Nitrate Starvation Induces Lateral Root Organogenesis in Triticum aestivum via Auxin Signaling
Abstract
:1. Introduction
2. Results
2.1. Plant Growth
2.2. Root System Architecture
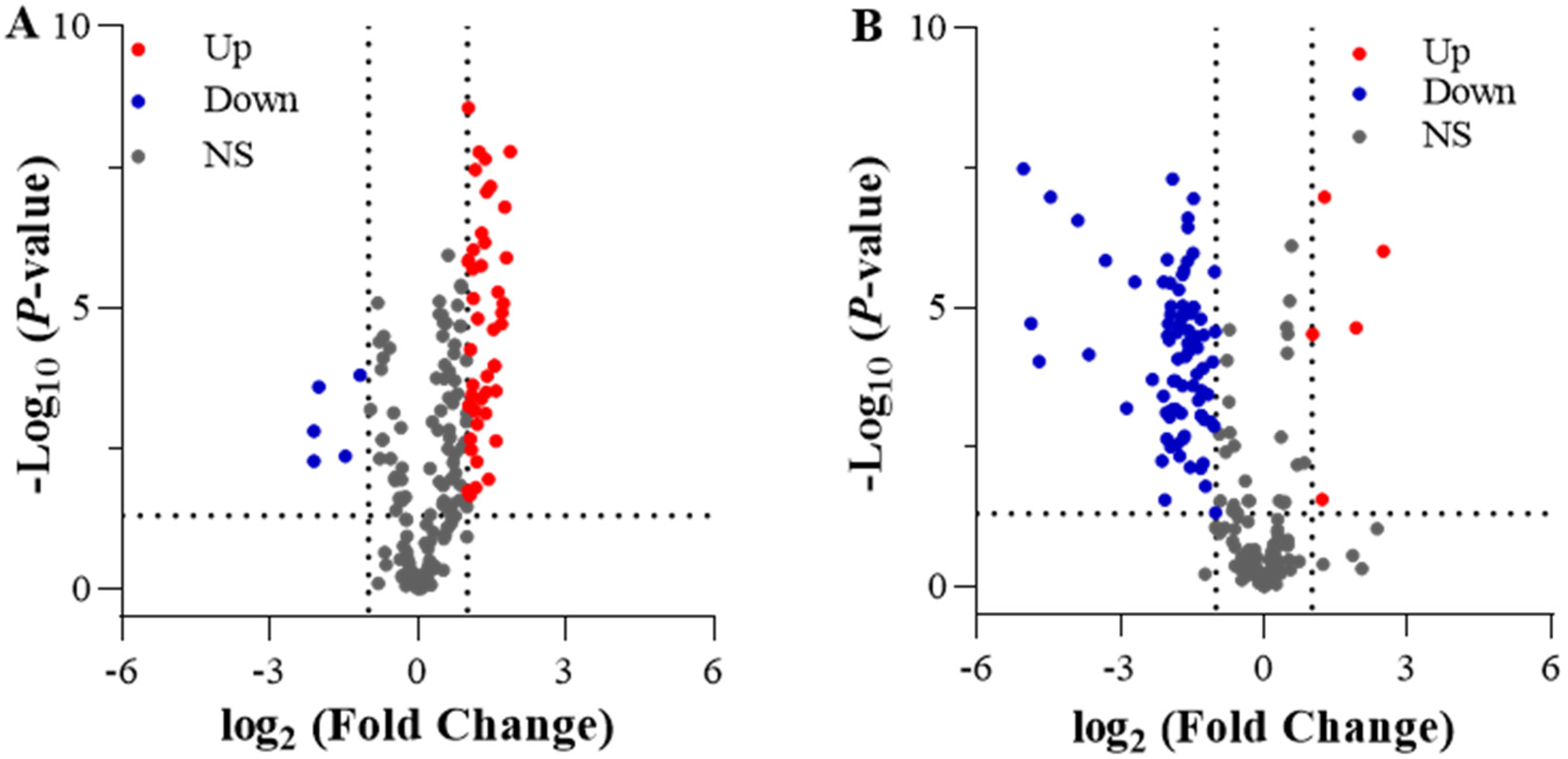
2.3. Differentially Expressed Genes (DEGs) under Different N Treatments
2.4. GO and KEGG Analysis of Differentially Expressed Genes
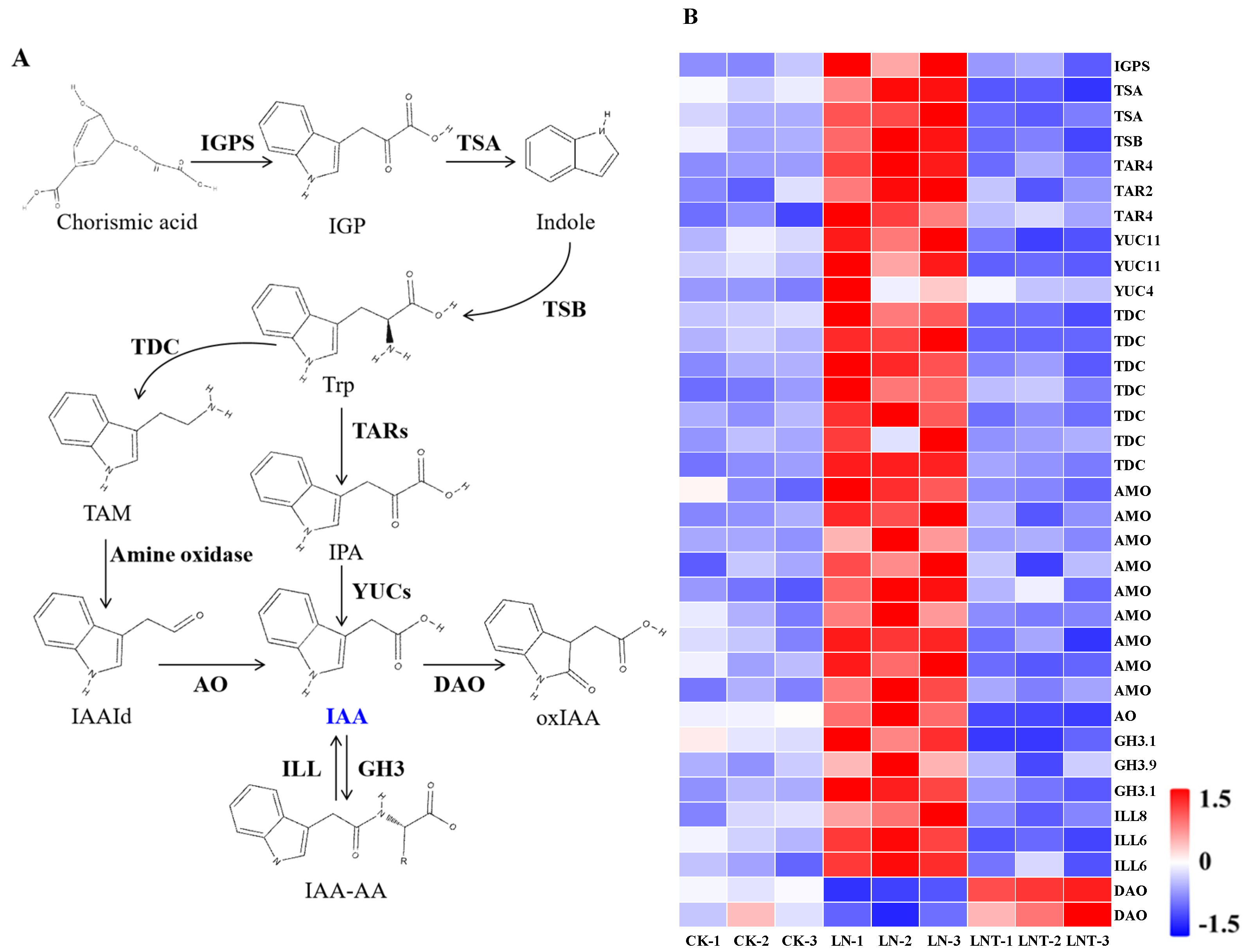
2.5. DEGs Related to IAA Synthesis, Oxidation, and Transport
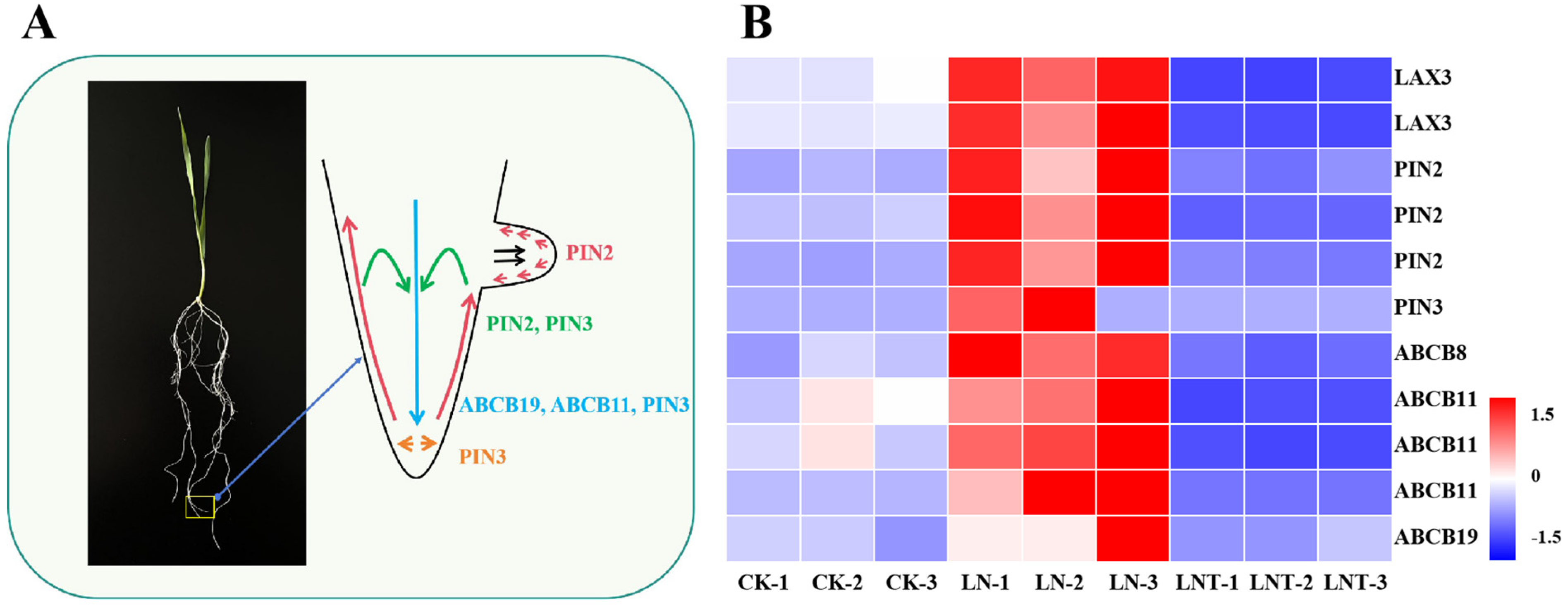
2.6. DEGs Involved in the Regulation of IAA Biosynthesis and Transport
2.7. DEGs Related to Cell Wall Biological Properties
2.8. Expression of Genes Mediated by Auxin and Potentially Involved in Cell Wall Remodeling and LR Development
2.9. DEGs Related to Cell Division, Elongation, and the Cell Cycle
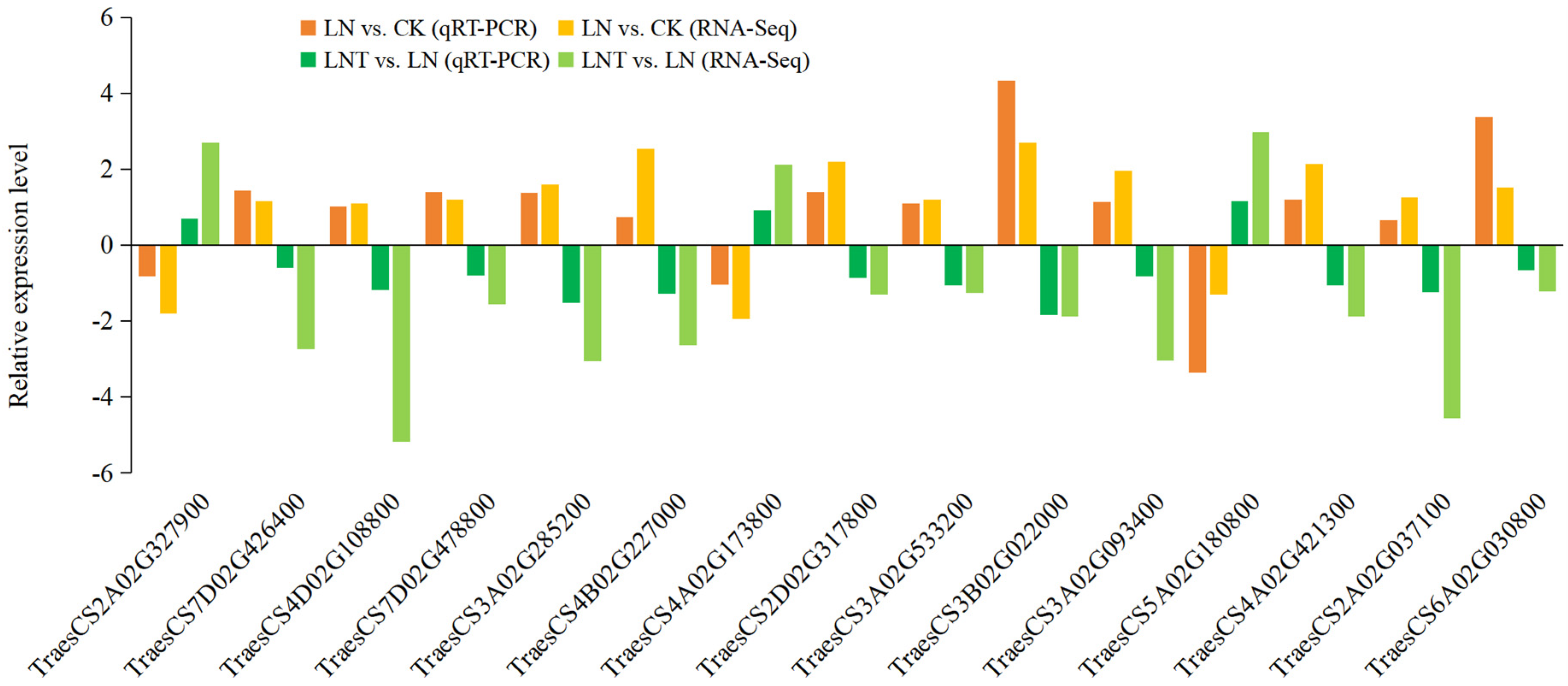
2.10. Verification of Hub DEGs via qRT-PCR
2.11. Root Tryptophan and IAA Content
2.12. Effects of Different N Treatments on the Activity of Cell Wall Remodeling Enzymes and Cell Wall Composition in Wheat Roots
2.13. Fourier-Transform Infrared (FTIR) Spectra of Cell Wall Composition
2.14. Cell Wall Plasticity
3. Discussion
3.1. Low NO3− Promotes Lateral Root Occurrence and Increases Root Surface Area
3.2. Low NO3– Promotes Root IAA Synthesis and Transport and Improves IAA Homeostasis
3.3. DEGs Involved in the Regulation of IAA Biosynthesis, Transport, and Signaling
3.4. Low NO3– Modulates Cell Wall Remodeling and Elasticity
3.5. Regulation of Low-NO3–-Induced Cell Wall Elasticity and LR Formation via Auxin Signaling
3.6. Low NO3– Promotes Root Cell Division and Elongation
4. Materials and Methods
4.1. Plant Materials and Plant Culture
4.2. Biomass Determination
4.3. Root Architecture Measurements
4.4. Transcriptome Sequencing
4.4.1. RNA Extraction and Detection
4.4.2. Library Construction and Quality Inspection
4.4.3. Sequencing
4.4.4. Data Quality Control
4.4.5. Analysis of Total Differentially Expressed Genes (DEGs)
4.4.6. GO Term and KEGG Enrichment Analysis
4.5. RNA Isolation and Reverse Transcription Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.6. 2,3,5-Triodobenzoic Acid (TIBA) Treatment
4.7. Determination of Tryptophan, IAA, and Cell Wall Components
4.8. Activity of Enzymes and Pectin Methylation Degree Involved in Cell Wall Remodeling
4.9. Atomic Force Microscopy
4.10. FTIR Analysis
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pascual, M.B.; El-Azaz, J.; De La Torre, F.N.; Cañas, R.A.; Avila, C.; Cánovas, F.M. Biosynthesis and Metabolic Fate of Phenylalanine in Conifers. Front. Plant Sci. 2016, 7, 1030. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The Microbial Nitrogen-Cycling Network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wu, J.; Yang, S.; Schiefelbein, J.; Gan, Y. Nitrate Regulation of Lateral Root and Root Hair Development in Plants. J. Exp. Bot. 2020, 71, 4405–4414. [Google Scholar] [CrossRef] [PubMed]
- Krouk, G.; Crawford, N.M.; Coruzzi, G.M.; Tsay, Y.-F. Nitrate Signaling: Adaptation to Fluctuating Environments. Curr. Opin. Plant Biol. 2010, 13, 265–272. [Google Scholar] [CrossRef]
- Alvarez, J.M.; Vidal, E.A.; Gutiérrez, R.A. Integration of Local and Systemic Signaling Pathways for Plant N Responses. Curr. Opin. Plant Biol. 2012, 15, 185–191. [Google Scholar] [CrossRef]
- Olas, J.J.; Van Dingenen, J.; Abel, C.; Działo, M.A.; Feil, R.; Krapp, A.; Schlereth, A.; Wahl, V. Nitrate Acts at the Arabidopsis Thaliana Shoot Apical Meristem to Regulate Flowering Time. New Phytol. 2019, 223, 814–827. [Google Scholar] [CrossRef]
- Asim, M.; Ullah, Z.; Oluwaseun, A.; Wang, Q.; Liu, H. Signalling Overlaps between Nitrate and Auxin in Regulation of The Root System Architecture: Insights from the Arabidopsis thaliana. Int. J. Mol. Sci. 2020, 21, 2880. [Google Scholar] [CrossRef]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant Acidification in Major Chinese Croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef]
- Wei, W.; Yang, M.; Liu, Y.; Huang, H.; Ye, C.; Zheng, J.; Guo, C.; Hao, M.; He, X.; Zhu, S. Fertilizer N Application Rate Impacts Plant-Soil Feedback in a Sanqi Production System. Sci. Total Environ. 2018, 633, 796–807. [Google Scholar] [CrossRef]
- Raza, S.; Miao, N.; Wang, P.; Ju, X.; Chen, Z.; Zhou, J.; Kuzyakov, Y. Dramatic Loss of Inorganic Carbon by Nitrogen-induced Soil Acidification in Chinese Croplands. Global Change Biol. 2020, 26, 3738–3751. [Google Scholar] [CrossRef]
- Gho, Y.S.; Song, M.Y.; Bae, D.Y.; Choi, H.; Jung, K.-H. Rice PIN Auxin Efflux Carriers Modulate the Nitrogen Response in a Changing Nitrogen Growth Environment. Int. J. Mol. Sci. 2021, 22, 3243. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Scheres, B. Lateral Root Formation and the Multiple Roles of Auxin. J. Exp. Bot. 2018, 69, 155–167. [Google Scholar] [CrossRef]
- Waidmann, S.; Sarkel, E.; Kleine-Vehn, J. Same Same, but Different: Growth Responses of Primary and Lateral Roots. J. Exp. Bot. 2020, 71, 2397–2411. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.Q.; Shu, J.Q.; Li, W.M.; Wang, G.Z. Role of Auxin and Nitrate Signaling in the Development of Root System Architecture. Front. Plant Sci. 2021, 12, 690363. [Google Scholar] [CrossRef]
