Inhibitory Effects of Surface Pre-Reacted Glass Ionomer Filler Eluate on Streptococcus mutans in the Presence of Sucrose
Abstract
1. Introduction
2. Results
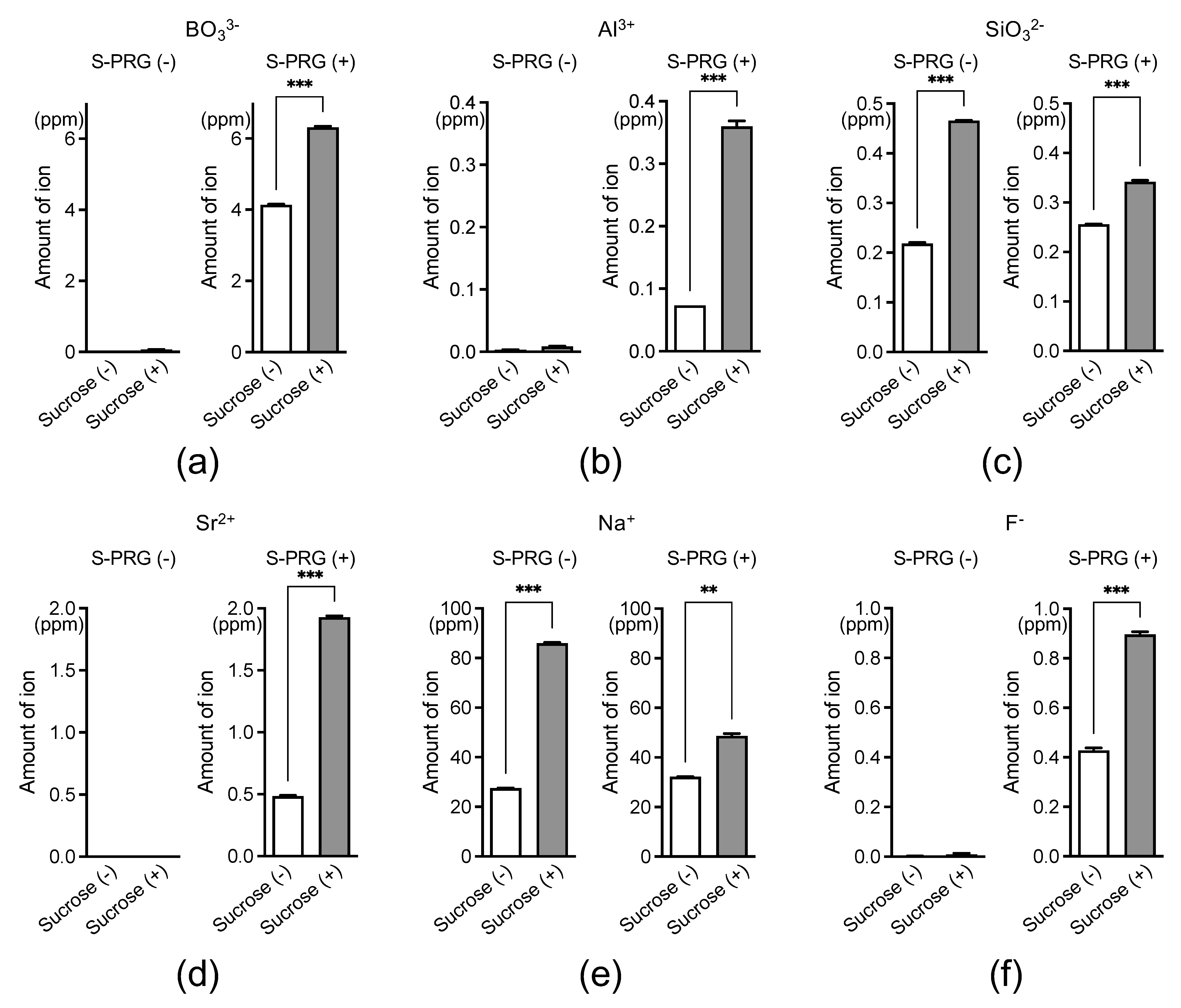
2.1. Ion Release from S-PRG Filler Eluate Surrounding S. mutans
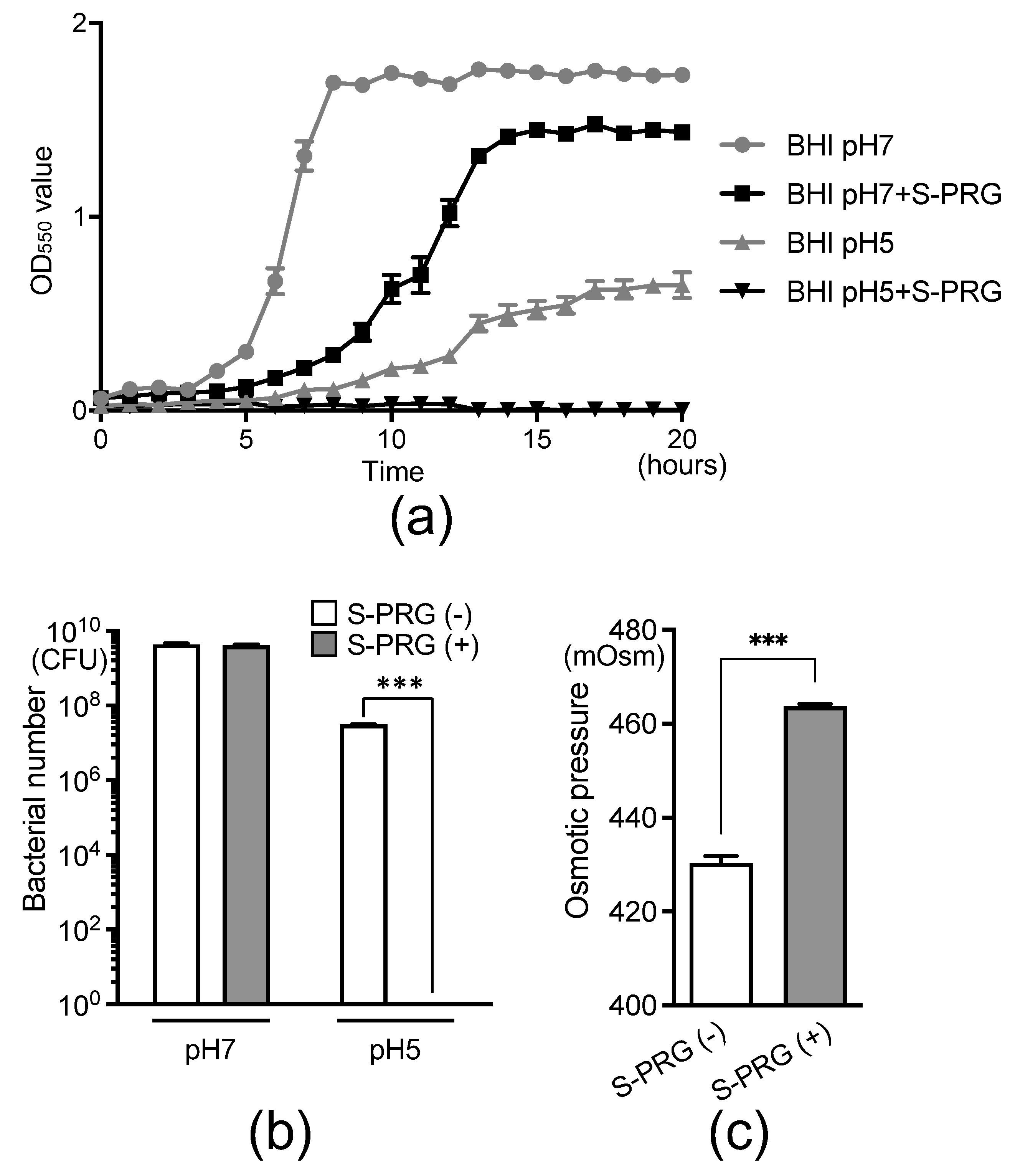
2.2. Effect of S-PRG Filler Eluate on Glucan Formation and pH in S. mutans Cultures
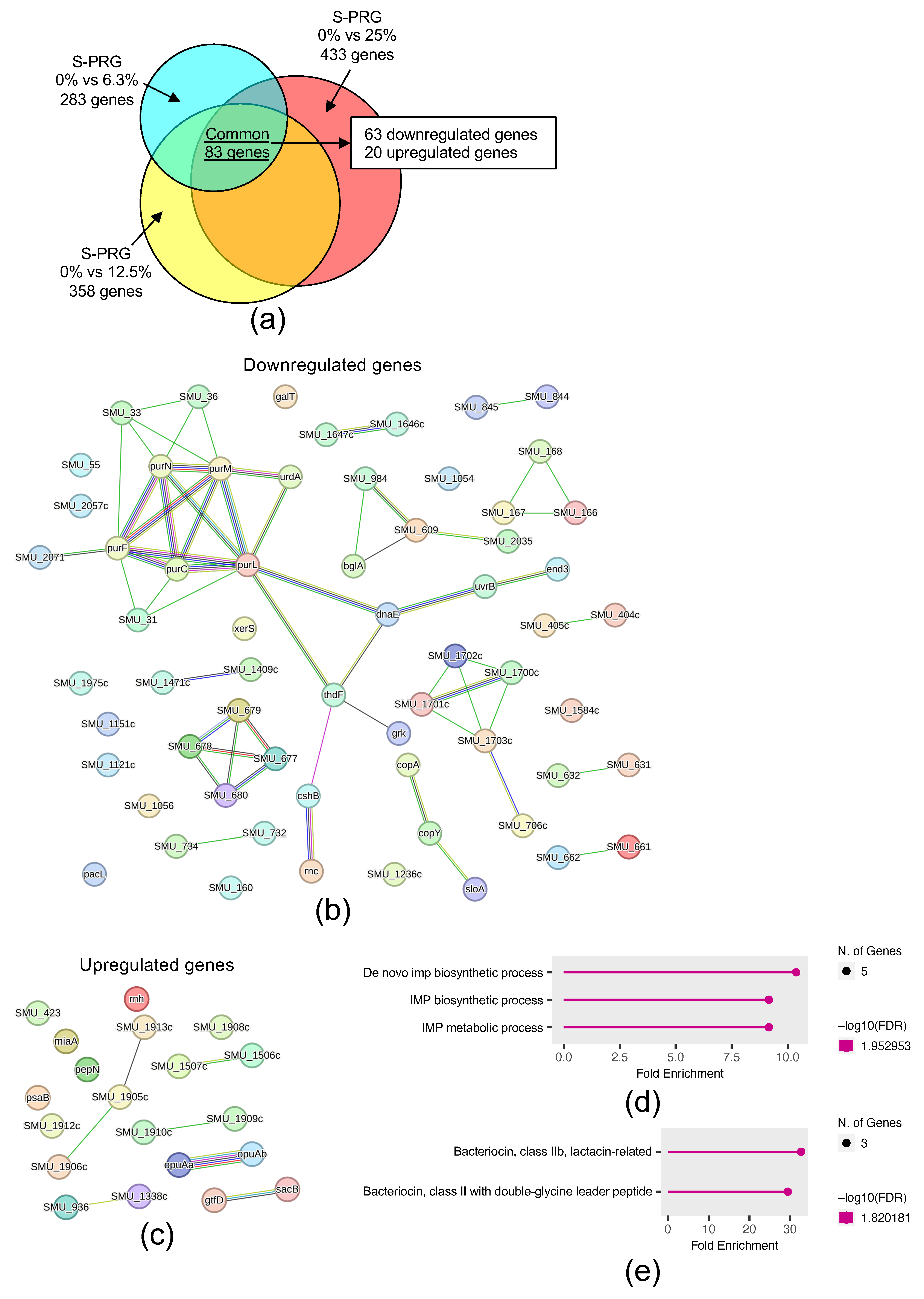
2.3. S-PRG Filler Eluate Affects S. mutans Gene Expression in the Presence of Sucrose
2.4. Effect of S-PRG Filler Eluate on S. mutans Survival in Acidic Environments and Osmotic Pressure on Bacteria
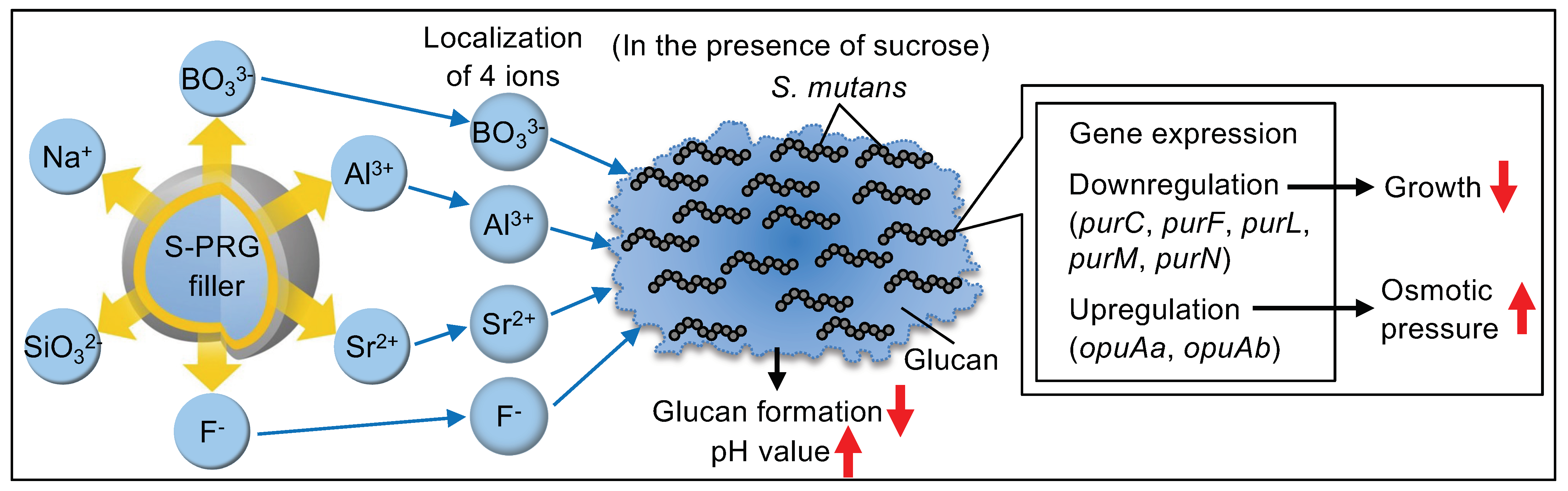
3. Discussion
4. Materials and Methods
4.1. Preparation of S-PRG Filler
4.2. Preparation of S-PRG Filler Eluate
4.3. Bacterial Strain and Growth Conditions
4.4. Measurement of Ion Concentrations Surrounding S. mutans
4.5. Electron Microscopy Observations
4.6. Glucan Quantification
4.7. DNA Microarray Analysis
4.8. Bioinformatic Analyses
4.9. Bacterial Growth Assay
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baty, J.J.; Stoner, S.N.; Scoffield, J.A. Oral Commensal Streptococci: Gatekeepers of the Oral Cavity. J. Bacteriol. 2022, 204, e0025722. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef]
- Simon-Soro, A.; Tomas, I.; Cabrera-Rubio, R.; Catalan, M.D.; Nyvad, B.; Mira, A. Microbial geography of the oral cavity. J. Dent. Res. 2013, 92, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Beighton, D.; Curtis, M.A.; Cury, J.A.; Dige, I.; Dommisch, H.; Ellwood, R.; Giacaman, R.A.; Herrera, D.; Herzberg, M.C.; et al. Role of microbial biofilms in the maintenance of oral health and in the development of dental caries and periodontal diseases. Consensus report of group 1 of the Joint EFP/ORCA workshop on the boundaries between caries and periodontal disease. J. Clin. Periodontol. 2017, 44 (Suppl. S18), S5–S11. [Google Scholar] [CrossRef]
