Plastome Evolution and Comparative Analyses of a Recently Radiated Genus Vanda (Aeridinae, Orchidaceae)
Abstract
1. Introduction
2. Result
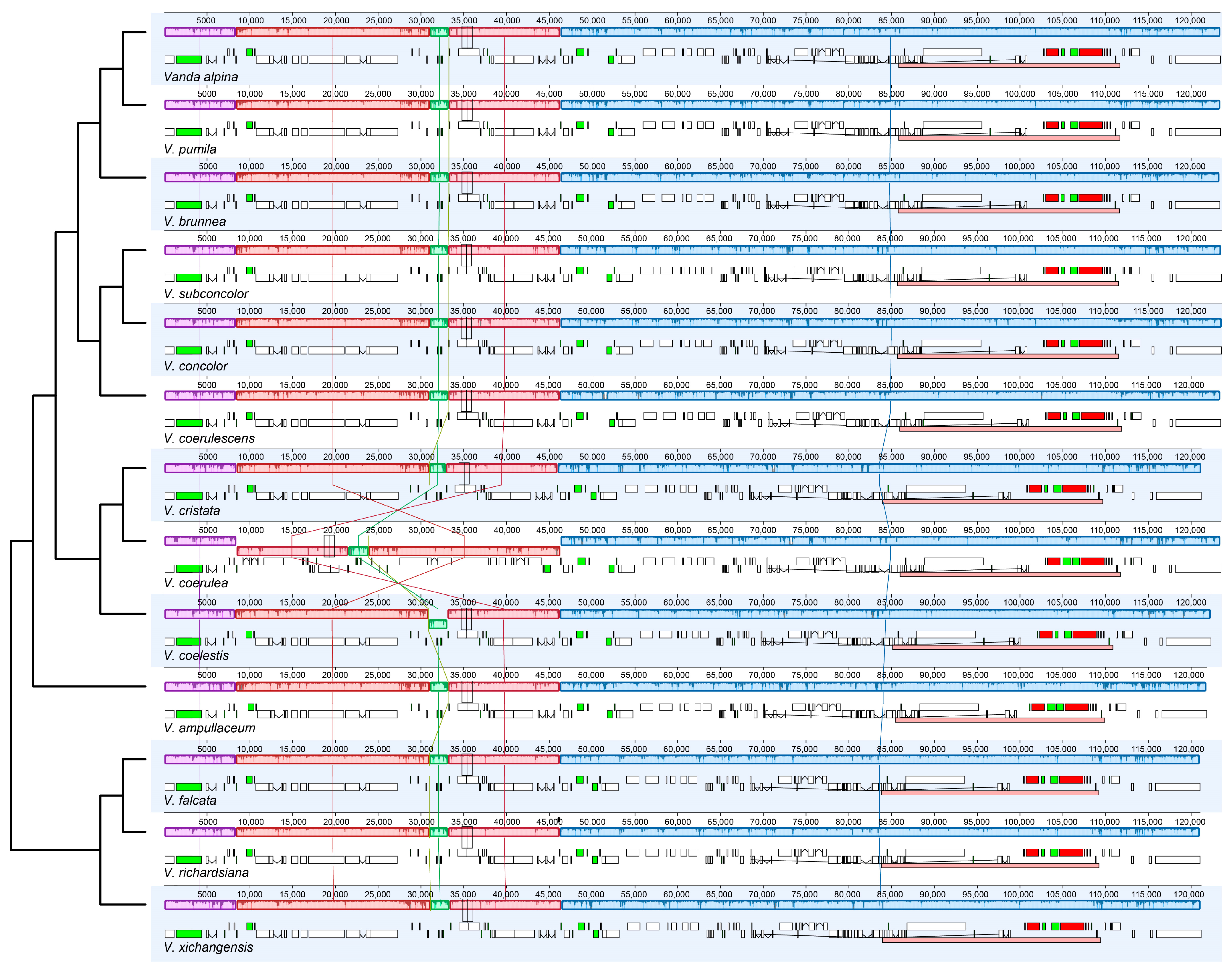
2.1. The Plastome Characters of Vanda
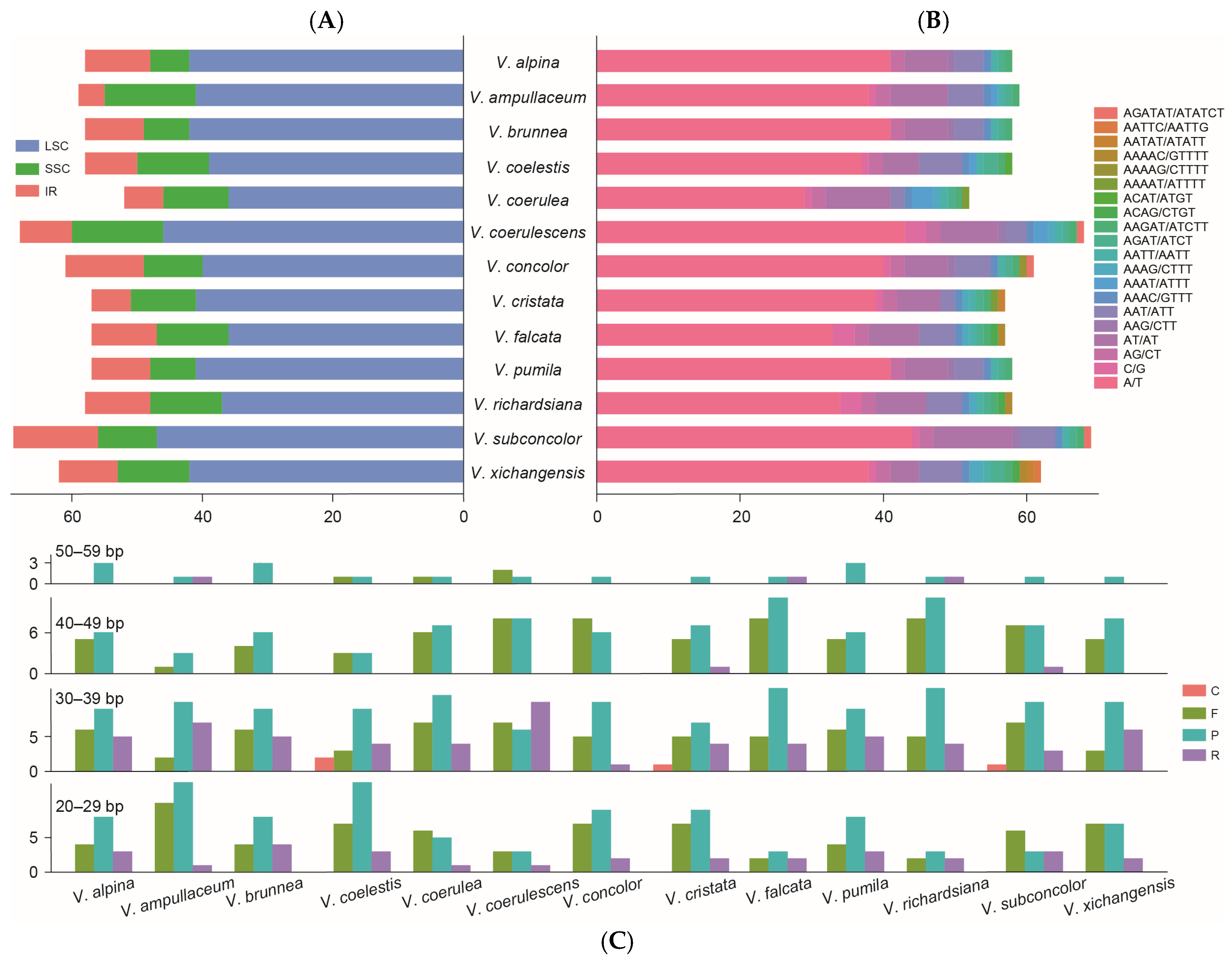
2.2. Repeated Analysis
2.3. Codon Usage Bias
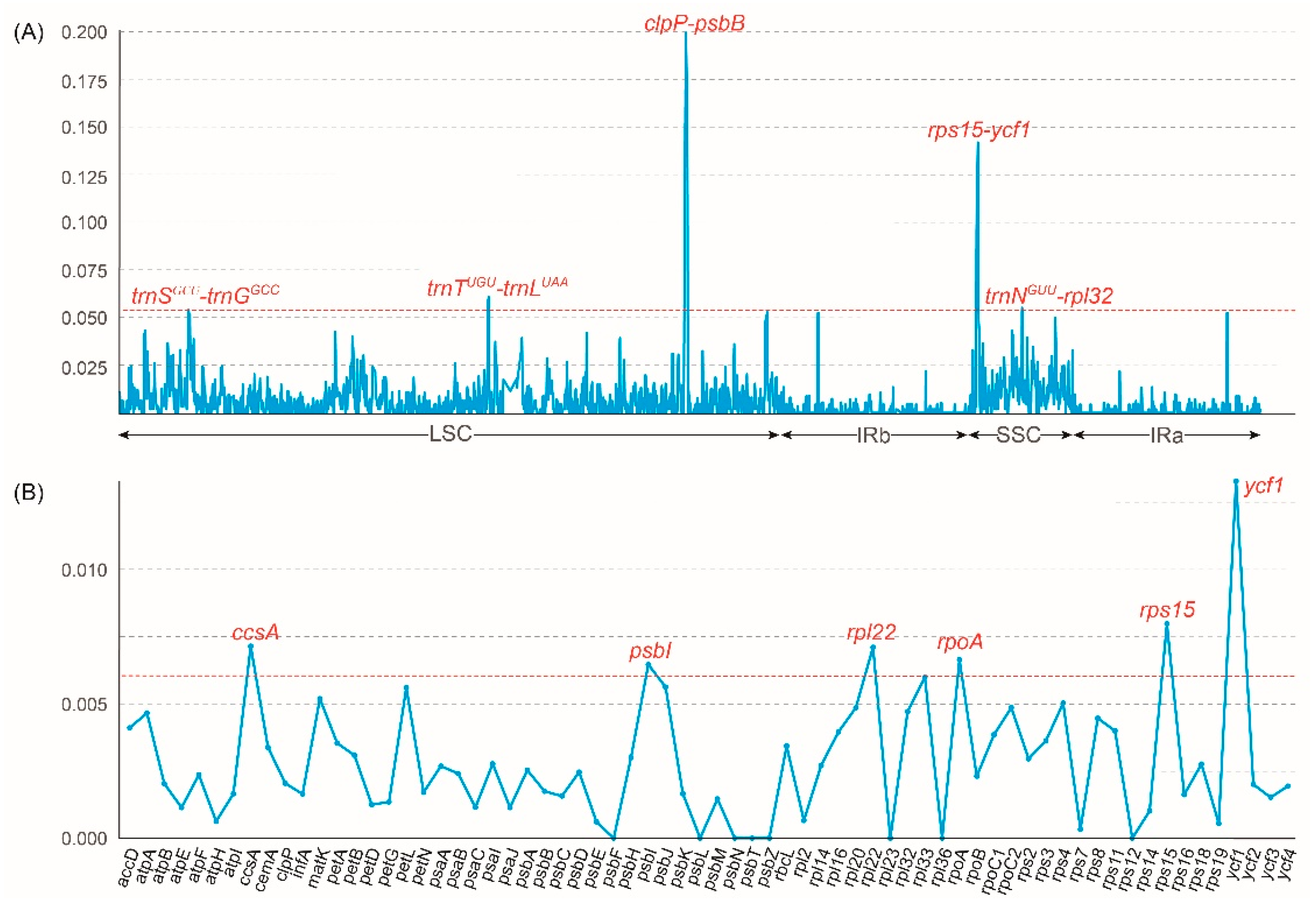
2.4. Plastome Sequence Divergence and DNA Marker Investigation
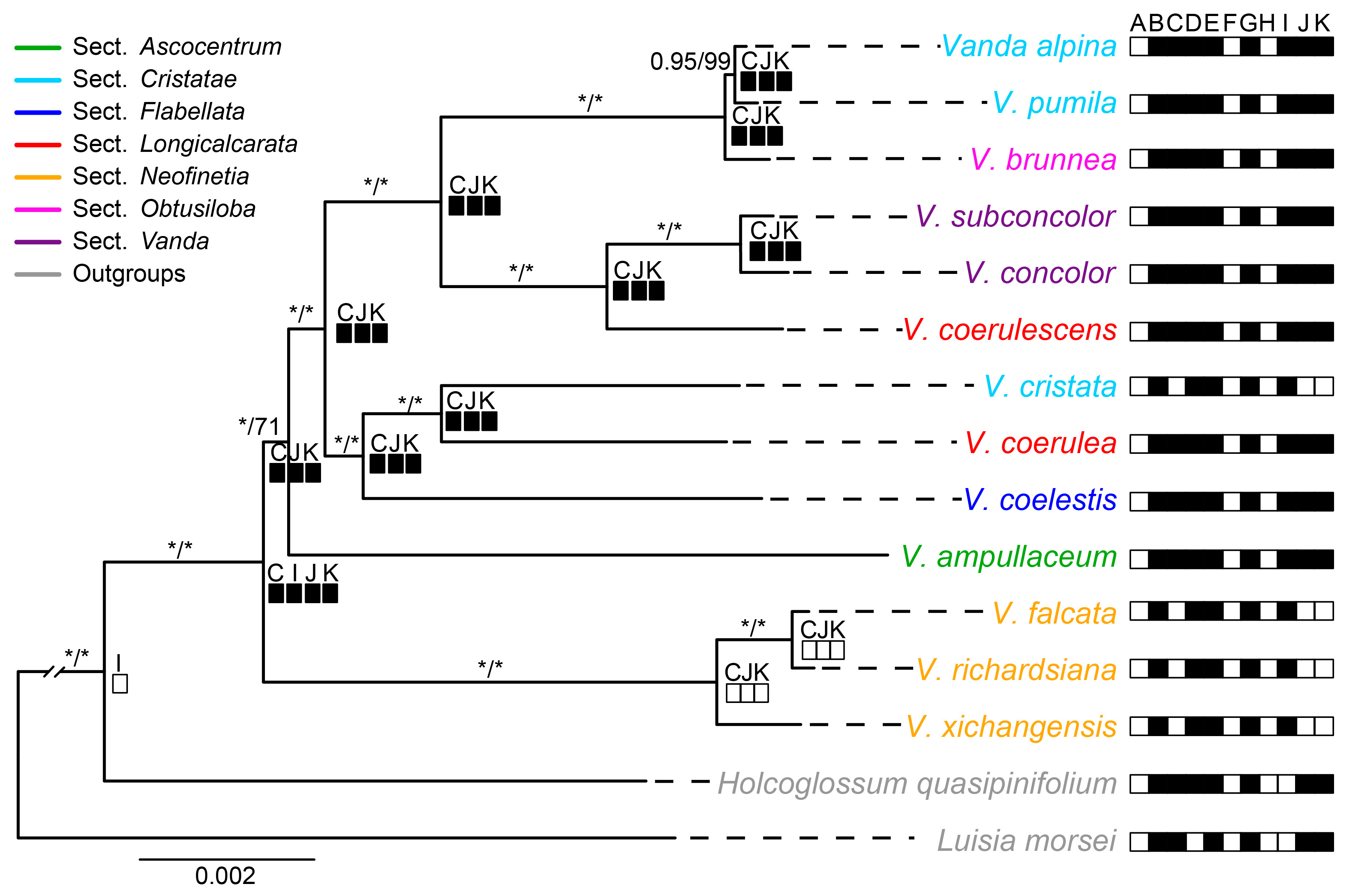
2.5. Phylogenetic Analyses and Character Reconstruction
3. Discussion
3.1. The Plastome Characteristics and Structural Evolution
3.2. The Barcoding Investigation and Phylogenetic Analyses
4. Materials and Methods
4.1. Sampling and Sequencing
4.2. Assembly and Annotation
4.3. Sequence and Structure Divergence Analyses
4.4. Repetitive Sequence and Codon Usage Analyses
4.5. Phylogenetic Analyses and Measurement of Divergence Variables
4.6. Character Reconstruction of Ndh Gene Status in Vanda
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gardiner, L.M.; Cribb, P.C. Renziana, Vol. 3: Vanda; The Swiss Orchid Foundation: Basel, Switzerland, 2013. [Google Scholar]
- Chase, M.W.; Cameron, K.M.; Freudenstein, J.V.; Pridgeon, A.M.; Salazar, G.; van den Berg, C.; Schuiteman, A. An updated classification of Orchidaceae. Bot. J. Linn. Soc. 2015, 177, 151–174. [Google Scholar] [CrossRef]
- Gardiner, L.M.; Kocyan, A.; Motes, M.; Roberts, D.L.; Emerson, B.C. Molecular phylogenetics of Vanda and related genera (Orchidaceae). Bot. J. Linn. Soc. 2013, 173, 549–572. [Google Scholar] [CrossRef]
- Motes, M. Vandas: Their Botany, History, and Culture; Timber Press: Portland, OR, USA, 1997. [Google Scholar]
- Pridgeon, A.M.; Cribb, P.J.; Chase, M.W.; Rasmussen, F.N. Genera Orchidacearum Vol. 6 Epidendroideae; Oxford University Press: New York, NY, USA, 2014. [Google Scholar]