- Zhang, H. Regulation of Arabidopsis Root Development by Nitrate Availability. J. Exp. Bot. 2000, 51, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Rong, H.; Pilbeam, D. Signalling Mechanisms Underlying the Morphological Responses of the Root System to Nitrogen in Arabidopsis thaliana. J. Exp. Bot. 2007, 58, 2329–2338. [Google Scholar] [CrossRef]
- Huang, R.; Zheng, R.; He, J.; Zhou, Z.; Wang, J.; Xiong, Y.; Xu, T. Noncanonical Auxin Signaling Regulates Cell Division Pattern during Lateral Root Development. Proc. Natl. Acad. Sci. USA 2019, 116, 21285–21290. [Google Scholar] [CrossRef] [PubMed]
- Nieves-Cordones, M.; Ródenas, R.; Lara, A.; Martínez, V.; Rubio, F. The Combination of K + Deficiency with Other Environmental Stresses: What Is the Outcome? Physiol. Plant. 2019, 165, 264–276. [Google Scholar] [CrossRef]
- Sustr, M.; Soukup, A.; Tylova, E. Potassium in Root Growth and Development. Plants 2019, 8, 435. [Google Scholar] [CrossRef]
- Ma, Z.; Bielenberg, D.G.; Brown, K.M.; Lynch, J.P. Regulation of Root Hair Density by Phosphorus Availability in Arabidopsis thaliana. Plant Cell Environ. 2001, 24, 459–467. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Wu, W. Potassium and Phosphorus Transport and Signaling in Plants. J. Integr. Plant Biol. 2021, 63, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, W.; Zhou, H.; Wang, R.; Zhang, P.; Wang, H.; Pan, X.; Xu, J. Manganese Toxicity Inhibited Root Growth by Disrupting Auxin Biosynthesis and Transport in Arabidopsis. Front. Plant Sci. 2017, 8, 272. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, Y.; Pan, Z.; Xie, S.; Peng, S. Boron Deficiency Alters Root Growth and Development and Interacts with Auxin Metabolism by Influencing the Expression of Auxin Synthesis and Transport Genes. Biotechnol. Biotechnol. Equip. 2016, 30, 661–668. [Google Scholar] [CrossRef]
- Olatunji, D.; Geelen, D.; Verstraeten, I. Control of Endogenous Auxin Levels in Plant Root Development. Int. J. Mol. Sci. 2017, 18, 2587. [Google Scholar] [CrossRef]
- Qin, H.; Huang, R. Auxin Controlled by Ethylene Steers Root Development. Int. J. Mol. Sci. 2018, 19, 3656. [Google Scholar] [CrossRef]
- Fonouni-Farde, C.; Miassod, A.; Laffont, C.; Morin, H.; Bendahmane, A.; Diet, A.; Frugier, F. Gibberellins Negatively Regulate the Development of Medicago Truncatula Root System. Sci. Rep. 2019, 9, 2335. [Google Scholar] [CrossRef] [PubMed]
- Placido, D.F.; Sandhu, J.; Sato, S.J.; Nersesian, N.; Quach, T.; Clemente, T.E.; Staswick, P.E.; Walia, H. The LATERAL ROOT DENSITY Gene Regulates Root Growth during Water Stress in Wheat. Plant Biotechnol. J. 2020, 18, 1955–1968. [Google Scholar] [CrossRef]
- Jing, H.; Strader, L.C. Interplay of Auxin and Cytokinin in Lateral Root Development. Int. J. Mol. Sci. 2019, 20, 486. [Google Scholar] [CrossRef]
- Enders, T.A.; Strader, L.C. Auxin Activity: Past, Present, and Future. Am. J. Bot. 2015, 102, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, J.; Ckurshumova, W.; Berleth, T. Auxin Signaling in Arabidopsis Leaf Vascular Development. Plant Physiol. 2003, 131, 1327–1339. [Google Scholar] [CrossRef]
- Tanaka, H.; Dhonukshe, P.; Brewer, P.B.; Friml, J. Spatiotemporal Asymmetric Auxin Distribution: A Means to Coordinate Plant Development. Cell. Mol. Life Sci. 2006, 63, 2738–2754. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Zhao, X.; Wu, B.; Wang, C.; Wu, C.; Yang, S.; Zhou, J.; Xue, Z. Auxin Response Factors Are Ubiquitous in Plant Growth and Development, and Involved in Crosstalk between Plant Hormones: A Review. Appl. Sci. 2022, 12, 1360. [Google Scholar] [CrossRef]
- Lv, X.M.; Zhang, Y.X.; Hu, L.; Zhang, Y.; Zhang, B.; Xia, H.Y.; Du, W.Y.; Fan, S.J.; Kong, L.A. Low-Nitrogen Stress Stimulates Lateral Root Initiation and Nitrogen Assimilation in Wheat: Roles of Phytohormone Signaling. J. Plant Growth Regul. 2021, 40, 436–450. [Google Scholar] [CrossRef]
- Krouk, G. Hormones and Nitrate: A Two-Way Connection. Plant Mol. Biol. 2016, 91, 599–606. [Google Scholar] [CrossRef]
- Sun, C.H.; Yu, J.Q.; Hu, D.G. Nitrate: A Crucial Signal during Lateral Roots Development. Front. Plant Sci. 2017, 8, 485. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; De Gernier, H.; Duan, X.; Xie, Y.; Geelen, D.; Hayashi, K.; Xuan, W.; Geisler, M.; Ten Tusscher, K.; Beeckman, T.; et al. GH3-mediated Auxin Inactivation Attenuates Multiple Stages of Lateral Root Development. New Phytol. 2023, 240, 1900–1912. [Google Scholar] [CrossRef]
- Guseman, J.M.; Hellmuth, A.; Lanctot, A.; Feldman, T.P.; Moss, B.L.; Klavins, E.; Calderón Villalobos, L.I.A.; Nemhauser, J.L. Auxin-Induced Degradation Dynamics Set the Pace for Lateral Root Development. Development 2015, 142, 905–909. [Google Scholar] [CrossRef]
- Stoeckle, D.; Thellmann, M.; Vermeer, J.E. Breakout—Lateral Root Emergence in Arabidopsis thaliana. Curr. Opin. Plant Biol. 2018, 41, 67–72. [Google Scholar] [CrossRef]
- Cowling, C.L.; Homayouni, A.L.; Callwood, J.B.; McReynolds, M.R.; Khor, J.; Ke, H.; Draves, M.A.; Dehesh, K.; Walley, J.W.; Strader, L.C.; et al. ZmPILS6 is an auxin efflux carrier required for maize root morphogenesis. Proc. Natl. Acad. Sci. USA 2024, 121, e2313216121. [Google Scholar] [CrossRef]
