Effect of Sodium Thiosulfate Pre-Treatment on Renal Ischemia-Reperfusion Injury in Kidney Transplantation
Abstract
:1. Introduction
2. Results
2.1. Effect of STS Pre-Treatment on Rat Renal Epithelial Cell Viability in an In Vitro Model of Hypoxia–Reoxygenation Injury
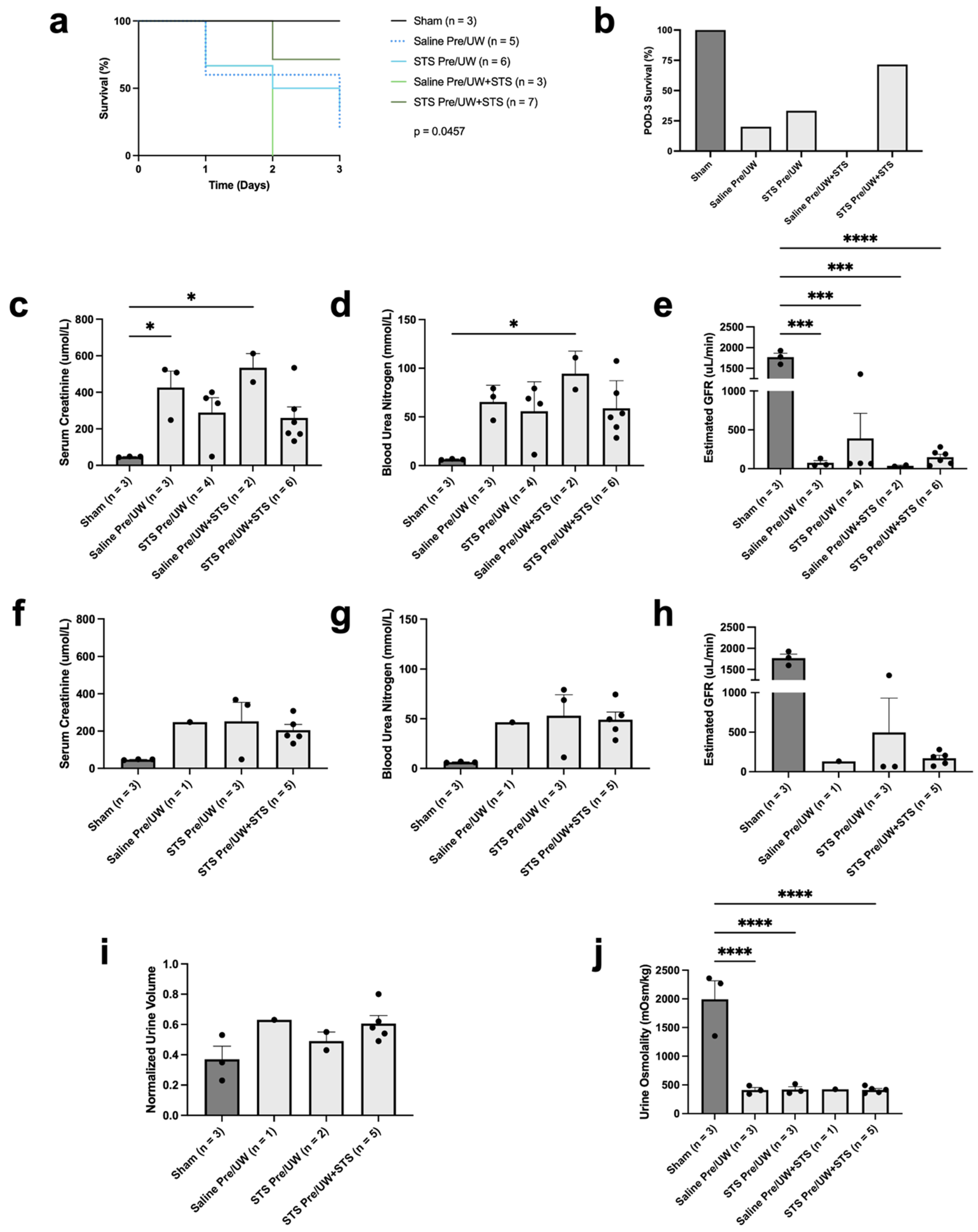
2.2. Effect of STS Pre-Treatment on Transplant Recipient Survival and Renal Function
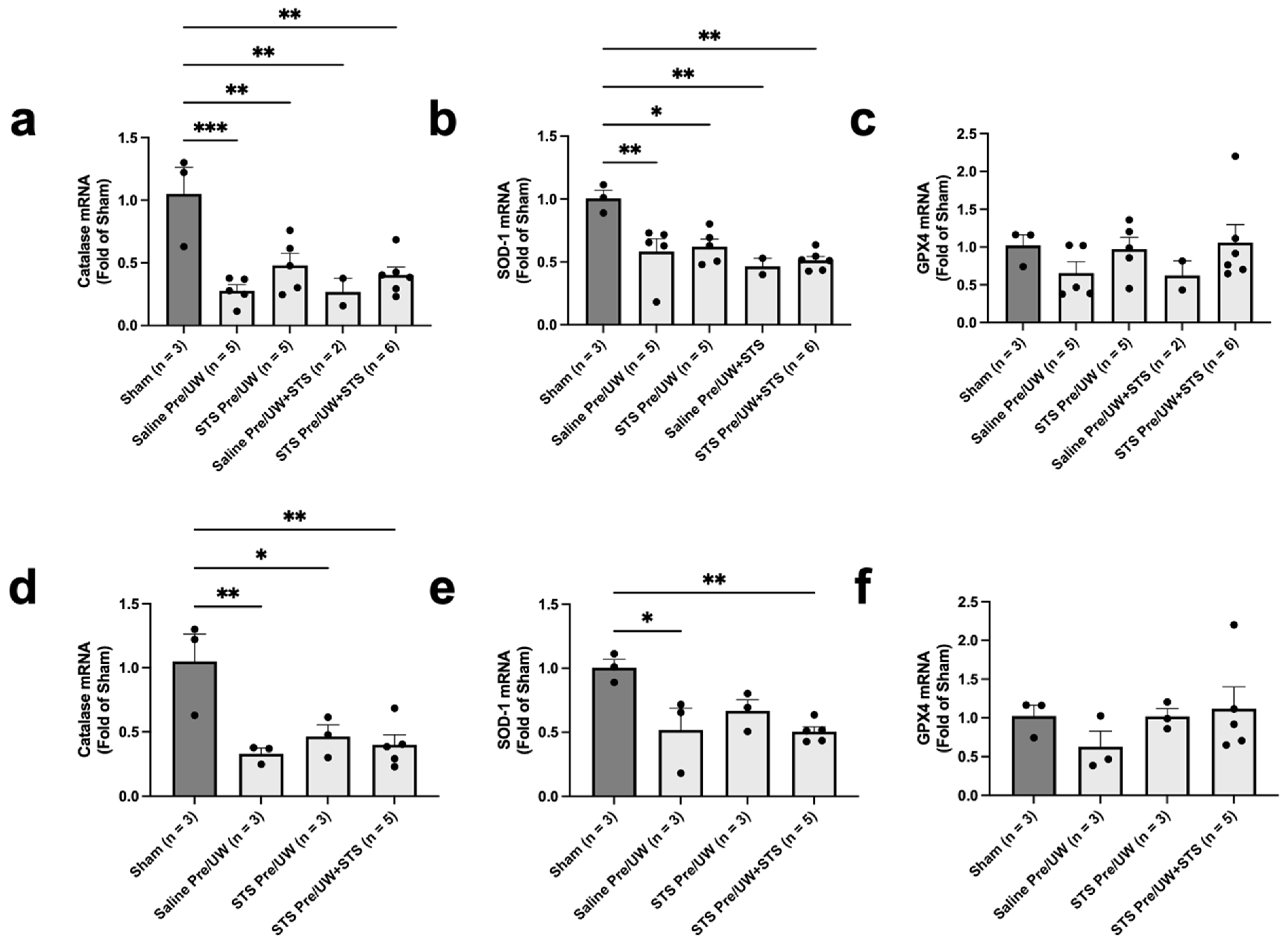
2.3. Effect of STS Pre-Treatment on Oxidative Stress in Renal Grafts
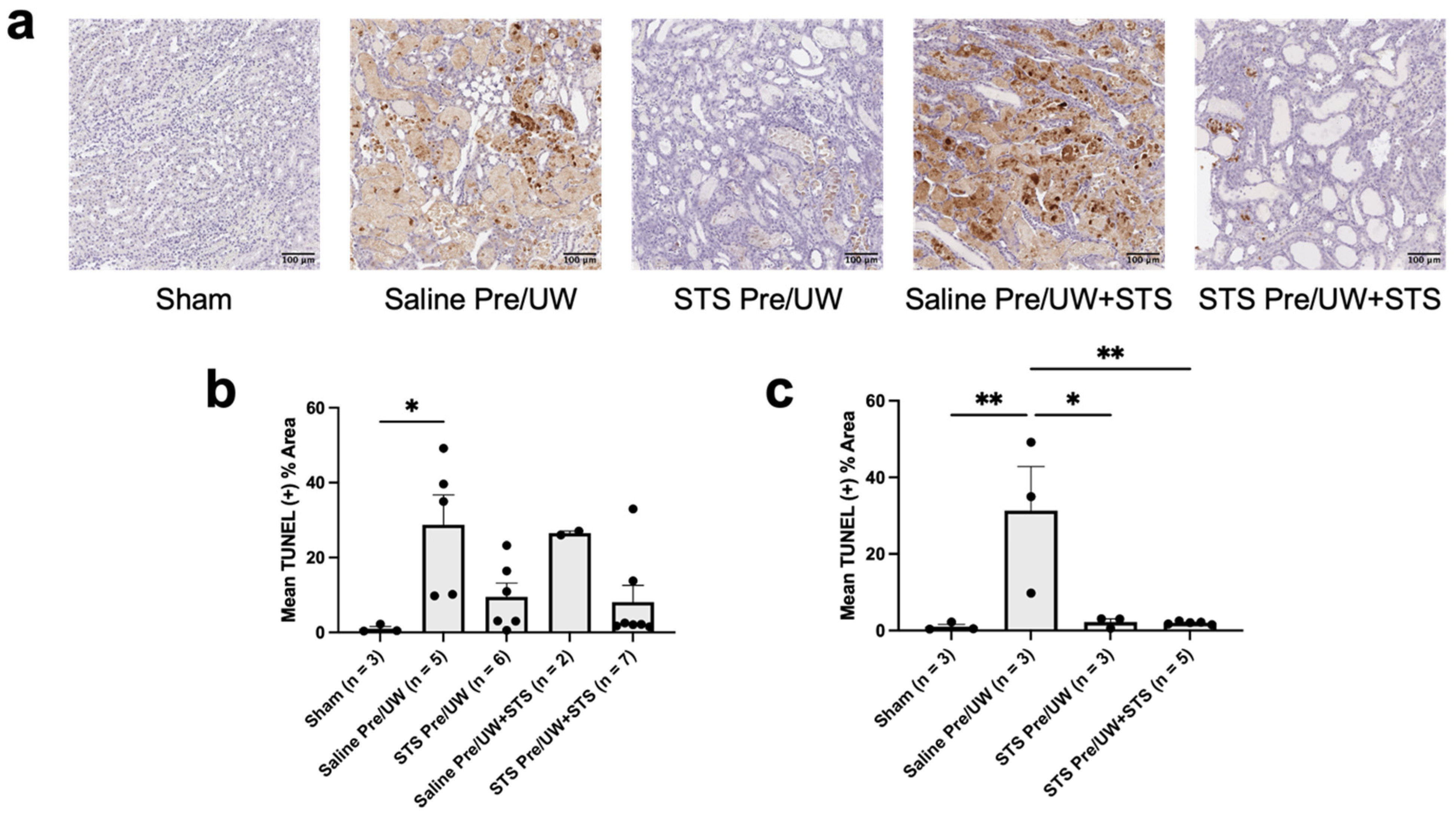
2.4. Effect of STS Pre-Treatment on Cell Death in Renal Grafts
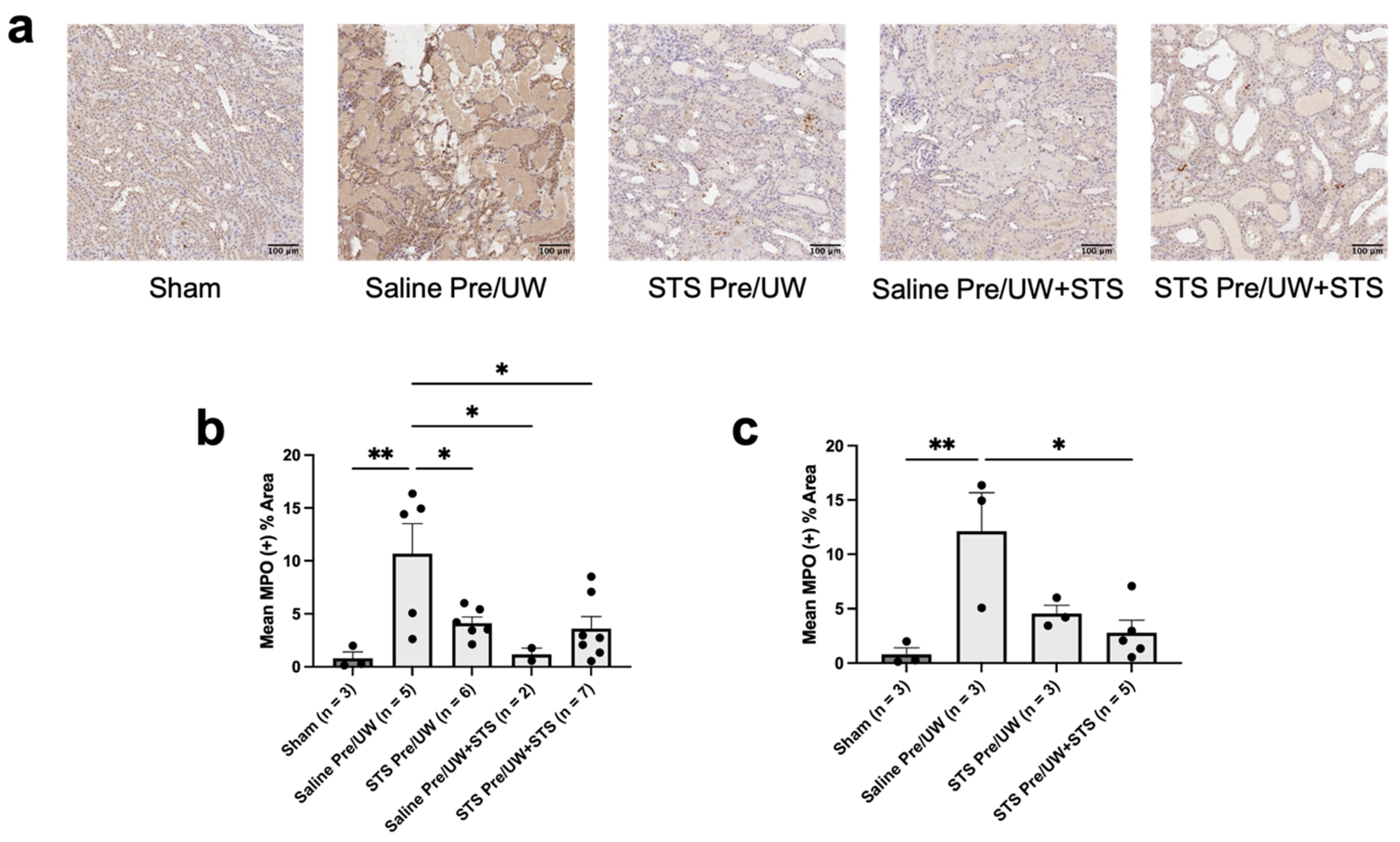
2.5. Effect of STS Pre-Treatment on Inflammation and Injury in Renal Grafts
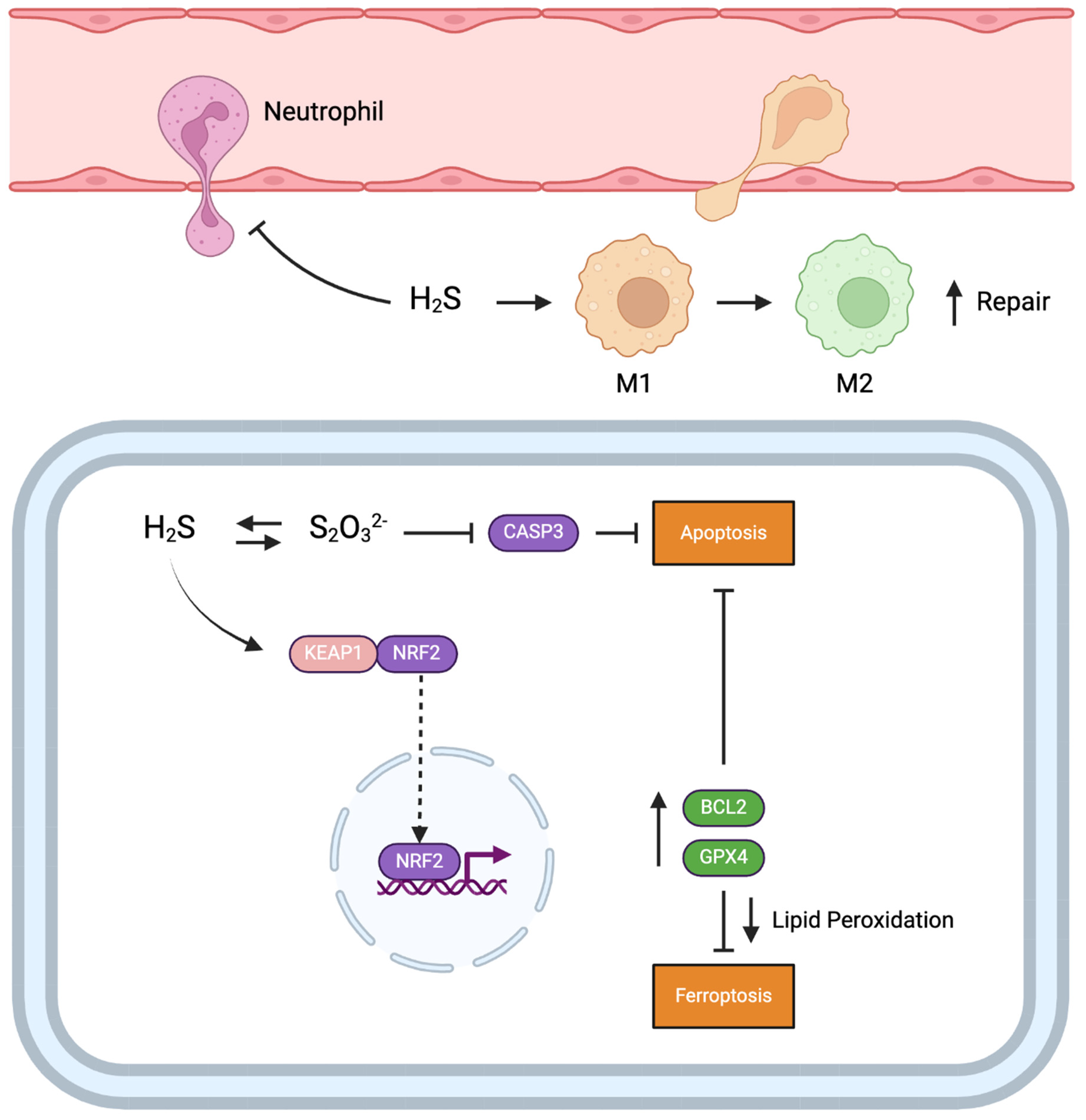
3. Discussion
4. Methods
4.1. In Vitro Model of Rat Renal Epithelial Cell Hypoxia–Reoxygenation Injury
4.2. Experimental Animals
4.3. Kidney Donor Pre-Treatment
4.4. Syngeneic Kidney Transplantation and Animal Monitoring
4.5. Serum Creatinine, BUN, and eGFR
4.6. Urine Osmolality
4.7. Renal Tissue Processing
4.8. Histological Staining
4.9. Whole-Slide Image Analysis
4.10. Quantitative PCR and Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liyanage, T.; Ninomiya, T.; Jha, V.; Neal, B.; Patrice, H.M.; Okpechi, I.; Zhao, M.; Lv, J.; Garg, A.X.; Knight, J.; et al. Worldwide Access to Treatment for End-Stage Kidney Disease: A Systematic Review. Lancet 2015, 385, 1975–1982. [Google Scholar] [CrossRef]
- GBD 2017 Causes of Death Collaborators. Global, Regional, and National Age-Sex-Specific Mortality for 282 Causes of Death in 195 Countries and Territories, 1980-2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Wiebe, N.; Knoll, G.; Bello, A.; Browne, S.; Jadhav, D.; Klarenbach, S.; Gill, J. Systematic review: Kidney transplantation compared with dialysis in clinically relevant outcomes. Am. J. Transpl. 2011, 11, 2093–2109. [Google Scholar] [CrossRef] [PubMed]
