MicroRNAs Participate in Morphological Acclimation of Sugar Beet Roots to Nitrogen Deficiency
Abstract
1. Introduction
2. Results
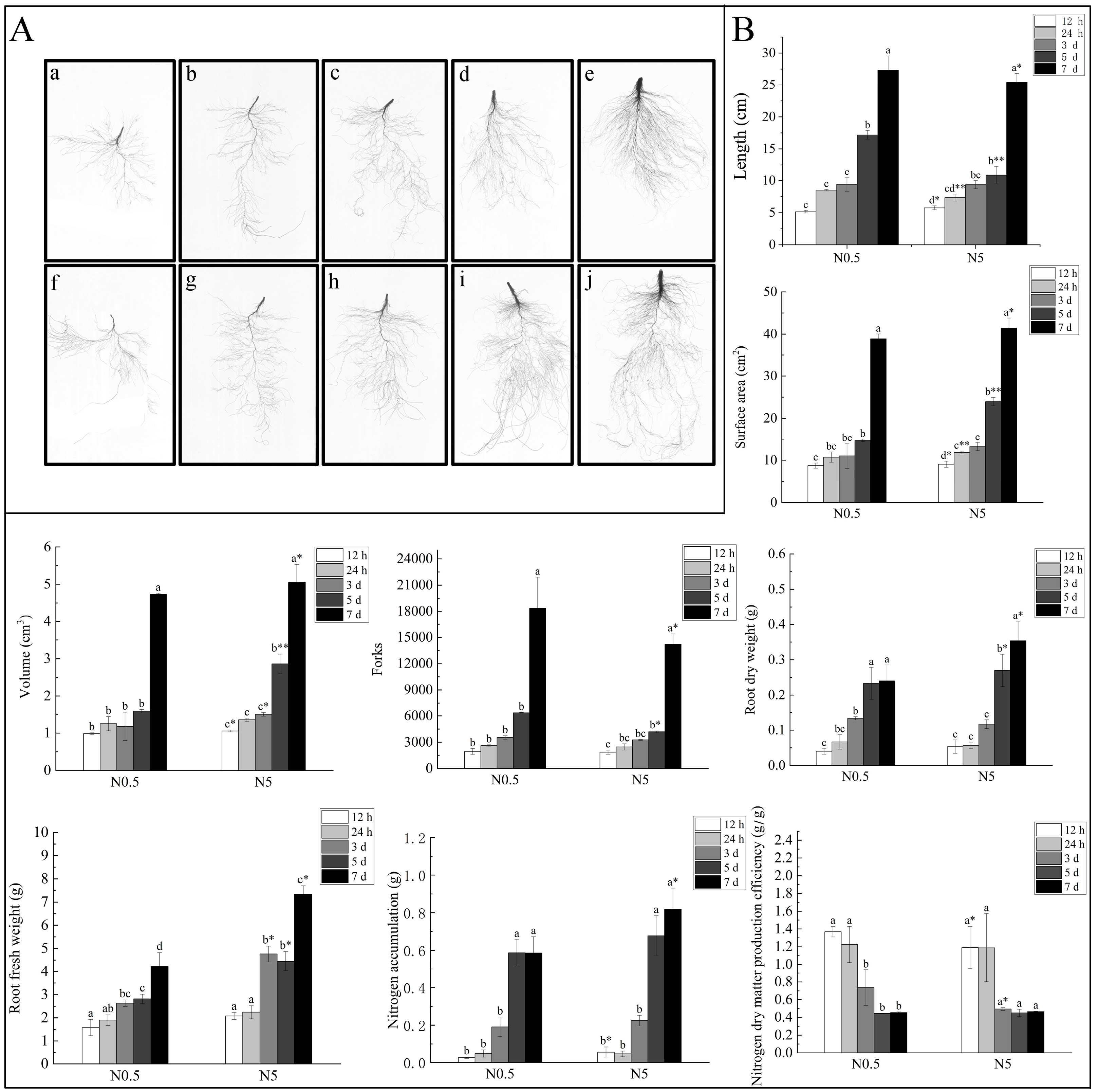
2.1. Effects of Low-Nitrogen Stress on Root Morphology of Sugar Beet Seedlings
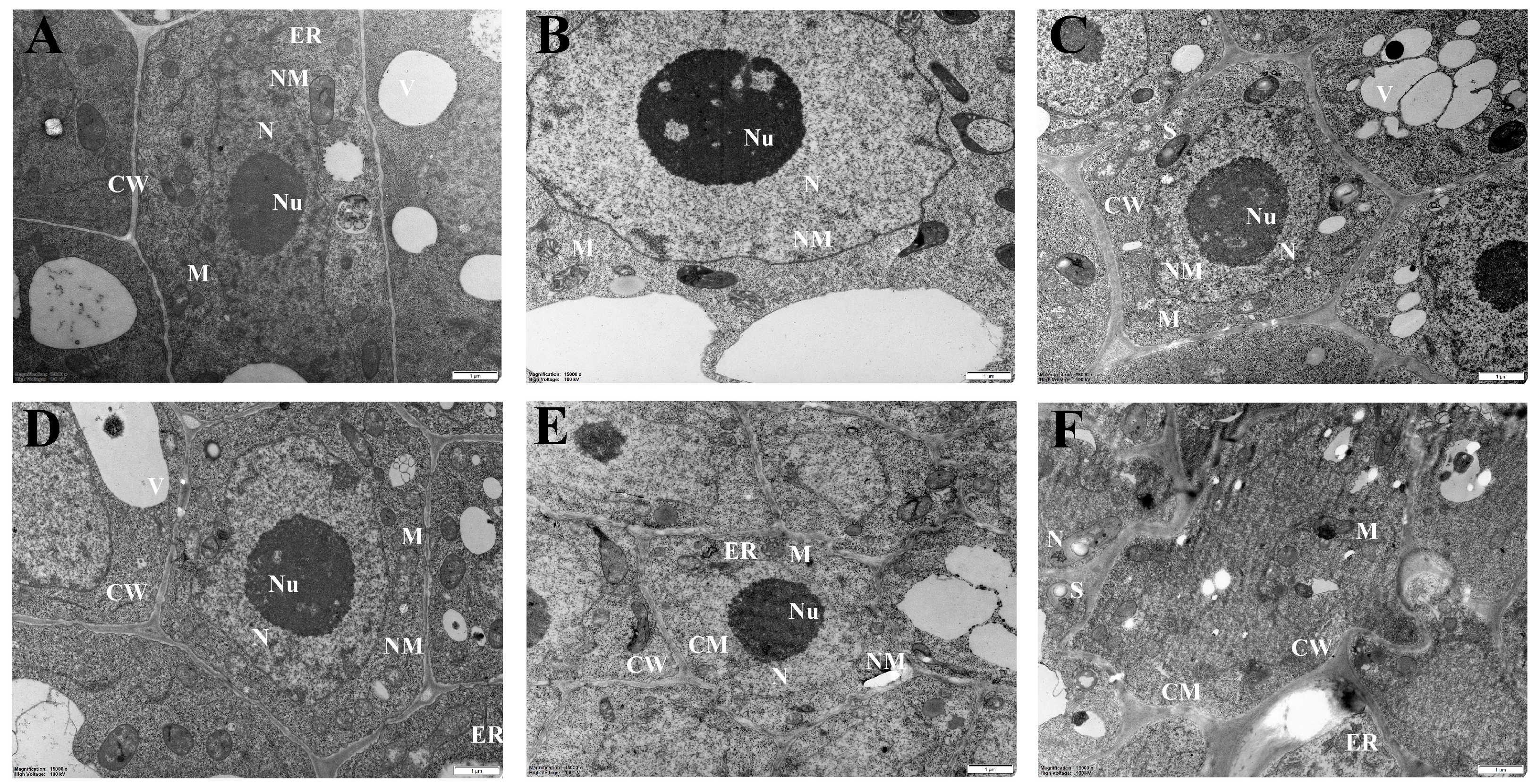
2.2. Ultrastructural Changes in Sugar Beet Roots under Low-Nitrogen Stress
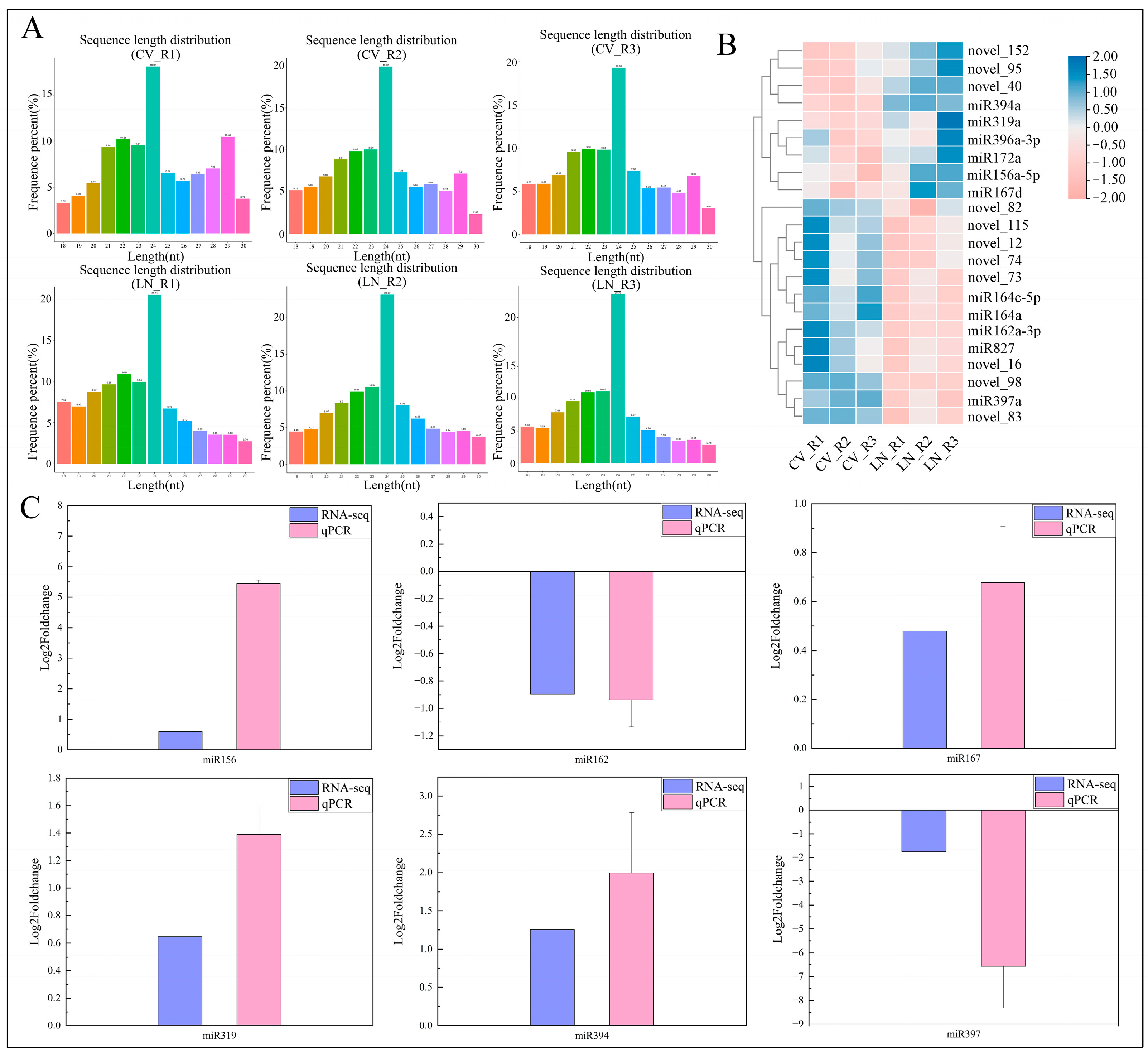
2.3. MicroRNA Identification after RNA-Seq in Sugar Beet Roots under Low-Nitrogen Stress
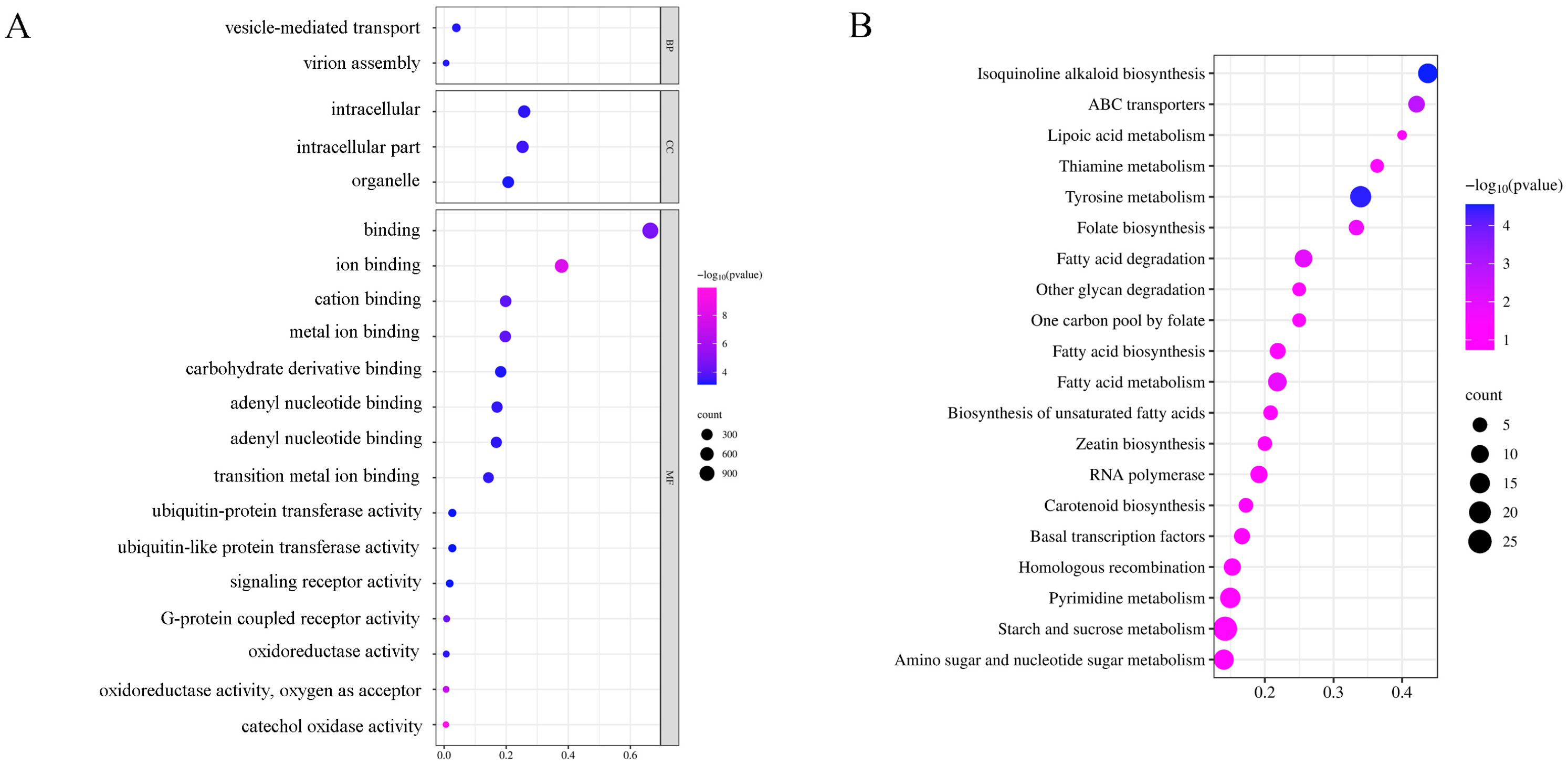
2.4. GO and KEGG Enrichment Analysis of Differentially Expressed microRNAs under Low-Nitrogen Stress
2.5. Regulation of Differentially Expressed microRNAs Correlated with Target Genes
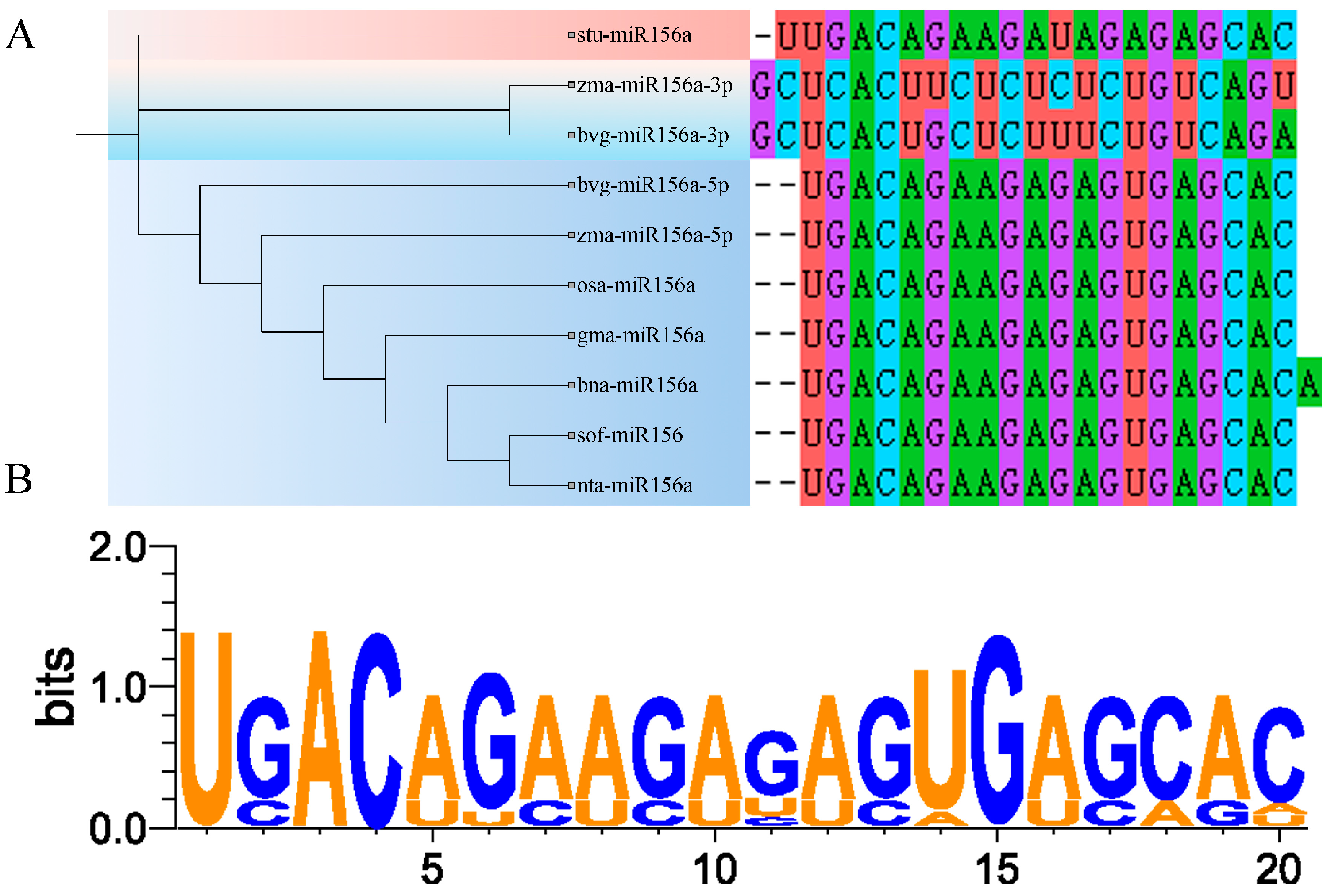
2.6. Sequence Analysis of miR156a Mature Bodies
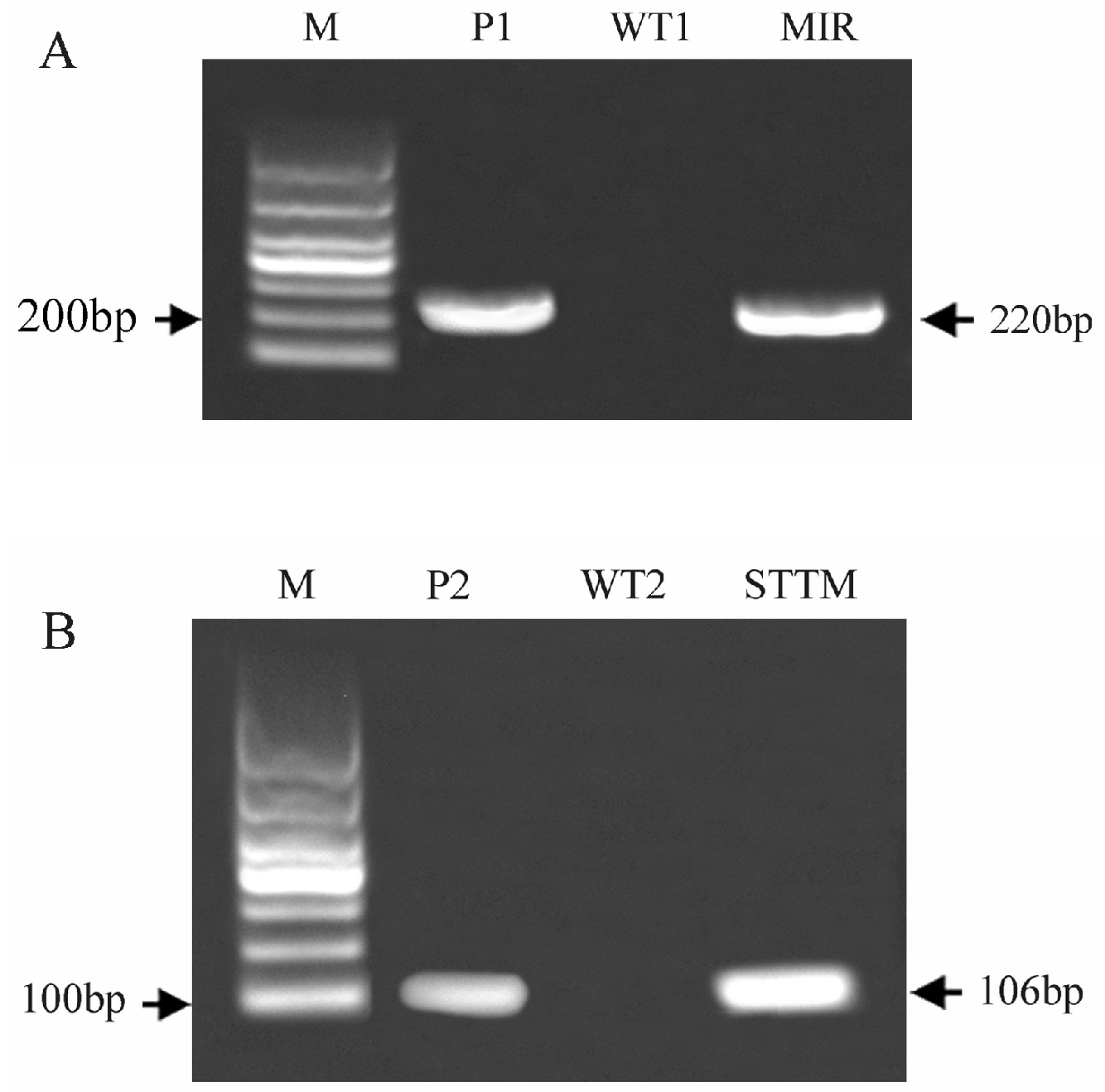
2.7. Generation and Identification of Transgenic Arabidopsis thaliana
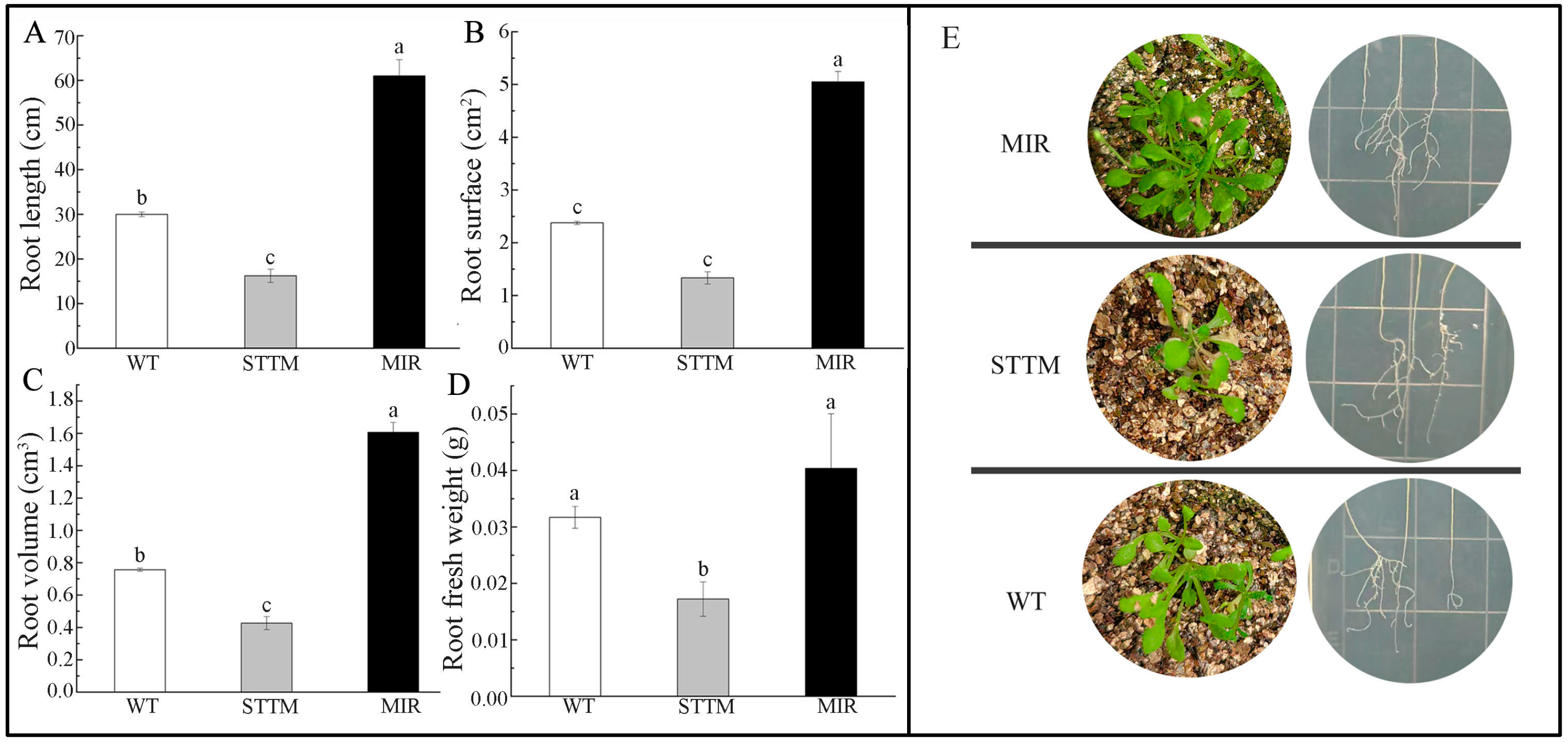
2.8. Characterization of Transgenic Arabidopsis
3. Discussion
3.1. Effect of Nitrogen Deficiency on Growth and Subcellular Structure of Sugar Beet Roots
3.2. microRNA Expression Diversity Involved in Low-Nitrogen Stress of Beet Roots
3.3. miR156 Improved the Potential Adaptability of Beet to Low-Nitrogen Stress
4. Materials and Methods
4.1. Plant Materials and Cultivation
4.2. Determination of Root Morphological Indices and N Content
4.3. Ultrastructural Observation of Sugar Beet Roots
4.4. cDNA Library Construction, RNA Sequencing, and Data Analysis
4.5. Differentially Expressed MicroRNA (DEM) Analysis and Transcriptome Data Validation
4.6. Prediction of DEM Target Genes and Construction of PPI Network
4.7. Bioinformatics Analysis of bvg-miR156a
4.8. bvg-MIR156a and bvg-STTM-miR156a Plant Expression Vector Construction
4.9. Acquisition, Identification, and Phenotypic Trait Analysis of Transgenic Arabidopsis thaliana
