The Facile Production of p-Chloroaniline Facilitated by an Efficient and Chemoselective Metal-Free N/S Co-Doped Carbon Catalyst
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials Synthesis
4.2. Material Characterization
4.3. Catalytic Tests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Könnecker, G.; Boehncke, A.; Schmidt, S. Ecotoxicological assessment of p-chloroaniline—Fate and effects in aquatic systems. Fresenius Environ. Bull. 2003, 12, 589–593. [Google Scholar]
- Dighe, S.U.; Juliá, F.; Luridiana, A.; Douglas, J.J.; Leonori, D. A photochemical dehydrogenative strategy for aniline synthesis. Nature 2020, 584, 75–81. [Google Scholar] [CrossRef]
- Raghav, A. Aniline Market Report 2022–2027, Size, Share, Growth, Price Trends and Forecast; IndustryARC: Hyderabad, India, 2022. [Google Scholar]
- Qiu, Z.; Lv, L.; Li, J.; Li, C.-C.; Li, C.-J. Direct conversion of phenols into primary anilines with hydrazine catalyzed by palladium. Chem. Sci. 2019, 10, 4775–4781. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, V.A. Game-changing recipe for chemical building blocks. Nature 2020, 584, 46–47. [Google Scholar] [CrossRef]
- Kadam, H.K.; Tilve, S.G. Advancement in methodologies for reduction of nitroarenes. RSC Adv. 2015, 5, 83391–83407. [Google Scholar] [CrossRef]
- Tokiwa, H.; Nakagawa, R.; Horikawa, K.; Ohkubo, A. The nature of the mutagenicity and carcinogenicity of nitrated, aromatic compounds in the environment. Environ. Heal. Perspect. 1987, 73, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, A.J.; Senthilkumar Perumal, W. Toxicity Values for p-Chloronitrobenzene (CASRN 100-00-5); EPA/690/R-15/004F Final 9-30-2015; United States Environmental Protection Agency, USEPA: Washington, DC, USA, 2015; pp. 1–9.
- Béchamp, A. De l’action des protosels de fer sur la nitronaphtaline et la nitrobenzine: Nouvelle méthode de formation des bases orgnaiques artificielles de zinin. Ann. Chim. Phys. 1854, 42, 186. [Google Scholar]
- Serna, P.; Corma, A. Transforming Nano Metal Nonselective Particulates into Chemoselective Catalysts for Hydrogenation of Substituted Nitrobenzenes. ACS Catal. 2015, 5, 7114–7121. [Google Scholar] [CrossRef]
- Jakab-Nácsa, A.; Sikora, E.; Prekob, Á.; Vanyorek, L.; Szőri, M.; Boros, R.Z.; Nehéz, K.; Szabó, M.; Farkas, L.; Viskolcz, B. Comparison of Catalysts with MIRA21 Model in Heterogeneous Catalytic Hydrogenation of Aromatic Nitro Compounds. Catalysts 2022, 12, 467. [Google Scholar] [CrossRef]
- Cárdenas-Lizana, F.; Gómez-Quero, S.; Perret, N.; Kiwi-Minsker, L.; Keane, M.A. β-Molybdenum nitride: Synthesis mechanism and catalytic response in the gas phase hydrogenation of p-chloronitrobenzene. Catal. Sci. Technol. 2011, 1, 794–801. [Google Scholar] [CrossRef]
- Turáková, M.; Salmi, T.; Eränen, K.; Wärnå, J.; Murzin, D.Y.; Králik, M. Liquid phase hydrogenation of nitrobenzene. Appl. Catal. A Gen. 2015, 499, 66–76. [Google Scholar] [CrossRef]
- Sahoo, B.; Formenti, D.; Topf, C.; Bachmann, S.; Scalone, M.; Junge, K.; Beller, M. Biomass-Derived Catalysts for Selective Hydrogenation of Nitroarenes. ChemSusChem 2017, 10, 3035–3039. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Wu, B.; Ren, J.; Wang, X.; Zou, X.; Lu, X. Efficient and recyclable bimetallic Co–Cu catalysts for selective hydrogenation of halogenated nitroarenes. J. Alloy. Compd. 2022, 897, 163143. [Google Scholar] [CrossRef]
- Formenti, D.; Ferretti, F.; Scharnagl, F.K.; Beller, M. Reduction of Nitro Compounds Using 3d-Non-Noble Metal Catalysts. Chem. Rev. 2018, 119, 2611–2680. [Google Scholar] [CrossRef]
- Campos, C.H.; Shanmugaraj, K.; Bustamante, T.M.; Leal-Villarroel, E.; Vinoth, V.; Aepuru, R.; Mangalaraja, R.V.; Torres, C.C. Catalytic production of anilines by nitro-compounds hydrogenation over highly recyclable platinum nanoparticles supported on halloysite nanotubes. Catal. Today 2021, 394–396, 510–523. [Google Scholar] [CrossRef]
