In Vitro and In Vivo Assessments of Newly Isolated N4-like Bacteriophage against ST45 K62 Capsular-Type Carbapenem-Resistant Klebsiella pneumoniae: vB_kpnP_KPYAP-1
Abstract
:1. Introduction
2. Results
2.1. Characteristics of K. pneumoniae Clinical Isolated Strains
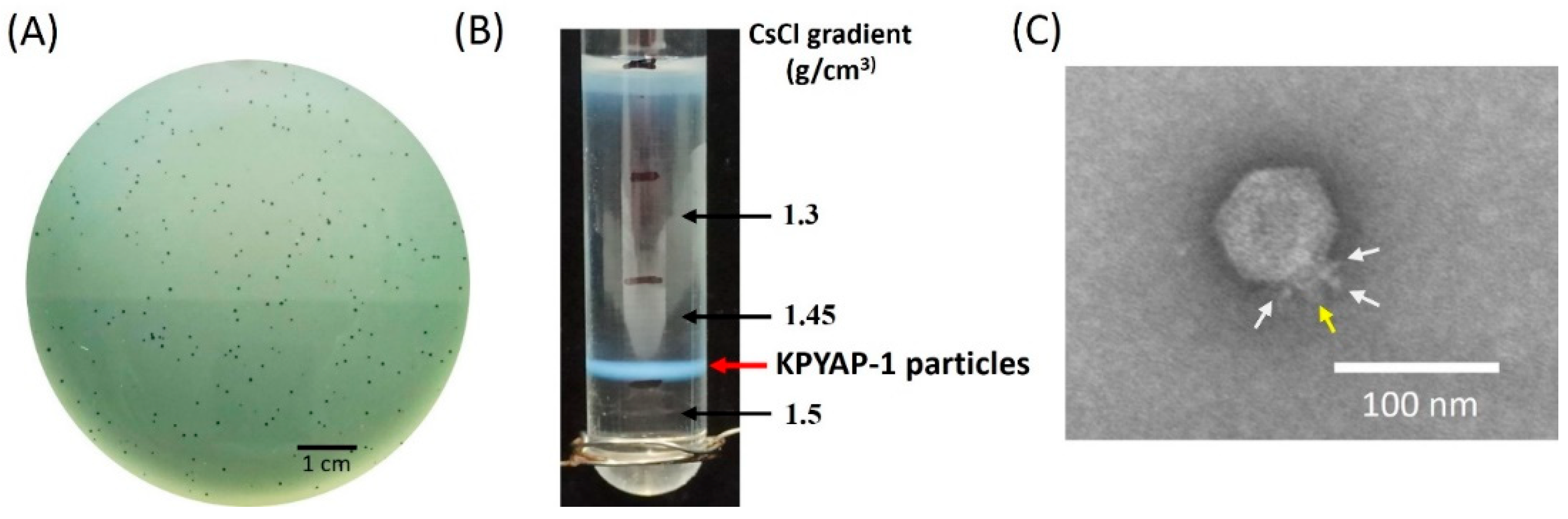
2.2. Isolation and Characterization of Phage vB_KpnP_KPYAP-1 (KPYAP-1)
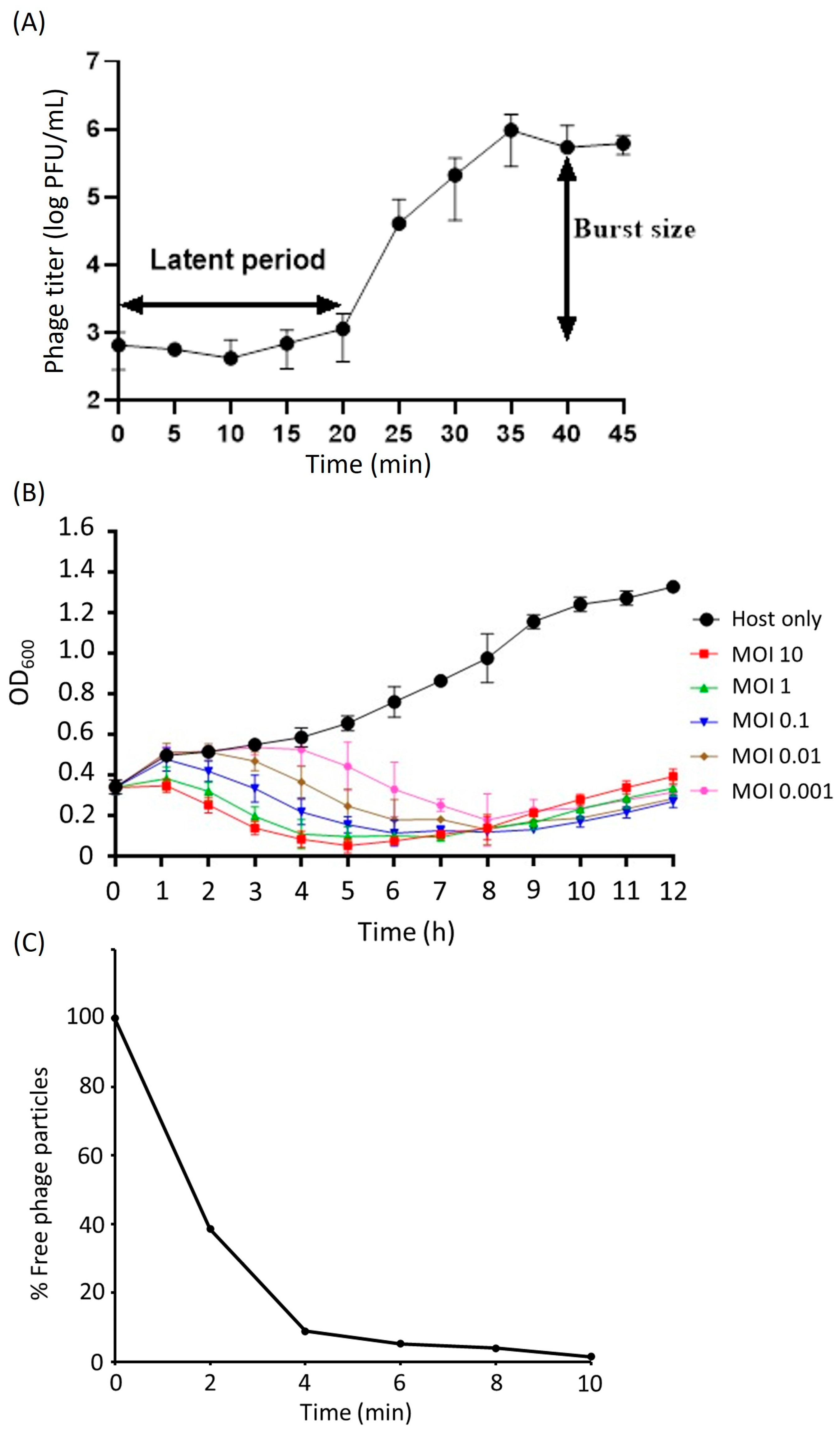
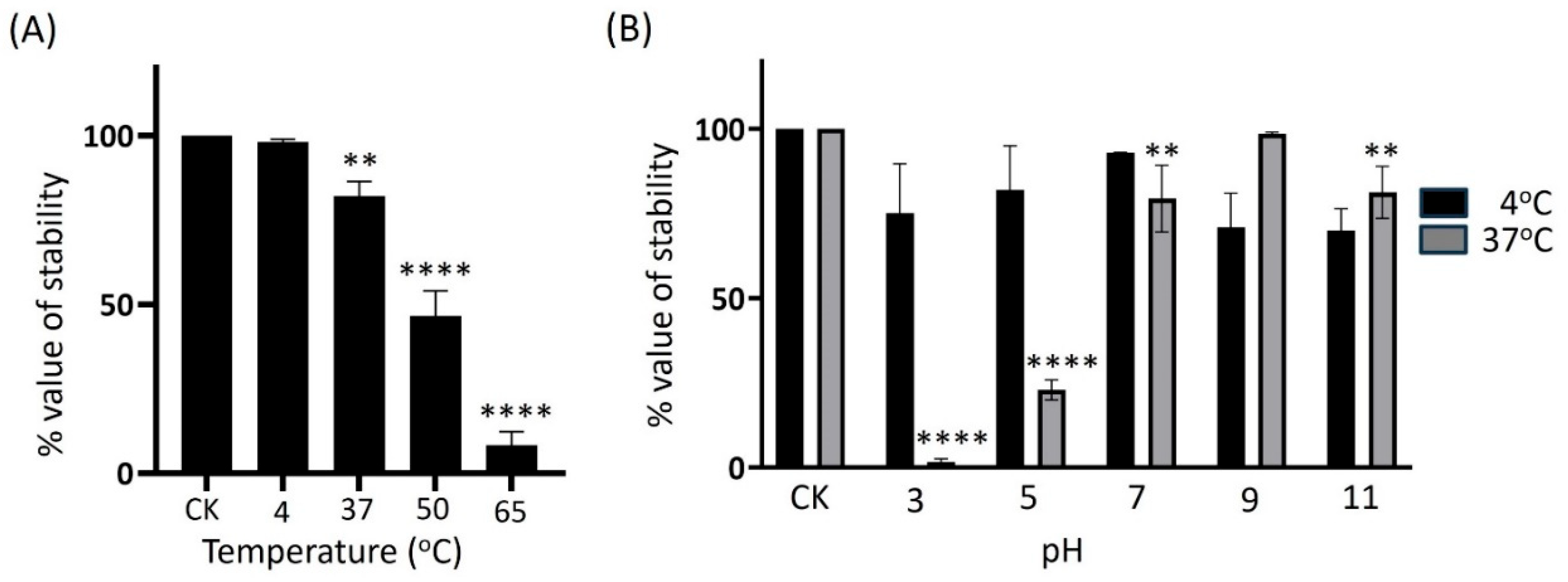
2.3. Biological Properties of KPYAP-1
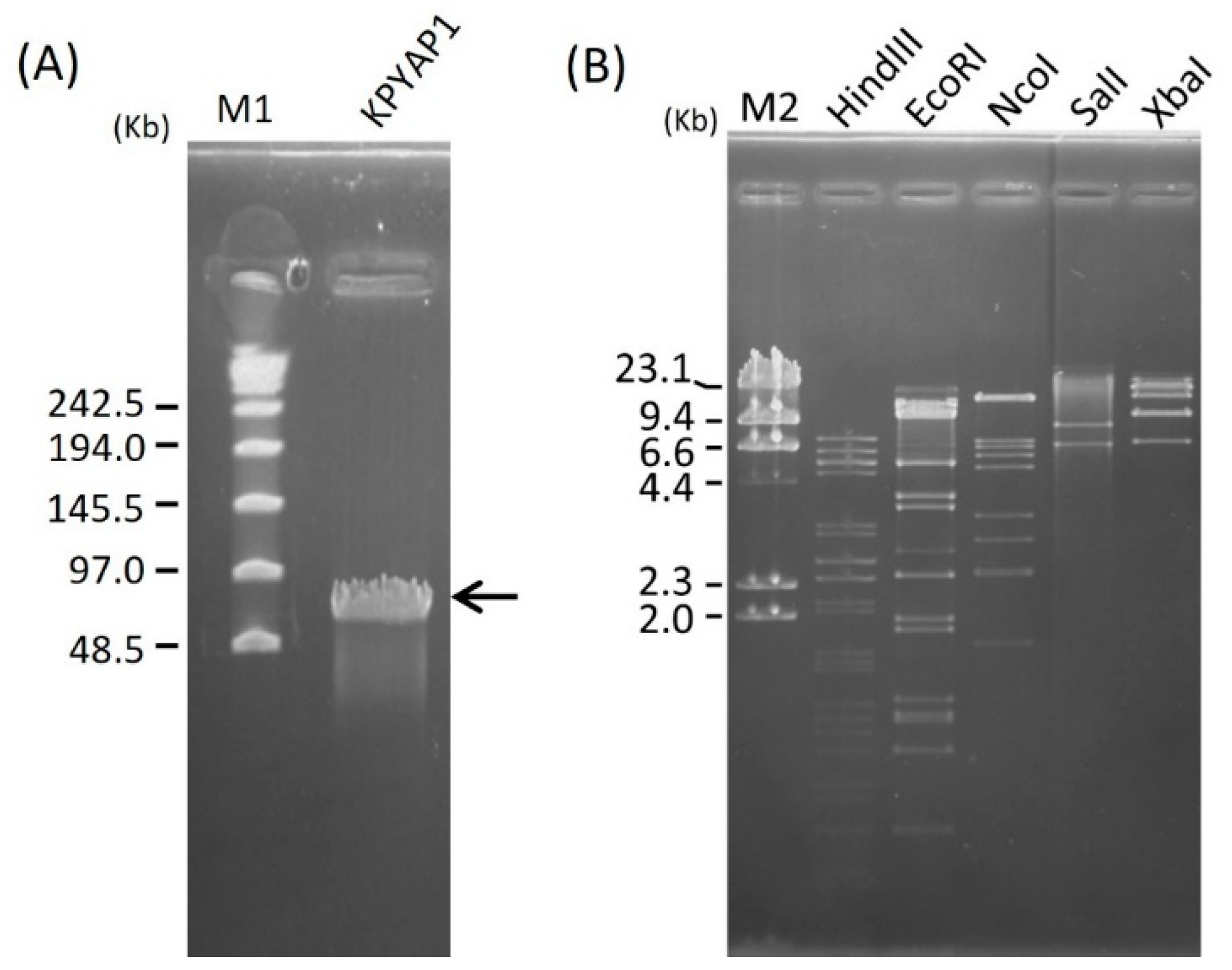
2.4. Genome Size Determination and Restriction Analysis of Phage DNA
2.5. Genome Analysis and Annotation
2.6. Proteomic Analysis
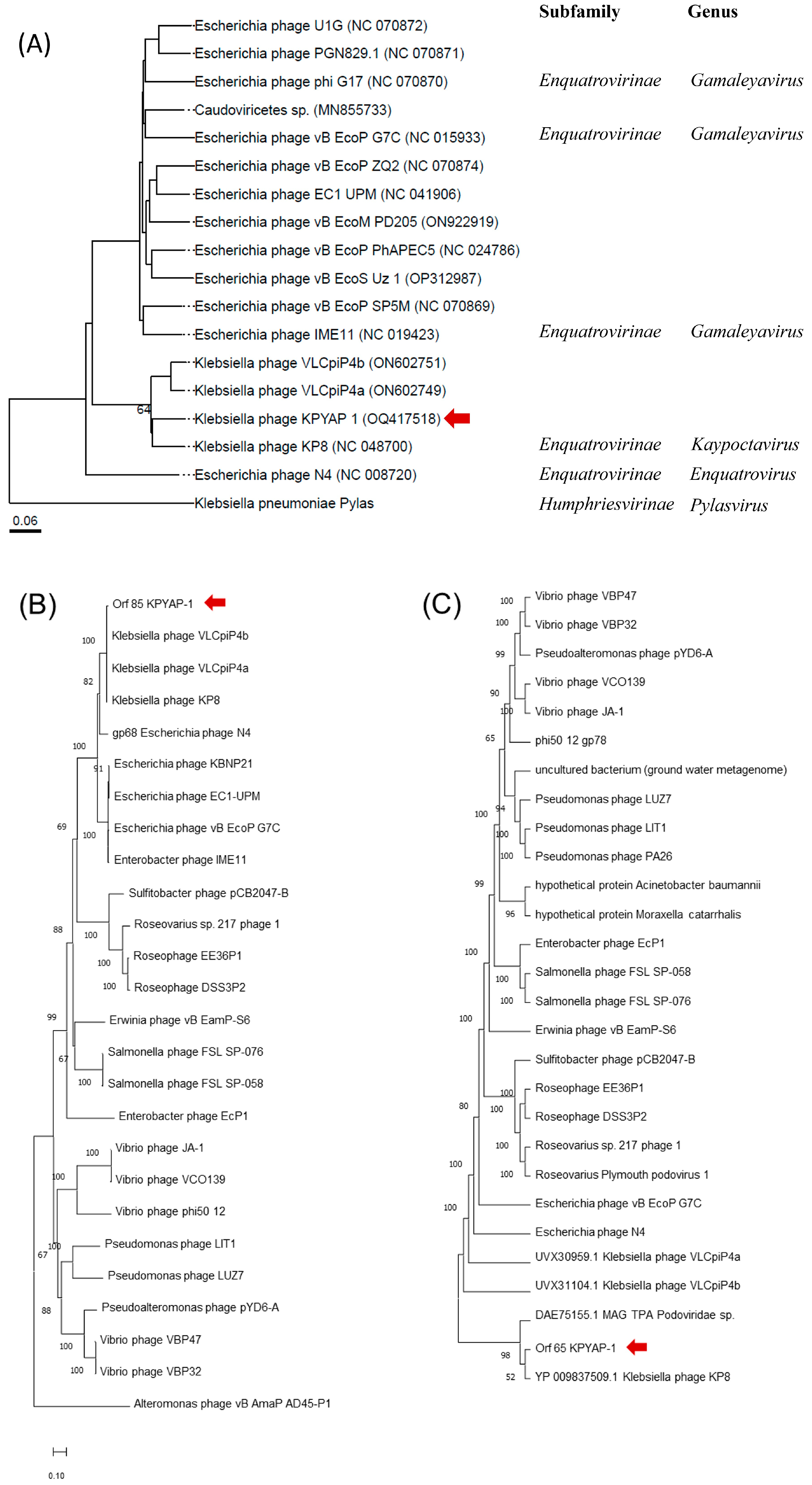
2.7. Phylogenetic Analysis of KPYAP-1
2.8. Genome Comparisons of KPYAP-1
2.9. Therapeutic Effect of KPYAP-1 on Kp20-Infected Zebrafish
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Screening for Carbapenemase Production
4.3. Detecting the blaOXA-48 Gene
4.4. Phage Isolation and Purification
4.5. Phage Characterization
4.5.1. Morphological Observation of Phage by TEM
4.5.2. Host Range Analysis of Phage
4.5.3. One-Step Growth and Adsorption Efficiency of Phage
4.5.4. Time-Dependent Bacteriolytic Effect of Phage
4.5.5. Phage Stability at Varying Temperatures and pH Values
4.5.6. Phage DNA Isolation and Determination of Genome Size and Restriction Patterns by PFGE
4.5.7. Phage Genome Sequencing and Characterization
4.5.8. Phage Structural Protein Analysis and Mass Spectrometry
4.5.9. Evaluating Phage Effectiveness against K. pneumoniae-Infected Zebrafish
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Effah, C.Y.; Sun, T.; Liu, S.; Wu, Y. Klebsiella pneumoniae: An increasing threat to public health. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 1. [Google Scholar] [CrossRef]
- Rodriguez-Bano, J.; Gutierrez-Gutierrez, B.; Machuca, I.; Pascual, A. Treatment of Infections Caused by Extended-Spectrum-Beta-Lactamase-, AmpC-, and Carbapenemase-Producing Enterobacteriaceae. Clin. Microbiol. Rev. 2018, 31, e00079-17. [Google Scholar] [CrossRef]