4.10. Statistics and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yousuf, P.Y.; Shabir, P.A.; Hakeem, K.R. miRNAomic approach to plant nitrogen starvation. Int. J. Genom. 2021, 2021, 8560323. [Google Scholar] [CrossRef]
- Tilman, D. Global environmental impacts of agricultural expansion: The need for sustainable and efficient practices. Proc. Natl. Acad. Sci. USA 1999, 96, 5995–6000. [Google Scholar] [CrossRef]
- Good, A.G.; Shrawat, A.K.; Muench, D.G. Can less yield more? Is reducing nutrient input into the environment compatible with maintaining crop production. Trends Plant Sci. 2004, 9, 597–605. [Google Scholar] [CrossRef]
- Han, M.; Okamoto, M.; Beatty, P.H.; Rothstein, S.J.; Good, A.G. The genetics of nitrogen use efficiency in crop plants. Annu. Rev. Genet. 2015, 49, 269–289. [Google Scholar] [CrossRef]
- Kiba, T.; Krapp, A. Plant nitrogen acquisition under low availability: Regulation of uptake and root architecture. Plant Cell Physiol. 2016, 57, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y. QTL Mapping and Proteomics Analysis for Seminal Root Length of Wheat Seedling under Control and Low Nitrogen Conditions. Ph.D. Thesis, Hebei Agricultural University, Baoding, China, 2019. [Google Scholar] [CrossRef]
- Niu, X. Effects of Partial Supply of Water and Nitrogen on Root Growth and Absorption Capacity. Ph.D. Thesis, Northwest A & F University, Shanxi, China, 2016. [Google Scholar]
- Li, W.; Jin, C.; Guan, D.; Wang, Q.; Wang, A.; Yuan, F.; Wu, J. The effects of simulated nitrogen deposition on plant root traits: A meta-analysis. Soil. Biol. Biochem. 2015, 82, 112–118. [Google Scholar] [CrossRef]
- Sun, X.; Chen, F.; Yuan, L.; Mi, G. The physiological mechanism underlying root elongation in response to nitrogen deficiency in crop plants. Planta 2020, 251, 84. [Google Scholar] [CrossRef]