- Romanazzi, G.; Fiore, A.M.; Mali, M.; Rizzuti, A.; Leonelli, C.; Nacci, A.; Mastrorilli, P.; Dell’anna, M.M. Polymer supported Nickel nanoparticles as recyclable catalyst for the reduction of nitroarenes to anilines in aqueous medium. Mol. Catal. 2018, 446, 31–38. [Google Scholar] [CrossRef]
- Petrelli, V.; Romanazzi, G.; Mortalò, C.; Leonelli, C.; Zapparoli, M.; De Giglio, E.; Calvano, C.D.; Dell’Anna, M.M.; Mastrorilli, P. N-doped resin supported cobalt nanoparticles for the catalytic reduction of nitroarenes to corresponding anilines in aqueous medium. Mol. Catal. 2023, 544, 11305. [Google Scholar] [CrossRef]
- Fiore, A.M.; Varvaro, G.; Agostinelli, E.; Mangone, A.; De Giglio, E.; Terzano, R.; Allegretta, I.; Dell’Anna, M.M.; Fiore, S.; Mastrorilli, P. Synthesis and Use in Catalysis of Hematite Nanoparticles Obtained from a Polymer Supported Fe(III) Complex. Eur. J. Inorg. Chem. 2022, 2022, e202100943. [Google Scholar] [CrossRef]
- Cárdenas-Lizana, F.; Hao, Y.; Crespo-Quesada, M.; Yuranov, I.; Wang, X.; Keane, M.A.; Kiwi-Minsker, L. Selective Gas Phase Hydrogenation of p-Chloronitrobenzene over Pd Catalysts: Role of the Support. ACS Catal. 2013, 3, 1386–1396. [Google Scholar] [CrossRef]
- Prekob, Á.; Szamosvölgyi, Á.; Muránszky, G.; Lakatos, J.; Kónya, Z.; Fiser, B.; Viskolcz, B.; Vanyorek, L. Palladium Decorated N-Doped Carbon Foam as a Highly Active and Selective Catalyst for Nitrobenzene Hydrogenation. Int. J. Mol. Sci. 2022, 23, 6423. [Google Scholar] [CrossRef]
- Cárdenas-Lizana, F.; Gómez-Quero, S.; Keane, M.A. Ultra-selective gas phase catalytic hydrogenation of aromatic nitro compounds over Au/Al2O3. Catal. Commun. 2008, 9, 475–481. [Google Scholar] [CrossRef]
- Yue, S.; Wang, X.; Li, S.; Sheng, Y.; Zou, X.; Lu, X.; Zhang, C. Highly selective hydrogenation of halogenated nitroarenes over Ru/CN nanocomposites by in situ pyrolysis. New J. Chem. 2020, 44, 11861–11869. [Google Scholar] [CrossRef]
- Dongil, A.; Rivera-Cárcamo, C.; Pastor-Pérez, L.; Sepúlveda-Escribano, A.; Reyes, P. Ir supported over carbon materials for the selective hydrogenation of chloronitrobenzenes. Catal. Today 2015, 249, 72–78. [Google Scholar] [CrossRef]
- Hu, Z.-N.; Liang, J.; Ding, K.; Ai, Y.; Liang, Q.; Sun, H.-B. Insight into the selectivity of nano-catalytic nitroarenes reduction over other active groups by exploring hydrogen sources and metal components. Appl. Catal. A Gen. 2021, 626, 118339. [Google Scholar] [CrossRef]
- Cheong, W.-C.; Yang, W.; Zhang, J.; Li, Y.; Zhao, D.; Liu, S.; Wu, K.; Liu, Q.; Zhang, C.; Wang, D.; et al. Isolated Iron Single-Atomic Site-Catalyzed Chemoselective Transfer Hydrogenation of Nitroarenes to Arylamines. ACS Appl. Mater. Interfaces 2019, 11, 33819–33824. [Google Scholar] [CrossRef] [PubMed]
- Villora-Picó, J.J.; Campello-Gómez, I.; Serrano-Ruiz, J.C.; Pastor-Blas, M.M.; Sepúlveda-Escribano, A.; Ramos-Fernández, E.V. Hydrogenation of 4-nitrochlorobenzene catalysed by cobalt nanoparticles supported on nitrogen-doped activated carbon. Catal. Sci. Technol. 2021, 11, 3845–3854. [Google Scholar] [CrossRef]
- Duran-Uribe, E.S.; Sepúlveda-Escribano, A.; Ramos-Fernandez, E.V. Microwave-assisted synthesis of CoxP@C catalysts: Application to the hydrogenation of 1-chloro-4-nitrobezene. Chem. Eng. J. 2023, 474, 145897. [Google Scholar] [CrossRef]
- Cárdenas-Lizana, F.; Gómez-Quero, S.; Keane, M.A. Clean production of chloroanilines by selective gas phase hydrogenation over supported Ni catalysts. Appl. Catal. A Gen. 2008, 334, 199–206. [Google Scholar] [CrossRef]
- Pothu, R.; Challa, P.; Rajesh, R.; Boddula, R.; Balaga, R.; Balla, P.; Perugopu, V.; Radwan, A.B.; Abdullah, A.M.; Al-Qahtani, N. Vapour-Phase Selective Hydrogenation of γ-Valerolactone to 2-Methyltetrahydrofuran Biofuel over Silica-Supported Copper Catalysts. Nanomaterials 2022, 12, 3414. [Google Scholar] [CrossRef]