- Brown, R. Vanda Roxburghii, a Chequer-Flowered Vanda; Botanical Register: Cambridgeshire, UK, 1820; Volume 6, p. 506. [Google Scholar]
- Lindley, J. Folia Orchidacea; Matthews: London, UK, 1853. [Google Scholar]
- Gardiner, L.M. New combinations in the genus Vanda (Orchidaceae). Phytotaxa 2012, 61, 47–54. [Google Scholar] [CrossRef]
- Zou, L.H.; Huang, J.X.; Zhang, G.Q.; Liu, Z.J.; Zhuang, X.Y. A molecular phylogeny of Aeridinae (Orchidaceae: Epidendroideae) inferred from multiple nuclear and chloroplast regions. Mol. Phylogenet. Evol. 2015, 85, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Q.; Liu, K.W.; Chen, L.J.; Xiao, X.J.; Zhai, J.W.; Li, L.Q.; Cai, J.; Hsiao, Y.Y.; Rao, W.H.; Huang, J.; et al. A new molecular phylogeny and a new genus, Pendulorchis, of the Aerides-Vanda alliance (Orchidaceae: Epidendroideae). PLoS ONE 2013, 8, e60097. [Google Scholar] [CrossRef]
- Zou, L.H.; Wu, X.Y.; Lin, M.; Chen, L.J.; Liu, Z.J. Vanda funingensis, a new species of Orchidaceae (Epidedroideae; Vandeae; Aeridinae) from China: Evidence from morphology and DNA. Phytotaxa 2016, 260, 1–13. [Google Scholar] [CrossRef]
- Zhang, G.J.; Hu, Y.; Huang, M.Z.; Huang, W.C.; Liu, D.K.; Zhang, D.Y.; Hu, H.H.; Downing, J.L.; Liu, Z.J.; Ma, H. Comprehensive phylogenetic analyses of Orchidaceae using nuclear genes and evolutionary insights into epiphytism. J. Integr. Plant Biol. 2023, 65, 1204–1225. [Google Scholar] [CrossRef]
- Li, Z.H.; Ma, X.; Wang, D.Y.; Li, Y.X.; Wang, C.W.; Jin, X.H. Evolution of plastid genomes of Holcoglossum (Orchidaceae) with recent radiation. BMC Evol. Biol. 2019, 19, 63. [Google Scholar] [CrossRef]