- Lee, Y.H.; Zimmerman, J.N.; Custodio, W.; Xiao, Y.; Basiri, T.; Hatibovic-Kofman, S.; Siqueira, W.L. Proteomic evaluation of acquired enamel pellicle during in vivo formation. PLoS ONE 2013, 8, e67919. [Google Scholar] [CrossRef] [PubMed]
- Nobbs, A.H.; Lamont, R.J.; Jenkinson, H.F. Streptococcus adherence and colonization. Microbiol. Mol. Biol. Rev. 2009, 73, 407–450. [Google Scholar] [CrossRef] [PubMed]
- de Soet, J.J.; Nyvad, B.; Kilian, M. Strain-related acid production by oral streptococci. Caries Res. 2000, 34, 486–490. [Google Scholar] [CrossRef]
- Marsh, P.D. Microbial ecology of dental plaque and its significance in health and disease. Adv. Dent. Res. 1994, 8, 263–271. [Google Scholar] [CrossRef]
- Takahashi, N.; Nyvad, B. Ecological Hypothesis of Dentin and Root Caries. Caries Res. 2016, 50, 422–431. [Google Scholar] [CrossRef]
- Lemos, J.A.; Palmer, S.R.; Zeng, L.; Wen, Z.T.; Kajfasz, J.K.; Freires, I.A.; Abranches, J.; Brady, L.J. The Biology of Streptococcus mutans. Microbiol. Spectr. 2019, 7, GPP3-0051-2018. [Google Scholar] [CrossRef]
- Imazato, S.; Nakatsuka, T.; Kitagawa, H.; Sasaki, J.I.; Yamaguchi, S.; Ito, S.; Takeuchi, H.; Nomura, R.; Nakano, K. Multiple-Ion Releasing Bioactive Surface Pre-Reacted Glass-Ionomer (S-PRG) Filler: Innovative Technology for Dental Treatment and Care. J. Funct. Biomater. 2023, 14, 236. [Google Scholar] [CrossRef] [PubMed]
