Aquaporin-3a Dysfunction Impairs Osmoadaptation in Post-Activated Marine Fish Spermatozoa
Abstract
:1. Introduction
2. Results and Discussion
2.1. Aqp3a Is Accumulated during Seabream Sperm Maturation and Localized at the Posterior Flagellum
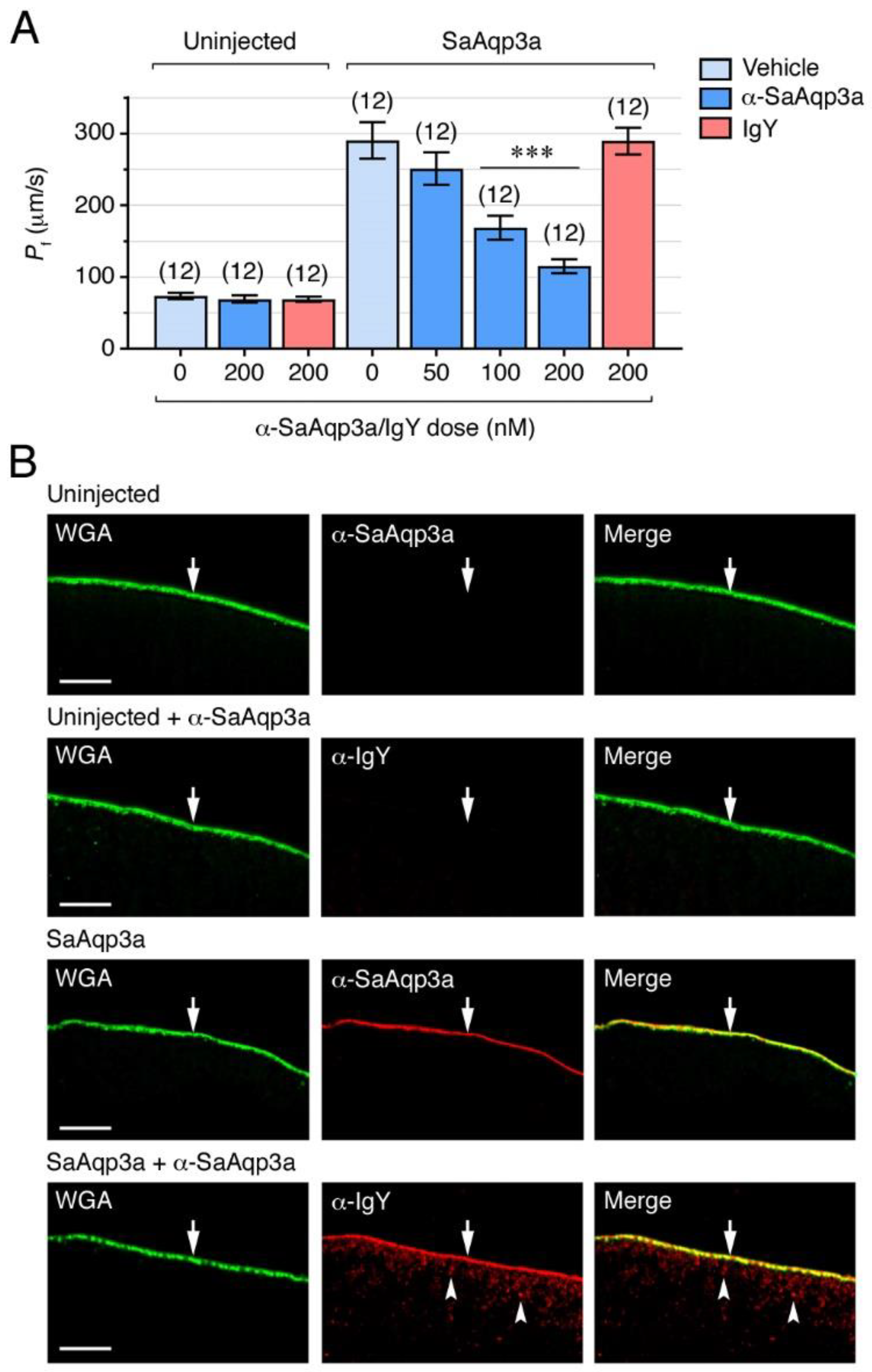
2.2. Specific Chemical and Immunological Inhibition of Seabream Aqp3a Channel Function
2.3. Aqp3a Inhibition Impairs Seabream Sperm Motility
2.4. Aqp3a Dysfunction Induces Tail Deformations in Post-Activated Spermatozoa
2.5. A Role of Aqp3a during Seabream Sperm Osmoadaptation
3. Materials and Methods
3.1. Animals and Sample Collection
3.2. Chemicals and Antibodies
3.3. Oocyte Swelling Assays
3.4. Sperm Motility Measurements
3.5. Protein Extraction and Immunoblotting
3.6. Immunofluorescence Microscopy
3.7. Electron Microscopy
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yeung, C.H.; Anapolski, M.; Depenbusch, M.; Zitzmann, M.; Cooper, T.G. Human sperm volume regulation. Response to physiological changes in osmolality, channel blockers and potential sperm osmolytes. Hum. Reprod. 2003, 18, 1029–1036. [Google Scholar] [CrossRef]
- Bondarenko, O.; Dzyuba, B.; Cosson, J.; Yamaner, G.; Prokopchuk, G.; Psenicka, M.; Linhart, O. Volume changes during the motility period of fish spermatozoa: Interspecies differences. Theriogenology 2013, 79, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.H.; Anapolski, M.; Sipilä, P.; Wagenfeld, A.; Poutanen, M.; Huhtaniemi, I.; Nieschlag, E.; Cooper, T.G. Sperm volume regulation: Maturational changes in fertile and infertile transgenic mice and association with kinematics and tail angulation. Biol. Reprod. 2002, 67, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Morisawa, M.; Suzuki, K. Osmolality and potassium ion: Their roles in initiation of sperm motility in teleosts. Science 1980, 210, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Cosson, J. Fish sperm physiology: Structure, factors regulating motility, and motility evaluation. Biol. Res. Aquat. Sci. 2019, 1, 1–26. [Google Scholar]
- Castro-Arnau, J.; Chauvigné, F.; Toft-Bertelsen, T.L.; Finn, R.N.; MacAulay, N.; Cerdà, J. Aqp4a and Trpv4 mediate regulatory cell volume increase for swimming maintenance of marine fish spermatozoa. Cell. Mol. Life Sci. 2024, 81, 285. [Google Scholar] [CrossRef]
- Chen, Q.; Peng, H.; Lei, L.; Zhang, Y.; Kuang, H.; Cao, Y.; Shi, Q.X.; Ma, T.; Duan, E. Aquaporin 3 is a sperm water channel essential for postcopulatory sperm osmoadaptation and migration. Cell Res. 2011, 21, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.H.; Callies, C.; Rojek, A.; Nielsen, S.; Cooper, T.G. Aquaporin isoforms involved in physiological volume regulation of murine spermatozoa. Biol. Reprod. 2009, 80, 350–357. [Google Scholar] [CrossRef]
- Carrageta, D.F.; Bernardino, R.L.; Soveral, G.; Calamita, G.; Alves, M.G.; Oliveira, P.F. Aquaporins and male (in) fertility: Expression and role throughout the male reproductive tract. Arch. Biochem. Biophys. 2020, 679, 108222. [Google Scholar] [CrossRef]
