Cystic Fibrosis: A Journey through Time and Hope
Abstract
:1. Introduction
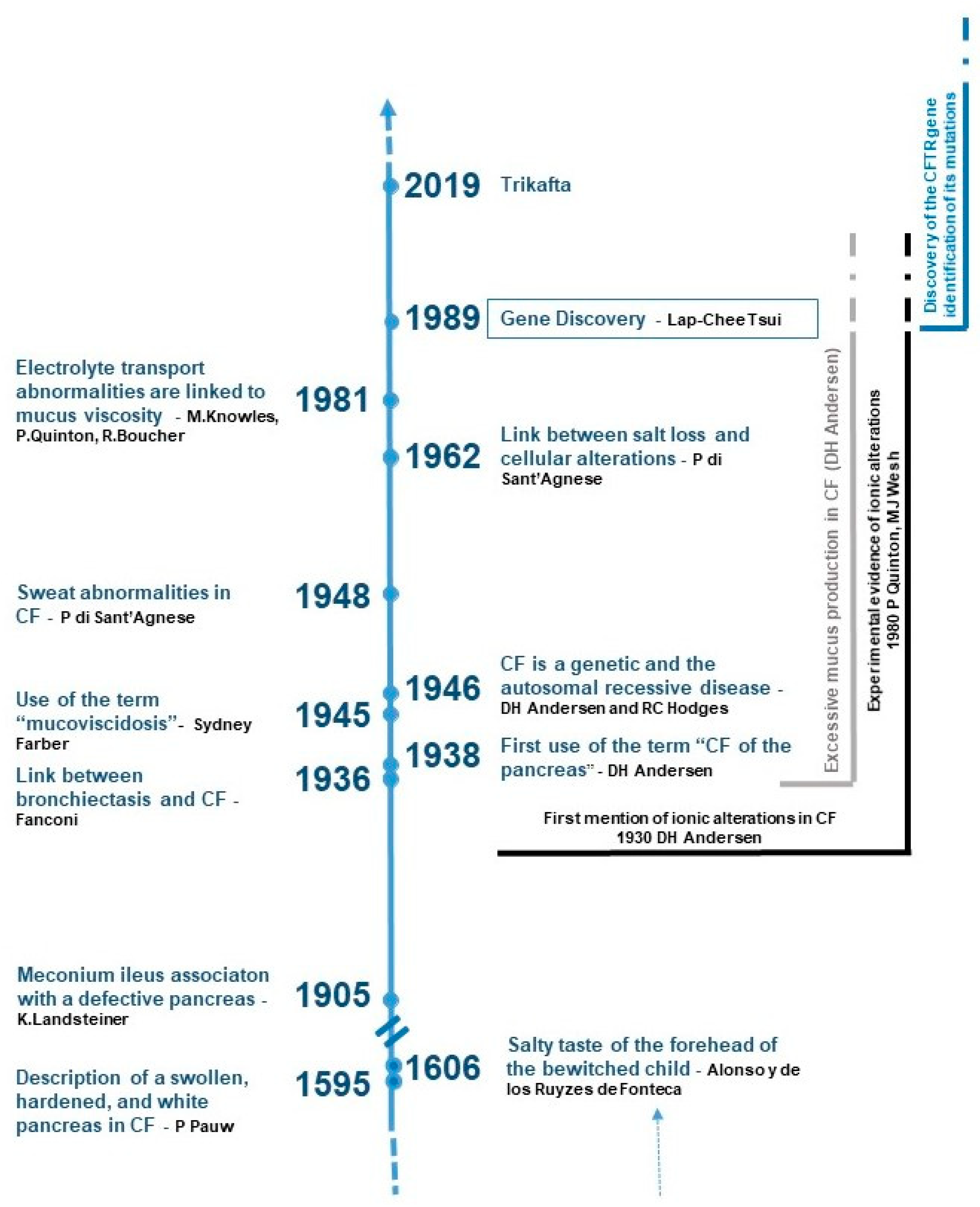
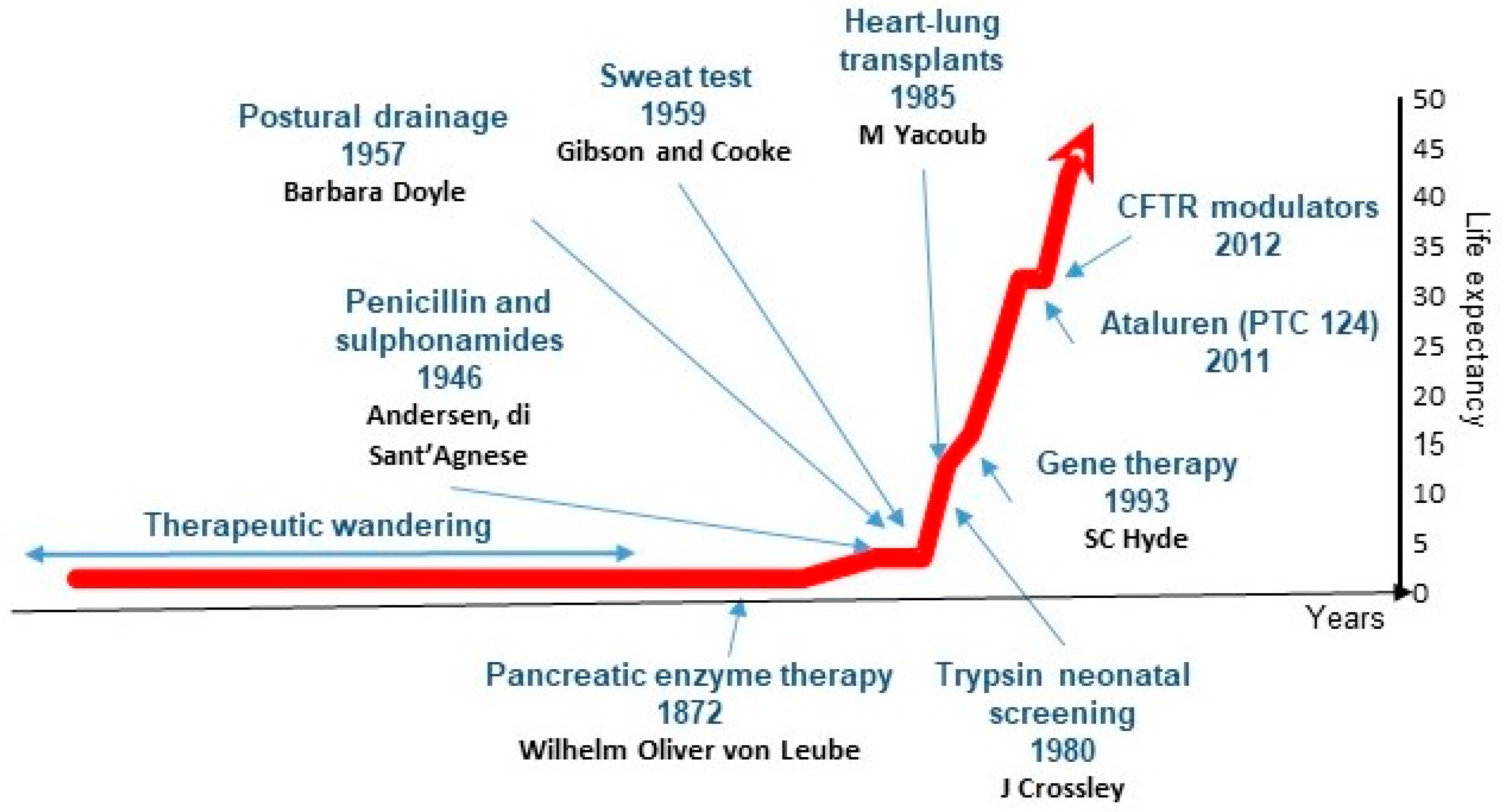
2. The History of CF: A Long March towards Hope
2.1. From Bewitchment Diagnosis to the Etiology of CF
2.2. Stuck in the Trail of Mucus
2.3. Tracing the Ionic Flux
2.4. Connecting the Dots by Unveiling the CFTR Gene
2.5. Autopsy of the CFTR Gene and Its Protein
3. Where We Are Today
3.1. Mutations, Their Impact on CFTR Function and the Patient’s Phenotype: A Complex Relationship
3.2. To Add Complications to Complexity, Here Are the Cftr-Related Diseases
3.3. From the Discovery of the CFTR Gene to Systematic Neonatal Screening
3.4. The Changing Epidemiological Data
3.5. Advancements in CF Treatment: A Beacon of Hope
3.6. CF and Precision Medicine
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kerem, B.S.; Rommens, J.M.; Buchanan, J.A.; Markiewicz, D.; Cox, T.K.; Chakravarti, A.; Buchwald, M.; Tsui, L.C. Identification of the cystic fibrosis gene: Genetic analysis. Science 1989, 245, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Tsui, L.-C.; Buchwald, M.; Barker, D.; Braman, J.C.; Knowlton, R.; Schumm, J.W.; Eiberg, H.; Mohr, J.; Kennedy, D.; Plavsic, N.; et al. Cystic Fibrosis Locus Defined by a Genetically Linked Polymorphic DNA Marker. Science 1985, 230, 1054–1057. [Google Scholar] [CrossRef] [PubMed]
- Rommens, J.M.; Iannuzzi, M.C.; Kerem, B.; Drumm, M.L.; Melmer, G.; Dean, M.; Rozmahel, R.; Cole, J.L.; Kennedy, D.; Hidaka, N.; et al. Identification of the cystic fibrosis gene: Chromosome walking and jumping. Science 1989, 245, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Riordan, J.R.; Rommens, J.M.; Kerem, B.