Revolutionizing Bone Regeneration with Grinder-Based Dentin Biomaterial: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Focus Question
2.2. Protocol
2.3. Eligibility Criteria
2.4. Information Sources, Search Strategy, and Study Selection
2.5. Data Collection Process and Data Items
2.6. Quality Assessment
3. Results
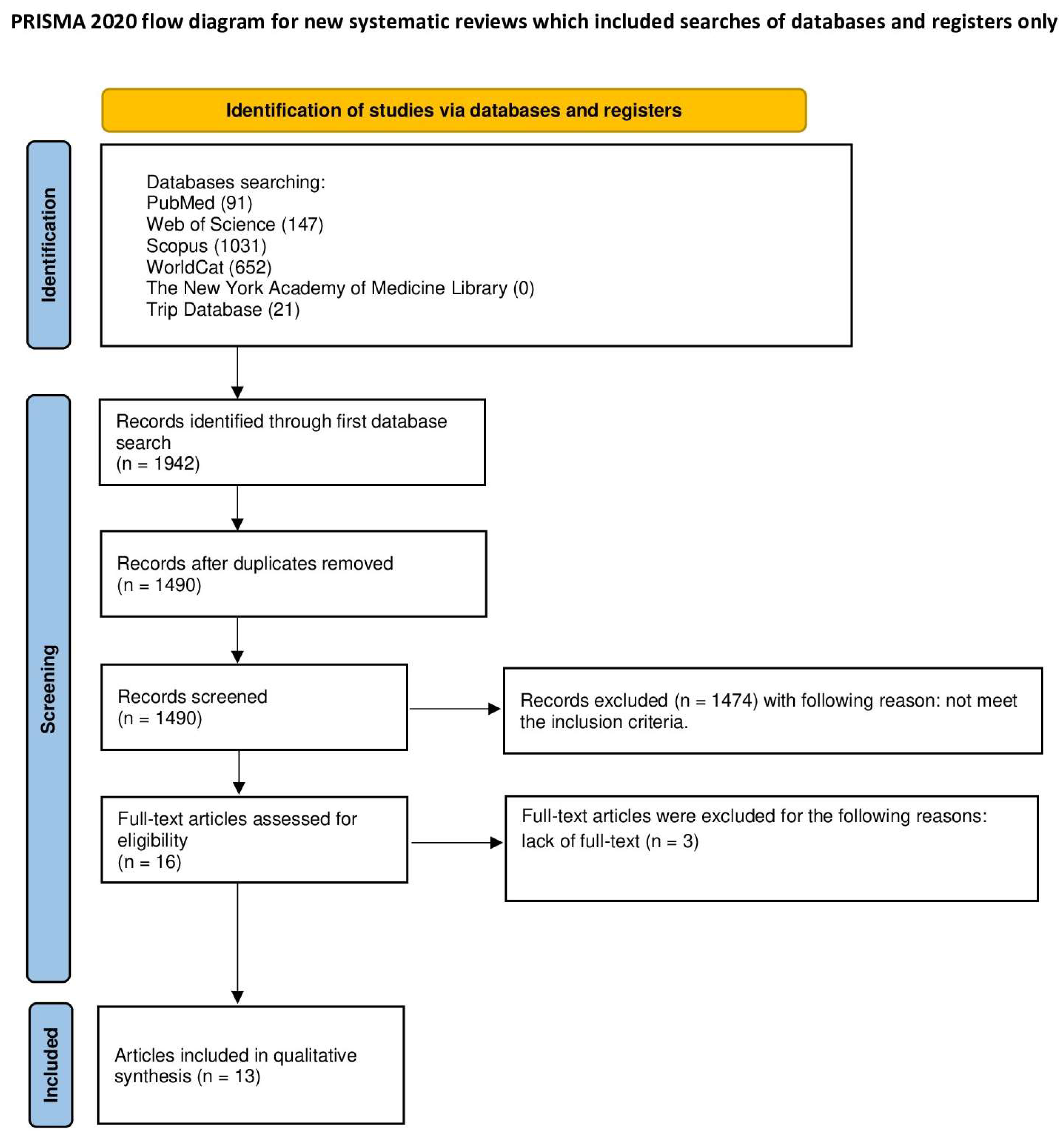
3.1. Study Selection
3.2. Molecular Aspects of Ground Dentin Grafts
3.2.1. Chemical Composition
3.2.2. Evaluation of Cellular Responses
3.3. Morphology
3.4. Histomorphometric Outcomes
3.5. Bacteriological Purity
3.6. Clinical Outcomes
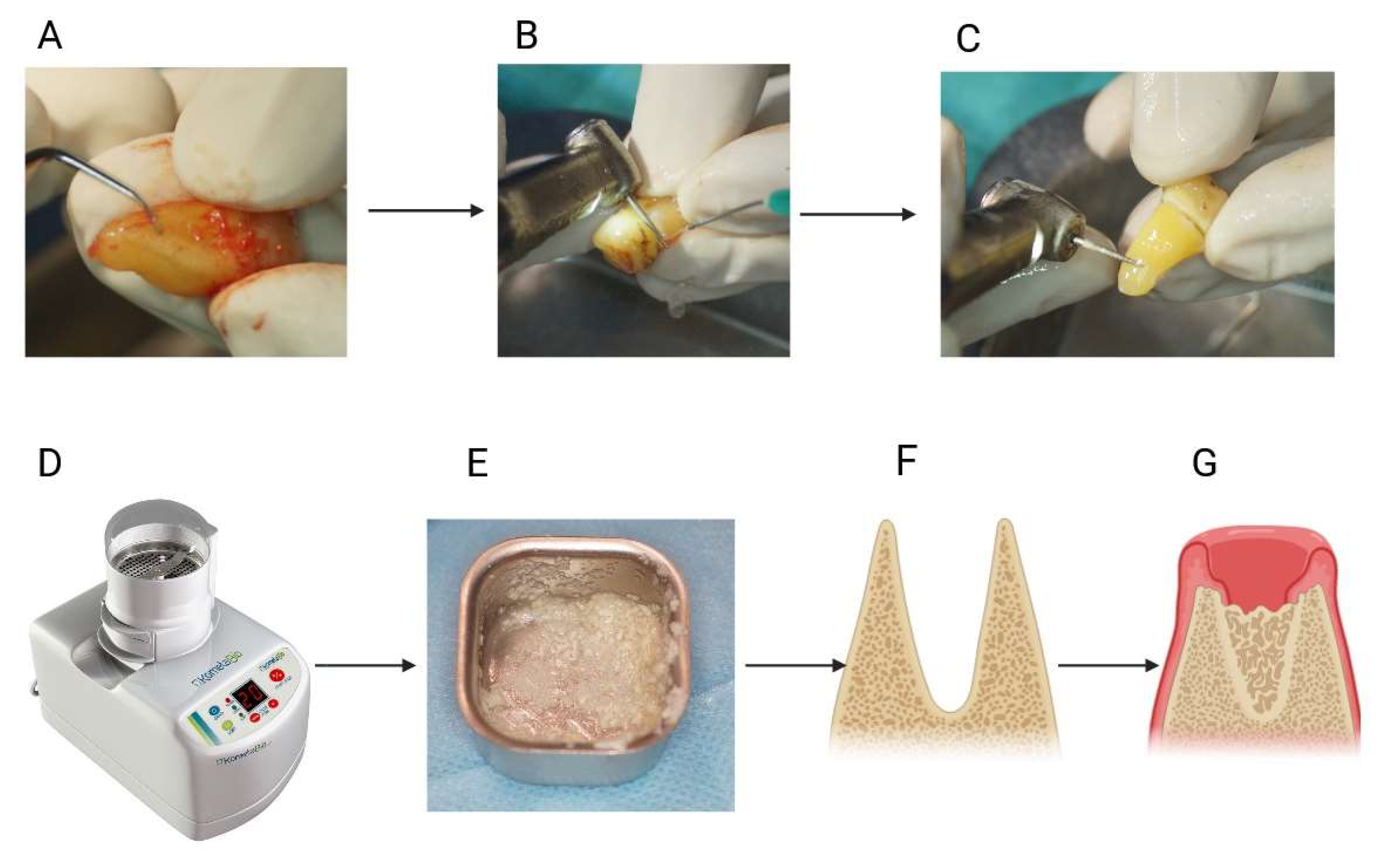
3.6.1. Bone Tissue Regeneration
3.6.2. Periprocedural and Long-Term Complications
3.6.3. Patients’ Reported Outcomes
3.7. Quality Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Santis, R.; Guarino, V.; Ambrosio, L. Composite biomaterials for bone repair. In Bone Repair Biomaterials; Woodhead Publishing: Cambridge, UK, 2019; pp. 273–299. [Google Scholar] [CrossRef]
- Sheikh, Z.; Hamdan, N.; Abdallah, M.-N.; Glogauer, M.; Grynpas, M. Natural and synthetic bone replacement graft materials for dental and maxillofacial applications. In Advanced Dental Biomaterials; Woodhead Publishing: Cambridge, UK, 2019; pp. 347–376. [Google Scholar] [CrossRef]
- Dominiak, M.; Hnitecka, S.; Olchowy, C.; Olchowy, A.; Gedrange, T. Analysis of alveolar ridge width in an area of central lower incisor using cone-beam computed tomography in vivo. Ann. Anat. 2021, 236, 151699. [Google Scholar] [CrossRef]
- Kinaci, A.; Neuhaus, V.; Ring, D.C. Trends in bone graft use in the United States. Orthopedics 2014, 37, e783–e788. [Google Scholar] [CrossRef]