- Nomura, R.; Kitamura, T.; Matayoshi, S.; Ohata, J.; Suehiro, Y.; Iwashita, N.; Okawa, R.; Nakano, K. Inhibitory effect of a gel paste containing surface pre-reacted glass-ionomer (S-PRG) filler on the cariogenicity of Streptococcus mutans. Sci. Rep. 2021, 11, 23495. [Google Scholar] [CrossRef]
- Ito, S.; Iijima, M.; Hashimoto, M.; Tsukamoto, N.; Mizoguchi, I.; Saito, T. Effects of surface pre-reacted glass-ionomer fillers on mineral induction by phosphoprotein. J. Dent. 2011, 39, 72–79. [Google Scholar] [CrossRef]
- Fujimoto, Y.; Iwasa, M.; Murayama, R.; Miyazaki, M.; Nagafuji, A.; Nakatsuka, T. Detection of ions released from S-PRG fillers and their modulation effect. Dent. Mater. J. 2010, 29, 392–397. [Google Scholar] [CrossRef]
- Uo, M.; Wada, T.; Asakura, K. Structural analysis of strontium in human teeth treated with surface pre-reacted glass-ionomer filler eluate by using extended X-ray absorption fine structure analysis. Dent. Mater. J. 2017, 36, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S.; Kohno, T.; Tsuboi, R.; Thongthai, P.; Xu, H.H.; Kitagawa, H. Cutting-edge filler technologies to release bio-active components for restorative and preventive dentistry. Dent. Mater. J. 2020, 39, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, K.; Ogata, K.; Karibe, H. Caries-preventive effect of fissure sealant containing surface reaction-type pre-reacted glass ionomer filler and bonded by self-etching primer. J. Clin. Pediatr. Dent. 2012, 36, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Imazato, S.; Chen, J.H.; Mayanagi, G.; Takahashi, N.; Ishimoto, T.; Nakano, T. Effects of a coating resin containing S-PRG filler to prevent demineralization of root surfaces. Dent. Mater. J. 2012, 31, 909–915. [Google Scholar] [CrossRef]