- Yang, C.; Wang, D.; Zhang, C.; Ye, M.; Kong, N.; Ma, H.; Chen, Q. Comprehensive Analysis and Expression Profiling of PIN, AUX/LAX, and ABCB Auxin Transporter Gene Families in Solanum Tuberosum under Phytohormone Stimuli and Abiotic Stresses. Biology 2021, 10, 127. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Y.; Zhao, D.; Tang, Z.; Zhang, T.; Zhang, K.; Dong, J.; Zhang, H. Genetic Regulation of Lateral Root Development. Plant Signaling Behav. 2023, 18, 2081397. [Google Scholar] [CrossRef] [PubMed]
- Aida, M.; Beis, D.; Heidstra, R.; Willemsen, V.; Blilou, I.; Galinha, C.; Nussaume, L.; Noh, Y.-S.; Amasino, R.; Scheres, B. The PLETHORA Genes Mediate Patterning of the Arabidopsis Root Stem Cell Niche. Cell 2004, 119, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Pinon, V.; Prasad, K.; Grigg, S.P.; Sanchez-Perez, G.F.; Scheres, B. Local Auxin Biosynthesis Regulation by PLETHORA Transcription Factors Controls Phyllotaxis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 110, 1107–1112. [Google Scholar] [CrossRef]
- Gutierrez, L.; Mongelard, G.; Floková, K.; Păcurar, D.I.; Novák, O.; Staswick, P.; Kowalczyk, M.; Păcurar, M.; Demailly, H.; Geiss, G.; et al. Auxin Controls Arabidopsis Adventitious Root Initiation by Regulating Jasmonic Acid Homeostasis. Plant Cell 2012, 24, 2515–2527. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Ma, W.; Yang, H.; Ma, G.; Mantyla, J.J.; Benning, C. The Arabidopsis WRINKLED1 Transcription Factor Affects Auxin Homeostasis in Roots. J. Exp. Bot. 2017, 68, 4627–4634. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.-H.; Miao, Z.-Q.; Qi, G.-F.; Wu, J.; Cai, X.-T.; Mao, J.-L.; Xiang, C.-B. MADS-Box Transcription Factor AGL21 Regulates Lateral Root Development and Responds to Multiple External and Physiological Signals. Mol. Plant 2014, 7, 1653–1669. [Google Scholar] [CrossRef]
- Alvarez-Buylla, E.R.; García-Ponce, B.; Sánchez, M.D.L.P.; Espinosa-Soto, C.; García-Gómez, M.L.; Piñeyro-Nelson, A.; Garay-Arroyo, A. MADS-box Genes Underground Becoming Mainstream: Plant Root Developmental Mechanisms. New Phytol. 2019, 223, 1143–1158. [Google Scholar] [CrossRef]
- Song, X.; Yu, Y.; Zhu, J.; Li, C. BRIP1 and BRIP2 Maintain Root Meristem by Affecting Auxin-Mediated Regulation. Planta 2024, 259, 8. [Google Scholar] [CrossRef]
- Yun, F.; Liu, H.; Deng, Y.; Hou, X.; Liao, W. The Role of Light-Regulated Auxin Signaling in Root Development. Int. J. Mol. Sci. 2023, 24, 5253. [Google Scholar] [CrossRef]
- Li, Q.F.; Lu, J.; Yu, J.W.; Zhang, C.Q.; He, J.X.; Liu, Q.Q. The Brassinosteroid-Regulated Transcription Factors BZR1/BES1 Function as a Coordinator in Multisignal-Regulated Plant Growth. Biochim. Biophys. Acta Gene Regul. Mech. 2018, 1861, 561–571. [Google Scholar] [CrossRef]
- Jia, Z.; Giehl, R.F.H.; Von Wirén, N. Local Auxin Biosynthesis Acts Downstream of Brassinosteroids to Trigger Root Foraging for Nitrogen. Nat. Commun. 2021, 12, 5437. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.Y.; Zhao, J.B.; Jing, Y.J.; Fan, M.Z.; Liu, J.; Wang, Z.C.; Xin, W.; Hu, Y.X. The Arabidopsis IDD14, IDD15, and IDD16 cooperatively regulate lateral organ morphogenesis and gravitropism by promoting auxin biosynthesis and transport. PLoS Genet. 2013, 9, e1003759. [Google Scholar] [CrossRef] [PubMed]
- Semeradova, H.; Montesinos, J.C.; Benkova, E. All Roads Lead to Auxin: Post-Translational Regulation of Auxin Transport by Multiple Hormonal Pathways. Plant Commun. 2020, 1, 100048. [Google Scholar] [CrossRef] [PubMed]
- Wakeel, A.; Ali, I.; Khan, A.R.; Wu, M.; Upreti, S.; Liu, D.; Liu, B.; Gan, Y. Involvement of Histone Acetylation and Deacetylation in Regulating Auxin Responses and Associated Phenotypic Changes in Plants. Plant Cell Rep. 2018, 37, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Weiste, C.; Dröge-Laser, W. The Arabidopsis Transcription Factor bZIP11 Activates Auxin-Mediated Transcription by Recruiting the Histone Acetylation Machinery. Nat. Commun. 2014, 5, 3883. [Google Scholar] [CrossRef]
- Gao, F.; Wang, K.; Liu, Y.; Chen, Y.; Chen, P.; Shi, Z.; Luo, J.; Jiang, D.; Fan, F.; Zhu, Y.; et al. Blocking miR396 Increases Rice Yield by Shaping Inflorescence Architecture. Nat. Plants 2015, 2, 15196. [Google Scholar] [CrossRef]
- Neuteboom, L.W. A Novel Subtilisin-like Protease Gene from Arabidopsis Thaliana Is Expressed at Sites of Lateral Root Emergence. DNA Res. 1999, 6, 13–19. [Google Scholar] [CrossRef]
- De Rybel, B.; Milad, A.; Alice, S.B.; Jos, R.W.; Margot, E.S.; Ondřej, N.; Nobutoshi, Y.; Saiko, Y.; Van Isterdael, G.; Joakim, P. Integration of growth and patterning during vascular tissue formation in Arabidopsis. Science 2014, 345. [Google Scholar] [CrossRef]
- Cuadrado-Pedetti, M.B.; Rauschert, I.; Sainz, M.M.; Amorim-Silva, V.; Botella, M.A.; Borsani, O.; Sotelo-Silveira, M. The Arabidopsis TETRATRICOPEPTIDE THIOREDOXIN-LIKE 1 Gene Is Involved in Anisotropic Root Growth during Osmotic Stress Adaptation. Genes 2021, 12, 236. [Google Scholar] [CrossRef]