- Yeung, E.; Bello, A.K.; Levin, A.; Lunney, M.; Osman, M.A.; Ye, F.; Ashuntantang, G.; Bellorin-Font, E.; Benghanem Gharbi, M.; Davison, S.; et al. Current Status of Health Systems Financing and Oversight for End-Stage Kidney Disease Care: A Cross-Sectional Global Survey. BMJ Open 2021, 11, e047245. [Google Scholar] [CrossRef] [PubMed]
- von der Lippe, N.; Waldum, B.; Brekke, F.B.; Amro, A.A.G.; Reisæter, A.V.; Os, I. From Dialysis to Transplantation: A 5-Year Longitudinal Study on Self-Reported Quality of Life. BMC Nephrol. 2014, 15, 191. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Eckle, T. Ischemia and Reperfusion—From Mechanism to Translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef]
- Chen, G.Y.; Nuñez, G. Sterile Inflammation: Sensing and Reacting to Damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef]
- Bon, D.; Chatauret, N.; Giraud, S.; Thuillier, R.; Favreau, F.; Hauet, T. New Strategies to Optimize Kidney Recovery and Preservation in Transplantation. Nat. Rev. Nephrol. 2012, 8, 339–347. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic Accumulation of Succinate Controls Reperfusion Injury through Mitochondrial ROS. Nature 2014, 515, 431–435. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, Y.; Li, L.; Liu, S.; Wang, C.; Yuan, Y.; Yang, G.; Chen, Y.; Cheng, J.; Lu, Y.; et al. Mitochondrial ROS Promote Mitochondrial Dysfunction and Inflammation in Ischemic Acute Kidney Injury by Disrupting TFAM-Mediated mtDNA Maintenance. Theranostics 2021, 11, 1845–1863. [Google Scholar] [CrossRef]
- Liang, H.L.; Sedlic, F.; Bosnjak, Z.; Nilakantan, V. SOD1 and MitoTEMPO Partially Prevent Mitochondrial Permeabil-ity Transition Pore Opening, Necrosis, and Mitochondrial Apoptosis after ATP Depletion Recovery. Free Radic. Biol. Med. 2010, 49, 1550–1560. [Google Scholar] [CrossRef] [PubMed]
