The Disease-Modifying Effects of a Single Intra-Articular Corticosteroid Injection during the Freezing Phase of Frozen Shoulder in an Animal Model
Abstract
:1. Introduction
2. Results
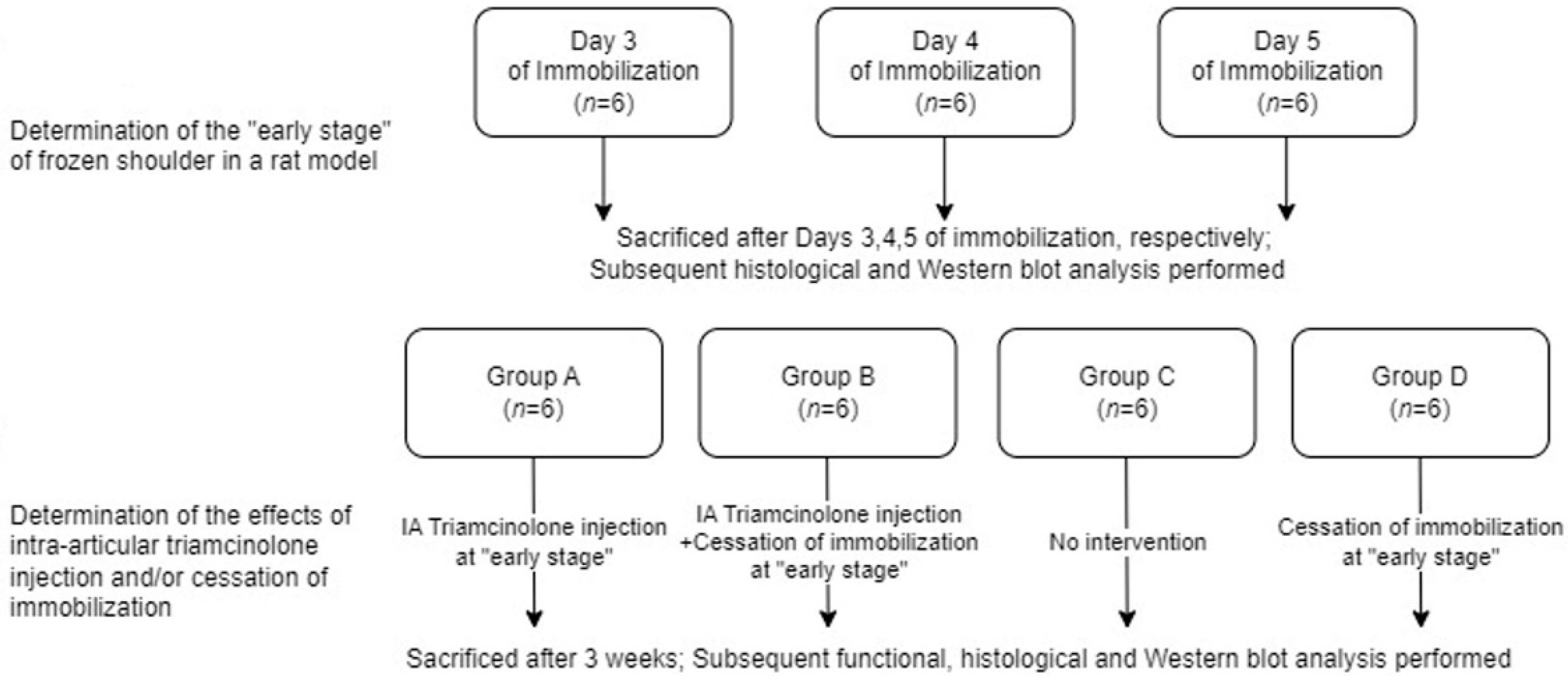
2.1. Study Design
2.2. Optimal Duration of Immobilization for Simulation of Freezing Phase
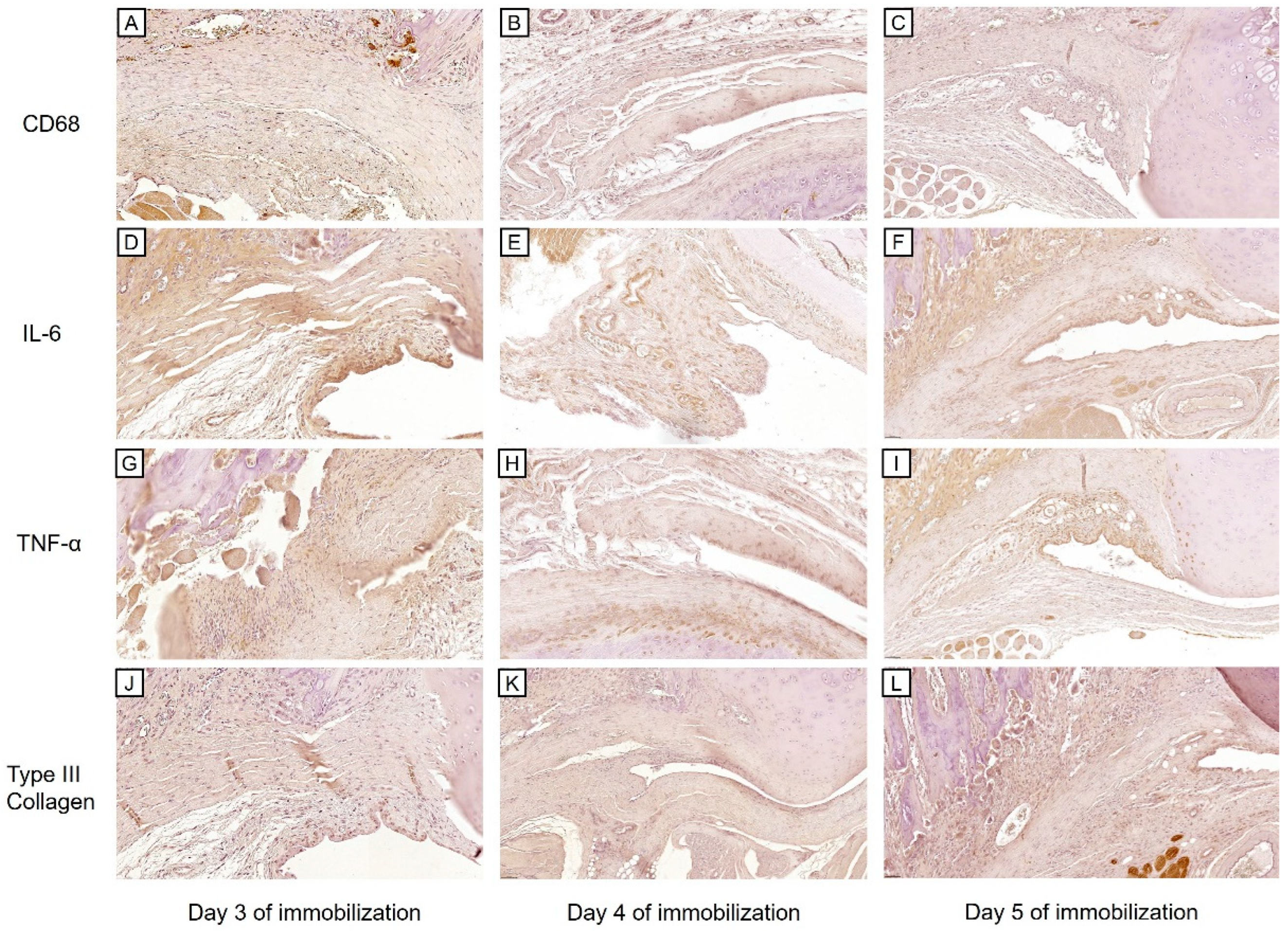
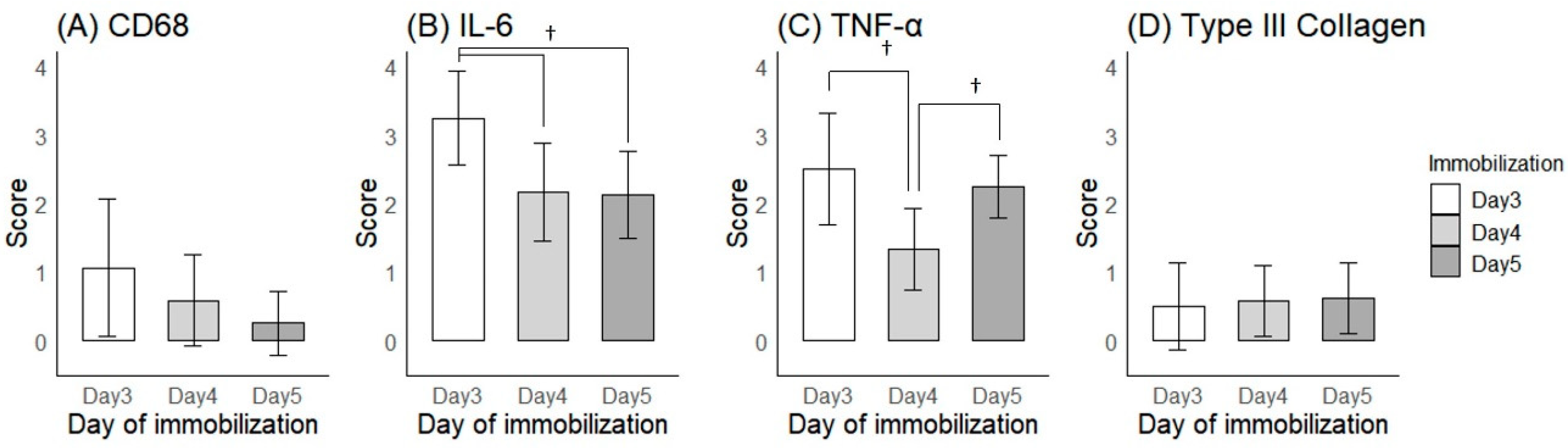
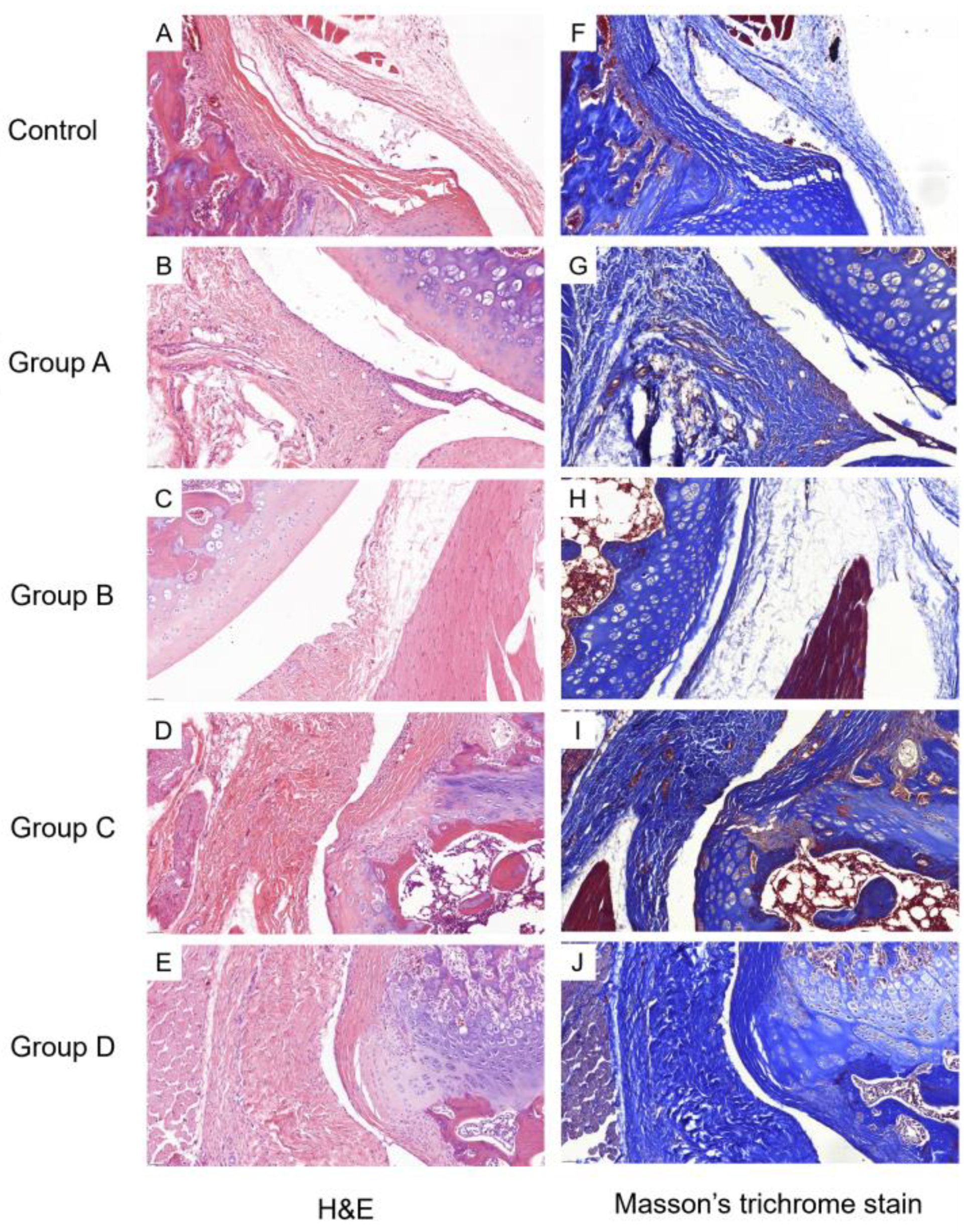
2.2.1. Histological Findings
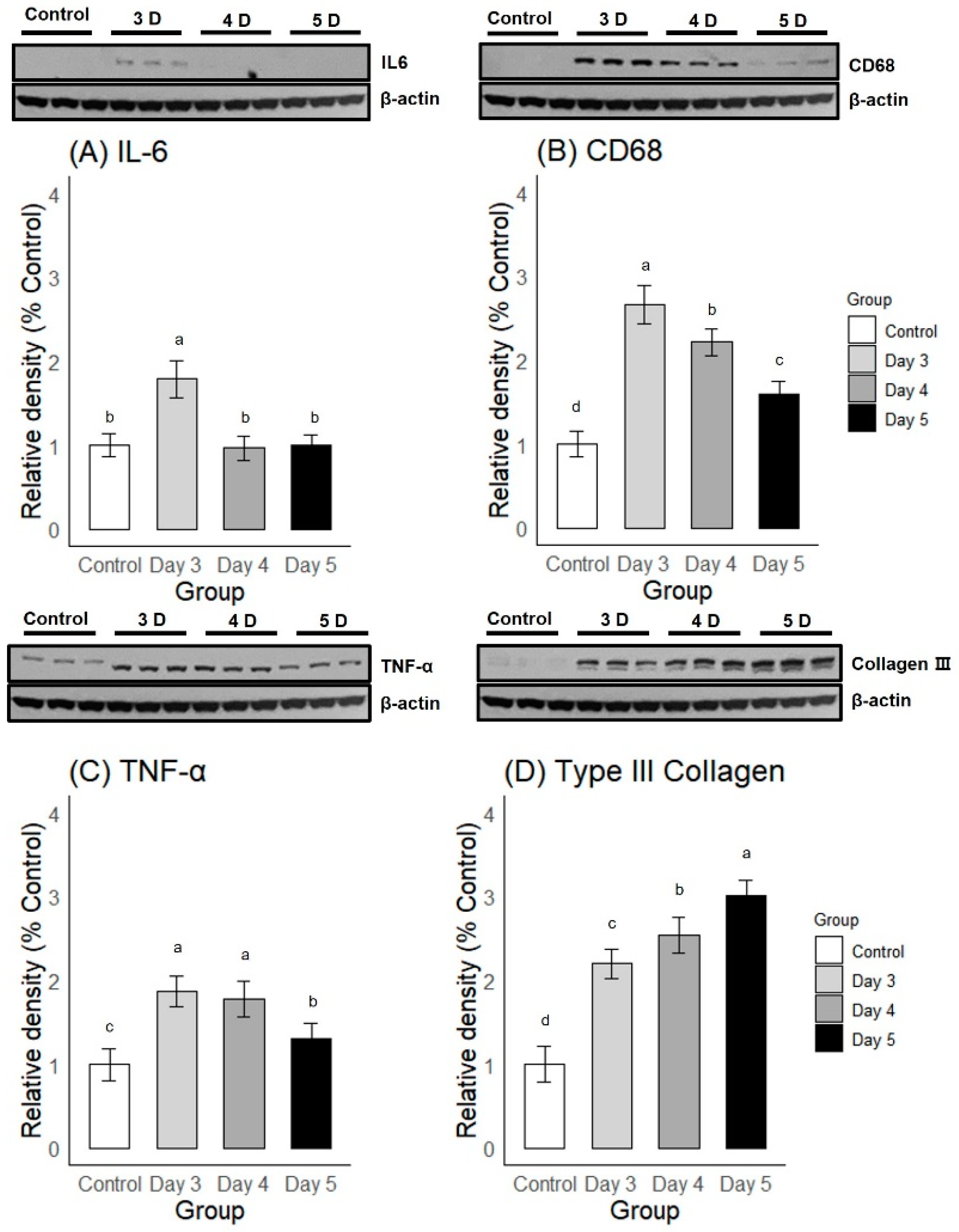
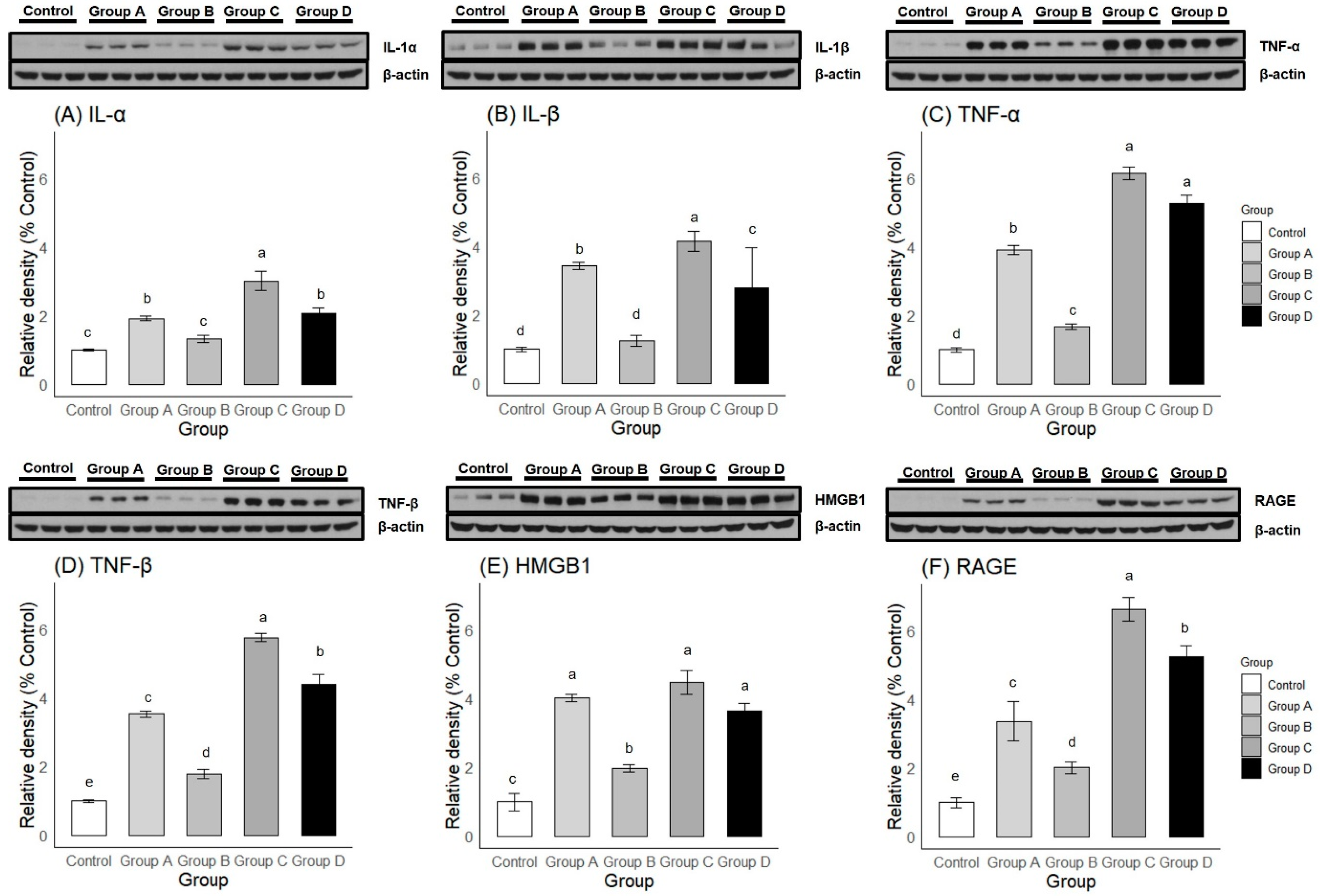
2.2.2. Western Blot
2.3. Efficacy of Intra-Articular Corticosteroid Injections during the Freezing Phase
2.3.1. Passive Shoulder ROM
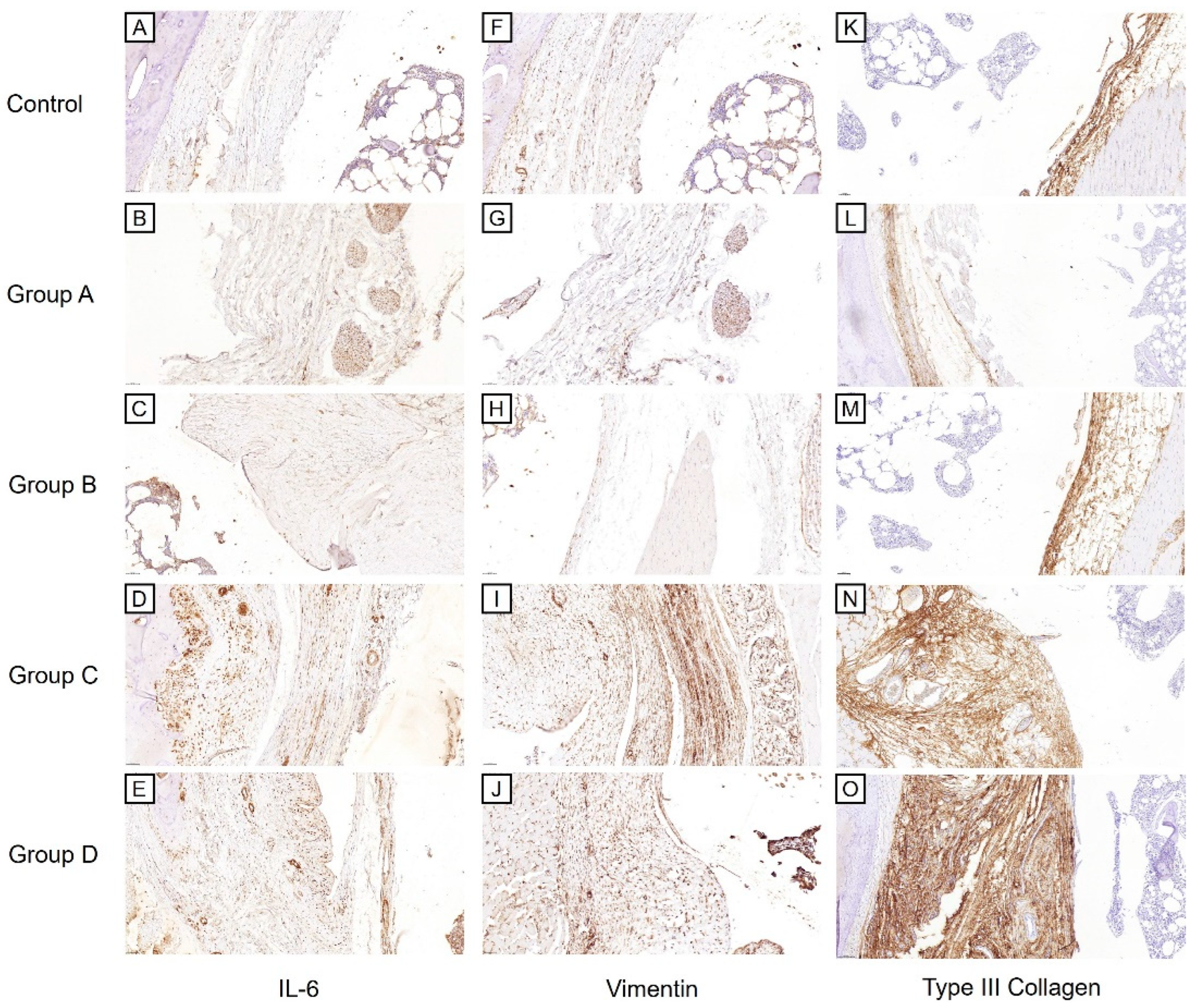
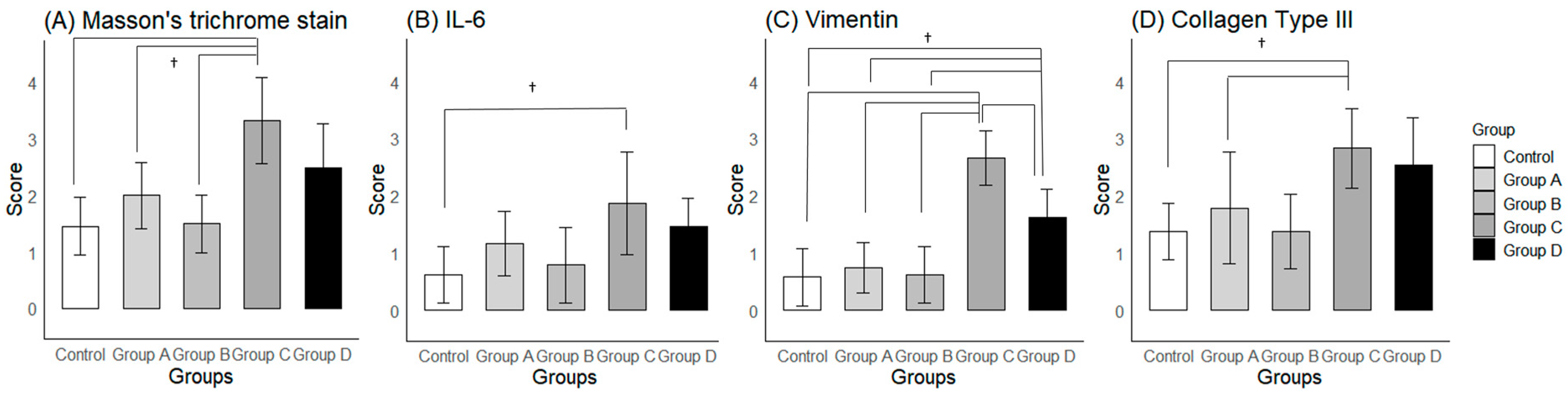
2.3.2. Histological Findings
2.3.3. Western Blot
3. Discussion
4. Materials and Methods
4.1. Measurement of Passive Shoulder ROM
4.2. Histological Evaluation
4.3. Western Blot
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, C.K.; Levine, W.N.; Deo, K.; Kesting, R.S.; Mercer, E.A.; Schram, G.A.; Strang, B.L. Natural history of frozen shoulder: Fact or fiction? A systematic review. Physiotherapy 2017, 103, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Schultheis, A.; Reichwein, F.; Nebelung, W. Frozen shoulder. Diagnosis and therapy. Orthopade 2008, 37, 1065–1066, 1068–1072. [Google Scholar] [CrossRef]