- Hager, B.A. Role of the Plasma Membrane H+-ATPase in Auxin-Induced Elongation Growth: Historical and New Aspects. J. Plant Res. 2003, 116, 483–505. [Google Scholar] [CrossRef]
- Péret, B.; Li, G.; Zhao, J.; Band, L.R.; Voß, U.; Postaire, O.; Luu, D.-T.; Da Ines, O.; Casimiro, I.; Lucas, M.; et al. Auxin Regulates Aquaporin Function to Facilitate Lateral Root Emergence. Nat. Cell Biol. 2012, 14, 991–998. [Google Scholar] [CrossRef]
- Vilches-Barro, A.; Maizel, A. Talking through Walls: Mechanisms of Lateral Root Emergence in Arabidopsis Thaliana. Curr. Opin. Plant Biol. 2015, 23, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Matsunami, M.; Toyofuku, K.; Ishikawa–Sakurai, J.; Ogawa, A.; Matsunami, T.; Kokubun, M. Root Development and the Expression of Aquaporin Genes in Rice Seedlings under Osmotic Stress. Plant Prod. Sci. 2016, 19, 315–322. [Google Scholar] [CrossRef]
- Lin, D.; Nagawa, S.; Chen, J.; Cao, L.; Chen, X.; Xu, T.; Li, H.; Dhonukshe, P.; Yamamuro, C.; Friml, J.; et al. A ROP GTPase-Dependent Auxin Signaling Pathway Regulates the Subcellular Distribution of PIN2 in Arabidopsis Roots. Curr. Biol. 2012, 22, 1319–1325. [Google Scholar] [CrossRef]
- Kirolinko, C.; Hobecker, K.; Cueva, M.; Botto, F.; Christ, A.; Niebel, A.; Ariel, F.; Blanco, F.A.; Crespi, M.; Zanetti, M.E. A Lateral Organ Boundaries Domain Transcription Factor Acts Downstream of the Auxin Response Factor 2 to Control Nodulation and Root Architecture in Medicago truncatula. New Phytol. 2024, 242, 2746–2762. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, M.; Rípodas, C.; Clúa, J.; Baudin, M.; Aguilar, O.M.; Niebel, A.; Zanetti, M.E.; Blanco, F.A. A Nuclear Factor Y Interacting Protein of the GRAS Family Is Required for Nodule Organogenesis, Infection Thread Progression, and Lateral Root Growth. Plant Physiol. 2014, 164, 1430–1442. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Renard, C.M.G.C.; Bureau, S.; Le Bourvellec, C. Revisiting the Contribution of ATR-FTIR Spectroscopy to Characterize Plant Cell Wall Polysaccharides. Carbohydr. Polym. 2021, 262, 117935. [Google Scholar] [CrossRef] [PubMed]
- Orazbekuly, Y.; Aitkaliyeva, G.; Yelubay, M. New Approaches to Sample Preparation and Integrated Spectroscopic Methods for The Identification of Polioxyethylene Triolate Sorbitane for Pharmaceutical Examination of Drugs. Indones. J. Pharm. 2020, 31, 181–186. [Google Scholar] [CrossRef]
- Zhang, H.; Jennings, A.; Barlow, P.W.; Forde, B.G. Dual Pathways for Regulation of Root Branching by Nitrate. Proc. Natl. Acad. Sci. USA 1999, 96, 6529–6534. [Google Scholar] [CrossRef]
- Saito, A.; Tanabata, S.; Tanabata, T.; Tajima, S.; Ueno, M.; Ishikawa, S.; Ohtake, N.; Sueyoshi, K.; Ohyama, T. Effect of Nitrate on Nodule and Root Growth of Soybean (Glycine max (L.) Merr.). Int. J. Mol. Sci. 2014, 15, 4464–4480. [Google Scholar] [CrossRef]
- Qin, L.; Walk, T.C.; Han, P.; Chen, L.; Zhang, S.; Li, Y.; Hu, X.; Xie, L.; Yang, Y.; Liu, J.; et al. Adaption of Roots to Nitrogen Deficiency Revealed by 3D Quantification and Proteomic Analysis. Plant Physiol. 2019, 179, 329–347. [Google Scholar] [CrossRef]
- Wang, Y.B.; Song, J.P.; Wang, Z.B. ASYMMETRIC LEAVES2-LIKE38, One Member of AS2/LOB Gene Family, Involves in Regulating Ab-Adaxial Patterning in Arabidopsis Lateral Organs. Acta Physiol. Plant. 2015, 37, 185. [Google Scholar] [CrossRef]
- Morffy, N.; Strader, L.C. Old Town Roads: Routes of Auxin Biosynthesis across Kingdoms. Curr. Opin. Plant Biol. 2020, 55, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Su, Y.; Dai, Z.; Lu, M.; Sun, W.; Yang, W.; Wu, S.S.; Wan, Z.T.; Wan, H.H.; Zhai, J. Integration of the Metabolome and Transcriptome Reveals Indigo Biosynthesis in Phaius flavus Flowers under Freezing Treatment. PeerJ 2022, 10, e13106. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Mashiguchi, K.; Chen, Q.; Kasahara, H.; Kamiya, Y.; Ojha, S.; DuBois, J.; Ballou, D.; Zhao, Y. The Biochemical Mechanism of Auxin Biosynthesis by an Arabidopsis YUCCA Flavin-Containing Monooxygenase. J. Biol. Chem. 2013, 288, 1448–1457. [Google Scholar] [CrossRef]
- Aoi, Y.; Oikawa, A.; Sasaki, R.; Huang, J.; Hayashi, K.; Kasahara, H. Arogenate Dehydratases Can Modulate the Levels of Phenylacetic Acid in Arabidopsis. Biochem. Biophys. Res. Commun. 2020, 524, 83–88. [Google Scholar] [CrossRef]
- Wu, J.; Kamanga, B.M.; Zhang, W.; Xu, Y.; Xu, L. Research Progress of Aldehyde Oxidases in Plants. Peer J 2022, 10, e13119. [Google Scholar] [CrossRef]
- Tivendale, N.D.; Ross, J.J.; Cohen, J.D. The Shifting Paradigms of Auxin Biosynthesis. Trends Plant Sci. 2014, 19, 44–51. [Google Scholar] [CrossRef]
- Stepanova, A.N.; Alonso, J.M. Auxin Catabolism Unplugged: Role of IAA Oxidation in Auxin Homeostasis. Proc. Natl. Acad. Sci. USA 2016, 113, 10742–10744. [Google Scholar] [CrossRef]
- Zhang, J.; Peer, W.A. Auxin Homeostasis: The DAO of Catabolism. J. Exp. Bot. 2017, 68, 3145–3154. [Google Scholar] [CrossRef]
- Hayashi, K.; Arai, K.; Aoi, Y.; Tanaka, Y.; Hira, H.; Guo, R.; Hu, Y.; Ge, C.; Zhao, Y.; Kasahara, H.; et al. The Main Oxidative Inactivation Pathway of the Plant Hormone Auxin. Nat. Commun. 2021, 12, 6752. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K.; Arai, K.; Tanaka, Y.; Aoi, Y.; Kukshal, V.; Jez, J.M.; Kubes, M.F.; Napier, R.; Zhao, Y.; Kasahara, H.; et al. Chemical Inhibition of the Auxin Inactivation Pathway Uncovers the Roles of Metabolic Turnover in Auxin Homeostasis. Proc. Natl. Acad. Sci. USA 2022, 119, e2206869119. [Google Scholar] [CrossRef] [PubMed]