- Minami, K.; Bae, S.; Uehara, H.; Zhao, C.; Lee, D.; Iske, J.; Fanger, M.W.; Reder, J.; Morrison, I.; Azuma, H.; et al. Targeting of Intragraft Reactive Oxygen Species by APP-103, a Novel Polymer Product, Mitigates Ischemia/Reperfusion Injury and Promotes the Survival of Renal Transplants. Am. J. Transpl. 2020, 20, 1527–1537. [Google Scholar] [CrossRef]
- Winterberg, P.D.; Wang, Y.; Lin, K.-M.; Hartono, J.R.; Nagami, G.T.; Zhou, X.J.; Shelton, J.M.; Richardson, J.A.; Lu, C.Y. Reactive Oxygen Species and IRF1 Stimulate IFNα Production by Proximal Tubules during Ischemic AKI. Am. J. Physiol. Renal. Physiol. 2013, 305, F164–F172. [Google Scholar] [CrossRef]
- Wang, Y.; John, R.; Chen, J.; Richardson, J.A.; Shelton, J.M.; Bennett, M.; Zhou, X.J.; Nagami, G.T.; Zhang, Y.; Wu, Q.Q.; et al. IRF-1 Promotes Inflammation Early after Ischemic Acute Kidney Injury. J. Am. Soc. Nephrol. 2009, 20, 1544–1555. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Huang, F.; Wang, Y.; Chen, C.; Wu, S.; Zhou, S.; Hei, Z.; Yuan, D. Connexin32 Plays a Crucial Role in ROS-Mediated Endoplasmic Reticulum Stress Apoptosis Signaling Pathway in Ischemia Reperfusion-Induced Acute Kidney Injury. J. Transl. Med. 2018, 16, 117. [Google Scholar] [CrossRef] [PubMed]
- Ponticelli, C. Ischaemia-Reperfusion Injury: A Major Protagonist in Kidney Transplantation. Nephrol. Dial. Transpl. 2014, 29, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, G.; Wyburn, K.R.; Yin, J.; Bertolino, P.; Eris, J.M.; Alexander, S.I.; Sharland, A.F.; Chadban, S.J. TLR4 Activation Mediates Kidney Ischemia/Reperfusion Injury. J. Clin. Investig. 2007, 117, 2847–2859. [Google Scholar] [CrossRef]
- Leemans, J.C.; Stokman, G.; Claessen, N.; Rouschop, K.M.; Teske, G.J.D.; Kirschning, C.J.; Akira, S.; van der Poll, T.; Weening, J.J.; Florquin, S. Renal-Associated TLR2 Mediates Ischemia/Reperfusion Injury in the Kidney. J. Clin. Investig. 2005, 115, 2894–2903. [Google Scholar] [CrossRef]
- Dugbartey, G.J. Cellular and molecular mechanisms of cell damage and cell death in ischemia–reperfusion injury in organ transplantation. Mol. Biol. Rep. 2024, 51, 473. [Google Scholar]