- Bernardi, S.; Macchiarelli, G.; Bianchi, S. Autologous Materials in Regenerative Dentistry: Harvested Bone, Platelet Concentrates and Dentin Derivates. Molecules 2020, 25, 5330. [Google Scholar] [CrossRef]
- Barone, A.; Toti, P.; Menchini-Fabris, G.B.; Felice, P.; Marchionni, S.; Covani, U. Early volumetric changes after vertical augmentation of the atrophic posterior mandible with interpositional block graft versus onlay bone graft: A retrospective radiological study. J. Cranio-Maxillofac. Surg. 2017, 45, 1438–1447. [Google Scholar] [CrossRef]
- Schmidt, A.H. Autologous bone graft: Is it still the gold standard? Injury 2021, 52, S18–S22. [Google Scholar] [CrossRef]
- Yaşar Mahsut, D. Bone Graft Types. In Bone Grafting; Raja, K., Ed.; IntechOpen: Rijeka, Croatia, 2018; Chapter 3. [Google Scholar] [CrossRef]
- Archunan, M.W.; Petronis, S. Bone Grafts in Trauma and Orthopaedics. Cureus 2021, 13, e17705. [Google Scholar] [CrossRef]
- Sohn, H.S.; Oh, J.K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater. Res. 2019, 23, 9. [Google Scholar] [CrossRef]
- Hinsenkamp, M.; Collard, J.F. Growth factors in orthopaedic surgery: Demineralized bone matrix versus recombinant bone morphogenetic proteins. Int. Orthop. 2015, 39, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Nezu, T.; Takebe, H.; Hirose, Y.; Okubo, N.; Saito, T.; Akazawa, T. Human dentin materials for minimally invasive bone regeneration: Animal studies and clinical cases. J. Oral Biosci. 