- Que, F.; Zhu, Y.; Liu, Q.; Wei, Q.; Ramakrishnan, M. Genome-Wide Identification, Expansion, Evolution, and Expression Analysis Reveals ABCB Genes Important for Secondary Cell Wall Development in Moso Bamboo (Phyllostachys edulis). Agronomy 2023, 13, 1828. [Google Scholar] [CrossRef]
- Devi, R.; Goyal, P.; Verma, B.; Hussain, S.; Chowdhary, F.; Arora, P.; Gupta, S. A Transcriptome-Wide Identification of ATP-Binding Cassette (ABC) Transporters Revealed Participation of ABCB Subfamily in Abiotic Stress Management of Glycyrrhiza glabra L. BMC Genom. 2024, 25, 315. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.X.; Liu, Y.; Chen, S.T.; Li, X.Q.; Xu, L.G.; Qi, Y.H.; Jiang, D.A.; Jin, S.H. The B Subfamily of Plant ATP Binding Cassette Transporters and Their Roles in Auxin Transport. Biol. Plant. 2014, 58, 401–410. [Google Scholar] [CrossRef]
- Reemmer, J.E. ABCB11 Functions with B1 and B19 to Regulate Rootward Auxin Transport. Ph.D. Thesis, Purdue University, West Lafayette, IN, USA, 2014. [Google Scholar]
- Yazaki, K.; Shitan, N.; Sugiyama, A.; Takanashi, K. Chapter 6 Cell and Molecular Biology of ATP-Binding Cassette Proteins in Plants. In International Review of Cell and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2009; Volume 276, pp. 263–299. ISBN 978-0-12-374807-2. [Google Scholar]
- Geisler, M.; Blakeslee, J.J.; Bouchard, R.; Lee, O.R.; Vincenzetti, V.; Bandyopadhyay, A.; Titapiwatanakun, B.; Peer, W.A.; Bailly, A.; Richards, E.L.; et al. Cellular Efflux of Auxin Catalyzed by the Arabidopsis MDR/PGP Transporter AtPGP1. Plant J. 2005, 44, 179–194. [Google Scholar] [CrossRef]
- Tian, Q.; Chen, F.; Liu, J.; Zhang, F.; Mi, G. Inhibition of Maize Root Growth by High Nitrate Supply Is Correlated with Reduced IAA Levels in Roots. J. Plant Physiol. 2008, 165, 942–951. [Google Scholar] [CrossRef]
- Wang, W.; Xin, W.; Chen, N.; Yang, F.; Li, J.; Qu, G.; Jiang, X.; Xu, L.; Zhao, S.; Liu, H.; et al. Transcriptome and Co-Expression Network Analysis Reveals the Molecular Mechanism of Rice Root Systems in Response to Low-Nitrogen Conditions. Int. J. Mol. Sci. 2023, 24, 5290. [Google Scholar] [CrossRef]
- Chen, J.; Liu, S.; Zhang, S.; Ge, C.; Shen, Q.; Ma, H.; Zhang, X.; Dong, H.; Zhao, X.; Pang, C. Nitrogen Modulates Cotton Root Morphology by Affecting Abscisic Acid (ABA) and Salicylic Acid (SA) Content. Arch. Agron. Soil Sci. 2021; 67, 1722–1738. [Google Scholar] [CrossRef]
- Forde, B.; Lorenzo, H. The Nutritional Control of Root Development. In Interactions in the Root Environment: An Integrated Approach; Powlson, D.S., Bateman, G.L., Davies, K.G., Gaunt, J.L., Hirsch, P.R., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 51–68. ISBN 978-94-010-3925-3. [Google Scholar]
- Mounier, E.; Pervent, M.; Ljung, K.; Gojon, A.; Nacry, P. Auxin-mediated Nitrate Signalling by NRT 1.1 Participates in the Adaptive Response of ARabidopsis Root Architecture to the Spatial Heterogeneity of Nitrate Availability. Plant Cell Environ. 2014, 37, 162–174. [Google Scholar] [CrossRef]
- Burton, R.A.; Gidley, M.J.; Fincher, G.B. Heterogeneity in the Chemistry, Structure and Function of Plant Cell Walls. Nat. Chem Biol. 2010, 6, 724–732. [Google Scholar] [CrossRef]
- Tian, H.; Song, H.; Wu, X.; Zhang, Z. Responses of Cell Wall Components to Low Nitrogen in Rapeseed Roots. Agronomy 2022, 12, 1044. [Google Scholar] [CrossRef]
- Peaucelle, A.; Braybrook, S.; Höfte, H. Cell Wall Mechanics and Growth Control in Plants: The Role of Pectins Revisited. Front. Plant Sci. 2012, 3, 121. [Google Scholar] [CrossRef]
- Wolf, S.; Hématy, K.; Höfte, H. Growth Control and Cell Wall Signaling in Plants. Annu. Rev. Plant Biol. 2012, 63, 381–407. [Google Scholar] [CrossRef] [PubMed]
- Majda, M. The Role of Auxin in Cell Wall Expansion. Int. J. Mol. Sci. 2018, 19, 951. [Google Scholar] [CrossRef] [PubMed]
- Levesque-Tremblay, G.; Pelloux, J.; Braybrook, S.A.; Müller, K. Tuning of Pectin Methylesterification: Consequences for Cell Wall Biomechanics and Development. Planta 2015, 242, 791–811. [Google Scholar] [CrossRef]
- Peaucelle, A.; Braybrook, S.A.; Le Guillou, L.; Bron, E.; Kuhlemeier, C.; Höfte, H. Pectin-Induced Changes in Cell Wall Mechanics Underlie Organ Initiation in Arabidopsis. Curr. Biol. 2011, 21, 1720–1726. [Google Scholar] [CrossRef] [PubMed]
- Braybrook, S.A.; Peaucelle, A. Mechano-Chemical Aspects of Organ Formation in Arabidopsis thaliana: The Relationship between Auxin and Pectin. PLoS ONE 2013, 8, e57813. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Laskowski, M.; Hawes, M. Cell separation in roots. In Plant Cell Separation and Adhesion; Roberts, J.A., Gonzalez-Carranza, Z., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2007; Volume 25, pp. 91–105. [Google Scholar]