- Hosgood, S.A.; Brown, R.J.; Nicholson, M.L. Advances in Kidney Preservation Techniques and Their Application in Clinical Practice. Transplantation 2021, 105, e202–e214. [Google Scholar] [CrossRef]
- Locke, J.E.; Segev, D.L.; Warren, D.S.; Dominici, F.; Simpkins, C.E.; Montgomery, R.A. Outcomes of Kidneys from Donors after Cardiac Death: Implications for Allocation and Preservation. Am. J. Transpl. 2007, 7, 1797–1807. [Google Scholar] [CrossRef]
- Wang, R. Two’s Company, Three’s a Crowd: Can H2S Be the Third Endogenous Gaseous Transmitter? FASEB J. 2002, 16, 1792–1798. [Google Scholar] [CrossRef] [PubMed]
- Majtan, T.; Krijt, J.; Sokolová, J.; Křížková, M.; Ralat, M.A.; Kent, J.; Gregory, J.F.; Kožich, V.; Kraus, J.P. Biogenesis of Hydrogen Sulfide and Thioethers by Cystathionine Beta-Synthase. Antioxid. Redox. Signal. 2018, 28, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Chiku, T.; Padovani, D.; Zhu, W.; Singh, S.; Vitvitsky, V.; Banerjee, R. H2S Biogenesis by Human Cystathionine Gamma-Lyase Leads to the Novel Sulfur Metabolites Lanthionine and Homolanthionine and Is Responsive to the Grade of Hyperhomocysteinemia. J. Biol. Chem. 2009, 284, 11601–11612. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Koike, S.; Tanaka, M.; Ishigami-Yuasa, M.; Kimura, Y.; Ogasawara, Y.; Fukui, K.; Nagahara, N.; Kimura, H. A Novel Pathway for the Production of Hydrogen Sulfide from D-Cysteine in Mammalian Cells. Nat. Commun. 2013, 4, 1366. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Tanaka, M.; Yoshida, M.; Ogasawara, Y.; Togawa, T.; Ishii, K.; Kimura, H. 3-Mercaptopyruvate Sul-furtransferase Produces Hydrogen Sulfide and Bound Sulfane Sulfur in the Brain. Antioxid. Redox. Signal 2009, 11, 703–714. [Google Scholar] [CrossRef]
- Health Canada Seacalphyx. Available online: https://health-products.canada.ca/dpd-bdpp/info.do?code=87223&lang=en#fn1 (accessed on 12 April 2024).