-S.; Alon, N.; Rozmahel, R.; Grzelczak, Z.; Zielenski, J.; Lok, S.; Plavsic, N.; Chou, J.-L.; et al. Identification of the Cystic Fibrosis Gene: Cloning and Characterization of Complementary DNA. Science 1989, 245, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Dodge, J.A.; Lewis, P.A. Cystic fibrosis is no longer an important cause of childhood death in the UK. Arch. Dis. Child. 2005, 90, 547. [Google Scholar]
- Farrell, P.M. Metabolic Diseases: Foundations of Clinical Management, Genetics and Pathology. Arch. Pediatr. Adolesc. Med. 2001, 155, 621–622. [Google Scholar] [CrossRef]
- Welsh, M.J.; Ramsey, B.W.; Accurso, F.; Cutting, G.R. Cystic Fibrosis. In The Online Metabolic and Molecular Bases of Inherited Disease; Valle, D.L., Antonarakis, S., Ballabio, A., Beaudet, A.L., Mitchell, G.A., Eds.; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Landsteiner, K. Darmverschluss durch Eingedticktes Meconium Pankreatitis. Zentralbl. Allg. Pathol. 1905, 16, 903–907. [Google Scholar]
- Fanconi, G.; Fanconi, G.; Uehlinger, E.; Knauer, C. Das coeliakiesyndrom bei angeborener zysticher pankreasfibromatose und bronchiektasien. Wien. Med. Wschr. 1936, 86, 753–756. [Google Scholar]
- Andersen, D.H. Cystic fibrosis of the pancreas and its relation to celiac disease: A clinical and pathologic study. Am. J. Dis. Child. 1938, 56, 344–399. [Google Scholar] [CrossRef]
- Farber: Some Organic Digestive Disturbances in Early Life–Google Scholar. Available online: https://scholar.google.com/scholar_lookup?hl=en&volume=44&publication_year=1945&pages=587-594&journal=J.+Mich.+State.+Med.+Soc.&author=S.+FARBER&title=Some+organic+digestive+disturbances+in+early+life (accessed on 1 August 2024).