- Barbosa, F.C.; Silvello, M.A.; Goldbeck, R. Cellulase and Oxidative Enzymes: New Approaches, Challenges and Perspectives on Cellulose Degradation for Bioethanol Production. Biotechnol. Lett. 2020, 42, 875–884. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005, 6, 850–861. [Google Scholar] [CrossRef]
- Duman, Z.; Eliyahu, A.; Abu-Abied, M.; Sadot, E. The Contribution of Cell Wall Remodeling and Signaling to Lateral Organs Formation. Israel J. Plant Sci. 2020, 67, 110–127. [Google Scholar] [CrossRef]
- Xu, Q.; Burgess, P.; Xu, J.; Meyer, W.; Huang, B. Osmotic Stress- and Salt Stress-Inhibition and Gibberellin-Mitigation of Leaf Elongation Associated with up-Regulation of Genes Controlling Cell Expansion. Environ. Exp. Bot. 2016, 131, 101–109. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, J. EXPANSINA17 Up-Regulated by LBD18/ASL20 Promotes Lateral Root Formation During the Auxin Response. Plant Cell Physiol. 2013, 54, 1600–1611. [Google Scholar] [CrossRef]
- Ramakrishna, P.; Ruiz Duarte, P.; Rance, G.A.; Schubert, M.; Vordermaier, V.; Vu, L.D.; Murphy, E.; Vilches Barro, A.; Swarup, K.; Moirangthem, K.; et al. EXPANSIN A1-Mediated Radial Swelling of Pericycle Cells Positions Anticlinal Cell Divisions during Lateral Root Initiation. Proc. Natl. Acad. Sci. USA 2019, 116, 8597–8602. [Google Scholar] [CrossRef] [PubMed]
- Spartz, A.K.; Lor, V.S.; Ren, H.; Olszewski, N.E.; Miller, N.D.; Wu, G.; Spalding, E.P.; Gray, W.M. Constitutive Expression of Arabidopsis SMALL AUXIN UP RNA19 (SAUR19) in Tomato Confers Auxin-Independent Hypocotyl Elongation. Plant Physiol. 2017, 173, 1453–1462. [Google Scholar] [CrossRef]
- Du, M.; Spalding, E.P.; Gray, W.M. Rapid Auxin-Mediated Cell Expansion. Annu. Rev. Plant Biol. 2020, 71, 379–402. [Google Scholar] [CrossRef]
- Verkman, A.S. Aquaporins. Curr. Biol. 2013, 23, R52–R55. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Tang, C.; Li, H.; Xia, H.; Fan, S.; Kong, L. Bicarbonate-Dependent Detoxification by Mitigating Ammonium-Induced Hypoxic Stress in Triticum Aestivum Root. Biology 2024, 13, 101. [Google Scholar] [CrossRef]
- Heinen, R.B.; Ye, Q.; Chaumont, F. Role of Aquaporins in Leaf Physiology. J. Exp. Bot. 2009, 60, 2971–2985. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, H.; Hachez, C.; Bienert, M.D.; Beebo, A.; Swarup, K.; Voß, U.; Bouhidel, K.; Frigerio, L.; Schjoerring, J.K.; Bennett, M.J.; et al. Tonoplast Aquaporins Facilitate Lateral Root Emergence. Plant Physiol. 2016, 170, 1640–1654. [Google Scholar] [CrossRef]
- Hu, F.; Fang, D.; Zhang, W.; Dong, K.; Ye, Z.; Cao, J. Lateral Root Primordium: Formation, Influencing Factors and Regulation. Plant Physiol. Biochem. 2024, 207, 108429. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Z.; Du, A.; Gao, H.; Liang, J.; Yu, W.; Yu, H.; Fan, S.; Chen, Q.; Guo, J.; et al. Transcription Factor LBD16 Targets Cell Wall Modification/Ion Transport Genes in Peach Lateral Root Formation. Plant Physiol. 2024, 194, 2472–2490. [Google Scholar] [CrossRef]
- Sharma, M.; Singh, D.; Saksena, H.B.; Sharma, M.; Tiwari, A.; Awasthi, P.; Botta, H.K.; Shukla, B.N.; Laxmi, A. Understanding the Intricate Web of Phytohormone Signalling in Modulating Root System Architecture. Int. J. Mol. Sci. 2021, 22, 5508. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Frugis, G.; Colgan, D.; Chua, N.-H. Arabidopsis NAC1 Transduces Auxin Signal Downstream of TIR1 to Promote Lateral Root Development. Genes Dev. 2000, 14, 3024–3036. [Google Scholar] [CrossRef]
- Huang, X.; Lu, Z.; Zhai, L.; Li, N.; Yan, H. The Small Auxin-Up RNA SAUR10 Is Involved in the Promotion of Seedling Growth in Rice. Plants 2023, 12, 3880. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.; Chen, Y.; Li, M.; Kong, Y.; Zhu, Y.; Han, N.; Bian, H.; Zhu, M.; Wang, J. The Tissue-Specific and Developmentally Regulated Expression Patterns of the SAUR41 Subfamily of SMALL AUXIN UP RNA Genes: Potential Implications. Plant Signaling Behav. 2013, 8, e25283. [Google Scholar] [CrossRef]
- Chen, X.; Naramoto, S.; Robert, S.; Tejos, R.; Löfke, C.; Lin, D.; Yang, Z.; Friml, J. ABP1 and ROP6 GTPase Signaling Regulate Clathrin-Mediated Endocytosis in Arabidopsis Roots. Curr. Biol. 2012, 22, 1326–1332. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, H.; Chen, M.; Li, X.; Wang, M.; Yang, Y.; Wang, C.; Huang, J.; Liu, G.; Liu, Y.; et al. ROP3 GTPase Contributes to Polar Auxin Transport and Auxin Responses and Is Important for Embryogenesis and Seedling Growth in Arabidopsis. Plant Cell 2014, 26, 3501–3518. [Google Scholar] [CrossRef]
- Dong, Q.; Zhang, Z.; Liu, Y.; Tao, L.; Liu, H. FERONIA Regulates Auxin-mediated Lateral Root Development and Primary Root Gravitropism. FEBS Lett. 2019, 593, 97–106. [Google Scholar] [CrossRef]
- Hirota, A.; Kato, T.; Fukaki, H.; Aida, M.; Tasaka, M. The Auxin-Regulated AP2/EREBP Gene PUCHI Is Required for Morphogenesis in the Early Lateral Root Primordium of Arabidopsis. Plant Cell 2007, 19, 2156–2168. [Google Scholar] [CrossRef]