- U.S. Food and Drug Administration Sodium Thiosulfate Injection, 250 Mg/mL. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/203923_sodium_thiosulfate_toc.cfm (accessed on 12 April 2024).
- Zhang, M.Y.; Dugbartey, G.J.; Juriasingani, S.; Akbari, M.; Liu, W.; Haig, A.; McLeod, P.; Arp, J.; Sener, A. Sodium Thiosulfate-Supplemented UW Solution Protects Renal Grafts against Prolonged Cold Ischemia-Reperfusion Injury in a Murine Model of Syngeneic Kidney Transplantation. Biomed. Pharmacother. 2022, 145, 112435. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, B.; Yu, J.; Wang, X.; Zhao, H.; Li, G.; Yin, Y.; Meng, C. Comparison of Inflation and Ventilation with Hydrogen Sulfide during the Warm Ischemia Phase on Ischemia-Reperfusion Injury in a Rat Model of Non-Heart-Beating Donor Lung Transplantation. Biomed. Res. Int. 2023, 2023, 3645304. [Google Scholar] [CrossRef]
- Meng, C.; Cui, X.; Qi, S.; Zhang, J.; Kang, J.; Zhou, H. Lung Inflation with Hydrogen Sulfide during the Warm Ischemia Phase Ameliorates Injury in Rat Donor Lungs via Metabolic Inhibition after Cardiac Death. Surgery 2017, 161, 1287–1298. [Google Scholar] [CrossRef]
- McFarlane, L.; Nelson, P.; Dugbartey, G.J.; Sener, A. Pre-Treatment of Transplant Donors with Hydrogen Sulfide to Protect against Warm and Cold Ischemia-Reperfusion Injury in Kidney and Other Transplantable Solid Organs. Int. J. Mol. Sci. 2023, 24, 3518. [Google Scholar] [CrossRef]
- Ni, J.; Jiang, L.; Shen, G.; Xia, Z.; Zhang, L.; Xu, J.; Feng, Q.; Qu, H.; Xu, F.; Li, X. Hydrogen Sulfide Reduces Pyroptosis and Alleviates Ischemia-Reperfusion-Induced Acute Kidney Injury by Inhibiting NLRP3 Inflammasome. Life Sci. 2021, 284, 119466. [Google Scholar] [CrossRef]
- Marutani, E.; Yamada, M.; Ida, T.; Tokuda, K.; Ikeda, K.; Kai, S.; Shirozu, K.; Hayashida, K.; Kosugi, S.; Hanaoka, K.; et al. Thiosulfate Mediates Cytoprotective Effects of Hydrogen Sulfide Against Neuronal Ischemia. J. Am. Heart Assoc. 2015, 4, e002125. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, S.; Boovarahan, S.R.; Shanmugam, K.; Vedarathinam, R.C.; Kurian, G.A. Sodium Thiosulfate Preconditioning Ameliorates Ischemia/Reperfusion Injury in Rat Hearts Via Reduction of Oxidative Stress and Apoptosis. Cardiovasc. Drugs Ther. 2017, 31, 511–524. [Google Scholar] [CrossRef]
- Juriasingani, S.; Akbari, M.; Chan, J.Y.; Whiteman, M.; Sener, A. H2S Supplementation: A Novel Method for Successful Organ Preservation at Subnormothermic Temperatures. Nitric. Oxide 2018, 81, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Abou Taka, M.; Dugbartey, G.J.; Richard-Mohamed, M.; McLeod, P.; Jiang, J.; Major, S.; Arp, J.; O’Neil, C.; Liu, W.; Gabril, M.; et al. Evaluating the Effects of Kidney Preservation at 10 °C with Hemopure and Sodium Thiosulfate in a Rat Model of Syngeneic Orthotopic Kidney Transplantation. Int. J. Mol. Sci. 2024, 25, 2210. [Google Scholar] [CrossRef]
- Khundmiri, S.J.; Chen, L.; Lederer, E.D.; Yang, C.-R.; Knepper, M.A. Transcriptomes of Major Proximal Tubule Cell Culture Models. J. Am. Soc. Nephrol. 2021, 32, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xu, M.; Tu, J.; Wang, H.; Niu, X. Relationship between Omnibus and Post-Hoc Tests: An Investigation of Performance of the F Test in ANOVA. Shanghai Arch. Psychiatry 2018, 30, 60–64. [Google Scholar] [CrossRef]
- Midway, S.; Robertson, M.; Flinn, S.; Kaller, M. Comparing Multiple Comparisons: Practical Guidance for Choosing the Best Multiple Comparisons Test. PeerJ 2020, 8, e10387. [Google Scholar] [CrossRef]
- Urbanellis, P.; Mazilescu, L.; Kollmann, D.; Linares-Cervantes, I.; Kaths, J.M.; Ganesh, S.; Oquendo, F.; Sharma, M.; Goto, T.; Noguchi, Y.; et al. Prolonged Warm Ischemia Time Leads to Severe Renal Dysfunction of Donation-after-Cardiac Death Kidney Grafts. Sci. Rep. 2021, 11, 17930. [Google Scholar] [CrossRef]
- Hall, I.E.; Doshi, M.D.; Poggio, E.D.; Parikh, C.R. A Comparison of Alternative Serum Biomarkers with Creatinine for Predicting Allograft Function after Kidney Transplantation. Transplantation 2011, 91, 48–56. [Google Scholar] [CrossRef]
- Reuter, S.; Schnöckel, U.; Edemir, B.; Schröter, R.; Kentrup, D.; Pavenstädt, H.; Schober, O.; Schlatter, E.; Gabriëls, G.; Schäfers, M. Potential of Noninvasive Serial Assessment of Acute Renal Allograft Rejection by 18F-FDG PET to Monitor Treatment Efficiency. J. Nucl. Med. 2010, 51, 1644–1652. [Google Scholar] [CrossRef]
- Bos, E.M.; Wang, R.; Snijder, P.M.; Boersema, M.; Damman, J.; Fu, M.; Moser, J.; Hillebrands, J.-L.; Ploeg, R.J.; Yang, G.; et al. Cystathionine γ-Lyase Protects against Renal Ischemia/Reperfusion by Modulating Oxidative Stress. J. Am. Soc. Nephrol. 2013, 24, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, S.F.; Rathore, H.A.; Sattar, M.A.; Johns, E.J.; Gan, C.-Y.; Chia, T.Y.; Ahmad, A. Hydrogen Sulphide Treatment Prevents Renal Ischemia-Reperfusion Injury by Inhibiting the Expression of ICAM-1 and NF-kB Concentration in Normotensive and Hypertensive Rats. Biomolecules 2021, 11, 1549. [Google Scholar] [CrossRef]