- Paczosa, M.K.; Mecsas, J. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Sun, X.; Ma, X. Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Gasink, L.B.; Edelstein, P.H.; Lautenbach, E.; Synnestvedt, M.; Fishman, N.O. Risk factors and clinical impact of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Infect. Control Hosp. Epidemiol. 2009, 30, 1180–1185. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.C.; Lin, J.C.; Chang, J.C.; Hiaso, Y.W.; Wang, C.H.; Chiu, S.K.; Fung, C.P.; Chang, F.Y.; Siu, L.K. Virulence among different types of hypervirulent Klebsiella pneumoniae with multi-locus sequence type (MLST)-11, Serotype K1 or K2 strains. Gut Pathog. 2021, 13, 40. [Google Scholar] [CrossRef] [PubMed]
- Volozhantsev, N.V.; Shpirt, A.M.; Kislichkina, A.A.; Shashkov, A.S.; Verevkin, V.V.; Fursova, N.K.; Knirel, Y.A. Structure and gene cluster of the capsular polysaccharide of multidrug resistant carbapenemase OXA-48-producing Klebsiella pneumoniae strain KPB536 of the genetic line ST147. Res. Microbiol. 2020, 171, 74–79. [Google Scholar] [CrossRef]
- Salerno, A.; Deletoile, A.; Lefevre, M.; Ciznar, I.; Krovacek, K.; Grimont, P.; Brisse, S. Recombining population structure of Plesiomonas shigelloides (Enterobacteriaceae) revealed by multilocus sequence typing. J. Bacteriol. 2007, 189, 7808–7818. [Google Scholar] [CrossRef]
- Brisse, S.; Passet, V.; Haugaard, A.B.; Babosan, A.; Kassis-Chikhani, N.; Struve, C.; Decre, D. wzi Gene sequencing, a rapid method for determination of capsular type for Klebsiella strains. J. Clin. Microbiol. 2013, 51, 4073–4078. [Google Scholar] [CrossRef]
- Tsai, C.C.; Lin, J.C.; Chen, P.C.; Liu, E.Y.; Tsai, Y.K.; Yu, C.P.; Li, J.J.; Wang, C.H.; Fung, C.P.; Lin, F.M.; et al. A 20-Yearstudy of capsular polysaccharide seroepidemiology, susceptibility profiles, and virulence determinants of Klebsiella pneumoniae from bacteremia patients in Taiwan. Microbiol. Spectr. 2023, 11, e0035923. [Google Scholar] [CrossRef]