- Andersen, D.H.; Hodges, R.G. Celiac syndrome; genetics of cystic fibrosis of the pancreas, with a consideration of etiology. Am. J. Dis. Child. 1946, 72, 62–80. [Google Scholar] [CrossRef]
- Di Sant’Agnese, P.A.; Darling, R.C.; Perera, G.A.; Shea, E. Abnormal electrolyte composition of sweat in cystic fibrosis of the pancreas; Clinical significance and relationship to the disease. Pediatrics 1953, 12, 549–563. [Google Scholar] [CrossRef] [PubMed]
- Gibson, L.E.; Cooke, R.E. A test for concentration of electrolytes in sweat in cystic fibrosis of the pancreas utilizing pilocarpine by iontophoresis. Pediatrics 1959, 23, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Mangos, J.A.; McSherry, N.R.; Benke, P.J. A Sodium Transport Inhibitory Factor in the Saliva of Patients with Cystic Fibrosis of the Pancreas. Pediatr. Res. 1967, 1, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Comstock, A.T.; Sajjan, U.S. Barrier function of airway tract epithelium. Tissue Barriers 2013, 1, e24997. [Google Scholar] [CrossRef] [PubMed]
- Frayman, K.B.; Armstrong, D.S.; Carzino, R.; Ferkol, T.W.; Grimwood, K.; Storch, G.A.; Teo, S.M.; Wylie, K.M.; Ranganathan, S.C. The lower airway microbiota in early cystic fibrosis lung disease: A longitudinal analysis. Thorax 2017, 72, 1104–1112. [Google Scholar] [CrossRef]
- Boucher, R.C. Muco-Obstructive Lung Diseases. N. Engl. J. Med. 2019, 380, 1941–1953. [Google Scholar] [CrossRef]
- Henderson, A.G.; Ehre, C.; Button, B.; Abdullah, L.H.; Cai, L.-H.; Leigh, M.W.; DeMaria, G.C.; Matsui, H.; Donaldson, S.H.; Davis, C.W.; et al. Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure. J. Clin. Investig. 2014, 124, 3047–3060. [Google Scholar] [CrossRef]
- Bush, A.; Payne, D.; Pike, S.; Jenkins, G.; Henke, M.O.; Rubin, B.K. Mucus properties in children with primary ciliary dyskinesia: Comparison with cystic fibrosis. Chest 2006, 129, 118–123. [Google Scholar] [CrossRef]
- Ghosh, A.; Boucher, R.C.; Tarran, R. Airway hydration and COPD. Cell. Mol. Life Sci. 2015, 72, 3637–3652. [Google Scholar] [CrossRef]
- Welsh, M.J. Intracellular chloride activities in canine tracheal epithelium. Direct evidence for sodium-coupled intracellular chloride accumulation in a chloride-secreting epithelium. J. Clin. Investig. 1983, 71, 1392–1401. [Google Scholar] [CrossRef]
- Welsh, M.J.; Widdicombe, J.H.; Nadel, J.A. Fluid transport across the canine tracheal epithelium. J. Appl. Physiol. 1980, 49, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Widdicombe, J.H.; Welsh, M.J.; Finkbeiner, W.E. Cystic fibrosis decreases the apical membrane chloride permeability of mon-olayers cultured from cells of tracheal epithelium. Proc. Natl. Acad. Sci. USA 1985, 82, 6167–6171. [Google Scholar] [CrossRef]
- Quinton, P.M. Chloride impermeability in cystic fibrosis. Nature 1983, 301, 421–422. [Google Scholar] [CrossRef] [PubMed]
- Quinton, P.M.; Bijman, J. Higher Bioelectric Potentials Due to Decreased Chloride Absorption in the Sweat Glands of Patients with Cystic Fibrosis. N. Engl. J. Med. 1983, 308, 1185–1189. [Google Scholar] [CrossRef] [PubMed]
- Knowles, M.R.; Stutts, M.J.; Spock, A.; Fischer, N.; Gatzy, J.T.; Boucher, R.C. Abnormal Ion Permeation Through Cystic Fibrosis Respiratory Epithelium. Science 1983, 221, 1067–1070. [Google Scholar] [CrossRef]
- Knowles, M.; Gatzy, J.; Boucher, R. Relative ion permeability of normal and cystic fibrosis nasal epithelium. J. Clin. Investig. 1983, 71, 1410–1417. [Google Scholar] [CrossRef] [PubMed]
- Boucher, R.C.; Cotton, C.U.; Gatzy, J.T.; Knowles, M.R.; Yankaskas, J.R. Evidence for reduced Cl− and increased Na+ permeability in cystic fibrosis human primary cell cultures. J. Physiol. 1988, 405, 77–103. [Google Scholar] [CrossRef] [PubMed]
- Cotton, C.U.; Stutts, M.J.; Knowles, M.R.; Gatzy, J.T.; Boucher, R.C. Abnormal apical cell membrane in cystic fibrosis respiratory epithelium. An in vitro electrophysiologic analysis. J. Clin. Investig. 1987, 79, 80–85. [Google Scholar] [CrossRef]
- Berschneider, H.M.; Knowles, M.R.; Azizkhan, R.G.; Boucher, R.C.; Tobey, N.A.; Orlando, R.C.; Powell, D.W. Altered intestinal chloride transport in cystic fibrosis. FASEB J. 1988, 2, 2625–2629. [Google Scholar] [CrossRef]
- Boucher, R.C.; Stutts, M.J.; Knowles, M.R.; Cantley, L.; Gatzy, J.T. Na+ transport in cystic fibrosis respiratory epithelia. Abnormal basal rate and response to adenylate cyclase activation. J. Clin. Investig. 1986, 78, 1245–1252. [Google Scholar] [CrossRef]
- Anderson, M.P.; Gregory, R.J.; Thompson, S.; Souza, D.W.; Paul, S.; Mulligan, R.C.; Smith, A.E.; Welsh, M.J. Demonstration That CFTR Is a Chloride Channel by Alteration of Its Anion Selectivity. Science 1991, 253, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.P.; Berger, H.A.; Rich, D.P.; Gregory, R.J.; Smith, A.E.; Welsh, M.J. Nucleoside triphosphates are required to open the CFTR chloride channel. Cell 1991, 67, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.P.; Welsh, M.J. Calcium and cAMP activate different chloride channels in the apical membrane of normal and cystic fibrosis epithelia. Proc. Natl. Acad. Sci. USA 1991, 88, 6003–6007. [Google Scholar] [CrossRef] [PubMed]
- Bear, C.E.; Reyes, E.F. cAMP-activated chloride conductance in the colonic cell line, Caco-2. Am. J. Physiol. 1992, 262, C251–C256. [Google Scholar] [CrossRef] [PubMed]
- Stutts, M.J.; Canessa, C.M.; Olsen, J.C.; Hamrick, M.; Cohn, J.A.; Rossier, B.C.; Boucher, R.C. CFTR as a cAMP-dependent regulator of sodium channels. Science 1995, 269, 847–850. [Google Scholar] [CrossRef]