- Laforenza, U.; Pellavio, G.; Marchetti, A.L.; Omes, C.; Todaro, F.; Gastaldi, G. Aquaporin-Mediated water and hydrogen peroxide transport is involved in normal human spermatozoa functioning. Int. J. Mol. Sci. 2016, 18, 66. [Google Scholar] [CrossRef]
- Pellavio, G.; Laforenza, U. Human sperm functioning is related to the aquaporin-mediated water and hydrogen peroxide transport regulation. Biochimie 2021, 188, 45–51. [Google Scholar] [CrossRef]
- Chauvigné, F.; Boj, M.; Finn, R.N.; Cerdà, J. Mitochondrial aquaporin-8-mediated hydrogen peroxide transport is essential for teleost spermatozoon motility. Sci. Rep. 2015, 5, 7789. [Google Scholar] [CrossRef] [PubMed]
- Chauvigné, F.; Ducat, C.; Ferré, A.; Hansen, T.; Carrascal, M.; Abián, J.; Finn, R.N.; Cerdà, J. A multiplier peroxiporin signal transduction pathway powers piscine spermatozoa. Proc. Natl. Acad. Sci. USA 2021, 118, e2019346118. [Google Scholar] [CrossRef] [PubMed]
- Boj, M.; Chauvigné, F.; Cerdà, J. Aquaporin biology of spermatogenesis and sperm physiology in mammals and teleosts. Biol. Bull. 2015, 229, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.H.; Wagenfeld, A.; Nieschlag, E.; Cooper, T.G. The cause of infertility of male c-ros tyrosine kinase receptor knockout mice. Biol. Reprod. 2000, 63, 612–618. [Google Scholar] [CrossRef]
- Chen, Q.; Duan, E.K. Aquaporins in sperm osmoadaptation: An emerging role for volume regulation. Acta Pharmacol. Sin. 2011, 32, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, O.; Ferré, A.; Nilsen, F.; Fjelldal, P.G.; Cerdà, J.; Finn, R.N. Unravelling the complex duplication history of deuterostome glycerol transporters. Cells 2020, 9, 1663. [Google Scholar] [CrossRef]
- Zhang, Y.; Guan, Y.; Zhang, T.; Yuan, C.; Liu, Y.; Wang, Z. Adult exposure to bisphenol A in rare minnow Gobiocypris rarus reduces sperm quality with disruption of testicular aquaporins. Chemosphere 2018, 193, 365–375. [Google Scholar] [CrossRef]
- Chauvigné, F.; Boj, M.; Vilella, S.; Finn, R.N.; Cerdà, J. Subcellular localization of selectively permeable aquaporins in the male germ line of a marine teleost reveals spatial redistribution in activated spermatozoa. Biol. Reprod. 2013, 89, 37. [Google Scholar] [CrossRef]
- Castro-Arnau, J.; Chauvigné, F.; Gómez-Garrido, J.; Esteve-Codina, A.; Dabad, M.; Alioto, T.; Finn, R.N.; Cerdà, J. Developmental RNA-Seq transcriptomics of haploid germ cells and spermatozoa uncovers novel pathways associated with teleost spermiogenesis. Sci. Rep. 2022, 12, 14162. [Google Scholar] [CrossRef]
- Ishibashi, K.; Sasaki, S.; Fushimi, K.; Uchida, S.; Kuwahara, M.; Saito, H.; Furukawa, T.; Nakajima, K.; Yamaguchi, Y.; Gojobori, T.; et al. Molecular cloning and expression of a member of the aquaporin family with permeability to glycerol and urea in addition to water expressed at the basolateral membrane of kidney collecting duct cells. Proc. Natl. Acad. Sci. USA 1994, 91, 6269–6273. [Google Scholar] [CrossRef] [PubMed]