- WHO. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Al-Ishaq, R.K.; Skariah, S.; Busselberg, D. Bacteriophage treatment: Critical evaluation of its application on World Health Organization priority pathogens. Viruses 2020, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Thiry, D.; Passet, V.; Danis-Wlodarczyk, K.; Lood, C.; Wagemans, J.; De Sordi, L.; van Noort, V.; Dufour, N.; Debarbieux, L.; Mainil, J.G.; et al. New bacteriophages against emerging lineages ST23 and ST258 of Klebsiella pneumoniae and efficacy assessment in Galleria mellonella Larvae. Viruses 2019, 11, 411. [Google Scholar] [CrossRef]
- Fanaei, V.; Validi, M.; Zamanzad, B.; Karimi, A. Isolation and identification of specific bacteriophages against methicillin-resistant Staphylococcus aureus, extended-spectrum beta-lactamases-producing Escherichia coli, extended-spectrum beta-lactamases-producing Klebsiella pneumoniae, and multidrug-resistant Acinetobacter baumannii in vitro. FEMS Microbiol. Lett. 2021, 368, fnab139. [Google Scholar] [CrossRef]
- Kondo, K.; Nakano, S.; Hisatsune, J.; Sugawara, Y.; Kataoka, M.; Kayama, S.; Sugai, M.; Kawano, M. Characterization of 29 newly isolated bacteriophages as a potential therapeutic agent against IMP-6-producing Klebsiella pneumoniae from clinical specimens. Microbiol. Spectr. 2023, 11, e0476122. [Google Scholar] [CrossRef] [PubMed]
- Laforet, F.; Antoine, C.; Blasdel Reuter, B.; Detilleux, J.; Pirnay, J.P.; Brisse, S.; Fall, A.; Duprez, J.N.; Delcenserie, V.; Thiry, D. In vitro and In vivo assessments of two newly isolated bacteriophages against an ST13 urinary tract infection Klebsiella pneumoniae. Viruses 2022, 14, 1079. [Google Scholar] [CrossRef] [PubMed]
- Martins, W.; Cino, J.; Lenzi, M.H.; Sands, K.; Portal, E.; Hassan, B.; Dantas, P.P.; Migliavacca, R.; Medeiros, E.A.; Gales, A.C.; et al. Diversity of lytic bacteriophages against XDR Klebsiella pneumoniae sequence type 16 recovered from sewage samples in different parts of the world. Sci. Total Environ. 2022, 839, 156074. [Google Scholar] [CrossRef] [PubMed]