- Reeves, B. The natural history of the frozen shoulder syndrome. Scand. J. Rheumatol. 1975, 4, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Hannafin, J.A.; Chiaia, T.A. Adhesive capsulitis. A treatment approach. Clin. Orthop. Relat. Res. 2000, 372, 95–109. [Google Scholar] [CrossRef]
- Cho, C.H.; Song, K.S.; Kim, B.S.; Kim, D.H.; Lho, Y.M. Biological Aspect of Pathophysiology for Frozen Shoulder. Biomed. Res. Int. 2018, 2018, 7274517. [Google Scholar] [CrossRef]
- Chan, H.B.Y.; Pua, P.Y.; How, C.H. Physical therapy in the management of frozen shoulder. Singapore Med. J. 2017, 58, 685–689. [Google Scholar] [CrossRef]
- Neviaser, R.J.; Neviaser, T.J. The frozen shoulder. Diagnosis and management. Clin. Orthop. Relat. Res. 1987, 223, 59–64. [Google Scholar] [CrossRef]
- Ahn, J.H.; Lee, D.H.; Kang, H.; Lee, M.Y.; Kang, D.R.; Yoon, S.H. Early Intra-articular Corticosteroid Injection Improves Pain and Function in Adhesive Capsulitis of the Shoulder: 1-Year Retrospective Longitudinal Study. PMR 2018, 10, 19–27. [Google Scholar] [CrossRef]
- Uva, L.; Miguel, D.; Pinheiro, C.; Antunes, J.; Cruz, D.; Ferreira, J.; Filipe, P. Mechanisms of action of topical corticosteroids in psoriasis. Int. J. Endocrinol. 2012, 2012, 561018. [Google Scholar] [CrossRef]
- Roh, Y.H.; Yi, S.R.; Noh, J.H.; Lee, S.Y.; Oh, J.H.; Gong, H.S.; Baek, G.H. Intra-articular corticosteroid injection in diabetic patients with adhesive capsulitis: A randomized controlled trial. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 1947–1952. [Google Scholar] [CrossRef]