- Lv, X.; Zhang, Y.; Hu, L.; Zhang, Y.; Zhang, B.; Xia, H.; Du, W.; Fan, S.; Kong, L. Low-nitrogen stress stimulates lateral root initiation and nitrogen assimilation in wheat: Roles of phytohormone signaling. J. Plant Growth Regul. 2021, 40, 436–450. [Google Scholar] [CrossRef]
- Shah, J.M.; Bukhari, S.A.H.; Zeng, J.; Quan, X.; Ali, E.; Muhammad, N.; Zhang, G. Nitrogen (N) metabolism related enzyme activities, cell ultrastructure and nutrient contents as affected by N level and narley genotype. J. Integr. Agric. 2017, 16, 190–198. [Google Scholar] [CrossRef]
- Sinha, S.K.; Srinivasan, R.; Mandal, P.K. MicroRNA-based approach to improve nitrogen use efficiency in crop plants. In Nutrient Use Efficiency: From Basics to Advances; Rakshit, A., Singh, H.B., Sen, A., Eds.; Springer: New Delhi, India, 2015; pp. 221–235. ISBN 978-81-322-2169-2. [Google Scholar]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Srivastava, S.; Suprasanna, P. MicroRNAs: Tiny, Powerful players of metal stress responses in plants. Plant Physiol Biochem. 2021, 166, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Guo, L.; Lu, W.; Li, X.; Chen, H.; Guo, C.; Xiao, K. Expression pattern analysis of microRNAs in root tissue of wheat (Triticum aestivum L.) under normal nitrogen and low nitrogen conditions. J. Plant Biochem. Biotechnol. 2015, 24, 143–153. [Google Scholar] [CrossRef]
- Gifford, M.L.; Dean, A.; Gutierrez, R.A.; Coruzzi, G.M.; Birnbaum, K.D. Cell-specific nitrogen responses mediate developmental plasticity. Proc. Natl. Acad. Sci. USA 2008, 105, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Roychoudhury, A.; Krishnamoorthi, S. Emerging techniques to decipher microRNAs (MiRNAs) and their regulatory role in conferring abiotic stress tolerance of plants. Plant Biotechnol. Rep. 2016, 10, 185–205. [Google Scholar] [CrossRef]
