assay. Recl. Des Trav. Chim. Des Pays-Bas 1979, 98, 214–220. [Google Scholar] [CrossRef]
- Israelachvili, J.N.; Mitchell, D.J.; Ninham, B.W. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1976, 72, 1525–1568. [Google Scholar] [CrossRef]
- Sharma, V.D.; Lees, J.; Hoffman, N.E.; Brailoiu, E.; Madesh, M.; Wunder, S.L.; Ilies, M.A. Modulation of pyridinium cationic lipid-DNA complex properties by pyridinium gemini surfactants and its impact on lipoplex transfection properties. Mol. Pharm. 2014, 11, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, R. Molecular Packing Parameter and Surfactant Self-Assembly: The Neglected Role of the Surfactant Tail. Langmuir 2002, 18, 31–38. [Google Scholar] [CrossRef]
- Draghici, B.; Ilies, M.A. Synthetic Nucleic Acid Delivery Systems: Present and Perspectives. J. Med. Chem. 2015, 58, 4091–4130. [Google Scholar] [CrossRef]
- Fujii, S.; Yamada, S.; Matsumoto, S.; Kubo, G.; Yoshida, K.; Tabata, E.; Miyake, R.; Sanada, Y.; Akiba, I.; Okobira, T.; et al. Platonic Micelles: Monodisperse Micelles with Discrete Aggregation Numbers Corresponding to Regular Polyhedra. Sci. Rep. 2017, 7, 44494. [Google Scholar] [CrossRef] [PubMed]
- Sufian, M.A.; Ilies, M.A. Lipid-based nucleic acid therapeutics with in vivo efficacy. WIREs Nanomed. Nanobiotechnol. 2023, 15, e1856. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.J.; Washburn, W.N.; Deems, R.A.; Dennis, E.A. [1] Assay strategies and methods for phospholipases. Methods Enzymol. 1991, 197, 3–23. [Google Scholar]
- Andring, J.T.; Fouch, M.; Akocak, S.; Angeli, A.; Supuran, C.T.; Ilies, M.A.; McKenna, R. Structural Basis of Nanomolar Inhibition of Tumor-Associated Carbonic Anhydrase IX: X-Ray Crystallographic and Inhibition Study of Lipophilic Inhibitors with Acetazolamide Backbone. J. Med. Chem. 2020, 63, 13064–13075. [Google Scholar] [CrossRef]
- Draghici, B.; Vullo, D.; Akocak, S.; Walker, E.A.; Supuran, C.T.; Ilies, M.A. Ethylene bis-imidazoles are highly potent and selective activators for isozymes VA and VII of carbonic anhydrase, with a potential nootropic effect. Chem. Commun. 2014, 50, 5980–5983. [Google Scholar] [CrossRef]
- Levene, P.A.; Rolf, I.P. Lysolecithins and lysocephalins. J. Biol. Chem. 1923, 55, 743–749. [Google Scholar] [CrossRef]
- Levene, P.A.; Rolf, I.P.; Simms, H.S. Lysolecithins and lysocephalins: ii. isolation and properties of lysolecithins and lysocephalins. J. Biol. Chem. 1924, 58, 859–871. [Google Scholar] [CrossRef]
- Stern, I.; Shapiro, B. A rapid and simple method for the determination of esterified fatty acids and for total fatty acids in blood. J. Clin. Pathol. 1953, 6, 158. [Google Scholar] [CrossRef] [PubMed]
- Magee, W.; Thompson, R. The estimation of phospholipase A activity in aqueous systems. Biochem. J. 1960, 77, 526. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.A.; Hanahan, D.J. [33] Phospholipase A from Crotalus adamanteus venom: EC 3.1.1.4 Phosphatide acyl-hydrolase. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1969; Volume 14, pp. 178–184. [Google Scholar]
- Bulos, B.A.; Sacktor, B. Assay for phospholipase A activity of snake venom with asolectin as substrate. Anal. Biochem. 1971, 42, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.J.; Hughes, L.L.; Dennis, E.A. Analysis of human synovial fluid phospholipase A2 on short chain phosphatidylcholine-mixed micelles: Development of a spectrophotometric assay suitable for a microtiterplate reader. Anal. Biochem. 1992, 204, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Dillard, R.D.; Bach, N.J.; Draheim, S.E.; Berry, D.R.; Carlson, D.G.; Chirgadze, N.Y.; Clawson, D.K.; Hartley, L.W.; Johnson, L.M.; Jones, N.D. Indole inhibitors of human nonpancreatic secretory phospholipase A2. 2. Indole-3-acetamides with additional functionality. J. Med. Chem. 1996, 39, 5137–5158. [Google Scholar] [CrossRef]