- MacIver, B.; Cutler, C.P.; Yin, J.; Hill, M.G.; Zeidel, M.L.; Hill, W.G. Expression and functional characterization of four aquaporin water channels from the European eel (Anguilla anguilla). J. Exp. Biol. 2009, 212, 2856–2863. [Google Scholar] [CrossRef]
- Tingaud-Sequeira, A.; Zapater, C.; Chauvigné, F.; Otero, D.; Cerdà, J. Adaptive plasticity of killifish (Fundulus heteroclitus) embryos: Dehydration-stimulated development and differential aquaporin-3 expression. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1041–R1052. [Google Scholar] [CrossRef]
- Tingaud-Sequeira, A.; Calusinska, M.; Finn, R.N.; Chauvigné, F.; Lozano, J.; Cerdà, J. The zebrafish genome encodes the largest vertebrate repertoire of functional aquaporins with dual paralogy and substrate specificities similar to mammals. BMC Evol. Biol. 2010, 10, 38. [Google Scholar] [CrossRef]
- Jung, D.; MacIver, B.; Jackson, B.P.; Barnaby, R.; Sato, J.D.; Zeidel, M.L.; Shaw, J.R.; Stanton, B.A. A novel aquaporin 3 in killifish (Fundulus heteroclitus) is not an arsenic channel. Toxicol. Sci. 2012, 127, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Chauvigné, F.; Parhi, J.; Ducat, C.; Ollé, J.; Finn, R.N.; Cerdà, J. The cellular localization and redistribution of multiple aquaporin paralogs in the spermatic duct epithelium of a maturing marine teleost. J. Anat. 2018, 233, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Madsen, S.S.; Engelund, M.B.; Cutler, C.P. Water transport and functional dynamics of aquaporins in osmoregulatory organs of fishes. Biol. Bull. 2015, 229, 70–92. [Google Scholar] [CrossRef] [PubMed]
- Takvam, M.; Wood, C.M.; Kryvi, H.; Nilsen, T.O. Ion transporters and osmoregulation in the kidney of teleost fishes as a function of salinity. Front. Physiol. 2021, 12, 664588. [Google Scholar] [CrossRef]
- Ribeiro, J.C.; Bernardino, R.L.; Gonçalves, A.; Barros, A.; Calamita, G.; Alves, M.G.; Oliveira, P.F. Aquaporin-7-mediated glycerol permeability is linked to human sperm motility in asthenozoospermia and during sperm capacitation. Cells 2023, 12, 2003. [Google Scholar] [CrossRef]
- Naz, R.K. Effect of actinomycin D and cycloheximide on human sperm function. Arch. Androl. 1998, 41, 135–142. [Google Scholar] [CrossRef]
- Gur, Y.; Breitbart, H. Mammalian sperm translate nuclear-encoded proteins by mitochondrial-type ribosomes. Genes Dev. 2006, 20, 411–416. [Google Scholar] [CrossRef]
- Rajamanickam, G.D.; Kastelic, J.P.; Thundathil, J.C. Content of testis-specific isoform of Na/K-ATPase (ATP1A4) is increased during bovine sperm capacitation through translation in mitochondrial ribosomes. Cell Tissue Res. 2017, 368, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Umehara, T.; Okazaki, T.; Goto, M.; Fujita, Y.; Hoque, S.A.M.; Kawai, T.; Zeng, W.; Shimada, M. Gene expression and protein synthesis in mitochondria enhance the duration of high-speed linear motility in boar sperm. Front. Physiol. 2019, 10, 252. [Google Scholar] [CrossRef]