- Chien, P.; Chiang, C.; Wang, Z.; Chiou, T. MicroRNA-mediated signaling and regulation of nutrient transport and utilization. Curr. Opin. Plant Biol. 2017, 39, 73–79. [Google Scholar] [CrossRef]
- Shin, S.; Jeong, J.; Lim, J.; Kim, T.; Park, J.; Kim, J.; Shin, C. Transcriptomic analyses of rice (Oryza sativa) genes and non-coding RNAs under nitrogen starvation using multiple omics technologies. BMC Genom. 2018, 19, 532. [Google Scholar] [CrossRef]
- Dong, J.; Jiang, W.; Gao, P.; Yang, T.; Zhang, W.; Huangfu, M.; Zhang, J.; Che, D. Comparison of betalain compounds in two Beta Vulgaris Var. Cicla and BvCYP76AD27 function identification in betalain biosynthesis. Plant Physiol. Biochem. 2023, 199, 107711. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, X.; Xu, L.; Li, W.; Yao, Q.; Yin, X.; Wang, Q.; Tan, W.; Xing, W.; Liu, D. Low nitrogen stress-induced transcriptome changes revealed the molecular response and tolerance characteristics in maintaining the C/N balance of sugar beet (Beta vulgaris L.). Front. Plant Sci. 2023, 14, 1164151. [Google Scholar] [CrossRef] [PubMed]
- Trimpler, K.; Stockfisch, N.; Märländer, B. Nitrogen fertilizer application and greenhouse gas emissions in sugar beet cultivation in Germany. Zuckerind. Sugar Ind. 2013, 138, 727–736. [Google Scholar] [CrossRef]
- Grzesiak, M.; Hura, T.; Grzesiak, S.; Kaczanowska, K.; Szczyrek, P. Influence of nitrogen deficiency or excess on a root system structure of maize and triticale seedlings grown under low and high soil density. J. Agron. Crop Sci. 2018, 204, 196–208. [Google Scholar] [CrossRef]
- Hadir, S.; Gaiser, T.; Hüging, H.; Athmann, M.; Pfarr, D.; Kemper, R.; Ewert, F.; Seidel, S. Sugar beet shoot and root phenotypic plasticity to nitrogen, phosphorus, potassium and lime omission. Agriculture 2021, 11, 21. [Google Scholar] [CrossRef]