- Zhao, H.; Qiu, Y.; Wu, Y.; Sun, H.; Gao, S. Protective Effects of GYY4137 on Renal Ischaemia/Reperfusion Injury through Nrf2-Mediated Antioxidant Defence. Kidney Blood Press. Res. 2021, 46, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Lobb, I.; Mok, A.; Lan, Z.; Liu, W.; Garcia, B.; Sener, A. Supplemental Hydrogen Sulphide Protects Transplant Kidney Function and Prolongs Recipient Survival after Prolonged Cold Ischaemia-Reperfusion Injury by Mitigating Renal Graft Apoptosis and Inflammation. BJU Int. 2012, 110, E1187–E1195. [Google Scholar] [CrossRef] [PubMed]
- Lobb, I.; Jiang, J.; Lian, D.; Liu, W.; Haig, A.; Saha, M.N.; Torregrossa, R.; Wood, M.E.; Whiteman, M.; Sener, A. Hydrogen Sulfide Protects Renal Grafts Against Prolonged Cold Ischemia-Reperfusion Injury via Specific Mitochondrial Actions. Am. J. Transplant. 2017, 17, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Pyeon, T.; Choi, J.I.; Kang, J.H.; Song, S.W.; Bae, H.-B.; Jeong, S. A Retrospective Study of the Relationship between Postoperative Urine Output and One Year Transplanted Kidney Function. BMC Anesthesiol. 2019, 19, 231. [Google Scholar] [CrossRef]
- Osman, Y.; El-Husseini, A.; Kamal, M.; Refaie, A.; Sheashaa, H.; Sobh, M. Does Timing of Post-Renal Transplant Diuresis Affect Graft Survival in Live-Donor Renal Transplants? BJU Int. 2011, 107, 284–287. [Google Scholar] [CrossRef]
- Jung, K.-J.; Jang, H.-S.; Kim, J.I.; Han, S.J.; Park, J.-W.; Park, K.M. Involvement of Hydrogen Sulfide and Homocysteine Transsulfuration Pathway in the Progression of Kidney Fibrosis after Ureteral Obstruction. Biochim. Biophys. Acta. 2013, 1832, 1989–1997. [Google Scholar] [CrossRef]
- Tang, Y.-H.; Wu, L.; Huang, H.-L.; Zhang, P.-P.; Zou, W.; Tang, X.-Q.; Tang, Y.-Y. Hydrogen Sulfide Antagonizes Formaldehyde-Induced Ferroptosis via Preventing Ferritinophagy by Upregulation of GDF11 in HT22 Cells. Toxicology 2023, 491, 153517. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, K.; Ju, Y.; Mani, S.; Cao, Q.; Puukila, S.; Khaper, N.; Wu, L.; Wang, R. Hydrogen Sulfide Protects against Cellular Senescence via S-Sulfhydration of Keap1 and Activation of Nrf2. Antioxid. Redox Signal. 2013, 18, 1906–1919. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Pan, J.; Huang, S.; Chen, X.; Chang, A.C.Y.; Wang, C.; Zhang, J.; Zhang, H. Hydrogen Sulfide Protects Cardiomyocytes from Doxorubicin-Induced Ferroptosis through the SLC7A11/GSH/GPx4 Pathway by Keap1 S-Sulfhydration and Nrf2 Activation. Redox Biol. 2024, 70, 103066. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M. Lipid Peroxidation and Ferroptosis: The Role of GSH and GPx4. Free Radic. Biol. Med. 2020, 152, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Calvert, J.W.; Jha, S.; Gundewar, S.; Elrod, J.W.; Ramachandran, A.; Pattillo, C.B.; Kevil, C.G.; Lefer, D.J. Hydrogen Sulfide Mediates Cardioprotection through Nrf2 Signaling. Circ. Res. 2009, 105, 365–374. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Zhang, A.; Yao, M.; Xin, W.; Guan, X.; Qin, S.; Liu, Y.; Xiong, J.; Yang, K.; Xiong, L.; et al. REST Contributes to AKI-to-CKD Transition through Inducing Ferroptosis in Renal Tubular Epithelial Cells. JCI Insight 2023, 8, e166001. [Google Scholar] [CrossRef]
- Song, J.; Sheng, J.; Lei, J.; Gan, W.; Yang, Y. Mitochondrial Targeted Antioxidant SKQ1 Ameliorates Acute Kidney Injury by Inhibiting Ferroptosis. Oxid. Med. Cell Longev. 2022, 2022, 2223957. [Google Scholar] [CrossRef]
- Wu, L.; Tian, X.; Zuo, H.; Zheng, W.; Li, X.; Yuan, M.; Tian, X.; Song, H. miR-124-3p Delivered by Exosomes from Heme Oxygenase-1 Modified Bone Marrow Mesenchymal Stem Cells Inhibits Ferroptosis to Attenuate Ischemia-Reperfusion Injury in Steatotic Grafts. J. Nanobiotechnology 2022, 20, 196. [Google Scholar] [CrossRef]
- Shi, S.; Bonaccorsi-Riani, E.; Schurink, I.; van den Bosch, T.; Doukas, M.; Lila, K.A.; Roest, H.P.; Xhema, D.; Gianello, P.; de Jonge, J.; et al. Liver Ischemia and Reperfusion Induce Periportal Expression of Necroptosis Executor pMLKL Which Is Associated with Early Allograft Dysfunction after Transplantation. Front. Immunol. 2022, 13, 890353. [Google Scholar] [CrossRef]
- Pefanis, A.; Bongoni, A.K.; McRae, J.L.; Salvaris, E.J.; Fisicaro, N.; Murphy, J.M.; Ierino, F.L.; Cowan, P.J. Dynamics of Necroptosis in Kidney Ischemia-Reperfusion Injury. Front. Immunol. 2023, 14, 1251452. [Google Scholar] [CrossRef]