- Matayoshi, S.; Nomura, R.; Kitamura, T.; Okawa, R.; Nakano, K. Inhibitory effect of toothbrush monofilament containing surface pre-reacted glass-ionomer (S-PRG) filler on Streptococcus mutans. Sci. Rep. 2021, 11, 211. [Google Scholar] [CrossRef]
- Nomura, R.; Morita, Y.; Matayoshi, S.; Nakano, K. Inhibitory effect of surface pre-reacted glass-ionomer (S-PRG) eluate against adhesion and colonization by Streptococcus mutans. Sci. Rep. 2018, 8, 5056. [Google Scholar] [CrossRef]
- Garcia, M.T.; Namba, A.M.; do Carmo, P.H.F.; Pedroso, L.L.C.; de Lima, P.M.N.; Goncale, J.C.; Junqueira, J.C. Antimicrobial effects of surface pre-reacted glass-ionomer (S-PRG) eluate against oral microcosm biofilm. Biofouling 2024, 40, 390–401. [Google Scholar] [CrossRef]
- Miki, S.; Kitagawa, H.; Kitagawa, R.; Kiba, W.; Hayashi, M.; Imazato, S. Antibacterial activity of resin composites containing surface pre-reacted glass-ionomer (S-PRG) filler. Dent. Mater. 2016, 32, 1095–1102. [Google Scholar] [CrossRef]
- Hamano, N.; Okada, S.; Hanaoka, K.; Ebihara, K.; Teranaka, T. Fluorine Uptake into Dentin from New Fluorine-Releasing Material “GIOMER”. Bull. Kanagawa Dent. Coll. 2001, 29, 31P–32P. [Google Scholar]
- Hotta, M.; Morikawa, T.; Tamura, D.; Kusakabe, S. Adherence of Streptococcus sanguinis and Streptococcus mutans to saliva-coated S-PRG resin blocks. Dent. Mater. J. 2014, 33, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Boisen, G.; Davies, J.R.; Neilands, J. Acid tolerance in early colonizers of oral biofilms. BMC Microbiol. 2021, 21, 45. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, J.; Zhang, S.; Zhou, W.; Zhang, L.; Huang, X. pH-activated antibiofilm strategies for controlling dental caries. Front. Cell. Infect. Microbiol. 2023, 13, 1130506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Morar, M.; Ealick, S.E. Structural biology of the purine biosynthetic pathway. Cell. Mol. Life Sci. 2008, 65, 3699–3724. [Google Scholar] [CrossRef]