- Wang, J.; Song, K.; Sun, L.; Qin, Q.; Sun, Y.; Pan, J.; Xue, Y. Morphological and transcriptome analysis of wheat seedlings response to low nitrogen stress. Plants 2019, 8, 98. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.L. Effects of soil nutrients and moisture on root/shoot ratios in Lolium perenne L. and Trifolium repens L. Ann. Bot. 1969, 33, 571–577. [Google Scholar] [CrossRef]
- Sattelmacher, B.; Gerendas, J.; Thoms, K.; Brück, H.; Bagdady, N.H. Interaction between root growth and mineral nutrition. Environ. Exp. Bot. 1993, 33, 63–73. [Google Scholar] [CrossRef]
- Taylor, N.L.; Day, D.A.; Millar, A.H. Targets of stress-induced oxidative damage in plant mitochondria and their impact on cell carbon/nitrogen metabolism. J. Exp. Bot. 2004, 55, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Reape, T.J.; McCabe, P.F. Apoptotic-like regulation of programmed cell death in plants. Apoptosis 2010, 15, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Rekha, T.; Baskar, V.; Maheswaran, E.; Hee, Y.C.; Muthu, T.; Seung-Hyun, K. Dynamic interplay of reactive oxygen and nitrogen species (ROS and RNS) in plant resilience: Unveiling the signaling pathways and metabolic responses to biotic and abiotic stresses. Plant Cell Rep. 2024, 43, 198. [Google Scholar] [CrossRef]
- Zhou, X.; Khare, T.; Kumar, V. Recent trends and advances in identification and functional characterization of plant miRNAs. Acta Physiol. Plant. 2020, 42, 25. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, N.; Bai, J.; Duan, X.; Zhang, L.; Liu, S.; Tang, X.; Jin, X.; Li, S.; Si, H. Stu-MiR827-Targeted StWRKY48 transcription factor negatively regulates drought tolerance of potato by increasing leaf stomatal density. Int. J. Mol. Sci. 2022, 23, 14805. [Google Scholar] [CrossRef]
- Ma, C.; Chen, Q.; Wang, S.; Lers, A. Downregulation of GeBP-like α factor by MiR827 suggests their involvement in senescence and phosphate homeostasis. BMC Biol. 2021, 19, 90. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Cheng, Y.; Du, X.; Teotia, S.; Miao, C.; Sun, H.; Fan, G.; Tang, G.; Xue, H.; et al. The MiR167-OsARF12 module regulates rice grain filling and grain size downstream of MiR159. Plant Commun. 2023, 4, 100604. [Google Scholar] [CrossRef]