- Yoon, S.H.; Lee, H.Y.; Lee, H.J.; Kwack, K.S. Optimal dose of intra-articular corticosteroids for adhesive capsulitis: A randomized, triple-blind, placebo-controlled trial. Am. J. Sports Med. 2013, 41, 1133–1139. [Google Scholar] [CrossRef]
- Prestgaard, T.; Wormgoor, M.E.A.; Haugen, S.; Harstad, H.; Mowinckel, P.; Brox, J.I. Ultrasound-guided intra-articular and rotator interval corticosteroid injections in adhesive capsulitis of the shoulder: A double-blind, sham-controlled randomized study. Pain 2015, 156, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Oki, S.; Shirasawa, H.; Yoda, M.; Matsumura, N.; Tohmonda, T.; Yuasa, K.; Nakamura, M.; Matsumoto, M.; Horiuchi, K. Generation and characterization of a novel shoulder contracture mouse model. J. Orthop. Res. 2015, 33, 1732–1738. [Google Scholar] [CrossRef] [PubMed]
- Kanno, A.; Sano, H.; Itoi, E. Development of a shoulder contracture model in rats. J. Shoulder Elb. Surg. 2010, 19, 700–708. [Google Scholar] [CrossRef]
- Liu, Y.L.; Ao, Y.F.; Cui, G.Q.; Zhu, J.X. Changes of histology and capsular collagen in a rat shoulder immobilization model. Chin Med. J. (Engl.) 2011, 124, 3939–3944. [Google Scholar]