- Xiong, W.; Wang, Z.; He, S.; Hao, F.; Yang, Y.; Lv, Y.; Zhang, W.; Liu, P.; Luo, H. Nitrogen-doped carbon nanotubes as a highly active metal-free catalyst for nitrobenzene hydrogenation. Appl. Catal. B Environ. 2019, 260, 118105. [Google Scholar] [CrossRef]
- Lara, P.; Philippot, K. The hydrogenation of nitroarenes mediated by platinum nanoparticles: An overview. Catal. Sci. Technol. 2014, 4, 2445–2465. [Google Scholar] [CrossRef]
- Gao, R.; Pan, L.; Lu, J.; Xu, J.; Zhang, X.; Wang, L.; Zou, J. Phosphorus-Doped and Lattice-Defective Carbon as Metal-like Catalyst for the Selective Hydrogenation of Nitroarenes. ChemCatChem 2017, 9, 4287–4294. [Google Scholar] [CrossRef]
- Song, J.; Huang, Z.-F.; Pan, L.; Li, K.; Zhang, X.; Wang, L.; Zou, J.-J. Review on selective hydrogenation of nitroarene by catalytic, photocatalytic and electrocatalytic reactions. Appl. Catal. B Environ. 2018, 227, 386–408. [Google Scholar] [CrossRef]
- Dasgupta, H.R.; Mukherjee, S.; Ghosh, P. A novel approach towards chemoselective reduction of nitro to amine. Tetrahedron Lett. 2019, 60, 151028. [Google Scholar] [CrossRef]
- Mayoral, E.P.; Ojer, M.G.; Ventura, M.; Matos, I. New Insights into N-Doped Porous Carbons as Both Heterogeneous Catalysts and Catalyst Supports: Opportunities for the Catalytic Synthesis of Valuable Compounds. Nanomaterials 2023, 13, 2013. [Google Scholar] [CrossRef]
- Quílez-Bermejo, J.; Morallón, E.; Cazorla-Amorós, D. Metal-free heteroatom-doped carbon-based catalysts for ORR: A critical assessment about the role of heteroatoms. Carbon 2020, 165, 434–454. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, H. Effects of Oxidation by Hydrogen Peroxide on the Structures of Multiwalled Carbon Nanotubes. Ind. Eng. Chem. Res. 2006, 45, 6483–6488. [Google Scholar] [CrossRef]
- Fujita, S.-I.; Watanabe, H.; Katagiri, A.; Yoshida, H.; Arai, M. Nitrogen and oxygen-doped metal-free carbon catalysts for chemoselective transfer hydrogenation of nitrobenzene, styrene, and 3-nitrostyrene with hydrazine. J. Mol. Catal. A Chem. 2014, 393, 257–262. [Google Scholar] [CrossRef]
- Zhao, A.; Masa, J.; Schuhmann, W.; Xia, W. Activation and Stabilization of Nitrogen-Doped Carbon Nanotubes as Electrocatalysts in the Oxygen Reduction Reaction at Strongly Alkaline Conditions. J. Phys. Chem. C 2013, 117, 24283–24291. [Google Scholar] [CrossRef]
- Paraknowitsch, J.P.; Thomas, A. Doping carbons beyond nitrogen: An overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications. Energy Environ. Sci. 2013, 6, 2839–2855. [Google Scholar] [CrossRef]
- Zhong, H.; Zhang, H.; Xu, Z.; Tang, Y.; Mao, J. A Nitrogen-Doped Polyaniline Carbon with High Electrocatalytic Activity and Stability for the Oxygen Reduction Reaction in Fuel Cells. ChemSusChem 2012, 5, 1698–1702. [Google Scholar] [CrossRef]
- Bosilj, M.; Rustam, L.; Thomann, R.; Melke, J.; Fischer, A.; White, R.J. Directing nitrogen-doped carbon support chemistry for improved aqueous phase hydrogenation catalysis. Catal. Sci. Technol. 2020, 10, 4794–4808. [Google Scholar] [CrossRef]
- Villora-Picó, J.J.; Pastor-Blas, M.M.; Sepúlveda-Escribano, A. N-Doped Activated Carbons from Polypyrrole-Effect of Steam Activation Conditions. Chem. Ing. Tech. 2021, 94, 94–100. [Google Scholar] [CrossRef]
- Zeng, L.; Li, X.; Fan, S.; Mu, J.; Qin, M.; Wang, X.; Gan, G.; Tadé, M.; Liu, S. Seaweed-Derived Nitrogen-Rich Porous Biomass Carbon as Bifunctional Materials for Effective Electrocatalytic Oxygen Reduction and High-Performance Gaseous Toluene Absorbent. ACS Sustain. Chem. Eng. 2019, 7, 5057–5064. [Google Scholar] [CrossRef]
- Su, C.; Guo, Y.; Chen, H.; Zou, J.; Zeng, Z.; Li, L. VOCs adsorption of resin-based activated carbon and bamboo char: Porous characterization and nitrogen-doped effect. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 601, 124983. [Google Scholar] [CrossRef]