- Pallavali, R.R.; Degati, V.L.; Narala, V.R.; Velpula, K.K.; Yenugu, S.; Durbaka, V.R.P. Lytic bacteriophages against bacterial biofilms formed by multidrug-resistant Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, and Staphylococcus aureus isolated from burn wounds. Ther. Appl. Res. 2021, 2, 120–130. [Google Scholar] [CrossRef]
- Torabi, L.R.; Naghavi, N.S.; Doudi, M.; Monajemi, R. Efficacious antibacterial potency of novel bacteriophages against ESBL-producing Klebsiella pneumoniae isolated from burn wound infections. Iran. J. Microbiol. 2021, 13, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Anand, T.; Virmani, N.; Kumar, S.; Mohanty, A.K.; Pavulraj, S.; Bera, B.C.; Vaid, R.K.; Ahlawat, U.; Tripathi, B.N. Phage therapy for treatment of virulent Klebsiella pneumoniae infection in a mouse model. J. Glob. Antimicrob. Resist. 2020, 21, 34–41. [Google Scholar] [CrossRef]
- Kuipers, S.; Ruth, M.M.; Mientjes, M.; de Sevaux, R.G.L.; van Ingen, J. A Dutch case report of successful treatment of chronic relapsing urinary tract infection with bacteriophages in a renal transplant patient. Antimicrob. Agents Chemother. 2019, 64, 10–1128. [Google Scholar] [CrossRef]
- Eskenazi, A.; Lood, C.; Wubbolts, J.; Hites, M.; Balarjishvili, N.; Leshkasheli, L.; Askilashvili, L.; Kvachadze, L.; van Noort, V.; Wagemans, J.; et al. Combination of pre-adapted bacteriophage therapy and antibiotics for treatment of fracture-related infection due to pandrug-resistant Klebsiella pneumoniae. Nat. Commun. 2022, 13, 302. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, H.; Ma, R.; Wu, Y.; Lun, H.; Wang, A.; He, K.; Yu, J.; He, P. A novel depolymerase specifically degrades the K62-type capsular polysaccharide of Klebsiella pneumoniae. One Health Adv. 2024, 2, 5. [Google Scholar] [CrossRef]
- Queenan, A.M.; Bush, K. Carbapenemases: The versatile beta-lactamases. Clin. Microbiol. Rev. 2007, 20, 440–458. [Google Scholar] [CrossRef] [PubMed]
- Yukgehnaish, K.; Rajandas, H.; Parimannan, S.; Manickam, R.; Marimuthu, K.; Petersen, B.; Clokie, M.R.J.; Millard, A.; Sicheritz-Ponten, T. PhageLeads: Rapid assessment of phage therapeutic suitability using an ensemble machine learning approach. Viruses 2022, 14, 342. [Google Scholar] [CrossRef]