- Chang, L.; Wang, Z.; Ma, F.; Tran, B.; Zhong, R.; Xiong, Y.; Dai, T.; Wu, J.; Xin, X.; Guo, W.; et al. ZYZ-803 Mitigates Endoplasmic Reticulum Stress-Related Necroptosis after Acute Myocardial Infarction through Downregulating the RIP3-CaMKII Signaling Pathway. Oxid. Med. Cell Longev. 2019, 2019, 6173685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, Y.; Cui, M.; Jin, L.; Wang, Y.; Lv, F.; Liu, Y.; Zheng, W.; Shang, H.; Zhang, J.; et al. CaMKII Is a RIP3 Substrate Mediating Ischemia- and Oxidative Stress-Induced Myocardial Necroptosis. Nat. Med. 2016, 22, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Hu, Q.; Tan, B.; Rose, P.; Zhu, D.; Zhu, Y.Z. Amelioration of Mitochondrial Dysfunction in Heart Failure through S-Sulfhydration of Ca2+/Calmodulin-Dependent Protein Kinase II. Redox Biol. 2018, 19, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Niture, S.K.; Jaiswal, A.K. Nrf2 Protein Up-Regulates Antiapoptotic Protein Bcl-2 and Prevents Cellular Apoptosis. J. Biol. Chem. 2012, 287, 9873–9886. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.X.G.; Kalbfleisch, M.; Yang, Y.X.; Bihari, R.; Lobb, I.; Davison, M.; Mok, A.; Cepinskas, G.; Lawendy, A.-R.; Sener, A. Detrimental Effects of Prolonged Warm Renal Ischaemia-Reperfusion Injury Are Abrogated by Supplemental Hydrogen Sulphide: An Analysis Using Real-Time Intravital Microscopy and Polymerase Chain Reaction. BJU Int. 2012, 110, E1218–E1227. [Google Scholar] [CrossRef]
- Dugbartey, G.J.; Juriasingani, S.; Richard-Mohamed, M.; Rasmussen, A.; Levine, M.; Liu, W.; Haig, A.; Whiteman, M.; Arp, J.; Luke, P.P.W.; et al. Static Cold Storage with Mitochondria-Targeted Hydrogen Sulfide Donor Improves Renal Graft Function in an Ex Vivo Porcine Model of Controlled Donation-after-Cardiac-Death Kidney Transplantation. Int. J. Mol. Sci. 2023, 24, 14017. [Google Scholar] [CrossRef]
- Lee, S.; Huen, S.; Nishio, H.; Nishio, S.; Lee, H.K.; Choi, B.-S.; Ruhrberg, C.; Cantley, L.G. Distinct Macrophage Phenotypes Contribute to Kidney Injury and Repair. J. Am. Soc. Nephrol. 2011, 22, 317–326. [Google Scholar] [CrossRef]
- Mao, R.; Wang, C.; Zhang, F.; Zhao, M.; Liu, S.; Liao, G.; Li, L.; Chen, Y.; Cheng, J.; Liu, J.; et al. Peritoneal M2 Macrophage Transplantation as a Potential Cell Therapy for Enhancing Renal Repair in Acute Kidney Injury. J. Cell Mol. Med. 2020, 24, 3314–3327. [Google Scholar] [CrossRef]
- Miao, L.; Shen, X.; Whiteman, M.; Xin, H.; Shen, Y.; Xin, X.; Moore, P.K.; Zhu, Y.-Z. Hydrogen Sulfide Mitigates My-ocardial Infarction via Promotion of Mitochondrial Biogenesis-Dependent M2 Polarization of Macrophages. Antioxid. Redox Signal. 2016, 25, 268–281. [Google Scholar] [CrossRef]
- Zhou, T.; Liu, W.; Lai, H.; Liu, Y.; Su, W.; Xu, Z. Hydrogen Sulfide Promotes Osteogenesis by Modulating Macrophage Polarization. Int. Immunopharmacol. 2023, 115, 109564. [Google Scholar] [CrossRef]
- Ball, C.J.; Reiffel, A.J.; Chintalapani, S.; Kim, M.; Spector, J.A.; King, M.R. Hydrogen Sulfide Reduces Neutrophil Re-cruitment in Hind-Limb Ischemia-Reperfusion Injury in an L-Selectin and ADAM-17-Dependent Manner. Plast. Reconstr. Surg. 2013, 131, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, T.; Bonventre, J.V.; Bailly, V.; Wei, H.; Hession, C.A.; Cate, R.L.; Sanicola, M. Kidney Injury Molecule-1 (KIM-1), a Putative Epithelial Cell Adhesion Molecule Containing a Novel Immunoglobulin Domain, Is up-Regulated in Renal Cells after Injury. J. Biol. Chem. 1998, 273, 4135–4142. [Google Scholar] [CrossRef]
- Liangos, O.; Perianayagam, M.C.; Vaidya, V.S.; Han, W.K.; Wald, R.; Tighiouart, H.; MacKinnon, R.W.; Li, L.; Bala-krishnan, V.S.; Pereira, B.J.G.; et al. Urinary N-Acetyl-Beta-(D)-Glucosaminidase Activity and Kidney Injury Molecule-1 Level Are Associated with Adverse Outcomes in Acute Renal Failure. J. Am. Soc. Nephrol. 2007, 18, 904–912. [Google Scholar] [CrossRef]
- Zhang, B.-H.; Liu, H.; Yuan, Y.; Weng, X.-D.; Du, Y.; Chen, H.; Chen, Z.-Y.; Wang, L.; Liu, X.-H. Knockdown of TRIM8 Protects HK-2 Cells Against Hypoxia/Reoxygenation-Induced Injury by Inhibiting Oxidative Stress-Mediated Apopto-sis and Pyroptosis via PI3K/Akt Signal Pathway. Drug. Des. Devel. Ther. 2021, 15, 4973–4983. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Y.; Han, S.; Liu, Y.; Gao, J.; Huang, Y.; Sun, W.; Wang, J.; Wang, C.; Zhao, J. Selenium Nanoparticles Alleviate Ischemia Reperfusion Injury-Induced Acute Kidney Injury by Modulating GPx-1/NLRP3/Caspase-1 Pathway. Theranostics 2022, 12, 3882–3895. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wan, S.; Liu, H.; Qiu, Q.; Chen, H.; Chen, Z.; Wang, L.; Liu, X. Naringenin Alleviates Renal Ischemia Reperfusion Injury by Suppressing ER Stress-Induced Pyroptosis and Apoptosis through Activating Nrf2/HO-1 Signaling Pathway. Oxid. Med. Cell Longev. 2022, 2022, 5992436. [Google Scholar] [CrossRef]