- Villora-Picó, J.-J.; Coloma-Pascual, F.; Sepúlveda-Escribano, A.; Pastor-Blas, M.M. N-doped activated carbons obtained from polyaniline for toluene and water adsorption. Inorg. Chem. Commun. 2024, 167, 112684. [Google Scholar] [CrossRef]
- Niu, J.; Zhang, H.; Li, L.; Guo, Y. Cost-effective activated carbon (AC) production from partial substitution of coal with red mud (RM) as additive for SO2 and NOx abatement at low temperature. Fuel 2021, 293, 120448. [Google Scholar] [CrossRef]
- Kang, K.Y.; Lee, B.I.; Lee, J.S. Hydrogen adsorption on nitrogen-doped carbon xerogels. Carbon 2009, 47, 1171–1180. [Google Scholar] [CrossRef]
- Liu, Z.; Du, Z.; Song, H.; Wang, C.; Subhan, F.; Xing, W.; Yan, Z. The fabrication of porous N-doped carbon from widely available urea formaldehyde resin for carbon dioxide adsorption. J. Colloid Interface Sci. 2013, 416, 124–132. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, N. A facile synthesis of nitrogen-doped porous carbons from lignocellulose and protein wastes for VOCs sorption. Environ. Res. 2020, 189, 109956. [Google Scholar] [CrossRef]
- Zhu, L.; Shen, D.; Luo, K.H. A critical review on VOCs adsorption by different porous materials: Species, mechanisms and modification methods. J. Hazard. Mater. 2020, 389, 122102. [Google Scholar] [CrossRef]
- Liu, S.; Cui, L.; Peng, Z.; Wang, J.; Hu, Y.; Yu, A.; Wang, H.; Peng, P.; Li, F.-F. Eco-friendly synthesis of N,S co-doped hierarchical nanocarbon as a highly efficient metal-free catalyst for the reduction of nitroarenes. Nanoscale 2018, 10, 21764–21771. [Google Scholar] [CrossRef] [PubMed]
- Kiciński, W.; Szala, M.; Bystrzejewski, M. Sulfur-doped porous carbons: Synthesis and applications. Carbon 2014, 68, 1–32. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, Y.; Li, D.; Werth, C.J.; Zhang, Y.; Zhou, X. Highly active Pd–In/mesoporous alumina catalyst for nitrate reduction. J. Hazard. Mater. 2015, 286, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Zhang, Y.; Yang, L.; Tan, Y.; Ma, M.; Xie, Q. Sulfur-doped porous carbon nanosheets as an advanced electrode material for supercapacitors. RSC Adv. 2015, 5, 13046–13051. [Google Scholar] [CrossRef]
- Chacón, F.J.; Cayuela, M.L.; Roig, A.; Sánchez-Monedero, M.A. Understanding, measuring and tuning the electrochemical properties of biochar for environmental applications. Rev. Environ. Sci. Bio/Technology 2017, 16, 695–715. [Google Scholar] [CrossRef]
- Hasegawa, G.; Deguchi, T.; Kanamori, K.; Kobayashi, Y.; Kageyama, H.; Abe, T.; Nakanishi, K. High-Level Doping of Nitrogen, Phosphorus, and Sulfur into Activated Carbon Monoliths and Their Electrochemical Capacitances. Chem. Mater. 2015, 27, 4703–4712. [Google Scholar] [CrossRef]
- Yan, Z.; Gao, L.; Dai, C.; Zhang, M.; Lv, X.; Shen, P.K. Metal-free mesoporous carbon with higher contents of active N and S codoping by template method for superior ORR efficiency to Pt/C. Int. J. Hydrogen Energy 2018, 43, 3705–3715. [Google Scholar] [CrossRef]
- Hua, Y.; Jiang, T.; Wang, K.; Wu, M.; Song, S.; Wang, Y.; Tsiakaras, P. Efficient Pt-free electrocatalyst for oxygen reduction reaction: Highly ordered mesoporous N and S co-doped carbon with saccharin as single-source molecular precursor. Appl. Catal. B Environ. 2016, 194, 202–208. [Google Scholar] [CrossRef]
- Yue, W.; Yu, Z.; Zhang, X.; Liu, H.; He, T.; Ma, X. Green activation method and natural N/O/S co-doped strategy to prepare biomass-derived graded porous carbon for supercapacitors. J. Anal. Appl. Pyrolysis 2024, 178, 106409. [Google Scholar] [CrossRef]
- Wu, W.; Wu, C.; Liu, J.; Yan, H.; Li, G.; Zhao, Y.; Bei, K.; Zhang, G. Synergistic effects of heteroatom doping and narrow micropores on carbon dioxide capture in bamboo shoot shell-based porous carbon. Sep. Purif. Technol. 2024, 339, 126690. [Google Scholar] [CrossRef]