- Smith, D.L.; Struck, D.K.; Scholtz, J.M.; Young, R. Purification and biochemical characterization of the lambda holin. J. Bacteriol. 1998, 180, 2531–2540. [Google Scholar] [CrossRef] [PubMed]
- Cubo, M.; Alías-Villegas, C.; Balsanelli, E.; Mesa, D.; Souza, E.; Espuny, M.R. Diversity of Sinorhizobium (Ensifer) meliloti bacteriophages in the rhizosphere of Medicago marina: Myoviruses, filamentous and N4-Like podovirus. Front. Microbiol. 2020, 11, 22. [Google Scholar] [CrossRef]
- Mitchell, M.S.; Matsuzaki, S.; Imai, S.; Rao, V.B. Sequence analysis of bacteriophage T4 DNA packaging/terminase genes 16 and 17 reveals a common ATPase center in the large subunit of viral terminases. Nucleic Acids Res. 2002, 30, 4009–4021. [Google Scholar] [CrossRef]
- Wang, B.; Pan, F.; Wang, C.; Zhao, W.; Sun, Y.; Zhang, T.; Shi, Y.; Zhang, H. Molecular epidemiology of carbapenem-resistant Klebsiella pneumoniae in a paediatric hospital in China. Int. J. Infect. Dis. 2020, 93, 311–319. [Google Scholar] [CrossRef]
- Hussain, A.; Mazumder, R.; Ahmed, A.; Saima, U.; Phelan, J.E.; Campino, S.; Ahmed, D.; Asadulghani, M.; Clark, T.G.; Mondal, D. Genome dynamics of high-risk resistant and hypervirulent Klebsiella pneumoniae clones in Dhaka, Bangladesh. Front. Microbiol. 2023, 14, 1184196. [Google Scholar] [CrossRef]
- Loc-Carrillo, C.; Abedon, S.T. Pros and cons of phage therapy. Bacteriophage 2011, 1, 111–114. [Google Scholar] [CrossRef]
- Morozova, V.; Babkin, I.; Kozlova, Y.; Baykov, I.; Bokovaya, O.; Tikunov, A.; Ushakova, T.; Bardasheva, A.; Ryabchikova, E.; Zelentsova, E.; et al. Isolation and characterization of a novel Klebsiella pneumoniae N4-like bacteriophage KP8. Viruses 2019, 11, 1115. [Google Scholar] [CrossRef]
- Knecht, L.E.; Veljkovic, M.; Fieseler, L. Diversity and function of phage encoded depolymerases. Front. Microbiol. 2019, 10, 2949. [Google Scholar] [CrossRef]