- Dillard, R.D.; Bach, N.J.; Draheim, S.E.; Berry, D.R.; Carlson, D.G.; Chirgadze, N.Y.; Clawson, D.K.; Hartley, L.W.; Johnson, L.M.; Jones, N.D. Indole inhibitors of human nonpancreatic secretory phospholipase A2. 1. Indole-3-acetamides. J. Med. Chem. 1996, 39, 5119–5136. [Google Scholar] [CrossRef] [PubMed]
- Draheim, S.E.; Bach, N.J.; Dillard, R.D.; Berry, D.R.; Carlson, D.G.; Chirgadze, N.Y.; Clawson, D.K.; Hartley, L.W.; Johnson, L.M.; Jones, N.D.; et al. Indole Inhibitors of Human Nonpancreatic Secretory Phospholipase A. 3. Indole-3-glyoxamides. J. Med. Chem. 1996, 39, 5159–5175. [Google Scholar] [CrossRef]
- Aarsman, A.; Van Deenen, L.; Van den Bosch, H. Studies on lysophospholipases: VII. Synthesis of acylthioester analogs of lysolecithin and their use in a continuous spectrophotometric assay for lysophospholipases, a method with potential applicability to other lipolytic enzymes. Bioorg. Chem. 1976, 5, 241–253. [Google Scholar] [CrossRef]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef]
- Ilies, M.A.; Seitz, W.A.; Johnson, B.H.; Ezell, E.L.; Miller, A.L.; Thompson, E.B.; Balaban, A.T. Lipophilic Pyrylium Salts in the Synthesis of Efficient Pyridinium-Based Cationic Lipids, Gemini Surfactants, and Lipophilic Oligomers for Gene Delivery. J. Med. Chem. 2006, 49, 3872–3887. [Google Scholar] [CrossRef]
- Ilies, M.A.; Seitz, W.A.; Ghiviriga, I.; Johnson, B.H.; Miller, A.; Thompson, E.B.; Balaban, A.T. Pyridinium Cationic Lipids in Gene Delivery: A Structure−Activity Correlation Study. J. Med. Chem. 2004, 47, 3744–3754. [Google Scholar] [CrossRef]
- Chang, H.-I.; Yeh, M.-K. Clinical development of liposome-based drugs: Formulation, characterization, and therapeutic efficacy. Int. J. Nanomed. 2012, 7, 49–60. [Google Scholar]
- Wnętrzak, A.; Łątka, K.; Dynarowicz-Łątka, P. Interactions of alkylphosphocholines with model membranes—The Langmuir monolayer study. J. Membr. Biol. 2013, 246, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A. Formation and characterization of mixed micelles of the nonionic surfactant Triton X-100 with egg, dipalmitoyl, and dimyristoyl phosphatidylcholines. Arch. Biochem. Biophys. 1974, 165, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A. Kinetic dependence of phospholipase A2 activity on the detergent Triton X-100. J. Lipid Res. 1973, 14, 152–159. [Google Scholar] [CrossRef]
- Yu, L.; Deems, R.; Hajdu, J.; Dennis, E. The interaction of phospholipase A2 with phospholipid analogues and inhibitors. J. Biol. Chem. 1990, 265, 2657–2664. [Google Scholar] [CrossRef]
- Jimenez, M.; Cabanes, J.; Gandίa-Herrero, F.; Escribano, J.; Garcίa-Carmona, F.; Perez-Gilabert, M. A continuous spectrophotometric assay for phospholipase A2 activity. Anal. Biochem. 2003, 319, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A. Phospholipase A2 activity towards phosphatidylcholine in mixed micelles: Surface dilution kinetics and the effect of thermotropic phase transitions. Arch. Biochem. Biophys. 1973, 158, 485–493. [Google Scholar] [CrossRef]
- Shimada, T.; Hasegawa, T. Determination of equilibrium structures of bromothymol blue revealed by using quantum chemistry with an aid of multivariate analysis of electronic absorption spectra. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 185, 104–110. [Google Scholar] [CrossRef]