2023, 65, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.M.; Um, I.W.; Kim, Y.K.; Woo, J.M.; Kim, S.M.; Lee, J.H. Autogenous demineralized dentin matrix from extracted tooth for the augmentation of alveolar bone defect: A prospective randomized clinical trial in comparison with anorganic bovine bone. Clin. Oral Implant. Res. 2017, 28, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Botelho, J.; Machado, V.; Borrecho, G.; Proença, L.; Mendes, J.J.; Mascarenhas, P.; Alcoforado, G. Autogenous Mineralized Dentin versus Xenograft granules in Ridge Preservation for Delayed Implantation in Post-extraction Sites: A Randomized controlled clinical trial with an 18 months follow-up. Clin. Oral Implant. Res. 2021, 32, 905–915. [Google Scholar] [CrossRef]
- Binderman, I.; Hallel, G.; Nardy, C.; Yaffe, A.; Sapoznikov, L. A Novel Procedure to Process Extracted Teeth for Immediate Grafting of Autogenous Dentin. J. Interdiscipl Med. Dent. Sci. 2014, 7, 1000154. [Google Scholar] [CrossRef]
- Amir-Behghadami, M.; Janati, A. Population, Intervention, Comparison, Outcomes and Study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg. Med. J. 2020, 37, 387. [Google Scholar] [CrossRef] [PubMed]
- De Biase, A.; Mazzucchi, G.; Di Nardo, D.; Lollobrigida, M.; Serafini, G.; Testarelli, L. Prevention of Periodontal Pocket Formation after Mandibular Third Molar Extraction Using Dentin Autologous Graft: A Split Mouth Case Report. Case Rep. Dent. 2020, 2020, 1762862. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Critical Appraisal Tools for Use in JBI Systematic Reviews. Joanna Briggs Institute (JBI). Available online: https://jbi.global/critical-appraisal-tools (accessed on 28 February 2024).