- Choi, K.H.; McPartland, J.; Kaganman, I.; Bowman, V.D.; Rothman-Denes, L.B.; Rossmann, M.G. Insight into DNA and protein transport in double-stranded DNA viruses: The structure of bacteriophage N4. J. Mol. Biol. 2008, 378, 726–736. [Google Scholar] [CrossRef]
- Stojkovic, E.A.; Rothman-Denes, L.B. Coliphage N4 N-acetylmuramidase defines a new family of murein hydrolases. J. Mol. Biol. 2007, 366, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Summer, E.J.; Berry, J.; Tran, T.A.; Niu, L.; Struck, D.K.; Young, R. Rz/Rz1 lysis gene equivalents in phages of Gram-negative hosts. J. Mol. Biol. 2007, 373, 1098–1112. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, L.; Gutierrez, D.; Garcia, P.; Rodriguez, A. The perfect bacteriophage for therapeutic applications—A quick guide. Antibiotics 2019, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.; Draper, L.A.; Ross, R.P.; Hill, C. The advantages and challenges of using endolysins in a clinical setting. Viruses 2021, 13, 680. [Google Scholar] [CrossRef]
- Fang, C.T.; Shih, Y.J.; Cheong, C.M.; Yi, W.C. Rapid and accurate determination of lipopolysaccharide O-antigen types in Klebsiella pneumoniae with a novel PCR-based O-genotyping method. J. Clin. Microbiol. 2016, 54, 666–675. [Google Scholar] [CrossRef]
- Diancourt, L.; Passet, V.; Verhoef, J.; Grimont, P.A.; Brisse, S. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J. Clin. Microbiol. 2005, 43, 4178–4182. [Google Scholar] [CrossRef]
- Poirel, L.; Heritier, C.; Tolun, V.; Nordmann, P. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2004, 48, 15–22. [Google Scholar] [CrossRef]
- Lin, N.T.; Chiou, P.Y.; Chang, K.C.; Chen, L.K.; Lai, M.J. Isolation and characterization of ϕAB2: A novel bacteriophage of Acinetobacter baumannii. Res. Microbiol. 2010, 161, 308–314. [Google Scholar] [CrossRef]
- Kropinski, A.M. Practical advice on the one-step growth curve. Methods Mol. Biol. 2018, 1681, 41–47. [Google Scholar] [CrossRef]
- Xie, Y.; Wahab, L.; Gill, J.J. Development and Validation of a Microtiter Plate-Based Assay for Determination of Bacteriophage Host Range and Virulence. Viruses 2018, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Overbeek, R.; Olson, R.; Pusch, G.D.; Olsen, G.J.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Parrello, B.; Shukla, M.; et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 2014, 42, D206–D214. [Google Scholar] [CrossRef] [PubMed]