- Bide, Y.; Jahromi, N.N. Nitrogen and sulfur dual doped porous carbon as metal-free catalyst for oxidative degradation of 4-nitrophenol by persulfate activation. Sci. Rep. 2023, 13, 1212. [Google Scholar] [CrossRef] [PubMed]
- Villora-Picó, J.-J.; Sepúlveda-Escribano, A.; Pastor-Blas, M.-M. Design and Synthesis of N-Doped Carbons as Efficient Metal-Free Catalysts in the Hydrogenation of 1-Chloro-4-Nitrobenzene. Int. J. Mol. Sci. 2024, 25, 2515. [Google Scholar] [CrossRef]
- Hussain, N.; Alwan, S.; Alshamsi, H.; Sahib, I. Green Synthesis of S- and N-Codoped Carbon Nanospheres and Application as Adsorbent of Pb (II) from Aqueous Solution. Int. J. Chem. Eng. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Tao, K.; Fu, S.; Yang, M.; Zhang, N.; Liu, K.; Liu, W.; Zhou, Y.; Li, S.; Sun, J. Nitrogen and sulfur co-doped carbon-coated Li4Ti5O12 composite to enhance lithium storage properties. Ionics 2024, 30, 2083–2091. [Google Scholar] [CrossRef]
- Zhong, G.; Yang, H.; Zeng, M.; Liu, S.; Chen, S.; Fan, Z.; Zhu, C.; Xu, J.; Yu, J. Enabling Heteroatom Doping of Flexible MXene Film in Seconds: A Microwave-Induced Targeted Thermal-Shock Method. Adv. Funct. Mater. 2024, 34, 2313845. [Google Scholar] [CrossRef]
- Horikawa, T.; Sakao, N.; Sekida, T.; Hayashi, J.; Do, D.; Katoh, M. Preparation of nitrogen-doped porous carbon by ammonia gas treatment and the effects of N-doping on water adsorption. Carbon 2011, 50, 1833–1842. [Google Scholar] [CrossRef]
- Luo, W.; Wang, B.; Heron, C.G.; Allen, M.J.; Morre, J.; Maier, C.S.; Stickle, W.F.; Ji, X. Pyrolysis of Cellulose under Ammonia Leads to Nitrogen-Doped Nanoporous Carbon Generated through Methane Formation. Nano Lett. 2014, 14, 2225–2229. [Google Scholar] [CrossRef] [PubMed]
- Skudin, V.; Andreeva, T.; Myachina, M.; Gavrilova, N. CVD-Synthesis of N-CNT Using Propane and Ammonia. Materials 2022, 15, 2241. [Google Scholar] [CrossRef]
- Wei, Q.; Tong, X.; Zhang, G.; Qiao, J.; Gong, Q.; Sun, S. Nitrogen-Doped Carbon Nanotube and Graphene Materials for Oxygen Reduction Reactions. Catalysts 2015, 5, 1574–1602. [Google Scholar] [CrossRef]
- Villora-Picó, J.-J.; Gil-Muñoz, G.; Sepúlveda-Escribano, A.; Pastor-Blas, M.M. Doped activated carbons obtained from nitrogen and sulfur-containing polymers as metal-free catalysts for application in nitroarenes hydrogenation. Int. J. Hydrogen Energy 2024, 53, 490–502. [Google Scholar] [CrossRef]
- Hu, X.; Sun, X.; Song, Q.; Zhu, Y.; Long, Y.; Dong, Z. N,S co-doped hierarchically porous carbon materials for efficient metal-free catalysis. Green Chem. 2019, 22, 742–752. [Google Scholar] [CrossRef]
- Pan, F.; Li, B.; Deng, W.; Du, Z.; Gang, Y.; Wang, G.; Li, Y. Promoting electrocatalytic CO2 reduction on nitrogen-doped carbon with sulfur addition. Appl. Catal. B Environ. 2019, 252, 240–249. [Google Scholar] [CrossRef]
- Bubanale, S.; Shivashankar, M.; Siddaganga Institute of Technology. History, Method of Production, Structure and Applications of Activated Carbon. Int. J. Eng. Res. 2017, V6, 495–498. [Google Scholar] [CrossRef]
- Sekirifa, M.L.; Hadj-Mahammed, M.; Pallier, S.; Baameur, L.; Richard, D.; Al-Dujaili, A.H. Preparation and characterization of an activated carbon from a date stones variety by physical activation with carbon dioxide. J. Anal. Appl. Pyrolysis 2013, 99, 155–160. [Google Scholar] [CrossRef]
- Dastgheib, S.A.; Salih, H.; Ilangovan, T.; Mock, J. NO Oxidation by Activated Carbon Catalysts: Impact of Carbon Characteristics, Pressure, and the Presence of Water. ACS Omega 2020, 5, 21172–21180. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wu, C.; Zhang, G.; Liu, J.; Li, Y.; Li, G. Synthesis and characterization of magnetic K2CO3-activated carbon produced from bamboo shoot for the adsorption of Rhodamine b and CO2 capture. Fuel 2023, 332, 126107. [Google Scholar] [CrossRef]