- Bianchi, S.; Mancini, L.; Torge, D.; Cristiano, L.; Mattei, A.; Varvara, G.; Macchiarelli, G.; Marchetti, E.; Bernardi, S. Bio-Morphological Reaction of Human Periodontal Ligament Fibroblasts to Different Types of Dentinal Derivates: In Vitro Study. Int. J. Mol. Sci. 2021, 22, 8681. [Google Scholar] [CrossRef] [PubMed]
- Khanijou, M.; Zhang, R.; Boonsiriseth, K.; Srisatjaluk, R.L.; Suphangul, S.; Pairuchvej, V.; Wongsirichat, N.; Seriwatanachai, D. Physicochemical and osteogenic properties of chairside processed tooth derived bone substitute and bone graft materials. Dent. Mater. J. 2021, 40, 173–183. [Google Scholar] [CrossRef]
- Sarna-Boś, K.; Boguta, P.; Skic, K.; Wiącek, D.; Maksymiuk, P.; Sobieszczański, J.; Chałas, R. Physicochemical Properties and Surface Characteristics of Ground Human Teeth. Molecules 2022, 27, 5852. [Google Scholar] [CrossRef]
- Cervera-Maillo, J.M.; Morales-Schwarz, D.; Morales-Melendez, H.; Mahesh, L.; Calvo-Guirado, J.L. Autologous Tooth Dentin Graft: A Retrospective Study in Humans. Medicina 2021, 58, 56. [Google Scholar] [CrossRef] [PubMed]
- Dłucik, R.; Orzechowska-Wylęgała, B.; Dłucik, D.; Puzzolo, D.; Santoro, G.; Micali, A.; Testagrossa, B.; Acri, G. Comparison of clinical efficacy of three different dentin matrix biomaterials obtained from different devices. Expert Rev. Med. Devices 2023, 20, 313–327. [Google Scholar] [CrossRef]
- Matsuzawa, Y.; Okubo, N.; Tanaka, S.; Kashiwazaki, H.; Kitagawa, Y.; Ohiro, Y.; Mikoya, T.; Akazawa, T.; Murata, M. Primary Teeth-Derived Demineralized Dentin Matrix Autograft for Unilateral Maxillary Alveolar Cleft during Mixed Dentition. J. Funct. Biomater. 2022, 13, 153. [Google Scholar] [CrossRef]
- Pohl, S.; Binderman, I.; Tomac, J. Maintenance of Alveolar Ridge Dimensions Utilizing an Extracted Tooth Dentin Particulate Autograft and PlateletRich Fibrin: A Retrospective Radiographic ConeBeam Computed Tomography Study. Materials 2020, 13, 1083. [Google Scholar] [CrossRef]
- Del Canto-Díaz, A.; de Elío-Oliveros, J.; Del Canto-Díaz, M.; Alobera-Gracia, M.A.; Del Canto-Pingarrón, M.; Martínez-González, J.M. Use of autologous tooth-derived graft material in the post-extraction dental socket. Pilot study. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e53–e60. [Google Scholar] [CrossRef]
- Dłucik, R.; Orzechowska-Wylęgała, B.; Dłucik, D.; Bogus, K. Histological examination of tooth-derived biomaterials obtained from different devices. Expert. Rev. Med. Devices 2023, 20, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.H.; Ahn, J.S.; Lee, J.I.; Ahn, K.J.; Yun, P.Y.; Kim, Y.K. A prospective study on the effectiveness of newly developed autogenous tooth bone graft material for sinus bone graft procedure. J. Adv. Prosthodont. 2014, 6, 528–538. [Google Scholar] [CrossRef]
- Zwittnig, K.; Mukaddam, K.; Vegh, D.; Herber, V.; Jakse, N.; Schlenke, P.; Zrnc, T.A.; Payer, M. Platelet-rich fibrin in oral surgery and implantology: A narrative review. Transfus. Med. Hemotherapy 2023, 50, 348–359. [Google Scholar] [CrossRef]
- Funato, A.; Moroi, H.; Ogawa, T. Guided bone regeneration assisted by tooth roots with periodontal ligament: Case reports of immediate and staged approaches to implant therapy. Int. J. Esthet. Dent. 2022, 17, 276–291. [Google Scholar]