- Brettin, T.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Olsen, G.J.; Olson, R.; Overbeek, R.; Parrello, B.; Pusch, G.D.; et al. RASTtk: A modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci. Rep. 2015, 5, 8365. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Garneau, J.R.; Depardieu, F.; Fortier, L.-C.; Bikard, D.; Monot, M. PhageTerm: A tool for fast and accurate determination of phage termini and packaging mechanism using next-generation sequencing data. Sci. Rep. 2017, 7, 8292. [Google Scholar] [CrossRef]
- Gurusaran, M.; Ravella, D.; Sekar, K. RepEx: Repeat extractor for biological sequences. Genomics 2013, 102, 403–408. [Google Scholar] [CrossRef]
- Fang, Q.; Feng, Y.; McNally, A.; Zong, Z. Characterization of phage resistance and phages capable of intestinal decolonization of carbapenem-resistant Klebsiella pneumoniae in mice. Commun. Biol. 2022, 5, 48. [Google Scholar] [CrossRef]
- Laslett, D.; Canback, B. ARAGORN, a program to detect tRNA genes and tmRNA genes in nucleotide sequences. Nucleic Acids Res. 2004, 32, 11–16. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Göker, M. VICTOR: Genome-based phylogeny and classification of prokaryotic viruses. Bioinformatics 2017, 33, 3396–3404. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.P.; Göker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef]
- Yu, G. Using ggtree to visualize data on tree-like structures. Curr. Protoc. Bioinform. 2020, 69, e96. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Hahnke, R.L.; Petersen, J.; Scheuner, C.; Michael, V.; Fiebig, A.; Rohde, C.; Rohde, M.; Fartmann, B.; Goodwin, L.A.; et al. Complete genome sequence of DSM 30083(T), the type strain (U5/41(T)) of Escherichia coli, and a proposal for delineating subspecies in microbial taxonomy. Stand. Genom. Sci. 2014, 9, 2. [Google Scholar] [CrossRef]