- Zhao, J.H.; Zhou, P.; Li, X.Q.; Zhang, L.G.; Jin, X.H.; Xiang, X.G. Temporal and spatial pattern of Holcoglossum Schltr. (Orchidaceae), an East Asian endemic genus, based on nuclear and chloroplast genes. Front. Ecol. Evol. 2020, 8, 245. [Google Scholar] [CrossRef]
- Li, E.Z.; Liu, K.J.; Deng, R.Y.; Gao, Y.W.; Liu, X.Y.; Dong, W.P.; Zhang, Z.X. Insights into the phylogeny and chloroplast genome evolution of Eriocaulon (Eriocaulaceae). BMC Plant Biol. 2023, 23, 32. [Google Scholar] [CrossRef]
- Wang, J.; He, W.C.; Liao, X.Z.; Ma, J.; Gao, W.; Wang, H.Q.; Wu, D.L.; Tembrock, R.; Wu, Z.Q.; Gu, C.H. Phylogeny, molecular evolution, and dating of divergences in Lagerstroemia using plastome sequences. Hortic. Plant J. 2023, 9, 345–355. [Google Scholar] [CrossRef]
- Chen, J.L.; Wang, F.; Zhou, C.Y.; Ahmad, S.; Zhou, Y.Z.; Li, M.H.; Liu, Z.J.; Peng, D.H. Comparative phylogenetic analysis for Aerides (Aeridinae, Orchidaceae) based on six complete plastid genomes. Int. J. Mol. Sci. 2023, 24, 12473. [Google Scholar] [CrossRef]
- Zhou, C.Y.; Lin, W.J.; Li, R.; Wu, Y.; Liu, Z.J.; Li, M.H. Characterization of Angraecum (Angraecinae, Orchidaceae) plastomes and utility of sequence variability hotspots. Int. J. Mol. Sci. 2023, 25, 184. [Google Scholar] [CrossRef]
- Martín, M.; Sabater, B. Plastid ndh genes in plant evolution. Plant Physiol. Biochem. 2010, 48, 636–645. [Google Scholar] [CrossRef]
- Silva, S.R.; Michael, T.P.; Meer, E.J.; Pinheiro, D.G.; Varani, A.M.; Miranda, V.F.O. Comparative genomic analysis of Genlisea (corkscrew plants–Lentibulariaceae) chloroplast genomes reveals an increasing loss of the ndh genes. PLoS ONE 2018, 13, e0190321. [Google Scholar] [CrossRef]
- Kim, H.T.; Kim, J.S.; Moore, M.J.; Neubig, K.M.; Williams, N.H.; Whitten, W.M.; Kim, J.H. Seven new complete plastome sequences reveal rampant independent loss of the ndh gene family across orchids and associated instability of the inverted repeat/small single-copy region boundaries. PLoS ONE 2015, 10, e0142215. [Google Scholar] [CrossRef]