- Picciotto, M.; Cohn, J.; Bertuzzi, G.; Greengard, P.; Nairn, A. Phosphorylation of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 1992, 267, 12742–12752. [Google Scholar] [CrossRef]
- Quinton, P.M. Cystic fibrosis: A disease in electrolyte transport. FASEB J. 1990, 4, 2709–2710. [Google Scholar] [CrossRef] [PubMed]
- Knowles, M.; Gatzy, J.; Boucher, R. Increased Bioelectric Potential Difference across Respiratory Epithelia in Cystic Fibrosis. N. Engl. J. Med. 1981, 305, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Knowles, M.R.; Carson, J.L.; Collier, A.M.; Gatzy, J.T.; Boucher, R.C. Measurements of nasal transepithelial electric potential differences in normal human subjects in vivo. Am. Rev. Respir. Dis. 1981, 124, 484–490. [Google Scholar]
- Canessa, C.M.; Schild, L.; Buell, G.; Thorens, B.; Gautschi, I.; Horisberger, J.-D.; Rossier, B.C. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 1994, 367, 463–467. [Google Scholar] [CrossRef]
- Snyder, P.; McDonald, F.; Stokes, J.; Welsh, M. Membrane topology of the amiloride-sensitive epithelial sodium channel. J. Biol. Chem. 1994, 269, 24379–24383. [Google Scholar] [CrossRef] [PubMed]
- Kunzelmann, K.; Kiser, G.; Schreiber, R.; Riordan, J. Inhibition of epithelial Na+ currents by intracellular domains of the cystic fibrosis transmembrane conductance regulator. FEBS Lett. 1997, 400, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Letz, B.; Korbmacher, C. cAMP stimulates CFTR-like Cl− channels and inhibits amiloride-sensitive Na+ channels in mouse CCD cells. Am. J. Physiol. Physiol. 1997, 272, C657–C666. [Google Scholar] [CrossRef] [PubMed]
- Berdiev, B.K.; Qadri, Y.J.; Benos, D.J. Assessment of the CFTR and ENaC association. Mol. Biosyst. 2008, 5, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Nagel, G.; Szellas, T.; Riordan, J.R.; Friedrich, T.; Hartung, K. Non-specific activation of the epithelial sodium channel by the CFTR chloride channel. EMBO Rep. 2001, 2, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Nagel, G.; Barbry, P.; Chabot, H.; Brochiero, E.; Hartung, K.; Grygorczyk, R. CFTR fails to inhibit the epithelial sodium channel ENaC expressed in Xenopus laevis oocytes. J. Physiol. 2005, 564, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.H.; Gregory, R.J.; Marshall, J.; Paul, S.; Souza, D.W.; White, G.A.; O’Riordan, C.R.; Smith, A.E. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell 1990, 63, 827–834. [Google Scholar] [CrossRef]
- Zielenski, J.; Tsui, L.-C. Cystic Fibrosis: Genotypic and Phenotypic Variations. Annu. Rev. Genet. 1995, 29, 777–807. [Google Scholar] [CrossRef]
- Lukacs, G.; Chang, X.; Bear, C.; Kartner, N.; Mohamed, A.; Riordan, J.; Grinstein, S. The delta F508 mutation decreases the stability of cystic fibrosis transmembrane conductance regulator in the plasma membrane. Determination of functional half-lives on transfected cells. J. Biol. Chem. 1993, 268, 21592–21598. [Google Scholar] [CrossRef]
- Farrell, P.; Férec, C.; Macek, M.; Frischer, T.; Renner, S.; Riss, K.; Barton, D.; Repetto, T.; Tzetis, M.; Giteau, K.; et al. Estimating the age of p.(Phe508del) with family studies of geographically distinct European populations and the early spread of cystic fibrosis. Eur. J. Hum. Genet. 2018, 26, 1832–1839. [Google Scholar] [CrossRef]
- Férec, C.; Audrezet, M.; Mercier, B.; Guillermit, H.; Moullier, P.; Quere, I.; Verlingue, C. Detection of over 98% cystic fibrosis mutations in a Celtic population. Nat. Genet. 1992, 1, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Levring, J.; Terry, D.S.; Kilic, Z.; Fitzgerald, G.; Blanchard, S.C.; Chen, J. CFTR function, pathology and pharmacology at single-molecule resolution. Nature 2023, 616, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Chappe, V.; Hinkson, D.A.; Howell, L.D.; Evagelidis, A.; Liao, J.; Chang, X.-B.; Riordan, J.R.; Hanrahan, J.W. Stimulatory and inhibitory protein kinase C consensus sequences regulate the cystic fibrosis transmembrane conductance regulator. Proc. Natl. Acad. Sci. USA 2003, 101, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Seavilleklein, G.; Amer, N.; Evagelidis, A.; Chappe, F.; Irvine, T.; Hanrahan, J.W.; Chappe, V. PKC phosphorylation modulates PKA-dependent binding of the R domain to other domains of CFTR. Am. J. Physiol. Physiol. 2008, 295, C1366–C1375. [Google Scholar] [CrossRef] [PubMed]
- Seibert, F.; Chang, X.-B.; Aleksandrov, A.; Clarke, D.; Hanrahan, J.; Riordan, J. Influence of phosphorylation by protein kinase A on CFTR at the cell surface and endoplasmic reticulum. Biochim. Biophys. Acta (BBA)—Biomembr. 1999, 1461, 275–283. [Google Scholar] [CrossRef]
- Guggino, W.B. The Cystic Fibrosis Transmembrane Regulator Forms Macromolecular Complexes with PDZ Domain Scaffold Proteins. Proc. Am. Thorac. Soc. 2004, 1, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Murabito, A.; Bhatt, J.; Ghigo, A. It Takes Two to Tango! Protein-Protein Interactions behind cAMP-Mediated CFTR Regulation. Int. J. Mol. Sci. 2023, 24, 10538. [Google Scholar] [CrossRef]
- Li, C.; Naren, A.P. Macromolecular complexes of cystic fibrosis transmembrane conductance regulator and its interacting partners. Pharmacol. Ther. 2005, 108, 208–223. [Google Scholar] [CrossRef] [PubMed]
- Estabrooks, S.; Brodsky, J.L. Regulation of CFTR Biogenesis by the Proteostatic Network and Pharmacological Modulators. Int. J. Mol. Sci. 2020, 21, 452. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.; Aissat, A.; Degrugillier, F.; Simonneau, B.; Fanen, P.; Arrigo, A.-P. Small Hsps as Therapeutic Targets of Cystic Fibrosis Transmembrane Conductance Regulator Protein. Int. J. Mol. Sci. 2021, 22, 4252. [Google Scholar] [CrossRef]