- Klotz, E.; Doyle, R.; Gross, E.; Mattson, B. The equilibrium constant for bromothymol blue: A general chemistry laboratory experiment using spectroscopy. J. Chem. Educ. 2011, 88, 637–639. [Google Scholar] [CrossRef]
- El Nahhal, I.M.; Zourab, S.M.; Kodeh, F.S.; Qudaih, A.I. Thin film optical BTB pH sensors using sol–gel method in presence of surfactants. Int. Nano Lett. 2012, 2, 1–9. [Google Scholar] [CrossRef]
- Price, J.A. A colorimetric assay for measuring phospholipase A2 degradation of phosphatidylcholine at physiological pH. J. Biochem. Biophys. Methods 2007, 70, 441–444. [Google Scholar] [CrossRef]
- Goldstein, D.A. Serum calcium. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Butterworths: Boston, MA, USA, 1990. [Google Scholar]
- Benzonana, G.; Desnuelle, P. Etude cinetique de l’action de la lipase pancreatique sur des triglycerides en emulsion. Essai d’une enzymologie en milieu heterogene. Biochim. Biophys. Acta (BBA) Biomembr. 1965, 105, 121–136. [Google Scholar] [CrossRef]
- Dennis, E.A. Interaction of enzymes with mixed micelles of phospholipid and detergent: Analysis of the phospholipase A2–dipalmitoyl phosphatidylcholine-triton X 100 system. J. Supramol. Struct. 1974, 2, 682–694. [Google Scholar] [CrossRef]
- Hope, M.J.; Bally, M.B.; Webb, G.; Cullis, P.R. Production of large unilamellar vesicles by a rapid extrusion procedure. Characterization of size distribution, trapped volume and ability to maintain a membrane potential. Biochim. Biophys. Acta (BBA) Biomembr. 1985, 812, 55–65. [Google Scholar] [CrossRef]
- MacDonald, R.C.; MacDonald, R.I.; Menco, B.P.M.; Takeshita, K.; Subbarao, N.K.; Hu, L.-R. Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. Biochim. Biophys. Acta (BBA) Biomembr. 1991, 1061, 297–303. [Google Scholar] [CrossRef]
- Torres-Flores, G.; Gonzalez-Horta, A.; Vega-Cantu, Y.I.; Rodriguez, C.; Rodriguez-Garcia, A. Preparation and Characterization of Liposomal Everolimus by Thin-Film Hydration Technique. Adv. Polym. Technol. 2020, 2020, 5462949. [Google Scholar] [CrossRef]

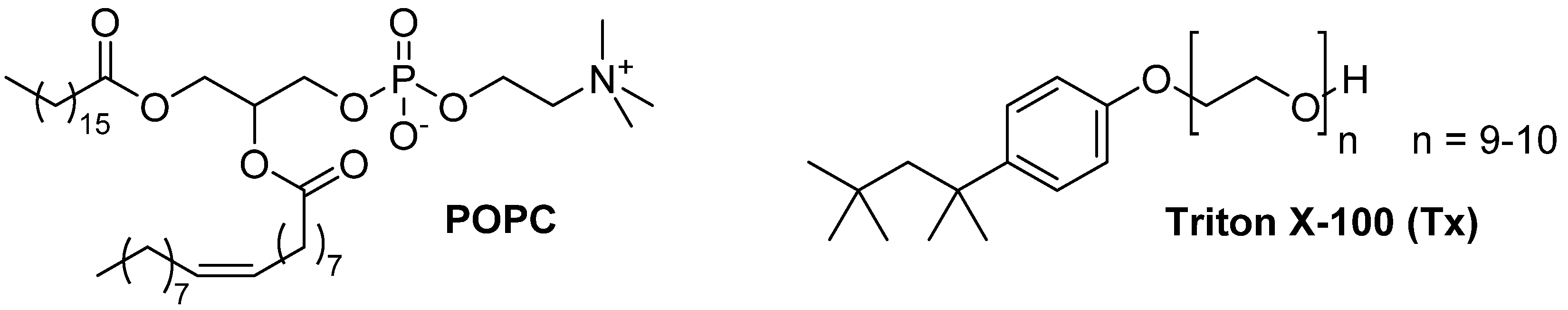



| Formulations | Z.avg (nm) | Main Peak (nm) | PDI | %Volume |
|---|---|---|---|---|
| POPC pure (liposomal) | 113.3 | 108.4 | 0.10 | 100% |
| Tx:POPC 2:1 mixed micelles | 70.0 | 50.3 | 0.22 | >95% |
| Tx:POPC 1:1 mixed micelles | 63.1 | 32.0 | 0.37 | >98% |
| Tx:POPC 1:2 mixed micelles | 63.7 | 33.4 | 0.41 | >98% |
| Tx:POPC 1:6.67 mixed micelles | 108.8 | 30.4 | 0.50 | >90% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, S.A.; Ilies, M.A. Development and Optimization of a Bromothymol Blue-Based PLA2 Assay Involving POPC-Based Self-Assemblies. Int. J. Mol. Sci. 2024, 25, 9517. https://doi.org/10.3390/ijms25179517
Khan SA, Ilies MA. Development and Optimization of a Bromothymol Blue-Based PLA2 Assay Involving POPC-Based Self-Assemblies. International Journal of Molecular Sciences. 2024; 25(17):9517. https://doi.org/10.3390/ijms25179517
Chicago/Turabian StyleKhan, Shibbir Ahmed, and Marc A. Ilies. 2024. "Development and Optimization of a Bromothymol Blue-Based PLA2 Assay Involving POPC-Based Self-Assemblies" International Journal of Molecular Sciences 25, no. 17: 9517. https://doi.org/10.3390/ijms25179517
APA StyleKhan, S. A., & Ilies, M. A. (2024). Development and Optimization of a Bromothymol Blue-Based PLA2 Assay Involving POPC-Based Self-Assemblies. International Journal of Molecular Sciences, 25(17), 9517. https://doi.org/10.3390/ijms25179517