- Chen, J.L.; Wang, F.; Zhao, Z.; Li, M.H.; Liu, Z.J.; Peng, D.H. Complete chloroplast genomes and comparative analyses of three Paraphalaenopsis (Aeridinae, Orchidaceae) species. Int. J. Mol. Sci. 2023, 24, 11167. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.Y.; Zeng, M.Y.; Gao, X.; Zhao, Z.; Li, R.; Wu, Y.; Liu, Z.J.; Zhang, D.; Li, M.H. Characteristics and comparative analysis of seven complete plastomes of Trichoglottis s.l. (Aeridinae, Orchidaceae). Int. J. Mol. Sci. 2023, 24, 14544. [Google Scholar] [CrossRef] [PubMed]
- Dugas, D.V.; Hernandez, D.; Koenen, E.J.; Schwarz, E.; Straub, S.; Hughes, C.E.; Jansen, R.K.; Nageswara-Rao, M.; Staats, M.; Trujillo, J.T.; et al. Mimosoid legume plastome evolution: IR expansion, tandem repeat expansions, and accelerated rate of evolution in clpP. Sci. Rep. 2015, 5, 16958. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.Q.; Yang, J.P.; Yu, W.H.; Wang, H.M.; Hou, Z.Y.; Li, C.; Xue, Q.Y.; Liu, W.; Ding, X.Y.; Niu, Z.T. The climate changes promoted the chloroplast genomic evolution of Dendrobium orchids among multiple photosynthetic pathways. BMC Plant Biol. 2023, 23, 189. [Google Scholar] [CrossRef]
- Zhao, Z.; Zeng, M.Y.; Wu, Y.W.; Li, J.W.; Zhou, Z.; Liu, Z.J.; Li, M.H. Characterization and comparative analysis of the complete plastomes of five Epidendrum (Epidendreae, Orchidaceae) species. Int. J. Mol. Sci. 2023, 24, 14437. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S.; Chen, J.J.; Huang, Y.T.; Chan, M.T.; Daniell, H.; Chang, W.J.; Hsu, C.T.; Liao, D.C.; Wu, F.H.; Lin, S.Y.; et al. The location and translocation of ndh genes of chloroplast origin in the Orchidaceae family. Sci. Rep. 2015, 5, 9040. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.T.; Pan, J.J.; Zhu, S.Y.; Li, L.D.; Xue, Q.Y.; Liu, W.; Ding, X.Y. Comparative analysis of the complete plastomes of Apostasia wallichii and Neuwiedia singapureana (Apostasioideae) reveals different evolutionary dynamics of IR/SSC boundary among photosynthetic orchids. Front. Plant Sci. 2017, 8, 1713. [Google Scholar] [CrossRef] [PubMed]
- Knill, T.; Reichelt, M.; Paetz, C.; Gershenzon, J.; Binder, S. Arabidopsis thaliana encodes a bacterial-type heterodimeric isopropylmalate isomerase involved in both Leu biosynthesis and the Met chain elongation pathway of glucosinolate formation. Plant Mol. Biol. 2009, 71, 227–239. [Google Scholar] [CrossRef]
- Iriarte, A.; Lamolle, G.; Musto, H. Codon usage bias: An endless tale. J. Mol. Evol. 2021, 89, 589–593. [Google Scholar] [CrossRef]
- Cavalier-Smith, T. Chloroplast evolution: Secondary symbiogenesis and multiple losses. Curr. Biol. 2002, 12, R62–R64. [Google Scholar] [CrossRef]
- Cui, Y.F.; Zhou, P.; Xiang, K.L.; Zhang, Q.; Yan, H.; Zhang, L.G.; Pan, B.; Huang, Y.S.; Guo, Z.Y.; Li, Z.Y.; et al. Plastome evolution and phylogenomics of Trichosporeae (Gesneriaceae) with its morphological characters appraisal. Front. Plant Sci. 2023, 14, 1160535. [Google Scholar] [CrossRef]
- Tao, K.F.; Tao, L.; Huang, J.L.; Duan, H.N.; Luo, Y.; Li, L. Complete chloroplast genome structural characterization of two Aerides (Orchidaceae) species with a focus on phylogenetic position of Aerides flabellata. BMC Genom. 2024, 25, 552. [Google Scholar] [CrossRef]