- Sun, J.; Jiang, X.; Luo, J.; Zhao, L.; Xu, Z.; Xiao, W. Effect of platelet-derived growth factor (PDGF-BB) and bone morphogenic protein 2 (BMP-2) transfection of rBMSCs compounded with platelet-rich plasma on adipogenic differentiation. Braz. J. Med. Biol. Res. 2020, 54, e9944. [Google Scholar] [CrossRef]
- Kim, B.J.; Kim, S.K.; Lee, J.H. Bone regeneration of demineralized dentin matrix with platelet-rich fibrin and recombinant human bone morphogenetic protein-2 on the bone defects in rabbit calvaria. Maxillofac. Plast. Reconstr. Surg. 2021, 43, 34. [Google Scholar] [CrossRef] [PubMed]
- Baseri, N.; Meysamie, A.; Campanile, F.; Hamidieh, A.A.; Jafarian, A. Bacterial Contamination of Bone Allografts in the Tissue Banks: A Systematic Review and Meta-Analysis. J. Hosp. Infect. 2022, 123, 156–173. [Google Scholar] [CrossRef] [PubMed]
- Ghanaati, S.; Barbeck, M.; Booms, P.; Lorenz, J.; Kirkpatrick, C.J.; Sader, R.A. Potential Lack of “Standardized” Processing Techniques for Production of Allogeneic and Xenogeneic Bone Blocks for Application in Humans. Acta Biomater. 2014, 10, 3557–3562. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, J.; Schlee, M.; Al-Maawi, S.; Chia, P.; Sader, R.A.; Ghanaati, S. Variant Purification of an Allogeneic Bone Block. Acta Stomatol. Croat. 2017, 51, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Fretwurst, T.; Spanou, A.; Nelson, K.; Wein, M.; Steinberg, T.; Stricker, A. Comparison of Four Different Allogeneic Bone Grafts for Alveolar Ridge Reconstruction: A Preliminary Histologic and Biochemical Analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 118, 424–431. [Google Scholar] [CrossRef]
- Lorenz, J.; Kubesch, A.; Al-Maawi, S.; Schwarz, F.; Sader, R.A.; Schlee, M.; Ghanaati, S. Allogeneic Bone Block for Challenging Augmentation—A Clinical, Histological, and Histomorphometrical Investigation of Tissue Reaction and New Bone Formation. Clin. Oral Investig. 2018, 22, 3159–3169. [Google Scholar] [CrossRef] [PubMed]
- Minetti, E.; Palermo, A.; Malcangi, G.; Inchingolo, A.D.; Mancini, A.; Dipalma, G.; Inchingolo, F.; Patano, A.; Inchingolo, A.M. Dentin, Dentin Graft, and Bone Graft: Microscopic and Spectroscopic Analysis. J. Funct. Biomater. 2023, 14, 272. [Google Scholar] [CrossRef]
- Tabatabaei, F.S.; Tatari, S.; Samadi, R.; Moharamzadeh, K. Different methods of dentin processing for application in bone tissue engineering: A systematic review. J. Biomed. Mater. Res. A 2016, 104, 2616–2627. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Population | Human studies, regardless of the age of the participants, experimental models, and in vitro studies | |
| Intervention | Dentin biomaterial used for bone regeneration produced with the dentin grinder | Dentin crushed or trimmed by other methods |
| Comparators | Any procedure of bone regeneration or none | |
| Outcomes of interest | Any | The technical aspect of material harvesting without investigating its biological activity and molecular aspects |
| Study designs | Clinical trials involving humans Case reports | Reviews Letters Technical report Proof-of-concept studies |
| Author | Study Design | Study Material */Tissue | Outcomes | |
|---|---|---|---|---|
| Physicochemical/Molecular | Clinical | |||
| Bianchi, 2021 [20] | In vitro; 4 teeth from 1 donor | Mineralized dentin (human) Deproteinized and demineralized dentin Demineralized dentin Deproteinized bovine bone Tissue: cell lines: human periodontal ligament fibroblasts |