- Crowley, P.J.; Gutierrez, J.A.; Hillman, J.D.; Bleiweis, A.S. Genetic and physiologic analysis of a formyl-tetrahydrofolate synthetase mutant of Streptococcus mutans. J. Bacteriol. 1997, 179, 1563–1572. [Google Scholar] [CrossRef][Green Version]
- Li, D.; Shibata, Y.; Takeshita, T.; Yamashita, Y. A novel gene involved in the survival of Streptococcus mutans under stress conditions. Appl. Environ. Microbiol. 2014, 80, 97–103. [Google Scholar] [CrossRef]
- Kovacs, C.J.; Faustoferri, R.C.; Quivey, R.G., Jr. RgpF Is Required for Maintenance of Stress Tolerance and Virulence in Streptococcus mutans. J. Bacteriol. 2017, 199, e00497-17. [Google Scholar] [CrossRef]
- Welin-Neilands, J.; Svensater, G. Acid tolerance of biofilm cells of Streptococcus mutans. Appl. Environ. Microbiol. 2007, 73, 5633–5638. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Srivastava, R.; Sharma, A.; Bharati, A.P.; Yadav, J.; Singh, A.K.; Tiwari, P.K.; Srivatava, A.K.; Chakdar, H.; Kashyap, P.L.; et al. Transcriptome Analysis to Understand Salt Stress Regulation Mechanism of Chromohalobacter salexigens ANJ207. Front. Microbiol. 2022, 13, 909276. [Google Scholar] [CrossRef]
- Abranches, J.; Lemos, J.A.; Burne, R.A. Osmotic stress responses of Streptococcus mutans UA159. FEMS Microbiol. Lett. 2006, 255, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Woudstra, C.; Brondsted, L. Producing Tailocins from Phages Using Osmotic Shock and Benzalkonium Chloride. Phage 2023, 4, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, K.; Mukai, Y.; Tominaga, T.; Iwaya, I.; Fujino, F.; Hirata, Y.; Teranaka, T. Fluoride release and recharge characteristics of denture base resins containing surface pre-reacted glass-ionomer filler. Dent. Mater. J. 2009, 28, 227–233. [Google Scholar] [CrossRef]
- Baucic, M.; Celebic, A.; Stipetic, J.; Mehulic, K.; Bozic, D. In vitro release of metal ions from a gold-platinum alloy in saliva-simulated conditions. Coll. Antropol. 2003, 27, 91–98. [Google Scholar]
- Raszewski, Z.; Chojnacka, K.; Mikulewicz, M.; Alhotan, A. Bioactive Glass-Enhanced Resins: A New Denture Base Material. Materials 2023, 16, 4363. [Google Scholar] [CrossRef]
- Suehiro, Y.; Nomura, R.; Matayoshi, S.; Otsugu, M.; Iwashita, N.; Nakano, K. Evaluation of the collagen-binding properties and virulence of killed Streptococcus mutans in a silkworm model. Sci. Rep. 2022, 12, 2800. [Google Scholar] [CrossRef]
- Sanders, M.K.; Duarte, S.; Ayoub, H.M.; Scully, A.C.; Vinson, L.A.; Gregory, R.L. Effect of titanium dioxide on Streptococcus mutans biofilm. J. Appl. Biomater. Funct. Mater. 2023, 21, 22808000221131892. [Google Scholar] [CrossRef]
- Zahurak, M.; Parmigiani, G.; Yu, W.; Scharpf, R.B.; Berman, D.; Schaeffer, E.; Shabbeer, S.; Cope, L. Pre-processing Agilent microarray data. BMC Bioinform. 2007, 8, 142. [Google Scholar] [CrossRef]
- Usuda, M.; Kametani, M.; Hamada, M.; Suehiro, Y.; Matayoshi, S.; Okawa, R.; Naka, S.; Matsumoto-Nakano, M.; Akitomo, T.; Mitsuhata, C.; et al. Inhibitory Effect of Adsorption of Streptococcus mutans onto Scallop-Derived Hydroxyapatite. Int. J. Mol. Sci. 2023, 24, 11371. [Google Scholar] [CrossRef] [PubMed]