- Ochiai, N.; Ohtori, S.; Kenmoku, T.; Yamazaki, H.; Ochiai, S.; Saisu, T.; Matsuki, K.; Takahashi, K. Sensory innervation of rat contracture shoulder model. J. Shoulder Elb. Surg. 2013, 22, 158–164. [Google Scholar] [CrossRef]
- Schollmeier, G.; Sarkar, K.; Fukuhara, K.; Uhthoff, H.K. Structural and functional changes in the canine shoulder after cessation of immobilization. Clin. Orthop. Relat. Res. 1996, 323, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Schollmeier, G.; Uhthoff, H.K.; Sarkar, K.; Fukuhara, K. Effects of immobilization on the capsule of the canine glenohumeral joint. A structural functional study. Clin. Orthop. Relat. Res. 1994, 304, 37–42. [Google Scholar] [CrossRef]
- Villa-Camacho, J.C.; Okajima, S.; Perez-Viloria, M.E.; Walley, K.C.; Zurakowski, D.; Rodriguez, E.K.; Nazarian, A. In Vivo kinetic evaluation of an adhesive capsulitis model in rats. J. Shoulder Elb. Surg. 2015, 24, 1809–1816. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, K.H.; Lho, Y.M.; Ha, E.; Hwang, I.; Song, K.S.; Cho, C.H. Characterization of a frozen shoulder model using immobilization in rats. J. Orthop. Surg. Res. 2016, 11, 160. [Google Scholar] [CrossRef]
- Çınar, B.M.; Battal, V.E.; Bal, N.; Güler, Ü.; Beyaz, S. Comparison of efficacy of Oral versus Intra-articular Corticosteroid Application in the treatment of Frozen Shoulder: An experimental study in rats. Acta Orthop. Traumatol. Turc. 2022, 56, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Moon, Y.S.; Park, G.Y.; Cho, S.C.; Lee, Y.J.; Kwon, D.R.; Lee, S.C. Efficacy of Intra-articular Triamcinolone and Hyaluronic Acid in a Frozen Shoulder Rat Model. Am. J. Sports Med. 2023, 51, 2881–2890. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.I.; Lee, Y.S.; Kim, J.Y.; Chung, S.W. Effect of diabetes and corticosteroid injection on glenohumeral joint capsule in a rat stiffness model. J. Shoulder Elb. Surg. 2021, 30, 2814–2823. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.H.; Lho, Y.M.; Hwang, I.; Kim, D.H. Role of matrix metalloproteinases 2 and 9 in the development of frozen shoulder: Human data and experimental analysis in a rat contracture model. J. Shoulder Elb. Surg. 2019, 28, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Karahan, N.; Ozdemir, G.; Kolukısa, D.; Duman, S.; Arslanoğlu, F.; Çetin, M. Can Collagenase Be Used in the Treatment of Adhesive Capsulitis? Med. Princ. Pract. 2020, 29, 174–180. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Carrington, C.B.; Gaensler, E.A.; Coutu, R.E.; FitzGerald, M.X.; Gupta, R.G. Natural history and treated course of usual and desquamative interstitial pneumonia. N. Engl. J. Med. 1978, 298, 801–809. [Google Scholar] [CrossRef]
- Scadding, J.G. Diffuse pulmonary alveolar fibrosis. Thorax 1974, 29, 271–281. [Google Scholar] [CrossRef]
- Castor, C.W.; Muirden, K.D. Collagen Formation in Monolayer Cultures of Human Fibroblasts. The Effects of Hydrocortisone. Lab. Investig. 1964, 13, 560–574. [Google Scholar]