|
|
| Khanijou et al., 2021 [21] | In vitro; 12 teeth | Tooth-derived bone substitute (human) Allografts (OraGRAFT, DO BONE) Xenograft (BioOss) Alloplast (BoneCeramic) Human mandibular ramus bone Tissue: cell lines: human fetal osteoblastic cells |
|
|
| Sarna-Boś et al., 2022 [22] | In vitro; 50 teeth 50 donors | Four groups (incisors, canines, premolars, molars) crushed without chemical processing |
|
|
| Binderman et al., 2014 [15] | 2 cases | Autogenous mineralized dentin matrix Tissue: alveolar bone regeneration after extraction |
|
|
| Cervera-Maillo et al., 2021 [23] | 10 patients | Autologous dentin with platelet-rich plasma Tissue: alveolar bone after tooth extraction |
|
|
| De Biase et al., 2020 [17] | Split-month case report | Autologous demineralized dentin matrix Tissue: alveolar bone regeneration after extraction |
|
|
| Del Canto-Díaz et al., 2019 [27] | 9 patients; split-mouth study | Autologous dental material Unfilled extraction sockets Tissue: alveolar bone regeneration after extraction |
|
|
| Dłucik et al., 2023 [24] | 21 patients in the SDG group | Autologous demineralized dentin matrix Tissue: alveolar bone regeneration after extraction, Sinus lift procedure |
|
|
| Dłucik et al., 2023 [28] | 13 patients in the SDG group | Autologous demineralized dentin matrix Tissue: alveolar bone regeneration after extraction |
|
|
| Matsuzawa et al., 2022 [25] | Case report | Autologous demineralized dentin matrix (primary teeth) Tissue: unilateral alveolar cleft |
|
|
| Pohl et al., 2020 [26] | 12 patients; 58 sockets | Autologous mineralized dentin with platelet-rich plasma Tissue: alveolar bone after tooth extraction |
|
|
| Santos et al., 2021 [14] | 52 patients; 66 implants | Autogenous mineralized dentin matrix Xenograft granules |
|
|
| Jun et al., 2014 [29] | 38 patients; 19 Bio-Oss; 19 AutoBT | Autogenous tooth bone graft (AutoBT) Xenograft (BioOss) |
|
|
| Case Reports | Binderman [15] | De Biase [17] | Matsuzawa [25] |
| Were the patient’s demographic characteristics clearly described? | No | Yes | Yes |
| Was the patient’s history clearly described and presented as a timeline? | No | Yes | Yes |
| Was the current clinical condition of the patient on presentation clearly described? | Yes | Yes | Yes |
| Were diagnostic tests or assessment methods and the results clearly described? | Yes | Yes | Yes |
| Was the intervention(s) or treatment procedure(s) clearly described? | Yes | Yes | Yes |
| Was the post-intervention clinical condition clearly described? | Yes | Yes | Yes |
| Were adverse events (harms) or unanticipated events identified and described? | Yes | No | Yes |
| Does the case report provide takeaway lessons? | Yes | No | Yes |
| Case Series | Pohl [26] | ||
| Were there clear criteria for inclusion in the case series? | Yes | ||
| Was the condition measured in a standard, reliable way for all participants included in the case series? | Yes | ||
| Were valid methods used for the identification of the condition for all participants included in the case series? | Yes | ||
| Did the case series have consecutive inclusion of participants? | Yes | ||
| Did the case series have a complete inclusion of participants? | Yes | ||
| Was there clear reporting of the demographics of the participants in the study? | No | ||