- Amaral, M.D. CFTR and Chaperones: Processing and Degradation. J. Mol. Neurosci. 2004, 23, 041–048. [Google Scholar] [CrossRef] [PubMed]
- Mall, M.A. ENaC inhibition in cystic fibrosis: Potential role in the new era of CFTR modulator therapies. Eur. Respir. J. 2020, 56, 2000946. [Google Scholar] [CrossRef] [PubMed]
- Mall, M.A.; Grubb, B.R.; Harkema, J.R.; O’Neal, W.K.; Boucher, R.C. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat. Med. 2004, 10, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Mall, M.; Hipper, A.; Greger, R.; Kunzelmann, K. Wild type but not deltaF508 CFTR inhibits Na+ conductance when coexpressed in Xenopus oocytes. FEBS Lett. 1996, 381, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Mornon, J.-P.; Hoffmann, B.; Jonic, S.; Lehn, P.; Callebaut, I. Full-open and closed CFTR channels, with lateral tunnels from the cytoplasm and an alternative position of the F508 region, as revealed by molecular dynamics. Cell. Mol. Life Sci. 2014, 72, 1377–1403. [Google Scholar] [CrossRef] [PubMed]
- Callebaut, I.; Hoffmann, B.; Mornon, J.-P. The implications of CFTR structural studies for cystic fibrosis drug development. Curr. Opin. Pharmacol. 2017, 34, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Callebaut, I.; Hoffmann, B.; Lehn, P.; Mornon, J.-P. Molecular modelling and molecular dynamics of CFTR. Cell. Mol. Life Sci. 2016, 74, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Cutting, G.R. Cystic fibrosis genetics: From molecular understanding to clinical application. Nat. Rev. Genet. 2014, 16, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Santis, G.; Osborne, L.; Knight, R.; Hodson, M. Independent genetic determinants of pancreatic and pulmonary status in cystic fibrosis. Lancet 1990, 336, 1081–1084. [Google Scholar] [CrossRef]
- Rowntree, R.K.; Harris, A. The Phenotypic Consequences of CFTR Mutations. Ann. Hum. Genet. 2003, 67, 471–485. [Google Scholar] [CrossRef]
- Claustres, M.; Thèze, C.; Georges, M.D.; Baux, D.; Girodon, E.; Bienvenu, T.; Audrezet, M.-P.; Dugueperoux, I.; Férec, C.; Lalau, G.; et al. CFTR-France, a national relational patient database for sharing genetic and phenotypic data associated with rare CFTR variants. Hum. Mutat. 2017, 38, 1297–1315. [Google Scholar] [CrossRef] [PubMed]
- Burgel, P.-R.; Bellis, G.; Olesen, H.V.; Viviani, L.; Zolin, A.; Blasi, F.; Elborn, J.S.; ERS/ECFS Task Force on Provision of Care for Adults with Cystic Fibrosisin Europe. Future trends in cystic fibrosis demography in 34 European countries. Eur. Respir. J. 2015, 46, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Mésinèle, J.; Ruffin, M.; Guillot, L.; Corvol, H. Modifier Factors of Cystic Fibrosis Phenotypes: A Focus on Modifier Genes. Int. J. Mol. Sci. 2022, 23, 14205. [Google Scholar] [CrossRef]
- Trouvé, P.; Génin, E.; Férec, C. In silico search for modifier genes associated with pancreatic and liver disease in Cystic Fibrosis. PLoS ONE 2017, 12, e0173822. [Google Scholar] [CrossRef]
- Simmonds, N.; Southern, K.; De Wachter, E.; De Boeck, K.; Bodewes, F.; Mainz, J.; Middleton, P.; Schwarz, C.; Vloeberghs, V.; Wilschanski, M.; et al. ECFS standards of care on CFTR-related disorders: Identification and care of the disorders. J. Cyst. Fibros. 2024, 23, 590–602. [Google Scholar] [CrossRef]
- Pagin, A.; Sermet-Gaudelus, I.; Burgel, P.-R. Genetic diagnosis in practice: From cystic fibrosis to CFTR-related disorders. Arch. Pediatr. 2020, 27, eS25–eS29. [Google Scholar] [CrossRef] [PubMed]
- Ferec, C.; Cutting, G.R. Assessing the Disease-Liability of Mutations in CFTR. Cold Spring Harb. Perspect. Med. 2012, 2, a009480. [Google Scholar] [CrossRef] [PubMed]
- Dumur, V.; Gervais, R.; Rigot, J.M.; Lafitte, J.J.; Manouvrier, S.; Biserte, J.; Mazeman, E.; Roussel, P. Abnormal distribution of CF delta F508 allele in azoospermic men with congenital aplasia of epididymis and vas deferens. Lancet 1990, 336, 512. [Google Scholar] [CrossRef]
- Anguiano, A.; Oates, R.D.; Amos, J.A.; Dean, M.; Gerrard, B.; Stewart, C.; Maher, T.A.; White, M.B.; Milunsky, A. Congenital bilateral absence of the vas deferens. A primarily genital form of cystic fibrosis. JAMA 1992, 267, 1794–1797. [Google Scholar] [CrossRef] [PubMed]
- Chillón, M.; Casals, T.; Mercier, B.; Bassas, L.; Lissens, W.; Silber, S.; Romey, M.-C.; Ruiz-Romero, J.; Verlingue, C.; Claustres, M.; et al. Mutations in the Cystic Fibrosis Gene in Patients with Congenital Absence of the Vas Deferens. N. Engl. J. Med. 1995, 332, 1475–1480. [Google Scholar] [CrossRef] [PubMed]
- Mercier, B.; Verlingue, C.; Lissens, W.; Silber, S.J.; Novelli, G.; Bonduelle, M.; Audrézet, M.P.; Férec, C. Is congenital bilateral absence of vas deferens a primary form of cystic fibrosis? Analyses of the CFTR gene in 67 patients. Am. J. Hum. Genet. 1995, 56, 272–277. [Google Scholar] [PubMed]
- Cohn, J.A.; Friedman, K.J.; Noone, P.G.; Knowles, M.R.; Silverman, L.M.; Jowell, P.S. Relation between Mutations of the Cystic Fibrosis Gene and Idiopathic Pancreatitis. N. Engl. J. Med. 1998, 339, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Masson, E.; Chen, J.-M.; Audrézet, M.-P.; Cooper, D.N.; Férec, C. A Conservative Assessment of the Major Genetic Causes of Idiopathic Chronic Pancreatitis: Data from a Comprehensive Analysis of PRSS1, SPINK1, CTRC and CFTR Genes in 253 Young French Patients. PLoS ONE 2013, 8, e73522. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-M.; Férec, C. Chronic Pancreatitis: Genetics and Pathogenesis. Annu. Rev. Genom. Hum. Genet. 2009, 10, 63–87. [Google Scholar] [CrossRef]