- Abdullah; Mehmood, F.; Rahim, A.; Heidari, P.; Ahmed, I.; Poczai, P. Comparative plastome analysis of Blumea, with implications for genome evolution and phylogeny of Asteroideae. Ecol. Evol. 2021, 11, 7810–7826. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Yang, J.X.; Bai, M.Z.; Zhang, G.Q.; Liu, Z.J. The chloroplast genome evolution of Venus slipper (Paphiopedilum): IR expansion, SSC contraction, and highly rearranged SSC regions. BMC Plant Biol. 2021, 21, 248. [Google Scholar] [CrossRef]
- Li, L.; Wu, Q.P.; Fang, L.; Wu, K.L.; Li, M.Z.; Zeng, S.J. Comparative chloroplast genomics and phylogenetic analysis of Thuniopsis and closely related genera within Coelogyninae (Orchidaceae). Front. Genet. 2022, 13, 850201. [Google Scholar] [CrossRef]
- Dong, W.P.; Sun, J.H.; Liu, Y.L.; Xu, C.; Wang, Y.H.; Suo, Z.L.; Zhou, S.L.; Zhang, Z.X.; Wen, J. Phylogenomic relationships and species identification of the olive genus Olea (Oleaceae). J. Syst. Evol. 2022, 60, 1263–1280. [Google Scholar] [CrossRef]
- Kocyan, A.; Vogel, E.F.; Conti, E.; Gravendeel, B. Molecular phylogeny of Aerides (Orchidaceae) based on one nuclear and two plastid markers: A step forward in understanding the evolution of the Aeridinae. Mol. Phylogenet. Evol. 2008, 48, 422–443. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small amounts of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Brown, J.; Pirrung, M.; McCue, L.A. FQC Dashboard: Integrates FastQC results into a web-based, interactive, and extensible FASTQ quality control tool. Bioinformatics 2017, 33, 3137–3139. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.J.; Yu, W.B.; Yang, J.B.; Song, Y.; dePamphilis, C.W.; Yi, T.S.; Li, D.Z. GetOrganelle: A fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 2020, 21, 241. [Google Scholar] [CrossRef]
- Wick, R.R.; Schultz, M.B.; Zobel, J.; Holt, K.E. Bandage: Interactive visualization of de novo genome assemblies. Bioinformatics 2015, 31, 3350–3352. [Google Scholar] [CrossRef]