| Was there clear reporting of the clinical information of the participants? | Yes | ||
| Were the outcomes or follow-up results of cases clearly reported? | Yes | ||
| Was there clear reporting of the presenting site(s)/clinic(s) demographic information? | Yes | ||
| Was statistical analysis appropriate? | Yes | ||
| Cohort Studies | Cervera-Maillo [23] | ||
| Were the two groups similar and recruited from the same population? | Yes | ||
| Were the exposures measured similarly to assign people to both exposed and unexposed groups? | Yes | ||
| Was the exposure measured in a valid and reliable way? | Yes | ||
| Were confounding factors identified? | No | ||
| Were strategies to deal with confounding factors stated? | No | ||
| Were the groups/participants free of the outcome at the start of the study (or at the moment of exposure)? | Yes | ||
| Were the outcomes measured in a valid and reliable way? | Yes | ||
| Was the follow-up time reported and sufficient to be long enough for outcomes to occur? | Yes | ||
| Was the follow up complete, and if not, were the reasons for the loss of follow-up described and explored? | Yes | ||
| Were strategies to address incomplete follow-up utilized? | NA | ||
| Was appropriate statistical analysis used? | Yes | ||
| Randomized Controlled Trials | Santos [14] | Jun [29] | |
| Was true randomization used for the assignment of participants to treatment groups? | Yes | Yes | |
| Was allocation to treatment groups concealed? | Yes | Unclear | |
| Were treatment groups similar at the baseline? | Yes | Yes | |
| Were participants blind to treatment assignment? | Yes | Unclear | |
| Were those delivering the treatment blind to treatment assignment? | No | Unclear | |
| Were treatment groups treated identically other than the intervention of interest? | Yes | Yes | |
| Were outcome assessors blind to treatment assignment? | Yes | Unclear | |
| Were outcomes measured in the same way for treatment groups? | Yes | Yes | |
| Were outcomes measured in a reliable way? | Yes | Yes | |
| Was follow-up complete, and if not, were differences between groups in terms of their follow-up adequately described and analyzed? | Yes | Unclear | |
| Were participants analyzed in the groups to which they were randomized? | Yes | Yes | |
| Was appropriate statistical analysis used? | Yes | Yes | |
| Was the trial design appropriate and were any deviations from the standard RCT design accounted for in the conduct and analysis of the trial? | Unclear | Unclear | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olchowy, A.; Olchowy, C.; Zawiślak, I.; Matys, J.; Dobrzyński, M. Revolutionizing Bone Regeneration with Grinder-Based Dentin Biomaterial: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 9583. https://doi.org/10.3390/ijms25179583
Olchowy A, Olchowy C, Zawiślak I, Matys J, Dobrzyński M. Revolutionizing Bone Regeneration with Grinder-Based Dentin Biomaterial: A Systematic Review. International Journal of Molecular Sciences. 2024; 25(17):9583. https://doi.org/10.3390/ijms25179583
Chicago/Turabian StyleOlchowy, Anna, Cyprian Olchowy, Ireneusz Zawiślak, Jacek Matys, and Maciej Dobrzyński. 2024. "Revolutionizing Bone Regeneration with Grinder-Based Dentin Biomaterial: A Systematic Review" International Journal of Molecular Sciences 25, no. 17: 9583. https://doi.org/10.3390/ijms25179583