- Zhou, M.; Luo, H. Role of microRNA319 in creeping bentgrass salinity and drought stress response. Plant Signal. Behav. 2014, 9, e28700. [Google Scholar] [CrossRef]
- Ma, Z.; Hu, L. MicroRNA: A dynamic player from signaling to abiotic tolerance in plants. Int. J. Mol. Sci. 2023, 24, 11364. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; He, S.; Zhao, N.; Zhai, H.; Liu, Q. A sucrose non-fermenting-1-related protein kinase-1 gene, IbSnRK1, improves starch content, composition, granule size, degree of crystallinity and gelatinization in transgenic sweet potato. Plant Biotechnol. J. 2019, 17, 21–32. [Google Scholar] [CrossRef]
- Huang, H.; Long, J.; Zheng, L.; Li, Y.; Hu, Y.; Yu, G.; Liu, H.; Liu, Y.; Huang, Z.; Zhang, J.; et al. Identification and characterization of microRNAs in maize endosperm response to exogenous sucrose using small RNA sequencing. Genomics 2016, 108, 216–223. [Google Scholar] [CrossRef]
- Xu, J.-J.; Fang, X.; Li, C.-Y.; Yang, L.; Chen, X.-Y. General and specialized tyrosine metabolism pathways in plants. aBIOTECH 2020, 1, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Alseekh, S.; Fernie, A.R.; Luo, J. The structure and function of major plant metabolite modifications. Mol. Plant 2019, 12, 899–919. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.; Niu, Q.-W.; Ng, K.-H.; Chua, N.-H. The role of miR156/SPLs modules in Arabidopsis lateral root development. Plant J. 2015, 83, 673–685. [Google Scholar] [CrossRef]