- Miller, A.C.; Comellas, A.P.; Hornick, D.B.; Stoltz, D.A.; Cavanaugh, J.E.; Gerke, A.K.; Welsh, M.J.; Zabner, J.; Polgreen, P.M. Cystic fibrosis carriers are at increased risk for a wide range of cystic fibrosis-related conditions. Proc. Natl. Acad. Sci. USA 2019, 117, 1621–1627. [Google Scholar] [CrossRef]
- Moran, A.; Oates, R.D.; Amos, J.A.; Dean, M.; Gerrard, B.; Stewart, C.; Maher, T.A.; White, M.B.; Milunsky, A. Cystic fibrosis-related diabetes: Current trends in prevalence, incidence, and mortality. Diabetes Care 2009, 32, 1626–1631. [Google Scholar] [CrossRef] [PubMed]
- Bombieri, C.; Claustres, M.; De Boeck, K.; Derichs, N.; Dodge, J.; Girodon, E.; Sermet, I.; Schwarz, M.; Tzetis, M.; Bareil, C.; et al. Recommendations for the classification of diseases as CFTR-related disorders. J. Cyst. Fibros. 2011, 10 (Suppl. S2), S86–S102. [Google Scholar] [CrossRef]
- Farrell, P.M.; Kosorok, M.R.; Rock, M.J.; Laxova, A.; Zeng, L.; Lai, H.-C.; Hoffman, G.; Laessig, R.H.; Splaingard, M.L.; the Wisconsin Cystic Fibrosis Neonatal Screening Study Group. Early Diagnosis of Cystic Fibrosis Through Neonatal Screening Prevents Severe Malnutrition and Improves Long-Term Growth. Pediatrics 2001, 107, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Scotet, V.; Dunitz, J.; Nathan, B.; Saeed, A.; Holme, B.; Thomas, W. Neonatal screening for cystic fibrosis in Brittany, France: Assessment of 10 years’ experience and impact on prenatal diagnosis. Lancet 2000, 356, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Scotet, V.; L’hostis, C.; Férec, C. The Changing Epidemiology of Cystic Fibrosis: Incidence, Survival and Impact of the CFTR Gene Discovery. Genes 2020, 11, 589. [Google Scholar] [CrossRef]
- Fajac, I.; Burgel, P.-R. Demographic growth and targeted therapies: The changing face of cystic fibrosis. Rev. Mal. Respir. 2016, 33, 645–647. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, A.L.; Stanojevic, S.; Sykes, J.; Burgel, P.-R. The changing epidemiology and demography of cystic fibrosis. Presse Med. 2017, 46, e87–e95. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.M.; Rock, M.J.; Baker, M.W. The Impact of the CFTR Gene Discovery on Cystic Fibrosis Diagnosis, Counseling, and Preventive Therapy. Genes 2020, 11, 401. [Google Scholar] [CrossRef]
- Southern, K.W.; Munck, A.; Pollitt, R.; Travert, G.; Zanolla, L.; Dankert-Roelse, J.; Castellani, C. A survey of newborn screening for cystic fibrosis in Europe. J. Cyst. Fibros. 2007, 6, 57–65. [Google Scholar] [CrossRef]
- Férec, C.; Scotet, V. Genetics of cystic fibrosis: Basics. Arch. Pediatr. 2020, 27, eS4–eS7. [Google Scholar] [CrossRef] [PubMed]
- Scotet, V.; Gutierrez, H.; Farrell, P.M. Newborn Screening for CF across the Globe-Where Is It Worthwhile? Int. J. Neonatal Screen. 2020, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- ECFS Patient Registry. Available online: https://www.ecfs.eu/sites/default/files/general-content-images/working-groups/ecfs-patient-registry/ECFSPR_Report_2019_v1_23Dec2021.pdf (accessed on 1 August 2024).
- Di Sant’agnese, P.E.A.; Andersen, D.H. Celiac syndrome; chemotherapy in infections of the respiratory tract associated with cystic fibrosis of the pancreas; observations with penicillin and drugs of the sulfonamide group, with special reference to penicillin aerosol. Am. J. Dis. Child. 1911 1946, 72, 17–61. [Google Scholar] [CrossRef]
- Doyle, B. Physical Therapy in the Treatment of Cystic Fibrosis. Phys. Ther. 1959, 39, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Crozier, D. Cystic Fibrosis: A Not-So-Fatal Disease. Pediatr. Clin. N. Am. 1974, 21, 935–950. [Google Scholar] [CrossRef] [PubMed]
- Crossle, J.; Elliot, R.; Smith, P. Dried-Blood Spot Screening for Cystic Fibrosis in the Newborn. Lancet 1979, 313, 472–474. [Google Scholar] [CrossRef]
- Hodson, M.; Penketh, A.; Batten, J. Aerosol Carbenicillin and Gentamicin Treatment of Pseudomonas aeruginosa Infection in Patients with Cystic Fibrosis. Lancet 1981, 318, 1137–1139. [Google Scholar] [CrossRef]
- Gilbert, J.; Robinson, T.; Littlewood, J.M. Home intravenous antibiotic treatment in cystic fibrosis. Arch. Dis. Child. 1988, 63, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, M.; Banner, N.; Khaghani, A.; Fitzgerald, M.; Madden, B.; Tsang, V.; Radleysmith, R.; Hodson, M. Heart-Lung Transplantation for Cystic-Fibrosis and Subsequent Domino Heart-Transplantation. J. Heart Transplant. 1990, 9, 459–467. [Google Scholar] [PubMed]
- Krauss, R.; Bubien, J.; Drumm, M.; Zheng, T.; Peiper, S.; Collins, F.; Kirk, K.; Frizzell, R.; Rado, T. Transfection of wild-type CFTR into cystic fibrosis lymphocytes restores chloride conductance at G1 of the cell cycle. EMBO J. 1992, 11, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Anguela, X.M.; High, K.A. Entering the Modern Era of Gene Therapy. Annu. Rev. Med. 2019, 70, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Belmadi, N.; Midoux, P.; Loyer, P.; Passirani, C.; Pichon, C.; Le Gall, T.; Jaffres, P.; Lehn, P.; Montier, T. Synthetic vectors for gene delivery: An overview of their evolution depending on routes of administration. Biotechnol. J. 2015, 10, 1370–1389. [Google Scholar] [CrossRef]
- Sun, Y.; Chatterjee, S.; Lian, X.; Traylor, Z.; Sattiraju, S.R.; Xiao, Y.; Dilliard, S.A.; Sung, Y.-C.; Kim, M.; Lee, S.M.; et al. In vivo editing of lung stem cells for durable gene correction in mice. Science 2024, 384, 1196–1202. [Google Scholar] [CrossRef]