- Castor, C.W. Adrenocorticoid suppression of mucopoly-saccharide formation in human connective tissue cell cultures. J. Lab. Clin. Med. 1962, 60, 788–798. [Google Scholar]
- Akbar, M.; Crowe, L.A.N.; McLean, M.; Garcia-Melchor, E.; MacDonald, L.; Carter, K.; Fazzi, U.G.; Martin, D.; Arthur, A.; Reilly, J.H.; et al. Translational targeting of inflammation and fibrosis in frozen shoulder: Molecular dissection of the T cell/IL-17A axis. Proc. Natl. Acad. Sci. USA 2021, 118, e2102715118. [Google Scholar] [CrossRef]
- Calis, M.; Demir, H.; Ulker, S.; Kirnap, M.; Duygulu, F.; Calis, H.T. Is intraarticular sodium hyaluronate injection an alternative treatment in patients with adhesive capsulitis? Rheumatol. Int. 2006, 26, 536–540. [Google Scholar] [CrossRef]
- Lee, P.N.; Lee, M.; Haq, A.M.; Longton, E.B.; Wright, V. Periarthritis of the shoulder. Trial of treatments investigated by multivariate analysis. Ann. Rheum. Dis. 1974, 33, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Kraal, T.; Sierevelt, I.; van Deurzen, D.; van den Bekerom, M.P.; Beimers, L. Corticosteroid injection alone vs additional physiotherapy treatment in early stage frozen shoulders. World J. Orthop. 2018, 9, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Dacre, J.E.; Beeney, N.; Scott, D.L. Injections and physiotherapy for the painful stiff shoulder. Ann. Rheum. Dis. 1989, 48, 322–325. [Google Scholar] [CrossRef]