- Treat, E.; Chow, E.K.H.; Peipert, J.D.; Waterman, A.; Kwan, L.; Massie, A.B.; Thomas, A.G.; Bowring, M.G.; Leeser, D.; Flechner, S.; et al. Shipping Living Donor Kidneys and Transplant Recipient Outcomes. Am. J. Transplant. 2018, 18, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Treat, E.G.; Miller, E.T.; Kwan, L.; Connor, S.E.; Maliski, S.L.; Hicks, E.M.; Williams, K.C.; Whitted, L.A.; Gritsch, H.A.; McGuire, S.M.; et al. Outcomes of Shipped Live Donor Kidney Transplants Compared with Traditional Living Donor Kidney Transplants. Transpl. Int. 2014, 27, 1175–1182. [Google Scholar] [CrossRef]
- Lobb, I.; Davison, M.; Carter, D.; Liu, W.; Haig, A.; Gunaratnam, L.; Sener, A. Hydrogen Sulfide Treatment Mitigates Renal Allograft Ischemia-Reperfusion Injury during Cold Storage and Improves Early Transplant Kidney Function and Survival Following Allogeneic Renal Transplantation. J. Urol. 2015, 194, 1806–1815. [Google Scholar] [CrossRef]
- Mengel, K.; Krämer, W.; Isert, B.; Friedberg, K.D. Thiosulphate and Hydroxocobalamin Prophylaxis in Progressive Cyanide Poisoning in Guinea-Pigs. Toxicology 1989, 54, 335–342. [Google Scholar] [CrossRef]
- Guo, Y.; Luke, P.; Sener, A. Organ Storage in Renal Transplantation. Curr. Opin. Urol. 2024, 34, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, P.E.; Monaco, A.P. Donation after Circulatory Death: Current Practices, Ongoing Challenges, and Potential Improvements. Transplantation 2014, 97, 258–264. [Google Scholar] [CrossRef]
- Sharfuddin, A.A.; Molitoris, B.A. Pathophysiology of Ischemic Acute Kidney Injury. Nat. Rev. Nephrol. 2011, 7, 189–200. [Google Scholar] [CrossRef]
- Besseling, P.J.; Pieters, T.T.; Nguyen, I.T.N.; de Bree, P.M.; Willekes, N.; Dijk, A.H.; Bovée, D.M.; Hoorn, E.J.; Rookmaaker, M.B.; Gerritsen, K.G.; et al. A Plasma Creatinine- and Urea-Based Equation to Estimate Glomerular Filtration Rate in Rats. Am. J. Physiol. Renal. Physiol. 2021, 320, F518–F524. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open Source Software for Digital Pathology Image Analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed]
- Vonbrunn, E.; Ries, T.; Söllner, S.; Müller-Deile, J.; Büttner-Herold, M.; Amann, K.; Daniel, C. Multiplex Gene Analysis Reveals T-Cell and Antibody-Mediated Rejection-Specific Upregulation of Complement in Renal Transplants. Sci. Rep. 2021, 11, 15464. [Google Scholar] [CrossRef]
- Li, D.-D.; Li, N.; Cai, C.; Wei, C.-M.; Liu, G.-H.; Wang, T.-H.; Xu, F.-R. A Molecular Network-Based Pharmacological Study on the Protective Effect of Panax Notoginseng Rhizomes against Renal Ischemia-Reperfusion Injury. Front. Pharmacol. 2023, 14, 1134408. [Google Scholar] [CrossRef] [PubMed]
- Sadi, G.; Baloğlu, M.C.; Pektaş, M.B. Differential Gene Expression in Liver Tissues of Streptozotocin-Induced Diabetic Rats in Response to Resveratrol Treatment. PLoS ONE 2015, 10, e0124968. [Google Scholar] [CrossRef]




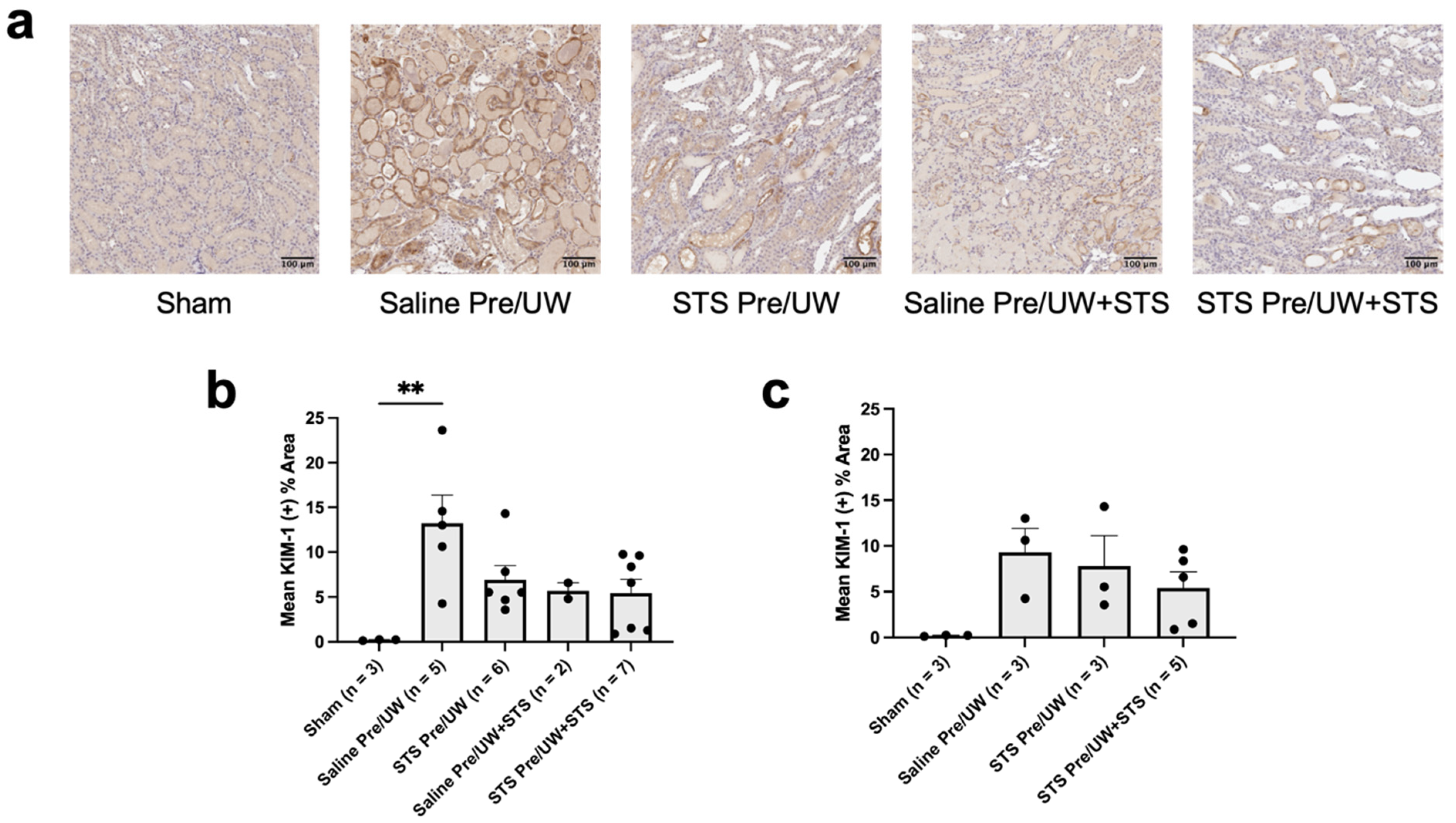
| Animal Group | Description ** |
|---|---|
| Sham * | Midline incision only |
| Saline Pre/UW (control) | PBS pre-treatment, graft storage in UW solution |
| STS Pre/UW | STS pre-treatment, graft storage in UW solution |
| Saline Pre/UW+STS | PBS pre-treatment, graft storage in UW+STS solution |
| STS Pre/UW+STS | STS pre-treatment, graft storage in UW+STS solution |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nelson, P.; Dugbartey, G.J.; McFarlane, L.; McLeod, P.; Major, S.; Jiang, J.; O’Neil, C.; Haig, A.; Sener, A. Effect of Sodium Thiosulfate Pre-Treatment on Renal Ischemia-Reperfusion Injury in Kidney Transplantation. Int. J. Mol. Sci. 2024, 25, 9529. https://doi.org/10.3390/ijms25179529
Nelson P, Dugbartey GJ, McFarlane L, McLeod P, Major S, Jiang J, O’Neil C, Haig A, Sener A. Effect of Sodium Thiosulfate Pre-Treatment on Renal Ischemia-Reperfusion Injury in Kidney Transplantation. International Journal of Molecular Sciences. 2024; 25(17):9529. https://doi.org/10.3390/ijms25179529
Chicago/Turabian StyleNelson, Pierce, George J. Dugbartey, Liam McFarlane, Patrick McLeod, Sally Major, Jifu Jiang, Caroline O’Neil, Aaron Haig, and Alp Sener. 2024. "Effect of Sodium Thiosulfate Pre-Treatment on Renal Ischemia-Reperfusion Injury in Kidney Transplantation" International Journal of Molecular Sciences 25, no. 17: 9529. https://doi.org/10.3390/ijms25179529
APA StyleNelson, P., Dugbartey, G. J., McFarlane, L., McLeod, P., Major, S., Jiang, J., O’Neil, C., Haig, A., & Sener, A. (2024). Effect of Sodium Thiosulfate Pre-Treatment on Renal Ischemia-Reperfusion Injury in Kidney Transplantation. International Journal of Molecular Sciences, 25(17), 9529. https://doi.org/10.3390/ijms25179529