- Bisconti, M.; Leroy, B.; Gallagher, M.T.; Senet, C.; Martinet, B.; Arcolia, V.; Wattiez, R.; Kirkman-Brown, J.C.; Simon, J.F.; Hennebert, E. The ribosome inhibitor chloramphenicol induces motility deficits in human spermatozoa: A proteomic approach identifies potentially involved proteins. Front. Cell Dev. Biol. 2022, 10, 965076. [Google Scholar] [CrossRef]
- Sonntag, Y.; Gena, P.; Maggio, A.; Singh, T.; Artner, I.; Oklinski, M.K.; Johanson, U.; Kjellbom, P.; Nieland, J.D.; Nielsen, S.; et al. Identification and characterization of potent and selective aquaporin-3 and aquaporin-7 inhibitors. J. Biol. Chem. 2019, 294, 7377–7387. [Google Scholar] [CrossRef]
- Boj, M.; Chauvigné, F.; Cerdà, J. Coordinated action of aquaporins regulates sperm motility in a marine teleost. Biol. Reprod. 2015, 93, 40. [Google Scholar] [CrossRef]
- Cosson, J.; Groison, A.L.; Suquet, M.; Fauvel, C.; Dreanno, C.; Billard, R. Marine fish spermatozoa: Racing ephemeral swimmers. Reproduction 2008, 136, 277–294. [Google Scholar] [CrossRef] [PubMed]
- Dzyuba, V.; Cosson, J. Motility of fish spermatozoa: From external signaling to flagella response. Reprod. Biol. 2014, 14, 165–175. [Google Scholar] [CrossRef]
- Dzyuba, B.; Bondarenko, O.; Gazo, I.; Prokopchuk, G.; Cosson, J. Energetics of fish spermatozoa: The proven and the possible. Aquaculture 2017, 472, 60–72. [Google Scholar] [CrossRef]
- Chauvigné, F.; Ferré, A.; Cerdà, J. The Xenopus oocyte as an expression system for functional analyses of fish aquaporins. Methods Mol. Biol. 2021, 2218, 11–28. [Google Scholar]
- Castro-Arnau, J.; Chauvigné, F.; Cerdà, J. Role of ion channels in the maintenance of sperm motility and swimming behavior in a marine teleost. Int. J. Mol. Sci. 2022, 23, 12113. [Google Scholar] [CrossRef] [PubMed]
- Tingaud-Sequeira, A.; Chauvigné, F.; Fabra, M.; Lozano, J.; Raldúa, D.; Cerdà, J. Structural and functional divergence of two fish aquaporin-1 water channels following teleost-specific gene duplication. BMC Evol. Biol. 2008, 8, 259. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chauvigné, F.; Castro-Arnau, J.; López-Fortún, N.; Sánchez-Chardi, A.; Rützler, M.; Calamita, G.; Finn, R.N.; Cerdà, J. Aquaporin-3a Dysfunction Impairs Osmoadaptation in Post-Activated Marine Fish Spermatozoa. Int. J. Mol. Sci. 2024, 25, 9604. https://doi.org/10.3390/ijms25179604
Chauvigné F, Castro-Arnau J, López-Fortún N, Sánchez-Chardi A, Rützler M, Calamita G, Finn RN, Cerdà J. Aquaporin-3a Dysfunction Impairs Osmoadaptation in Post-Activated Marine Fish Spermatozoa. International Journal of Molecular Sciences. 2024; 25(17):9604. https://doi.org/10.3390/ijms25179604
Chicago/Turabian StyleChauvigné, François, Júlia Castro-Arnau, Noelia López-Fortún, Alejandro Sánchez-Chardi, Michael Rützler, Giuseppe Calamita, Roderick Nigel Finn, and Joan Cerdà. 2024. "Aquaporin-3a Dysfunction Impairs Osmoadaptation in Post-Activated Marine Fish Spermatozoa" International Journal of Molecular Sciences 25, no. 17: 9604. https://doi.org/10.3390/ijms25179604