- Qu, X.J.; Moore, M.J.; Li, D.Z.; Yi, T.S. PGA: A software package for rapid, accurate, and flexible batch annotation of plastomes. Plant. Methods 2019, 15, 50. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Brudno, M.; Malde, S.; Poliakov, A.; Do, C.B.; Couronne, O.; Dubchak, I.; Batzoglou, S. Glocal alignment: Finding rearrangements during alignment. Bioinformatics 2003, 19, i54–i62. [Google Scholar] [CrossRef] [PubMed]
- Amiryousefi, A.; Hyvönen, J.; Poczai, P. IRscope: An online program to visualize the junction sites of chloroplast genomes. Bioinformatics 2018, 34, 3030–3031. [Google Scholar] [CrossRef]
- Darling, A.E.; Mau, B.; Perna, N.T. Progressive Mauve: Multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE 2010, 5, e11147. [Google Scholar] [CrossRef] [PubMed]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Beier, S.; Thiel, T.; Münch, T.; Scholz, U.; Mascher, M. MISA-web: A web server for microsatellite prediction. Bioinformatics 2017, 33, 2583–2585. [Google Scholar] [CrossRef]
- Kumar, S.; Nei, M.; Dudley, J.; Tamura, K. MEGA: A biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief. Bioinform. 2008, 9, 299–306. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nuclc. Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]

| Taxa | Length (bp) | GC Content (%) | Gene Numbers | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Plastome | LSC | IR | SSC | Total | CDS | tRNA | rRNA | ||
| V. alpina | 149,273 | 85,819 | 25,860 | 11,734 | 36.7 | 120 | 74 | 38 | 8 |
| V. ampullaceum | 146,340 | 85,436 | 24,523 | 11,858 | 36.5 | 120 | 74 | 38 | 8 |
| V. brunnea | 149,216 | 85,783 | 25,860 | 11,713 | 36.7 | 120 | 74 | 38 | 8 |
| V. coelestis | 148,073 | 85,105 | 25,772 | 11,424 | 36.8 | 120 | 74 | 38 | 8 |
| V. coerulea | 149,268 | 85,982 | 25,787 | 11,712 | 36.6 | 120 | 74 | 38 | 8 |