- Takatsuka, H.; Umeda, M. Hormonal Control of Cell Division and Elongation along Differentiation Trajectories in Roots. J. Exp. Bot. 2014, 65, 2633–2643. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, Y.; Zou, X.; Li, F.; Zhang, J.; Kang, Z.; Li, X.; Yin, C.; Lin, Y. Nitrogen Deficiency-Induced Decrease in Cytokinins Content Promotes Rice Seminal Root Growth by Promoting Root Meristem Cell Proliferation and Cell Elongation. Cells 2020, 9, 916. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Zhang, L.; Gao, J.; Zhang, W.; Yi, J.; Zhen, X.; Bi, C.; He, D.; Liu, S.; Zhao, X. Adaptation Mechanism of Roots to Low and High Nitrogen Revealed by Proteomic Analysis. Rice 2021, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Stals, H.; Inzé, D. When Plant Cells Decide to Divide. Trends Plant Sci. 2001, 6, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Himanen, K.; Boucheron, E.; Vanneste, S.; De Almeida Engler, J.; Inzé, D.; Beeckman, T. Auxin-Mediated Cell Cycle Activation during Early Lateral Root Initiation. Plant Cell 2002, 14, 2339–2351. [Google Scholar] [CrossRef]
- Wasteneys, G.O.; Yang, Z. New Views on the Plant Cytoskeleton. Plant Physiol. 2004, 136, 3884–3891. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, M.; Yang, Y.; Xuan, W.; Zou, Z.W.; Arkorful, E.; Chen, Y.; Ma, Q.P.; Jeyaraj, A.; Chen, X.; et al. A novel insight into nitrogen and auxin signaling in lateral root formation in tea plant [Camellia sinensis (L.) O. Kuntze]. BMC Plant Biol. 2020, 20, 232. [Google Scholar] [CrossRef]
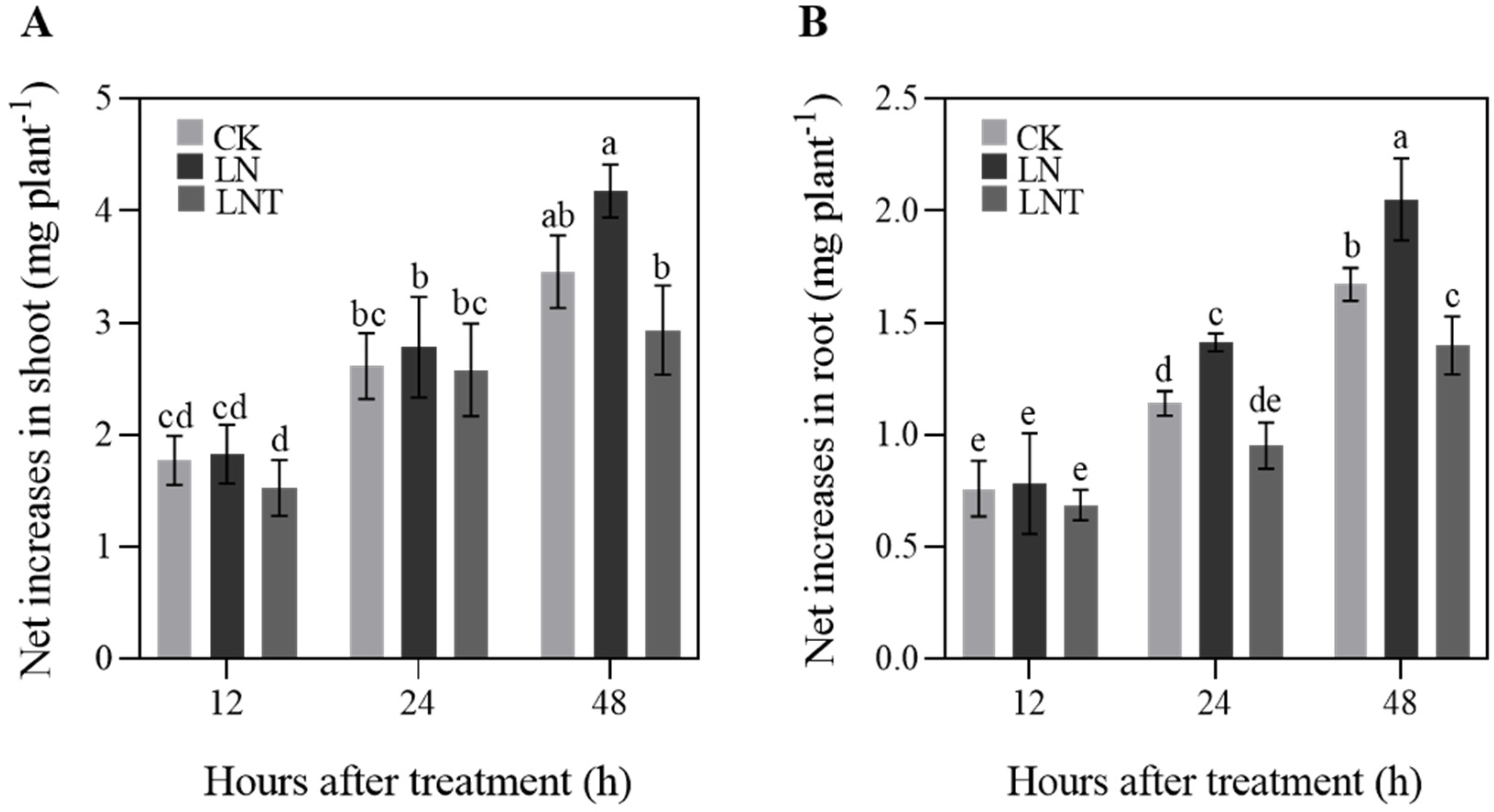
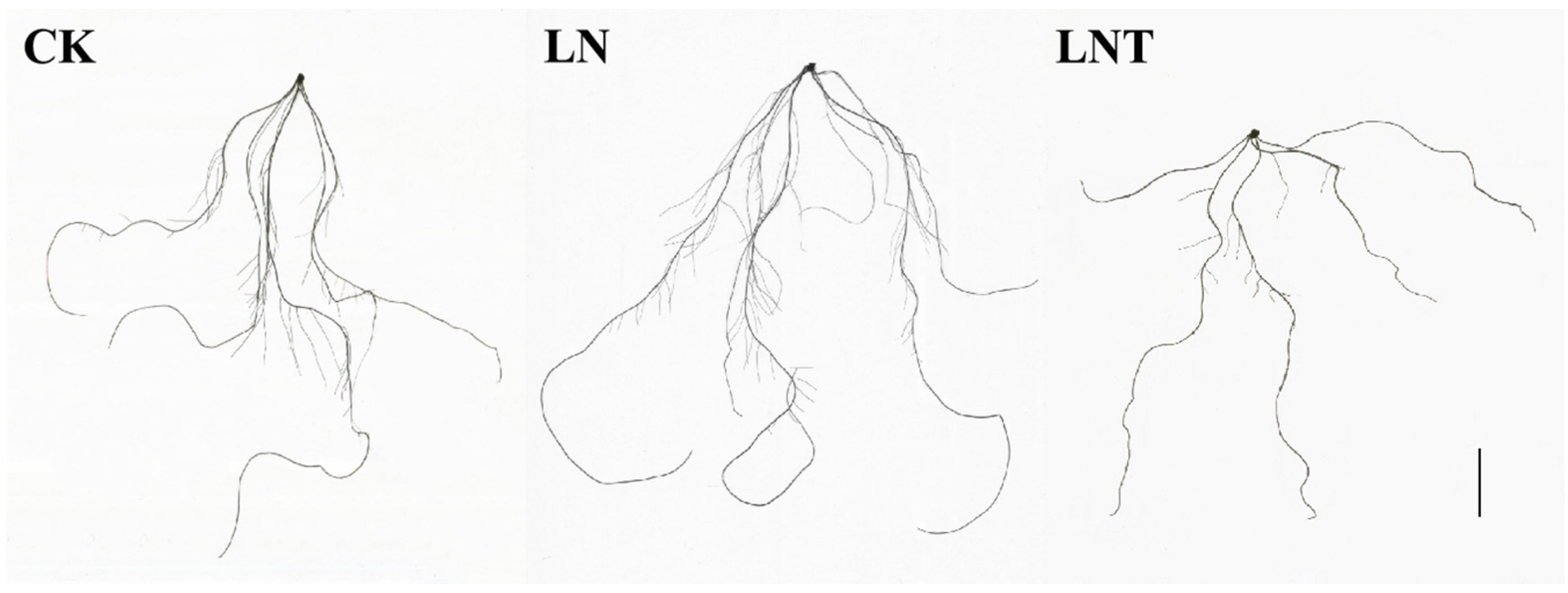
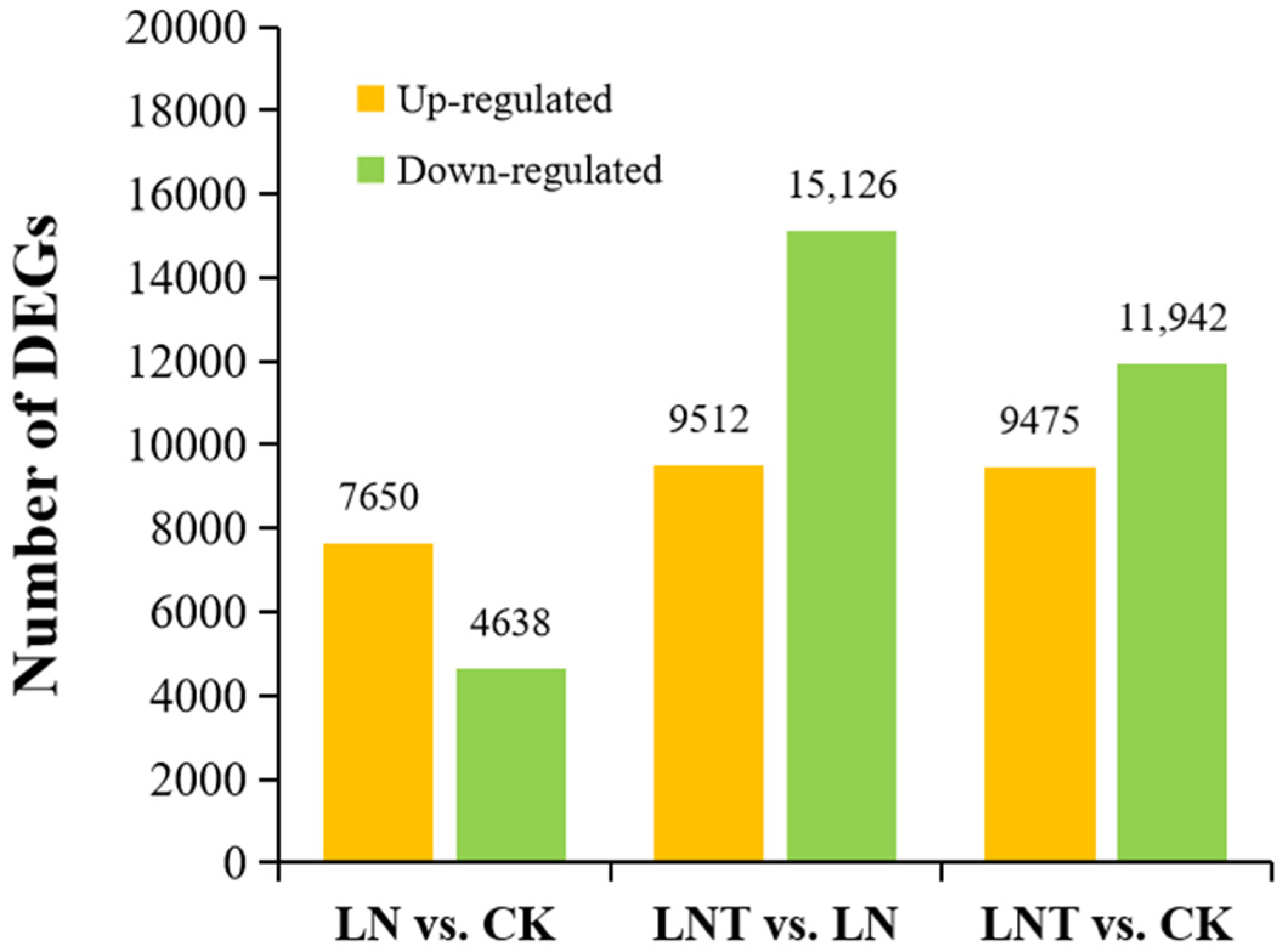
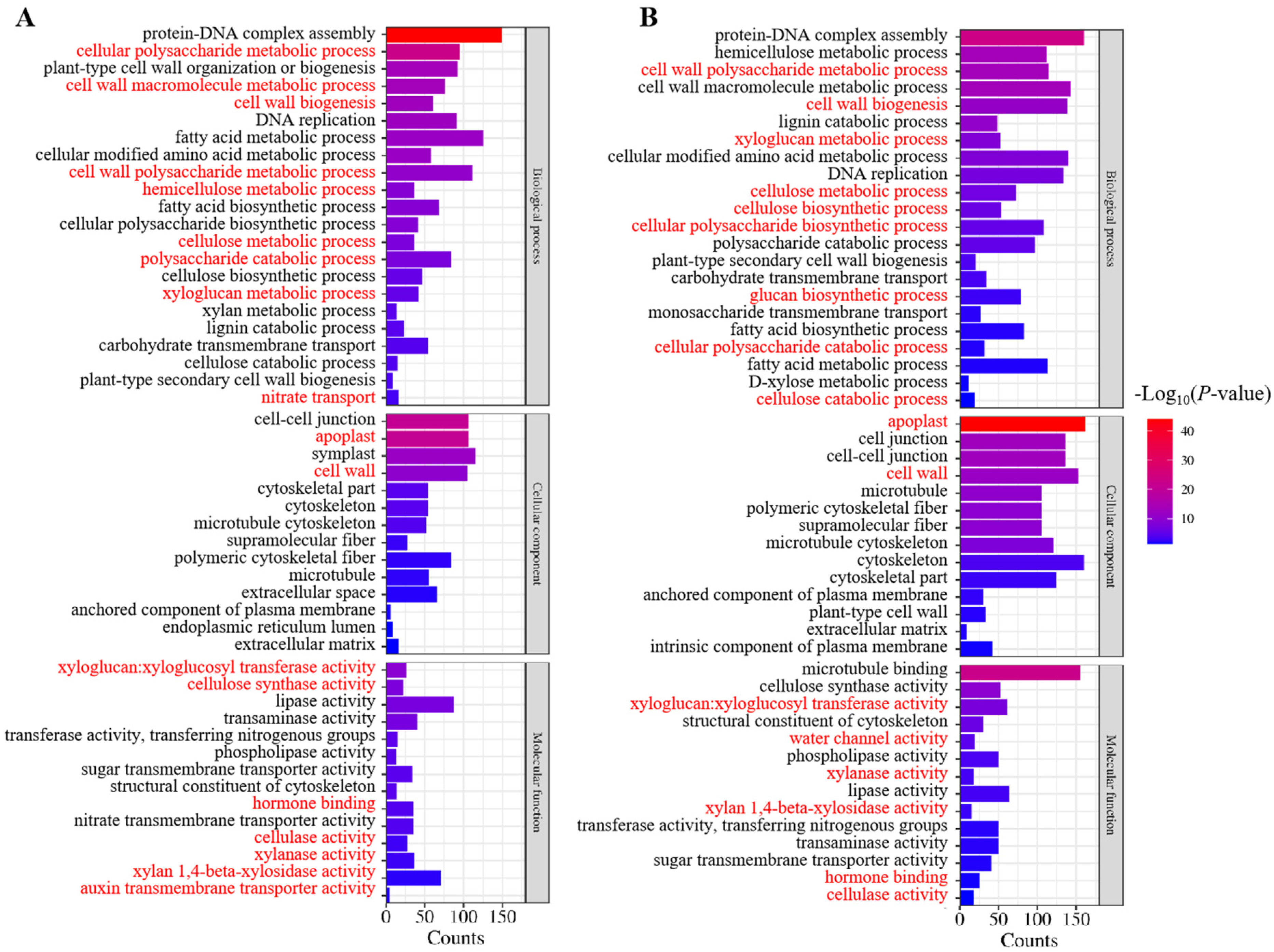



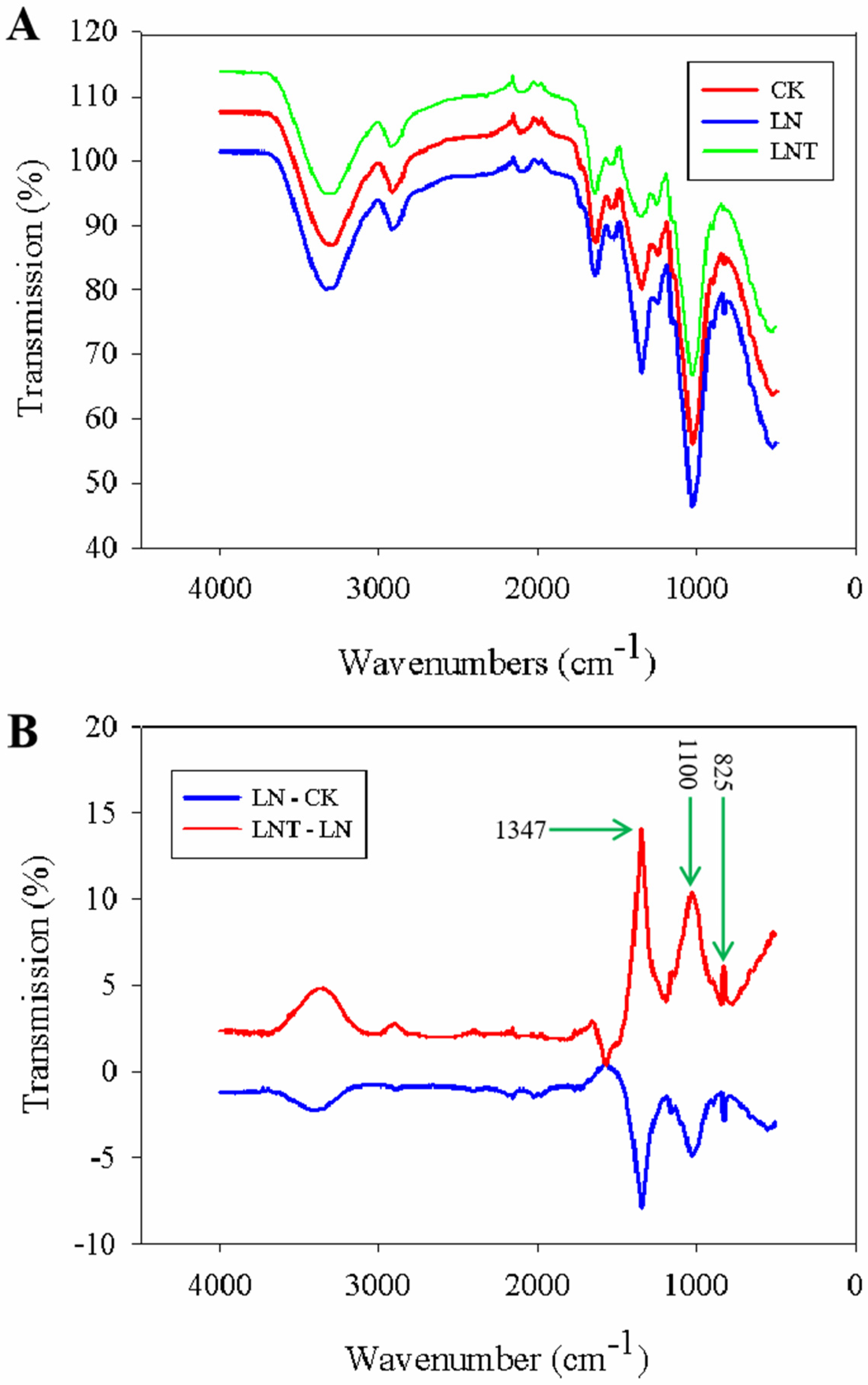
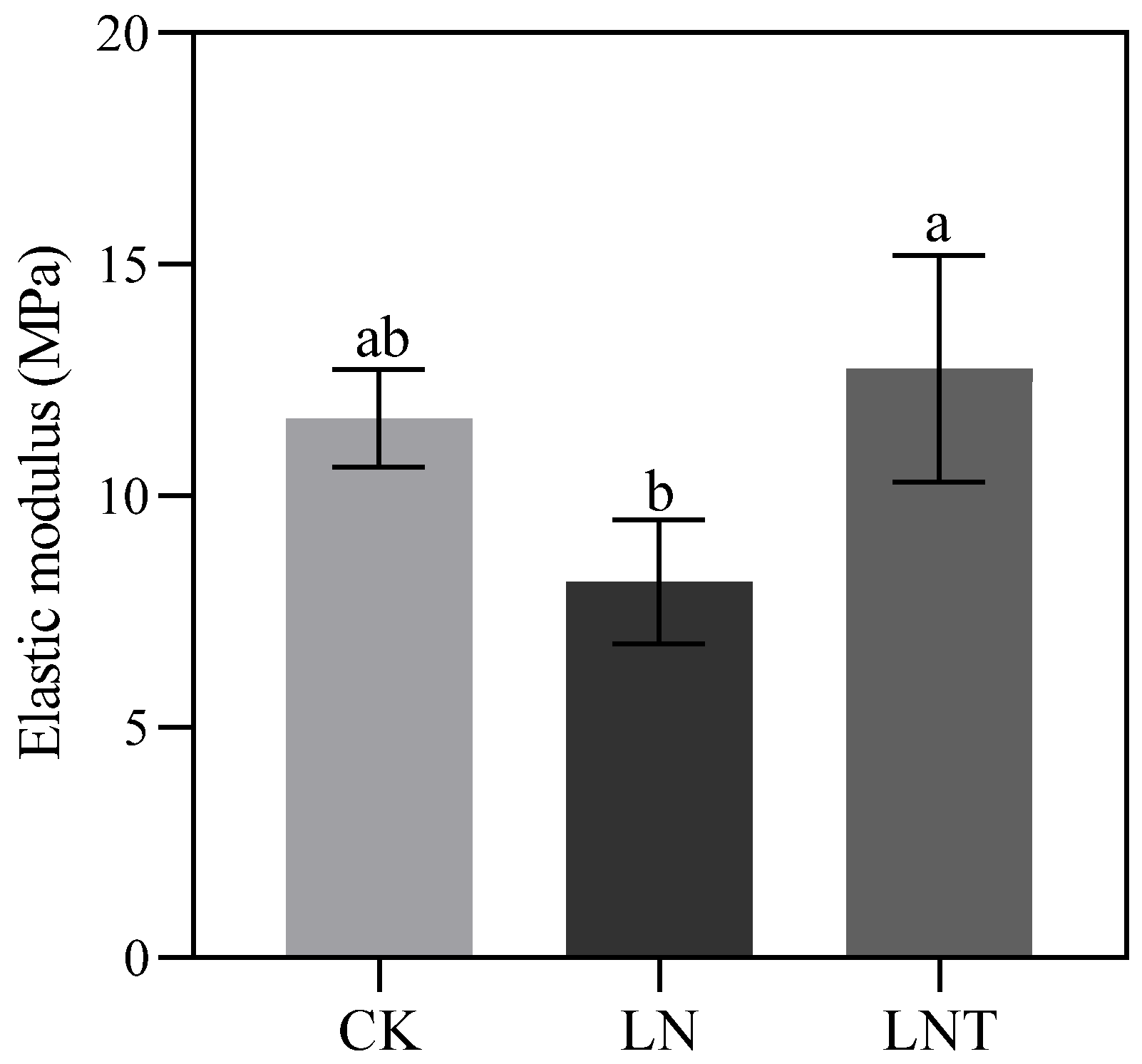
| Treatment | Root Tips | Total Root Length (cm) | Root Surface (cm2) |
|---|---|---|---|
| 5 mM NO3− (CK) | 103.60 ± 17.32 b | 123.85 ± 19.60 b | 12.28 ± 1.75 b |
| 0.1 mM NO3− (LN) | 150.02 ± 25.49 a | 149.04 ± 15.61 a | 14.01 ± 1.53 a |
| 0.1 mM NO3− + 60 mg/L TIBA (LNT) | 61.84 ± 20.00 c | 77.32 ± 11.23 c | 8.48 ± 0.93 c |
| Gene ID | Gene Expression | Gene Description | Function | ||
|---|---|---|---|---|---|
| TraesCS3A02G341400 | Protein PIN-LIKES 6 | Regulating the spatial redistribution of IAA in roots [39]. | |||
| TraesCS2A02G430400 | Auxin-responsive protein SAUR71 | Regulating cell expansion downstream of auxin transport and auxin signaling [40]. | |||
| TraesCS6B02G044300 | High-affinity nitrate transporter 2.1 | Polar auxin transport [35,36]. | |||
| novel.9662 | High-affinity nitrate transporter 2.1 | ||||
| TraesCS6D02G035900 | High-affinity nitrate transporter 2.1 | ||||
| TraesCS2A02G323900 | Putative multidrug resistance protein | Polar auxin transport [41]. | |||
| TraesCS4A02G370600 | Putative multidrug resistance protein | ||||
| TraesCS2D02G198800 | Putative polyol transporter 2 | Transcription factors of auxin biosynthesis and key molecular triggers for de novo organ patterning during LR formation [42,43]. | |||
| TraesCS2A02G188900 | Putative polyol transporter 2 | ||||
| TraesCS2D02G199100 | Putative polyol transporter 5 | ||||
| TraesCS5A02G141700 | Ethylene-responsive transcription factor WRI1 | Regulating primary root growth through alterations in auxin homeostasis [44,45]. | |||
| novel.7503 | Ethylene-responsive transcription factor WRI1 | ||||
| TraesCS5B02G002500 | Agamous-like MADS-box protein AGL21 | Positive regulation of auxin accumulation [46,47]. | |||
| novel.12069 | Agamous-like MADS-box protein AGL21 | ||||
| TraesCS5D02G359700 | Fanconi anemia group J protein homolog | Regulation of auxin output [48]. | |||
| TraesCS5A02G352700 | Fanconi anemia group J protein homolog | ||||
| TraesCS5B02G354900 | Fanconi anemia group J protein homolog | ||||
| TraesCS7A02G373800 | Transcription factor HY5 | Negatively affecting PIN3 and LAX3 levels [49]. Transcription factors of brassinosteroid [50]; upregulating the expression of TAA1 and YUC5/7/8 [51]. | |||
| TraesCS2A02G187800 | BES1/BZR1 plant transcription factor | ||||
| TraesCS2D02G199900 | BES1/BZR1 plant transcription factor | ||||
| TraesCS3A02G123500 | BES1/BZR1 plant transcription factor | ||||
| TraesCS5A02G233700 | Protein indeterminate-domain 14 | Binding to promoter regions of YUC5, TAA1, and PIN1 to activate their expression [52]. | |||
| TraesCS5D02G240600 | Protein indeterminate-domain 14 | ||||
| TraesCS4A02G082200 | Serine/threonine-protein kinase WAG1 | Controlling the phosphorylation of PIN proteins [53]. | |||
| TraesCS3A02G425700 | Probable histone acetyltransferase HAC-like 1 | Targeting auxin influx carrier LAX2 and inducing auxin-responsive genes [54,55]. | |||
| TraesCS5D02G193200 | Histone acetyl transferase HAT1 | ||||
| TraesCS5B02G186000 | Histone acetyl transferase HAT1 | ||||
| Gene ID | Gene Description | Log2 (FoldChange) | |
|---|---|---|---|
| LN vs. CK | LNT vs. LN | ||
| TraesCS5D02G145900 | Cellulase (glycosyl hydrolase family 5) | 1.34 | −3.08 |
| TraesCS3A02G241000 | Cellulase (glycosyl hydrolase family 5) | 2.90 | −2.23 |
| TraesCS5D02G500000 | Pectin acetylesterase 3 | 1.95 | −6.42 |
| TraesCS3D02G538500 | Pectin acetylesterase 5 | 1.43 | −6.25 |
| TraesCS3A02G533800 | Pectin acetylesterase 5 | 1.62 | −7.31 |
| TraesCS2B02G117500 | Probable pectinesterase 53 | 2.76 | −5.11 |
| TraesCS1A02G143600 | Probable pectate lyase 12 | 1.21 | −1.28 |
| TraesCS7B02G230600 | Polygalacturonase | 2.15 | −3.45 |