- Hassani, S.S.; Ganjali, M.R.; Samiee, L.; Rashidi, A.M.; Tasharrofi, S.; Yadegari, A.; Shoghi, F.; Martel, R. Comparative Study of Various Types of Metal-Free N and S Co-Doped Porous Graphene for High Performance Oxygen Reduction Reaction in Alkaline Solution. J. Nanosci. Nanotechnol. 2018, 18, 4565–4579. [Google Scholar] [CrossRef]
- Mansour, S.A. Thermal decomposition of calcium citrate tetrahydrate. Thermochim. Acta 1994, 233, 243–256. [Google Scholar] [CrossRef]
- Chalermnon, M.; Cherdchom, S.; Sereemaspun, A.; Rojanathanes, R.; Khotavivattana, T. Biguanide-Based Synthesis of 1,3,5-Triazine Derivatives with Anticancer Activity and 1,3,5-Triazine Incorporated Calcium Citrate Nanoparticles. Molecules 2021, 26, 1028. [Google Scholar] [CrossRef]
- Lee, S.; Choi, J.; Chung, Y.S.; Kim, J.; Moon, S.; Lee, S. Understanding the catalytic mechanism of calcium compounds for enhancing crystallinity in carbon fiber. Chem. Eng. J. 2024, 479, 147728. [Google Scholar] [CrossRef]
- Li, X.; Kurasch, S.; Kaiser, U.; Antonietti, M. Synthesis of Monolayer-Patched Graphene from Glucose. Angew. Chem. Int. Ed. 2012, 51, 9689–9692. [Google Scholar] [CrossRef]
- Wang, K.; Li, Q.; Liu, B.; Cheng, B.; Ho, W.; Yu, J. Sulfur-doped g-C3N4 with enhanced photocatalytic CO2-reduction performance. Appl. Catal. B Environ. 2015, 176, 44–52. [Google Scholar] [CrossRef]
- Ojha, N.; Bajpai, A.; Kumar, S. Visible light-driven enhanced CO2 reduction by water over Cu modified S-doped g-C3N4. Catal. Sci. Technol. 2019, 9, 4598–4613. [Google Scholar] [CrossRef]
- Pan, F.; Jin, J.; Fu, X.; Liu, Q.; Zhang, J. Advanced Oxygen Reduction Electrocatalyst Based on Nitrogen-Doped Graphene Derived from Edible Sugar and Urea. ACS Appl. Mater. Interfaces 2013, 5, 11108–11114. [Google Scholar] [CrossRef] [PubMed]
- Shcherban, N.D.; Filonenko, S.M.; Ovcharov, M.L.; Mishura, A.M.; Skoryk, M.A.; Aho, A.; Murzin, D.Y. Simple method for preparing of sulfur–doped graphitic carbon nitride with superior activity in CO2 photoreduction. ChemistrySelect 2016, 1, 4987–4993. [Google Scholar] [CrossRef]
- Guo, X.; Wang, R.; Ni, L.; Qiu, S.; Zhang, Z. Synthesis of Li4Ti5O12 with Tunable Morphology Using l-Cysteine and Its Enhanced Lithium Storage Properties. Chempluschem 2018, 84, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Tsubouchi, N.; Ohtsuka, Y. Nitrogen release during high temperature pyrolysis of coals and catalytic role of calcium in N2 formation. Fuel 2002, 81, 2335–2342. [Google Scholar] [CrossRef]
- Ejaz, A.; Jeon, S. The individual role of pyrrolic, pyridinic and graphitic nitrogen in the growth kinetics of Pd NPs on N-rGO followed by a comprehensive study on ORR. Int. J. Hydrogen Energy 2018, 43, 5690–5702. [Google Scholar] [CrossRef]
- Liu, P.; Gao, S.; Wang, Y.; Huang, Y.; He, W.; Huang, W.; Luo, J. Carbon nanocages with N-doped carbon inner shell and Co/N-doped carbon outer shell as electromagnetic wave absorption materials. Chem. Eng. J. 2019, 381, 122653. [Google Scholar] [CrossRef]
- Ferrero, G.; Sevilla, M.; Fuertes, A. Mesoporous carbons synthesized by direct carbonization of citrate salts for use as high-performance capacitors. Carbon 2015, 88, 239–251. [Google Scholar] [CrossRef]
- Bokobza, L.; Bruneel, J.-L.; Couzi, M. Raman Spectra of Carbon-Based Materials (from Graphite to Carbon Black) and of Some Silicone Composites. C J. Carbon Res. 2015, 1, 77–94. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Cho, S.-R.; Cho, H.-G. Characterization of Black Carbon Collected from Candle Light and Automobile Exhaust Pipe. J. Korean Chem. Soc. 2013, 57, 691–696. [Google Scholar] [CrossRef]
- Ayiania, M.; Weiss-Hortala, E.; Smith, M.; McEwen, J.-S.; Garcia-Perez, M. Microstructural analysis of nitrogen-doped char by Raman spectroscopy: Raman shift analysis from first principles. Carbon 2020, 167, 559–574. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Hu, X.; Long, Y.; Fan, M.; Yuan, M.; Zhao, H.; Ma, J.; Dong, Z. Two-dimensional covalent organic frameworks as self-template derived nitrogen-doped carbon nanosheets for eco-friendly metal-free catalysis. Appl. Catal. B Environ. 2018, 244, 25–35. [Google Scholar] [CrossRef]