- Sullivan, M.J.; Petty, N.K.; Beatson, S.A. Easyfig: A genome comparison visualizer. Bioinformatics 2011, 27, 1009–1010. [Google Scholar] [CrossRef]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef]
- Zankari, E.; Allesoe, R.; Joensen, K.G.; Cavaco, L.M.; Lund, O.; Aarestrup, F.M. PointFinder: A novel web tool for WGS-based detection of antimicrobial resistance associated with chromosomal point mutations in bacterial pathogens. J. Antimicrob. Chemother. 2017, 72, 2764–2768. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.F.; Teh, S.H.; Yang, H.H.; Tsai, Y.C.; Chao, H.J.; Peng, S.S.; Chen, S.C.; Lin, L.C.; Lin, N.T. Therapeutic potential of a novel lytic phage, vB_EclM_ECLFM1, against carbapenem-resistant Enterobacter cloacae. Int. J. Mol. Sci. 2024, 25, 854. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Lee, Y.P.; Lin, N.T.; Yang, H.H.; Teh, S.H.; Lin, L.C. Therapeutic effect and anti-biofilm ability assessment of a novel phage, phiPA1-3, against carbapenem-resistant Pseudomonas aeruginosa. Virus Res. 2023, 335, 199178. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natarajan, S.P.; Teh, S.-H.; Lin, L.-C.; Lin, N.-T. In Vitro and In Vivo Assessments of Newly Isolated N4-like Bacteriophage against ST45 K62 Capsular-Type Carbapenem-Resistant Klebsiella pneumoniae: vB_kpnP_KPYAP-1. Int. J. Mol. Sci. 2024, 25, 9595. https://doi.org/10.3390/ijms25179595
Natarajan SP, Teh S-H, Lin L-C, Lin N-T. In Vitro and In Vivo Assessments of Newly Isolated N4-like Bacteriophage against ST45 K62 Capsular-Type Carbapenem-Resistant Klebsiella pneumoniae: vB_kpnP_KPYAP-1. International Journal of Molecular Sciences. 2024; 25(17):9595. https://doi.org/10.3390/ijms25179595
Chicago/Turabian StyleNatarajan, Shanmuga Priya, Soon-Hian Teh, Ling-Chun Lin, and Nien-Tsung Lin. 2024. "In Vitro and In Vivo Assessments of Newly Isolated N4-like Bacteriophage against ST45 K62 Capsular-Type Carbapenem-Resistant Klebsiella pneumoniae: vB_kpnP_KPYAP-1" International Journal of Molecular Sciences 25, no. 17: 9595. https://doi.org/10.3390/ijms25179595