| TraesCS3B02G020300 | Probable polygalacturonase | 3.91 | −7.45 |
| TraesCS3D02G010500 | Probable xyloglucan glycosyltransferase 3 | 1.44 | −3.15 |
| TraesCSU02G175000 | Xyloglucan endotransglucosylase/hydrolase | 2.40 | −5.16 |
| TraesCS2A02G498500 | Xyloglucan endotransglucosylase/hydrolase 15 | 1.64 | −5.56 |
| TraesCS3B02G045700 | Endo-1,4-beta-xylanase 1 | 2.55 | −2.47 |
| TraesCS4D02G227700 | Endo-1,4-beta-xylanase 4 | 1.20 | −2.45 |
| TraesCS4B02G227000 | Endo-1,4-beta-xylanase 4 | 2.55 | −2.63 |
| TraesCS3D02G039400 | Xylanase inhibitor C-terminal | −2.13 | 3.56 |
| TraesCS1D02G064500 | Probable beta-1,4-xylosyltransferase | 1.30 | −1.05 |
| TraesCS7B02G311200 | Beta-1,2-xylosyltransferase | 1.83 | −5.36 |
| TraesCS7D02G405200 | Beta-1,2-xylosyltransferase | 1.89 | −3.93 |
| Gene ID | Gene Expression | Gene Description | Function | ||
|---|---|---|---|---|---|
| TraesCS4B02G060000 | Growth-regulating factor 6 | Activating OsYUC1 and auxin biosynthesis [56]. | |||
| TraesCS4D02G059600 | Growth-regulating factor 6 | ||||
| TraesCS2A02G037100 | Subtilisin-like protease SBT5.3 | Activated by NAC and mediating auxin signaling to promote LR development by weakening connections among cells during root development [57]. | |||
| TraesCS3B02G286900 | Subtilisin-like protease SBT5.3 | ||||
| TraesCS4B02G350600 | NAC domain-containing protein 86 | Transcription activator of genes involved in the biosynthesis of secondary cell walls, activating AIR3, and mediating auxin signaling to promote LR development [57]. | |||
| andTraesCS3B02G439600 | NAC domain-containing protein 48 | ||||
| TraesCS7A02G068000 | NAC domain-containing protein 43 | ||||
| TraesCS4A02G421300 | NAC domain-containing protein 43 | ||||
| TraesCS2D02G199900 | Brassinosteroid (BR)-regulated growth response | Inducing the expression of cyclin D genes in LR initiation and elongation [58]. | |||
| TraesCS5A02G473800 | APETALA2-like protein 3 | Defining the primordium boundary during LR outgrowth [12]. | |||
| TraesCS2D02G515800 | APETALA2-like protein 5 | ||||
| TraesCS3B02G251600 | Tetratricopeptide repeat-containing thioredoxin TTL1 | Regulating microtubule structure and cell wall elasticity [59]. | |||
| TraesCS3B02G162100 | Tetratricopeptide repeat-containing thioredoxin TTL1 | ||||
| TraesCS6D02G305000 | Glycosyl hydrolase family 17 | Exhibiting glucan 1,3-beta-D-glucoside activity and relaxing the plant cell wall [41]. | |||
| TraesCS3A02G374000 | Glycosyl hydrolase family 17 | ||||
| TraesCS4A02G005000 | Plasma membrane ATPase 10 | Excreting H+ ions into the cell wall compartment, lowering the cell wall pH, and initiating cell wall loosening and extended growth [60]. | |||
| TraesCS4B02G300000 | Plasma membrane ATPase 10 | ||||
| TraesCS2D02G206300 | Aquaporin PIP2-1 | Promoting water transport from overlying tissue to the primordium [61,62,63]. | |||
| TraesCS6A02G082900 | Aquaporin PIP2-5 | ||||
| TraesCS2B02G497800 | CRIB domain-containing protein RIC10 | Propagation of ROP GTPase signals [64]. | |||
| TraesCS6D02G126000 | CRIB domain-containing protein RIC10 | ||||
| TraesCS4B02G113300 | Protein NODULATION SIGNALING PATHWAY 2 | Transcriptional regulator essential for Nod-factor-induced gene expression [65,66]. | |||
| TraesCS4D02G110800 | Protein NODULATION SIGNALING PATHWAY 2 | ||||
| CK | LN | LNT | |
|---|---|---|---|
| Activity of cell-wall-remodeling-associated enzymes (μg min−1 g−1 FW) | |||
| pectinase | 25.78 ± 0.30 b | 34.97 ± 4.58 a | 26.12 ± 1.37 b |
| β-1,3-glucanase | 183.63 ± 7.95 b | 735.34 ± 39.37 a | 226.22 ± 11.30 b |
| Polygalacturonase | 0.81 ± 0.03 c | 3.32 ± 0.23 a | 1.97 ± 0.16 b |
| exo-β-1,4-glucanase | 19.85 ± 0.52 c | 22.59 ± 0.92 a | 21.16 ± 0.19 b |
| Cellulase | 507.43 ± 3.94 b | 576.20 ± 11.05 a | 505.95 ± 12.57 b |
| neutral xylanase | 96.84 ± 4.38 b | 235.52 ± 23.28 a | 117.03 ± 4.28 b |
| pectinate lyases | 112.50 ± 4.69 b | 152.71 ± 4.96 a | 85.31 ± 3.57 c |
| Pectinesterase | 43.19 ± 1.55 b | 47.54 ± 2.08 a | 44.65 ± 1.64 ab |
| Cell wall components | |||
| galacturonic acid (μmol g−1 FW) | 5.18 ± 0.16 b | 5.70 ± 0.24 a | 5.10 ± 0.23 b |
| cellulose (μmol g−1 FW) | 4077.76 ± 45.73 a | 2277.95 ± 76.69 c | 2857.38 ± 40.26 b |
| DPM (%) | 1.61 ± 0.06 a | 1.32 ± 0.05 b | 1.68 ± 0.06 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, C.; Zhang, Y.; Liu, X.; Zhang, B.; Si, J.; Xia, H.; Fan, S.; Kong, L. Nitrate Starvation Induces Lateral Root Organogenesis in Triticum aestivum via Auxin Signaling. Int. J. Mol. Sci. 2024, 25, 9566. https://doi.org/10.3390/ijms25179566
Tang C, Zhang Y, Liu X, Zhang B, Si J, Xia H, Fan S, Kong L. Nitrate Starvation Induces Lateral Root Organogenesis in Triticum aestivum via Auxin Signaling. International Journal of Molecular Sciences. 2024; 25(17):9566. https://doi.org/10.3390/ijms25179566
Chicago/Turabian StyleTang, Chengming, Yunxiu Zhang, Xiao Liu, Bin Zhang, Jisheng Si, Haiyong Xia, Shoujin Fan, and Lingan Kong. 2024. "Nitrate Starvation Induces Lateral Root Organogenesis in Triticum aestivum via Auxin Signaling" International Journal of Molecular Sciences 25, no. 17: 9566. https://doi.org/10.3390/ijms25179566