- Carette, S.; Moffet, H.; Tardif, J.; Bessette, L.; Morin, F.; Frémont, P.; Bykerk, V.; Thorne, C.; Bell, M.; Bensen, W.; et al. Intraarticular corticosteroids, supervised physiotherapy, or a combination of the two in the treatment of adhesive capsulitis of the shoulder: A placebo-controlled trial. Arthritis Rheum. 2003, 48, 829–838. [Google Scholar] [CrossRef]
- Ryans, I.; Montgomery, A.; Galway, R.; Kernohan, W.G.; McKane, R. A randomized controlled trial of intra-articular triamcinolone and/or physiotherapy in shoulder capsulitis. Rheumatology 2005, 44, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, K.L.; Rasmussen, B.B.; Lykkesfeldt, A.E.; Møller, S.; Ejlertsen, B.; Mouridsen, H.T. Semi-quantitative scoring of potentially predictive markers for endocrine treatment of breast cancer: A comparison between whole sections and tissue microarrays. J. Clin. Pathol. 2007, 60, 397–404. [Google Scholar] [CrossRef]
- Han, C.P.; Kok, L.F.; Wang, P.H.; Wu, T.S.; Tyan, Y.S.; Cheng, Y.W.; Lee, M.Y.; Yang, S.F. Scoring of p16(INK4a) immunohistochemistry based on independent nuclear staining alone can sufficiently distinguish between endocervical and endometrial adenocarcinomas in a tissue microarray study. Mod. Pathol. 2009, 22, 797–806. [Google Scholar] [CrossRef]

| Passive Shoulder Abduction Angle | |||||
|---|---|---|---|---|---|