- Aslam, A.A.; Sinha, I.P.; Southern, K.W. Ataluren and similar compounds (specific therapies for premature termination codon class I mutations) for cystic fibrosis. Cochrane Database Syst. Rev. 2023, 3, CD012040. [Google Scholar]
- Laselva, O.; Eckford, P.D.; Bartlett, C.; Ouyang, H.; NA Gunawardena, T.; Gonska, T.; Moraes, T.J.; Bear, C.E. Functional rescue of c.3846G>A (W1282X) in patient-derived nasal cultures achieved by inhibition of nonsense mediated decay and protein modulators with complementary mechanisms of action. J. Cyst. Fibros. 2019, 19, 717–727. [Google Scholar] [CrossRef]
- Kerem, E.; Konstan, M.W.; De Boeck, K.; Accurso, F.J.; Sermet-Gaudelus, I.; Wilschanski, M.; Elborn, J.S.; Melotti, P.; Bronsveld, I.; Fajac, I.; et al. Ataluren for the treatment of nonsense-mutation cystic fibrosis: A randomised, double-blind, placebo-controlled phase 3 trial. Lancet Respir. Med. 2014, 2, 539–547. [Google Scholar] [CrossRef]
- McElroy, S.P.; Nomura, T.; Torrie, L.S.; Warbrick, E.; Gartner, U.; Wood, G.; McLean, W.H.I. A Lack of Premature Termination Codon Read-Through Efficacy of PTC124 (Ataluren) in a Diverse Array of Reporter Assays. PLoS Biol. 2013, 11, e1001593. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Jones, J.R.; Lanier, J.; Keeling, K.M.; Lindsey, R.J.; Tousson, A.; Bebök, Z.; Whitsett, J.A.; Dey, C.R.; Colledge, W.H.; et al. Aminoglycoside suppression of a premature stop mutation in a Cftr−/− mouse carrying a human CFTR-G542X transgene. J. Mol. Med. Berl. Ger. 2002, 80, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.; Frizzell, R.A.; Bedwell, D.M. Aminoglycoside antibiotics restore CFTR function by overcoming premature stop mutations. Nat. Med. 1996, 2, 467–469. [Google Scholar] [CrossRef]
- Ng, M.Y.; Li, H.; Ghelfi, M.D.; Goldman, Y.E.; Cooperman, B.S. Ataluren and aminoglycosides stimulate read-through of non-sense codons by orthogonal mechanisms. Proc. Natl. Acad. Sci. USA 2021, 118, e2020599118. [Google Scholar] [CrossRef]
- Lueck, J.D.; Yoon, J.S.; Perales-Puchalt, A.; Mackey, A.L.; Infield, D.T.; Behlke, M.A.; Pope, M.R.; Weiner, D.B.; Skach, W.R.; McCray, P.B.; et al. Engineered transfer RNAs for suppression of premature termination codons. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Harrison, P.T. CFTR RNA- and DNA-based therapies. Curr. Opin. Pharmacol. 2022, 65, 102247. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.; Porter, J.J.; Sipple, M.T.; Edwards, K.M.; Lueck, J.D. Efficient suppression of endogenous CFTR nonsense mutations using anticodon-engineered transfer RNAs. Mol. Ther.–Nucleic Acids 2022, 28, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.J.; Mao, Y.; White, T.R.; Foye, C.; Oliver, K.E. Features of CFTR mRNA and implications for therapeutics development. Front. Genet. 2023, 14, 1166529. [Google Scholar] [CrossRef] [PubMed]
- Van Goor, F.; Burton, B.; Hadida, S.; Grootenhuis, P.; Olson, E.; Wine, J.; Frizzell, R.; Ashlock, M.; Negulescu, P. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. J. Cyst. Fibros. 2009, 8, S17. [Google Scholar] [CrossRef]
- Ramsey, B.W.; Davies, J.; McElvaney, N.G.; Tullis, E.; Bell, S.C.; Dřevínek, P.; Griese, M.; McKone, E.F.; Wainwright, C.E.; Konstan, M.W.; et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N. Engl. J. Med. 2011, 365, 1663–1672. [Google Scholar] [CrossRef]
- Farinha, C.M.; Canato, S. From the endoplasmic reticulum to the plasma membrane: Mechanisms of CFTR folding and trafficking. Cell. Mol. Life Sci. 2017, 74, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Farinha, C.M.; Amaral, M.D. Most F508del-CFTR Is Targeted to Degradation at an Early Folding Checkpoint and Independently of Calnexin. Mol. Cell. Biol. 2005, 25, 5242–5252. [Google Scholar] [CrossRef] [PubMed]
- Pedemonte, N.; Lukacs, G.L.; Du, K.; Caci, E.; Zegarra-Moran, O.; Galietta, L.J.; Verkman, A.S. Small-molecule correctors of defective DeltaF508-CFTR cellular processing identified by high-throughput screening. J. Clin. Investig. 2005, 115, 2564–2571. [Google Scholar] [CrossRef]
- Li, H.; Pesce, E.; Sheppard, D.N.; Singh, A.K.; Pedemonte, N. Therapeutic approaches to CFTR dysfunction: From discovery to drug development. J. Cyst. Fibros. 2018, 17, S14–S21. [Google Scholar] [CrossRef]
- Farinha, C.M.; Sousa, M.; Canato, S.; Schmidt, A.; Uliyakina, I.; Amaral, M.D. Increased efficacy of VX-809 in different cellular systems results from an early stabilization effect of F508del-CFTR. Pharmacol. Res. Perspect. 2015, 3, e00152. [Google Scholar] [CrossRef] [PubMed]
- Fiedorczuk, K.; Chen, J. Mechanism of CFTR correction by type I folding correctors. Cell 2022, 185, 158–168.e11. [Google Scholar] [CrossRef] [PubMed]
- Aalbers, B.L.; De Winter-de Groot, K.M.; Arets, H.G.; Hofland, R.W.; de Kiviet, A.C.; van Oirschot-van de Ven, M.M.M.; Kruijswijk, M.A.; Schotman, S.; Michel, S.; van der Ent, C.K.; et al. Clinical effect of lumacaftor/ivacaftor in F508del homozygous CF patients with FEV1 ≥ 90% predicted at baseline. J. Cyst. Fibros. 2020, 19, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Connett, G.J. Lumacaftor-ivacaftor in the treatment of cystic fibrosis: Design, development and place in therapy. Drug Des. Dev. Ther. 2019, 13, 2405–2412. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, C.E.; Elborn, J.S.; Ramsey, B.W.; Marigowda, G.; Huang, X.; Cipolli, M.; Colombo, C.; Davies, J.C.; De Boeck, K.; Flume, P.A.; et al. Lumacaftor–Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del CFTR. N. Engl. J. Med. 2015, 373, 220–231. [Google Scholar] [CrossRef]