| V. coerulescens | 149,410 | 85,954 | 25,965 | 11,526 | 36.7 | 120 | 74 | 38 | 8 |
| V. concolor | 149,474 | 85,678 | 25,897 | 12,002 | 36.6 | 120 | 74 | 38 | 8 |
| V. cristata | 146,993 | 83,927 | 25,772 | 11,522 | 36.7 | 120 | 74 | 38 | 8 |
| V. falcata | 146,497 | 83,808 | 25,457 | 11,775 | 36.6 | 120 | 74 | 38 | 8 |
| V. pumila | 149,273 | 85,819 | 25,860 | 11,734 | 36.7 | 120 | 74 | 38 | 8 |
| V. richardsiana | 146,498 | 83,809 | 25,457 | 11,775 | 36.6 | 120 | 74 | 38 | 8 |
| V. subconcolor | 149,490 | 85,691 | 25,912 | 11,975 | 36.6 | 120 | 74 | 38 | 8 |
| V. xichangensis | 146,681 | 83,920 | 25,505 | 11,751 | 36.6 | 120 | 74 | 38 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, W.; Zhou, P.; Pei, Z.; Liu, Y.; Luo, Y.; Xiang, X. Plastome Evolution and Comparative Analyses of a Recently Radiated Genus Vanda (Aeridinae, Orchidaceae). Int. J. Mol. Sci. 2024, 25, 9538. https://doi.org/10.3390/ijms25179538
Lei W, Zhou P, Pei Z, Liu Y, Luo Y, Xiang X. Plastome Evolution and Comparative Analyses of a Recently Radiated Genus Vanda (Aeridinae, Orchidaceae). International Journal of Molecular Sciences. 2024; 25(17):9538. https://doi.org/10.3390/ijms25179538
Chicago/Turabian StyleLei, Wanshun, Peng Zhou, Zelong Pei, Yizhen Liu, Yan Luo, and Xiaoguo Xiang. 2024. "Plastome Evolution and Comparative Analyses of a Recently Radiated Genus Vanda (Aeridinae, Orchidaceae)" International Journal of Molecular Sciences 25, no. 17: 9538. https://doi.org/10.3390/ijms25179538
APA StyleLei, W., Zhou, P., Pei, Z., Liu, Y., Luo, Y., & Xiang, X. (2024). Plastome Evolution and Comparative Analyses of a Recently Radiated Genus Vanda (Aeridinae, Orchidaceae). International Journal of Molecular Sciences, 25(17), 9538. https://doi.org/10.3390/ijms25179538