- Liu, N.; Song, H.; Chen, X. Morphology control of ordered mesoporous carbons by changing HCl concentration. J. Mater. Chem. 2011, 21, 5345–5351. [Google Scholar] [CrossRef]
- Yang, Y.; Gu, L.; Guo, S.; Shao, S.; Li, Z.; Sun, Y.; Hao, S. N-Doped Mesoporous Carbons: From Synthesis to Applications as Metal-Free Reduction Catalysts and Energy Storage Materials. Front. Chem. 2019, 7, 761. [Google Scholar] [CrossRef]
- Lv, Q.; Si, W.; He, J.; Sun, L.; Zhang, C.; Wang, N.; Yang, Z.; Li, X.; Wang, X.; Deng, W.; et al. Selectively nitrogen-doped carbon materials as superior metal-free catalysts for oxygen reduction. Nat. Commun. 2018, 9, 3376. [Google Scholar] [CrossRef]
- Han, Y.; Yan, D.; Ma, Z.; Wang, Q.; Wang, X.; Li, Y.; Sun, G. Lignin-derived sulfonate base metal-free N, S co-doped carbon microspheres doped with different nitrogen sources as catalysts for oxygen reduction reactions. Int. J. Biol. Macromol. 2023, 244, 125363. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Takada, Y.; Takada, R.; Taniguchi, Y.; Miyake, K.; Uchida, Y.; Kong, C.Y.; Nishiyama, N. Experimental and Theoretical Elucidation of Metal-Free Sulfur and Nitrogen Co-Doped Porous Carbon Materials with an Efficient Synergistic Effect on the Oxygen Reduction Reaction. Adv. Mater. Interfaces 2023, 10, 2300088. [Google Scholar] [CrossRef]
- Sun, P.; Wang, X.; Zhu, M.; Ahmad, N.; Zhang, K.; Xu, X. Nitrogen Self-Doped Metal Free Catalysts Derived from Chitin via One Step Method for Efficient Electrocatalytic CO2 Reduction to CO. Catalysts 2023, 13, 904. [Google Scholar] [CrossRef]
- Liu, Z.; Peng, F.; Wang, H.; Yu, H.; Zheng, W.; Yang, J. Phosphorus-Doped Graphite Layers with High Electrocatalytic Activity for the O2 Reduction in an Alkaline Medium. Angew. Chem. Int. Ed. 2011, 50, 3257–3261. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, S.; Zhao, Y.; Zhu, L.; Chen, S.; Wang, X.; Wu, Q.; Ma, J.; Ma, Y.; Hu, Z. Boron-Doped Carbon Nanotubes as Metal-Free Electrocatalysts for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2011, 50, 7132–7135. [Google Scholar] [CrossRef]
- Yang, D.-S.; Bhattacharjya, D.; Inamdar, S.; Park, J.; Yu, J.-S. Phosphorus-Doped Ordered Mesoporous Carbons with Different Lengths as Efficient Metal-Free Electrocatalysts for Oxygen Reduction Reaction in Alkaline Media. J. Am. Chem. Soc. 2012, 134, 16127–16130. [Google Scholar] [CrossRef]
- Quílez-Bermejo, J.; González-Gaitán, C.; Morallón, E.; Cazorla-Amorós, D. Effect of carbonization conditions of polyaniline on its catalytic activity towards ORR. Some insights about the nature of the active sites. Carbon 2017, 119, 62–71. [Google Scholar] [CrossRef]
- Rangraz, Y.; Heravi, M.M. Recent advances in metal-free heteroatom-doped carbon heterogonous catalysts. RSC Adv. 2021, 11, 23725–23778. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, D.; Zhou, Y.; Qian, J.; Wen, X.; Jiang, P.; Ma, L.; Lu, C.; Feng, F.; Li, X. Preparation of Heteroatom-Doped Carbon Materials and Applications in Selective Hydrogenation. ChemistrySelect 2022, 7, e202102581. [Google Scholar] [CrossRef]
- Denis, P.A.; Faccio, R.; Mombru, A.W. Is It Possible to Dope Single-Walled Carbon Nanotubes and Graphene with Sulfur? Chemphyschem 2009, 10, 715–722. [Google Scholar] [CrossRef]
- Yang, Z.; Yao, Z.; Li, G.; Fang, G.; Nie, H.; Liu, Z.; Zhou, X.; Chen, X.; Huang, S. Sulfur-Doped Graphene as an Efficient Metal-free Cathode Catalyst for Oxygen Reduction. ACS Nano 2012, 6, 205–211. [Google Scholar]
- Gao, K.; Wang, B.; Tao, L.; Cunning, B.V.; Zhang, Z.; Wang, S.; Ruoff, R.S.; Qu, L. Efficient Metal-Free Electrocatalysts from N-Doped Carbon Nanomaterials: Mono-Doping and Co-Doping. Adv. Mater. 2018, 31, e1805121. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Duan, Y.; Zhang, X.; Zhang, J. A Facile Synthesis of Nitrogen/Sulfur Co-Doped Graphene for the Oxygen Reduction Reaction. ChemCatChem 2015, 8, 163–170. [Google Scholar] [CrossRef]