| Baseline | Day 3 | Week 3 | Δ | P | |
| Group A | 159 ± 3 | 135 ± 6 | 138 ± 8 | 3 ± 3 | 0.048 |
| Group B | 160 ± 3 | 135 ± 4 | 146 ± 5 | 11 ± 4 | <0.001 |
| Group C | 159 ± 4 | 135 ± 5 | 95 ± 11 | −40 ± 7 | <0.001 |
| Group D | 158 ± 5 | 129 ± 2 | 132 ± 8 | 3 ± 7 | 0.334 |
| Control | 158 ± 4 | 158 ± 3 | 158 ± 2 | −0 ± 3 | 0.880 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, Y.; Lee, S.-J.; Moon, Y.S.; Lee, Y.-J.; Park, J.H.; Chun, Y.; Kwon, D.R.; Lee, S.C. The Disease-Modifying Effects of a Single Intra-Articular Corticosteroid Injection during the Freezing Phase of Frozen Shoulder in an Animal Model. Int. J. Mol. Sci. 2024, 25, 9585. https://doi.org/10.3390/ijms25179585
Ahn Y, Lee S-J, Moon YS, Lee Y-J, Park JH, Chun Y, Kwon DR, Lee SC. The Disease-Modifying Effects of a Single Intra-Articular Corticosteroid Injection during the Freezing Phase of Frozen Shoulder in an Animal Model. International Journal of Molecular Sciences. 2024; 25(17):9585. https://doi.org/10.3390/ijms25179585
Chicago/Turabian StyleAhn, Yongjin, Sun-Jae Lee, Yong Suk Moon, Yoon-Jin Lee, Jung Hyun Park, Yongmin Chun, Dong Rak Kwon, and Sang Chul Lee. 2024. "The Disease-Modifying Effects of a Single Intra-Articular Corticosteroid Injection during the Freezing Phase of Frozen Shoulder in an Animal Model" International Journal of Molecular Sciences 25, no. 17: 9585. https://doi.org/10.3390/ijms25179585