- Nomura, R.; Ohata, J.; Otsugu, M.; Okawa, R.; Naka, S.; Matsumoto-Nakano, M.; Nakano, K. Inhibitory effects of flavedo, albedo, fruits, and leaves of Citrus unshiu extracts on Streptococcus mutans. Arch. Oral. Biol. 2021, 124, 105056. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kametani, M.; Akitomo, T.; Hamada, M.; Usuda, M.; Kaneki, A.; Ogawa, M.; Ikeda, S.; Ito, Y.; Hamaguchi, S.; Kusaka, S.; et al. Inhibitory Effects of Surface Pre-Reacted Glass Ionomer Filler Eluate on Streptococcus mutans in the Presence of Sucrose. Int. J. Mol. Sci. 2024, 25, 9541. https://doi.org/10.3390/ijms25179541
Kametani M, Akitomo T, Hamada M, Usuda M, Kaneki A, Ogawa M, Ikeda S, Ito Y, Hamaguchi S, Kusaka S, et al. Inhibitory Effects of Surface Pre-Reacted Glass Ionomer Filler Eluate on Streptococcus mutans in the Presence of Sucrose. International Journal of Molecular Sciences. 2024; 25(17):9541. https://doi.org/10.3390/ijms25179541
Chicago/Turabian StyleKametani, Mariko, Tatsuya Akitomo, Masakazu Hamada, Momoko Usuda, Ami Kaneki, Masashi Ogawa, Shunya Ikeda, Yuya Ito, Shuma Hamaguchi, Satoru Kusaka, and et al. 2024. "Inhibitory Effects of Surface Pre-Reacted Glass Ionomer Filler Eluate on Streptococcus mutans in the Presence of Sucrose" International Journal of Molecular Sciences 25, no. 17: 9541. https://doi.org/10.3390/ijms25179541
APA StyleKametani, M., Akitomo, T., Hamada, M., Usuda, M., Kaneki, A., Ogawa, M., Ikeda, S., Ito, Y., Hamaguchi, S., Kusaka, S., Asao, Y., Iwamoto, Y., Mitsuhata, C., Suehiro, Y., Okawa, R., Nakano, K., & Nomura, R. (2024). Inhibitory Effects of Surface Pre-Reacted Glass Ionomer Filler Eluate on Streptococcus mutans in the Presence of Sucrose. International Journal of Molecular Sciences, 25(17), 9541. https://doi.org/10.3390/ijms25179541