- Levy, A.; Szwerdszarf, D.; Abu-Abied, M.; Mordehaev, I.; Yaniv, Y.; Riov, J.; Arazi, T.; Sadot, E. Profiling microRNAs in eucalyptus grandis reveals no mutual relationship between alterations in MiR156 and MiR172 expression and adventitious root induction during development. BMC Genom. 2014, 15, 524. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Yang, J.; Liu, S.; Li, S.; Si, H.; Zhang, N. Control of plant height and lateral root development via stu-miR156 regulation of SPL9 transcription factor in potato. Plants 2024, 13, 723. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Ye, M.; Sang, M.; Wu, R. A regulatory network for miR156-SPL module in Arabidopsis thaliana. Int. J. Mol. Sci. 2019, 20, 6166. [Google Scholar] [CrossRef]
- Cock, P.J.A.; Fields, C.J.; Goto, N.; Heuer, M.L.; Rice, P.M. The Sanger FASTQ file format for sequences with quality scores, and the Solexa/Illumina FASTQ variants. Nucleic Acids Res. 2010, 38, 1767–1771. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Schlesinger, F.; Davis, C.A.; Zhang, Y.; Li, R.; Salit, M.; Gingeras, T.R.; Oliver, B. Synthetic spike-in standards for RNA-Seq experiments. Genome Res. 2011, 21, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Moxon, S.; Schwach, F.; MacLean, D.; Dalmay, T.; Studholme, D.; Moulton, V. A toolkit for analysing large-scale plant small RNA datasets. Bioinformatics 2008, 24, 2252–2253. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, J.; Li, Z.; Li, X.; Hu, X.; Huang, Y.; Zhao, X.; Liang, C.; Wang, Y.; Sun, L.; et al. Integrated profiling of microRNAs and mRNAs: microRNAs located on Xq27.3 associate with clear cell renal cell carcinoma. PLoS ONE 2010, 5, e15224. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-Seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Goseq: Gene Ontology testing for RNA-seq datasets. R. Bioconductor 2012, 8, 1–25. [Google Scholar] [CrossRef]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2007, 36, 480–484. [Google Scholar] [CrossRef]
- Abel, Y.; Rederstorff, M. Stem-Loop qRT-PCR–Based Quantification of miRNAs; Humana: New York, NY, USA, 2021; p. 2300. [Google Scholar] [CrossRef]