- Sutharsan, S.; McKone, E.F.; Downey, D.G.; Duckers, J.; MacGregor, G.; Tullis, E.; Van Braeckel, E.E.; Wainwright, C.; Watson, D.; Ahluwalia, N.; et al. Efficacy and safety of elexacaftor plus tezacaftor plus ivacaftor versus tezacaftor plus ivacaftor in people with cystic fibrosis homozygous for F508del-CFTR: A 24-week, multicentre, randomised, double-blind, active-controlled, phase 3b trial. Lancet Respir. Med. 2021, 10, 267–277. [Google Scholar] [CrossRef]
- Ridley, K.; Condren, M. Elexacaftor-Tezacaftor-Ivacaftor: The First Triple-Combination Cystic Fibrosis Transmembrane Conductance Regulator Modulating Therapy. J. Pediatr. Pharmacol. Ther. 2020, 25, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Bacalhau, M.; Camargo, M.; Magalhães-Ghiotto, G.A.V.; Drumond, S.; Castelletti, C.H.M.; Lopes-Pacheco, M. Elexacaftor-Tezacaftor-Ivacaftor: A Life-Changing Triple Combination of CFTR Modulator Drugs for Cystic Fibrosis. Pharmaceuticals 2023, 16, 410. [Google Scholar] [CrossRef] [PubMed]
- Becq, F.; Mirval, S.; Carrez, T.; Lévêque, M.; Billet, A.; Coraux, C.; Sage, E.; Cantereau, A. The rescue of F508del-CFTR by elexacaftor/tezacaftor/ivacaftor (Trikafta) in human airway epithelial cells is underestimated due to the presence of ivacaftor. Eur. Respir. J. 2021, 59, 2100671. [Google Scholar] [CrossRef] [PubMed]
| Name and Mode of Action | Clinical Trial Phase | Targeted Mutations | Clinical Trial Results |
|---|---|---|---|
| Ivacaftor (Kalydeco®)/VX-770 Binds CFTR within the cleft formed by TM 4, 5, and 8 on the TMD2. Increases the probability of CFTR channel opening. | Marketed | G551D, S1251N, and other gating mutations | ~10% improvement in FEV1, ~48 mmol/L reduction in sweat chloride concentration |
| Deutivacaftor/VX-561 Derivates from VX-770 with enhanced in vitro stability and plasma half-life. | Phase 2 | Gating mutations | Improved lung function |
| GLPG-1837 Competitively binds to the same binding site as Ivacaftor. Necessitates a twice daily dosing regimen. | Phase 2 | G551D, S1251N | Improved lung function |
| GLPG-2451 Greater potency than GLPG-1837, supports a once-daily dosing regimen. | Phase 2 | Gating mutations | Improved lung function |
| PTI-808 Structurally resembling Ivacaftor. | Phase 2 | F508del and other mutations | Improved lung function, reduced sweat chloride |
| VX-121/Tezacaftor/Ivacaftor | Phase 3 | F508del and other mutations | Not yet published |
| ABBV-3067 | Phase 2 | Gating mutations | Not yet published |
| QBW251 | Phase 2 | Gating mutations | Not yet published |
| Name | Clinical Trial Phase | Clinical Trial Results |
|---|---|---|
| Lumacaftor (Orkambi®®)/VX-809 | Marketed | ~4–6% improvement in FEV1, ~30–40 mmol/L reduction in sweat chloride concentration1 |
| Tezacaftor (Symdeko®®)/VX-661 | Marketed | ~6–8% improvement in FEV1, ~40 mmol/L reduction in sweat chloride concentration2 |
| Elexacaftor (Trikafta®®/Kaftrio®®)/VX-445 | Marketed | ~14% improvement in FEV1, ~45–50 mmol/L reduction in sweat chloride concentration3 |
| ABBV-2222 | Phase 2 | Not yet published |
| GLPG-2222 | Phase 2 | Not yet published |
| PTI-801 | Phase 2 | Not yet published |
| Molecule Name | EU Market Name (Year) | CF Mutations |
|---|---|---|
| Ivacaftor | Kalydeco 2014 | Classes III and IV, gating and conduction mutations, residual function mutations G551D, S549N, G1244E, G178R, S1251N, G551S, G1349D, S1255P, R117H, E56K, K1060T, P67L, E193K, A1067T, R74W, L206W, G1069R, D110E, R347H, D579G, R1070Q, D1270N, D110H, R352Q, S945L, R1070W, R117C, A455E, S977F, F1074L, F1052V, D115H; 3849+10 kb C>T, 2789+5G>A, 3273-26A>G, 711+3A>G, E831X |
| Lumacaftor/Ivacaftor | Orkambi 2018 | Class II, p. Phe508del homozygous |
| Tezacaftor/Ivacaftor | Symkevi 2018 | Two copies of P.Phe508del One copy of P.Phe508del in association with E56K, K1060T, P67L, E193K, A1067T, R74W, L206W, D110E, D110H, R347H, D579G, R1070Q, D1270N, R352Q, S945L, R1070W, R117C, A455E, S977F, F1074L, F1052V, D1152H, 3849+10 kb C>T, 2789+5G>A, 327326A>G, 711+3A>G |
| Elexacaftor/Tezacaftor/ Ivacaftor | Kaftrio 2020 | Class II, at least one copy of P.Phe508del mutation and one copy with residual function mutation |
| Name | % Increase in FEV1 | Sweat Chloride Concentration | Age | Targeted Mutations |
|---|---|---|---|---|
| Kalydeco®® (Ivacaftor) | ~10% | Reduction of ~48 mmol/L | 6 months and older | G551D, S1251N, and other gating mutations |
| Orkambi®® (Lumacaftor/Ivacaftor) | ~4–6% | Reduction of ~30–40 mmol/L | 2 years and older | p.Phe508del homozygous |
| Symdeko®® (Tezacaftor/Ivacaftor) | ~6–8% | Reduction of ~40 mmol/L | 6 years and older | p.Phe508del homozygous or one F508del and one residual function mutation |
| Trikafta®®/Kaftrio®® (Elexacaftor/Tezacaftor/Ivacaftor) | ~14% | Reduction of ~45–50 mmol/L | 6 years and older | At least one p.Phe508del mutation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trouvé, P.; Saint Pierre, A.; Férec, C. Cystic Fibrosis: A Journey through Time and Hope. Int. J. Mol. Sci. 2024, 25, 9599. https://doi.org/10.3390/ijms25179599
Trouvé P, Saint Pierre A, Férec C. Cystic Fibrosis: A Journey through Time and Hope. International Journal of Molecular Sciences. 2024; 25(17):9599. https://doi.org/10.3390/ijms25179599
Chicago/Turabian StyleTrouvé, Pascal, Aude Saint Pierre, and Claude Férec. 2024. "Cystic Fibrosis: A Journey through Time and Hope" International Journal of Molecular Sciences 25, no. 17: 9599. https://doi.org/10.3390/ijms25179599