- Fahlgren, N.; Howell, M.D.; Kasschau, K.D.; Chapman, E.J.; Sullivan, C.M.; Cumbie, J.S.; Carrington, J.C. High-Throughput sequencing of Arabidopsis microRNAs: Evidence for frequent birth and death of miRNA genes. PLoS ONE 2007, 2, e219. [Google Scholar] [CrossRef]
- Qi, Y.-L.; Xue, L.-J.; El-Kassaby, Y.A.; Guo, Y. Identification and comparative analysis of conserved and species-specific microRNAs in four Populus sections. Forests 2022, 13, 873. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of life (ITOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Crooks, G.E.; Hon, G.; Chandonia, J.-M.; Brenner, S.E. WebLogo: A sequence logo generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Lu, Z.; Yao, Q.; Xu, L.; Fu, J.; Yin, X.; Bai, Q.; Liu, D.; Xing, W. MicroRNAs Participate in Morphological Acclimation of Sugar Beet Roots to Nitrogen Deficiency. Int. J. Mol. Sci. 2024, 25, 9027. https://doi.org/10.3390/ijms25169027
Liu X, Lu Z, Yao Q, Xu L, Fu J, Yin X, Bai Q, Liu D, Xing W. MicroRNAs Participate in Morphological Acclimation of Sugar Beet Roots to Nitrogen Deficiency. International Journal of Molecular Sciences. 2024; 25(16):9027. https://doi.org/10.3390/ijms25169027
Chicago/Turabian StyleLiu, Xinyu, Zhenqiang Lu, Qi Yao, Lingqing Xu, Jingjing Fu, Xilong Yin, Qing Bai, Dali Liu, and Wang Xing. 2024. "MicroRNAs Participate in Morphological Acclimation of Sugar Beet Roots to Nitrogen Deficiency" International Journal of Molecular Sciences 25, no. 16: 9027. https://doi.org/10.3390/ijms25169027
APA StyleLiu, X., Lu, Z., Yao, Q., Xu, L., Fu, J., Yin, X., Bai, Q., Liu, D., & Xing, W. (2024). MicroRNAs Participate in Morphological Acclimation of Sugar Beet Roots to Nitrogen Deficiency. International Journal of Molecular Sciences, 25(16), 9027. https://doi.org/10.3390/ijms25169027