- Liang, C.; Wang, Y.; Li, T. Synthesis of sulfur-doped p-type graphene by annealing with hydrogen sulfide. Carbon 2015, 82, 506–512. [Google Scholar] [CrossRef]
- Li, X.; Lau, S.P.; Tang, L.; Ji, R.; Yang, P. Sulphur doping: A facile approach to tune the electronic structure and optical properties of graphene quantum dots. Nanoscale 2014, 6, 5323–5328. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Sulfur and Nitrogen Dual-Doped Mesoporous Graphene Electrocatalyst for Oxygen Reduction with Synergistically Enhanced Performance. Angew. Chem. Int. Ed. 2012, 51, 11496–11500. [Google Scholar] [CrossRef]
- Li, W.; Bandosz, T.J. Role of Heteroatoms in S,N-Codoped Nanoporous Carbon Materials in CO2 (Photo)electrochemical Reduction. ChemSusChem 2018, 11, 2987–2999. [Google Scholar] [CrossRef] [PubMed]
- Qu, K.; Zheng, Y.; Jiao, Y.; Zhang, X.; Dai, S.; Qiao, S. Polydopamine-Inspired, Dual Heteroatom-Doped Carbon Nanotubes for Highly Efficient Overall Water Splitting. Adv. Energy Mater. 2016, 7, 1602068. [Google Scholar] [CrossRef]
- Lu, C.; Zhu, Q.; Zhang, X.; Ji, H.; Zhou, Y.; Wang, H.; Liu, Q.; Nie, J.; Han, W.; Li, X. Decoration of Pd Nanoparticles with N and S Doped Carbon Quantum Dots as a Robust Catalyst for the Chemoselective Hydrogenation Reaction. ACS Sustain. Chem. Eng. 2019, 7, 8542–8553. [Google Scholar] [CrossRef]
- Bag, S.; Mondal, B.; Das, A.K.; Raj, C.R. Nitrogen and Sulfur Dual-Doped Reduced Graphene Oxide: Synergistic Effect of Dopants Towards Oxygen Reduction Reaction. Electrochimica Acta 2015, 163, 16–23. [Google Scholar] [CrossRef]
- Dinadayalane, T.; Lazare, J.; Alzaaqi, N.F.; Herath, D.; Hill, B.; Campbell, A.E. Structures, Properties, and Applications of Nitrogen-Doped Graphene, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2022; Volume 21. [Google Scholar] [CrossRef]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on Recent Progress in Nitrogen-Doped Graphene: Synthesis, Characterization, and Its Potential Applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Biddinger, E.J.; von Deak, D.; Ozkan, U.S. Nitrogen-Containing Carbon Nanostructures as Oxygen-Reduction Catalysts. Top. Catal. 2009, 52, 1566–1574. [Google Scholar] [CrossRef]
- Yang, X.; Chen, D.; Liao, S.; Song, H.; Li, Y.; Fu, Z.; Su, Y. High-performance Pd–Au bimetallic catalyst with mesoporous silica nanoparticles as support and its catalysis of cinnamaldehyde hydrogenation. J. Catal. 2012, 291, 36–43. [Google Scholar] [CrossRef]
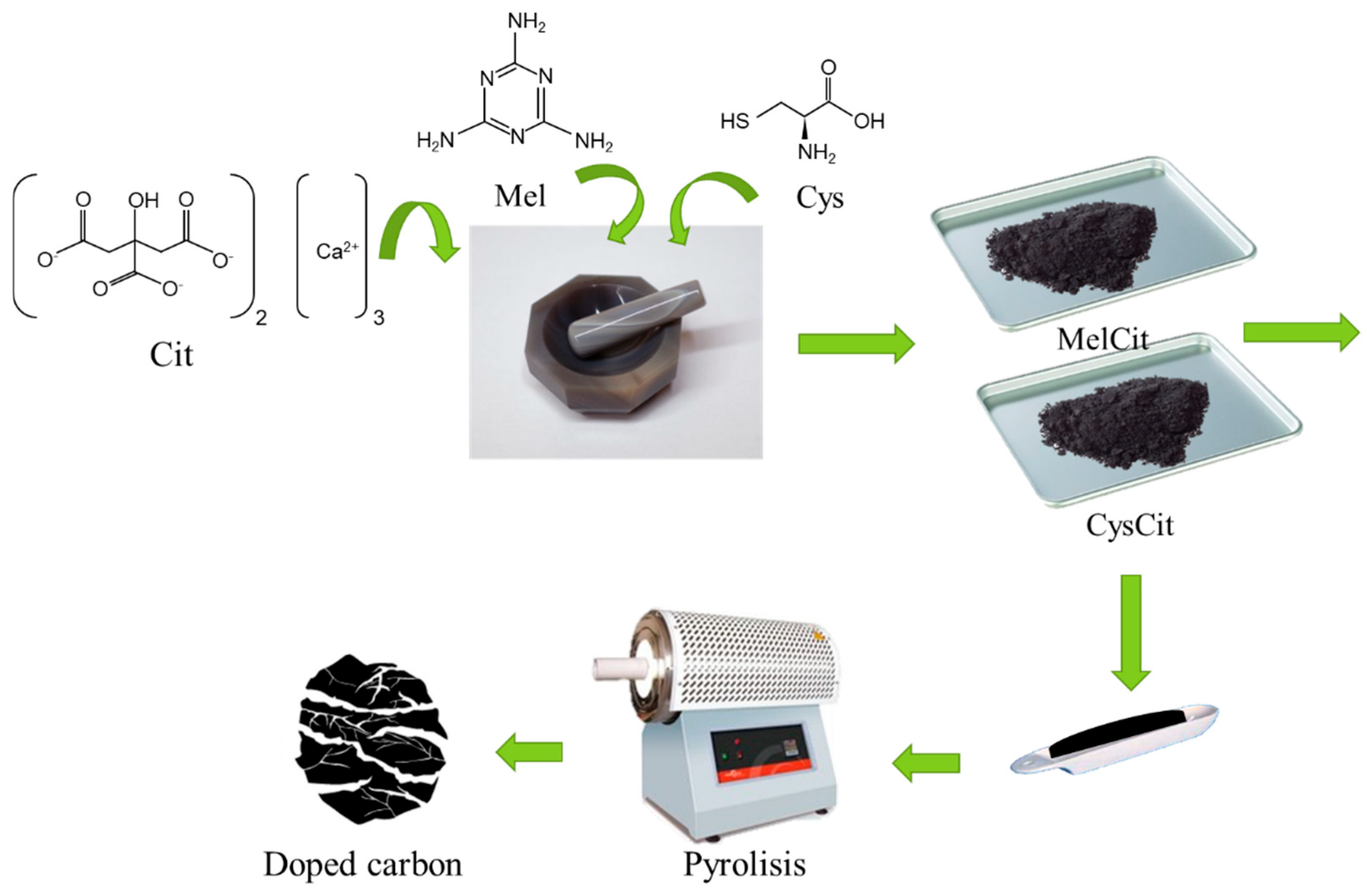

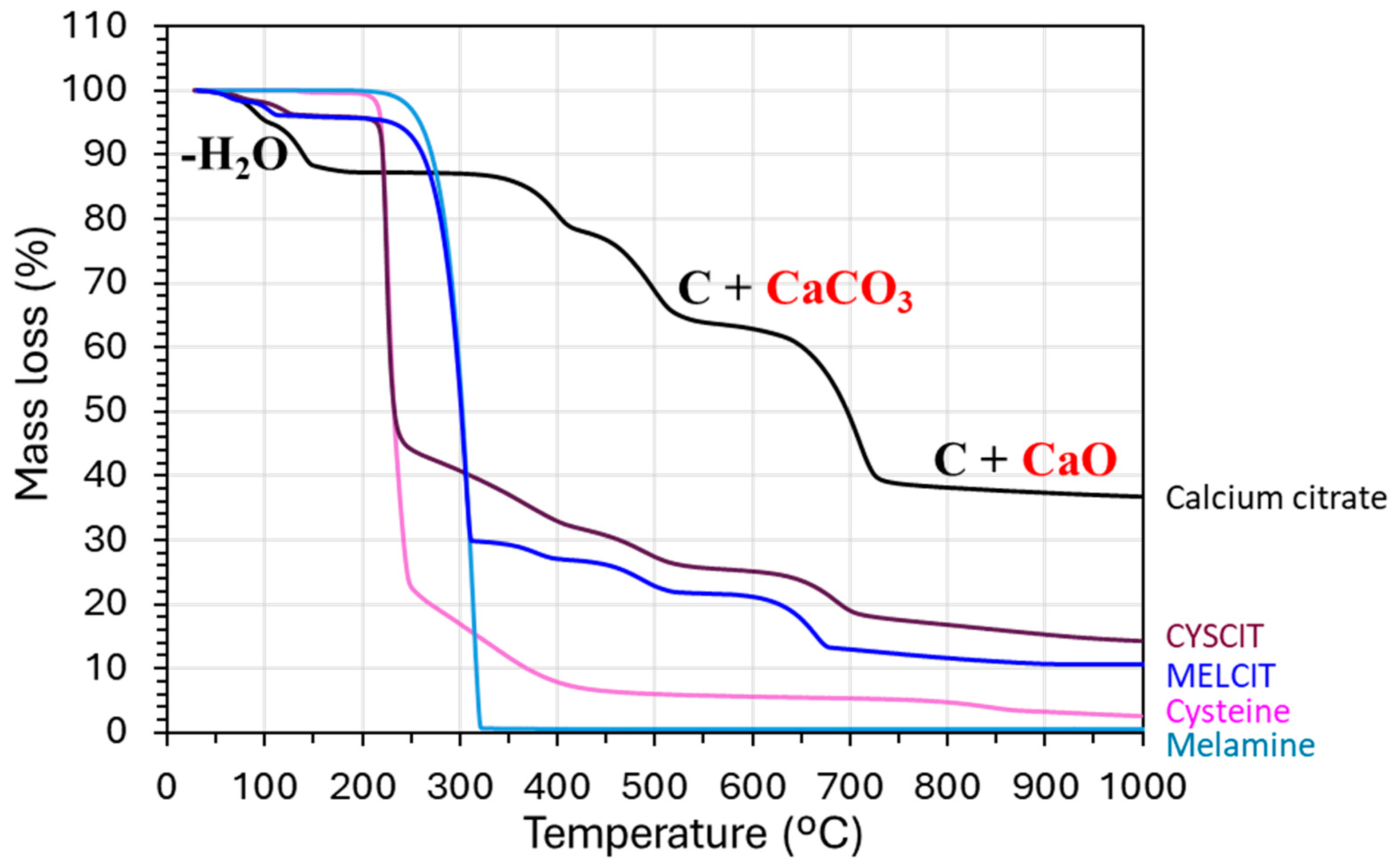


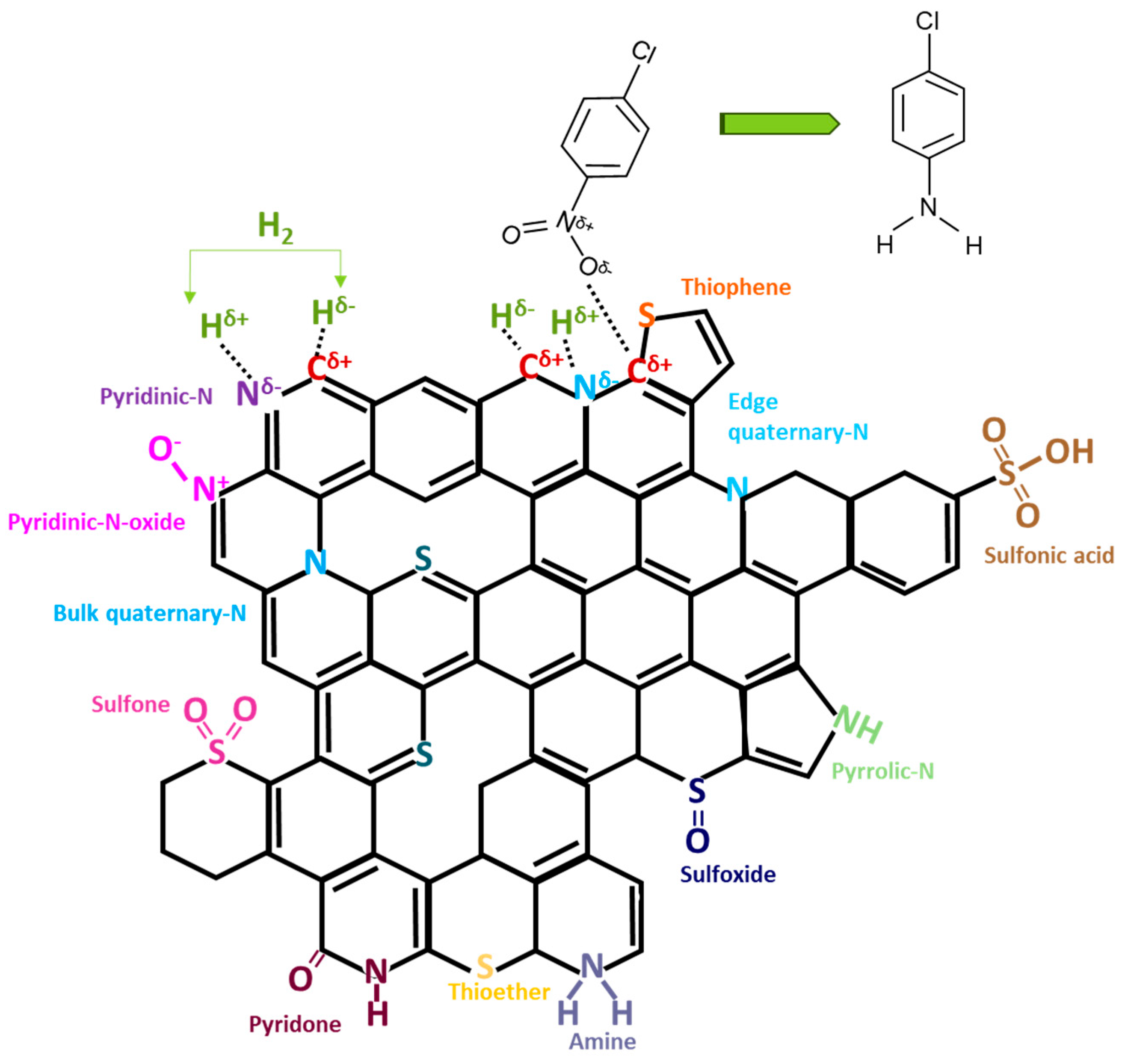
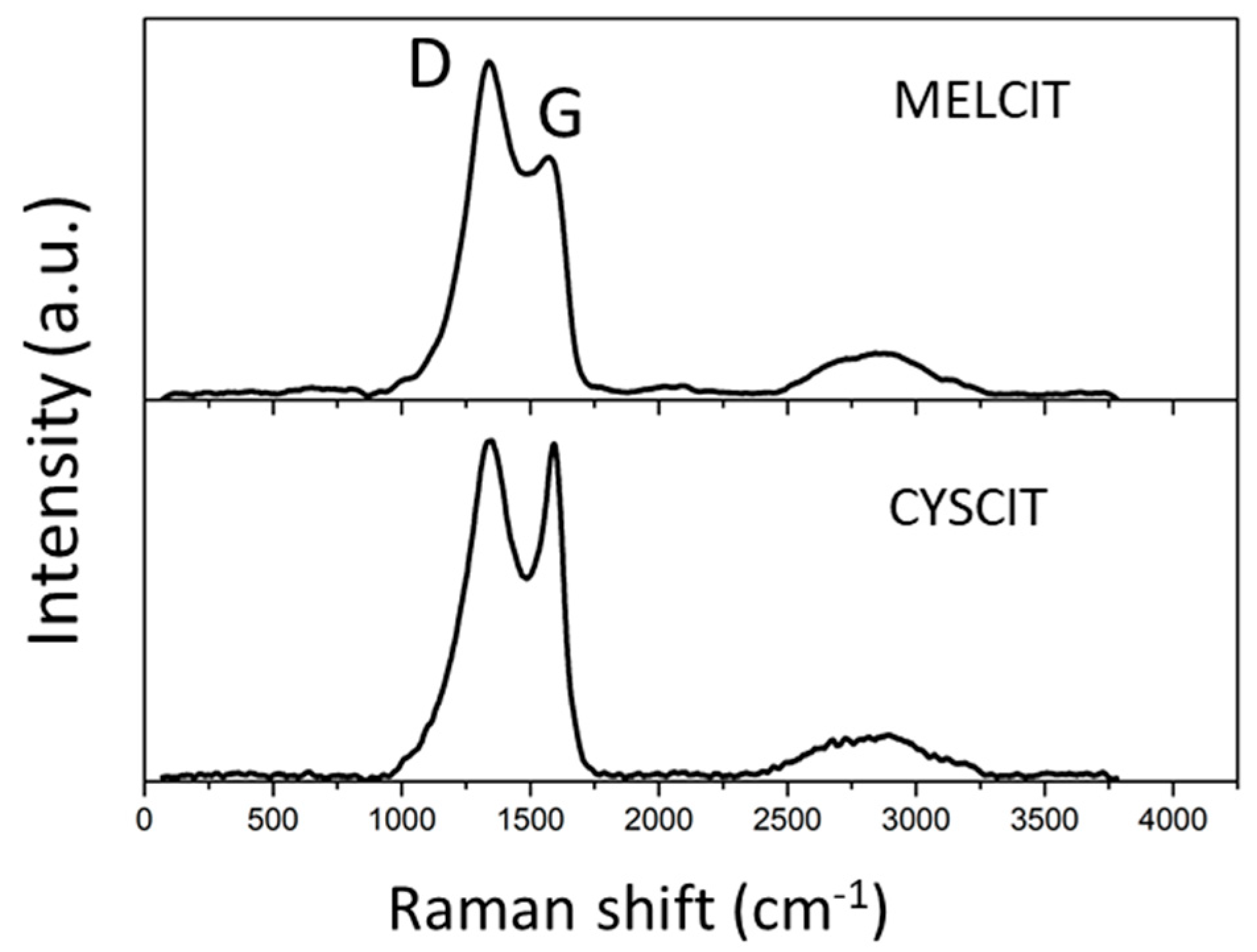
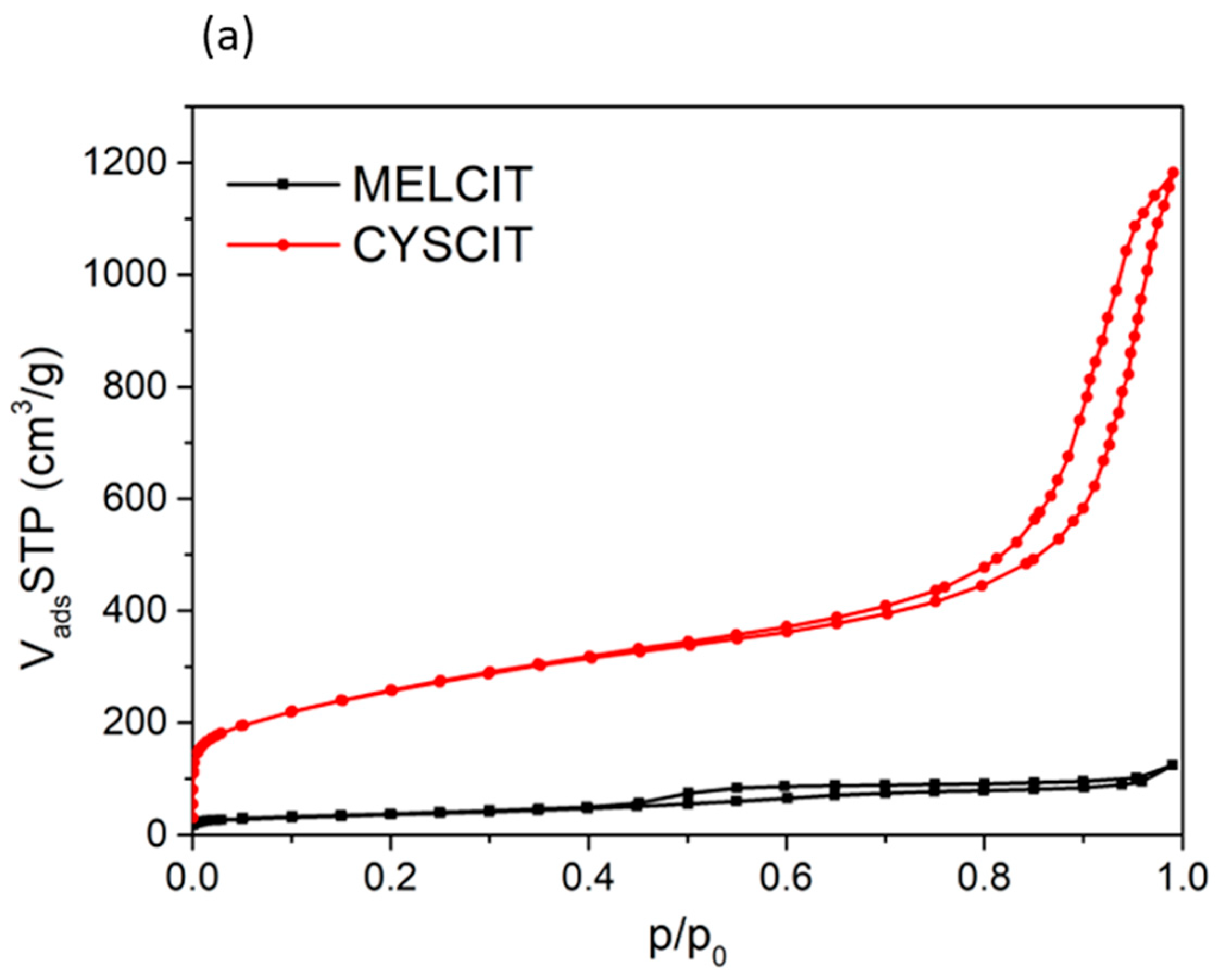

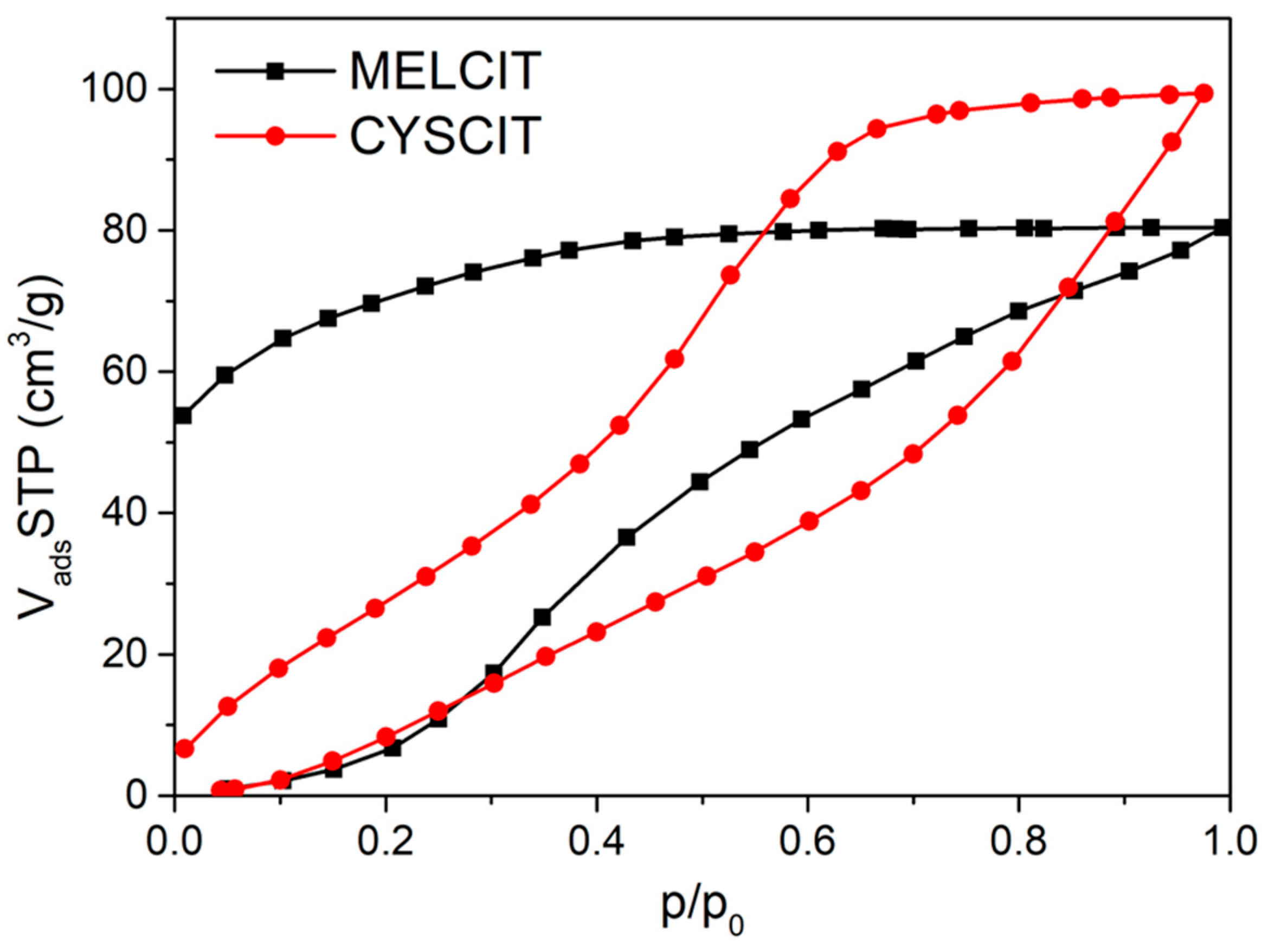
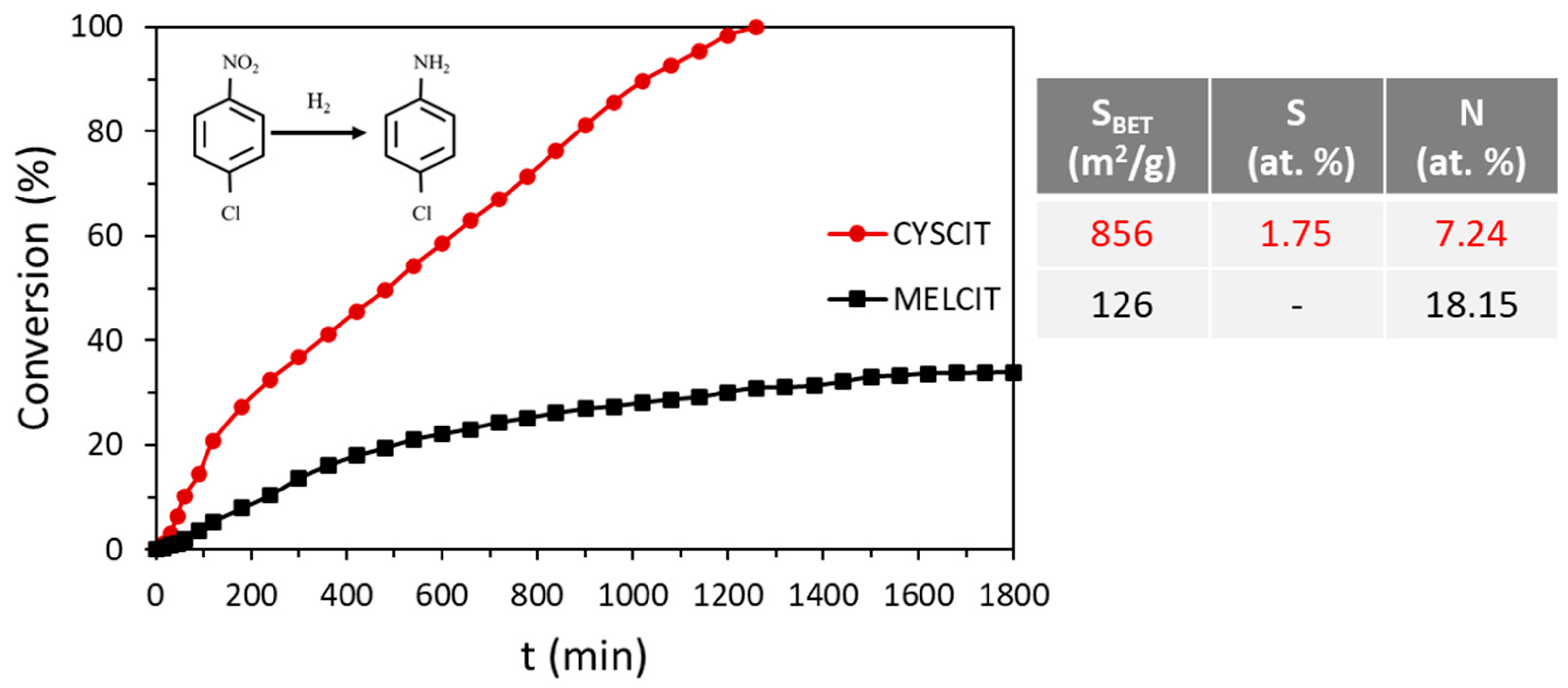
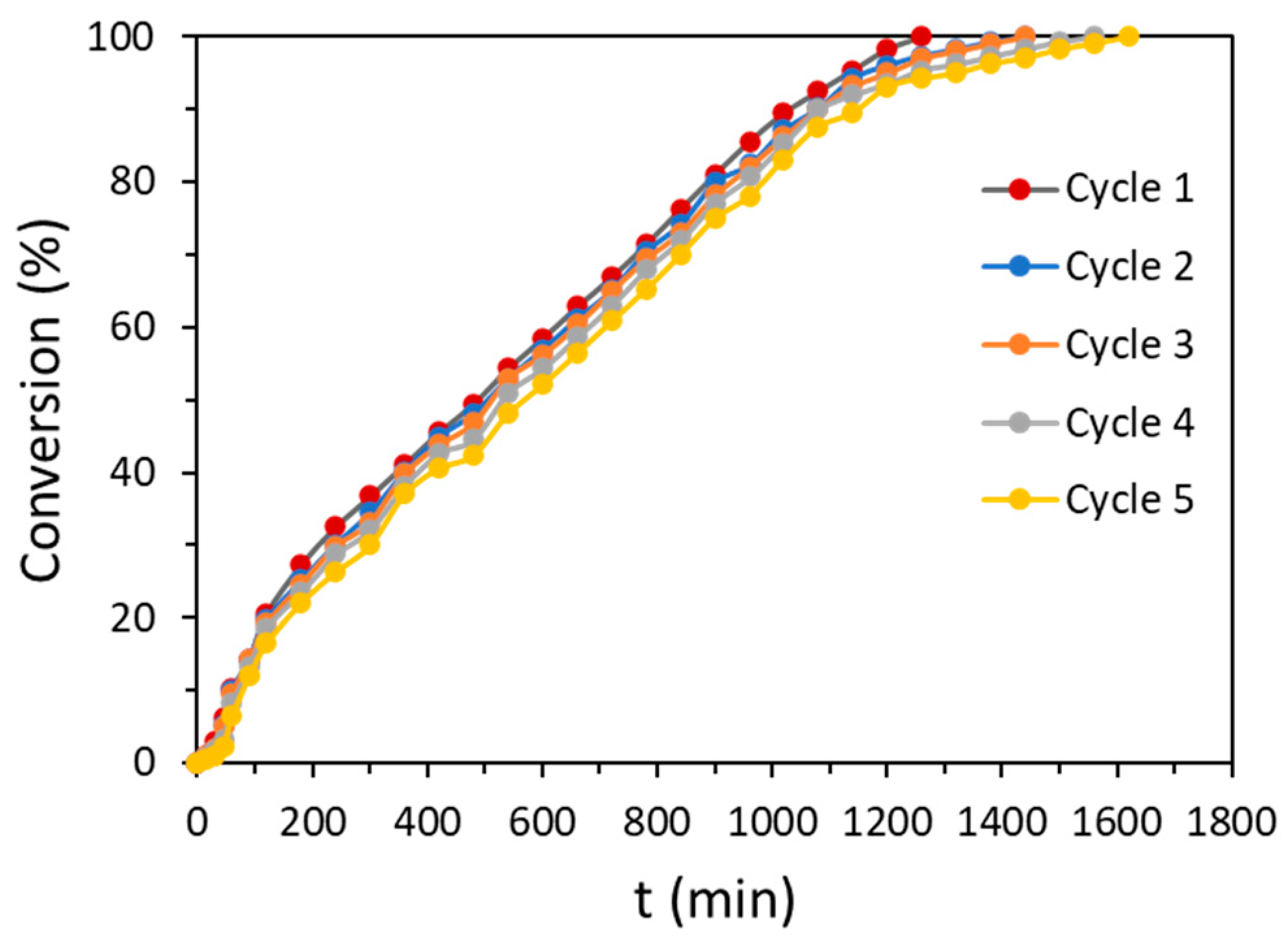
| Carbon Sample | C | H | N | S |
|---|---|---|---|---|
| MELCIT | 51.72 | 1.41 | 17.85 | 0.00 |
| CYSCIT | 62.45 | 1.74 | 6.97 | 7.61 |
| Carbon Sample | C 1s | O 1s | S 2p | Ca 2p | N 1s |
|---|---|---|---|---|---|
| MELCIT | 73.80 | 6.48 | 0.00 | 1.57 | 18.15 |
| CYSCIT | 86.35 | 4.5 | 1.75 | 0.15 | 7.24 |
| Carbon Sample | ID/IG |
|---|---|
| MELCIT | 1.41 |
| CYSCIT | 1.02 |
| Sample | Vtotal (cm3/g) | Vmicro (cm3/g) | Vmeso (cm3/g) | SBET (m2/g) |
|---|---|---|---|---|
| MELCIT | 0.15 | 0.05 | 0.099 | 126 |
| CYSCIT | 1.38 | 0.33 | 1.05 | 856 |
| Sample | Vmicro (N2) (cm3/g) | Vmicro (CO2) (cm3/g) |
|---|---|---|
| MELCIT | 0.05 | 0.55 |
| CYSCIT | 0.33 | 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villora-Picó, J.-J.; Gil-Muñoz, G.; Sepúlveda-Escribano, A.; Pastor-Blas, M.M. The Facile Production of p-Chloroaniline Facilitated by an Efficient and Chemoselective Metal-Free N/S Co-Doped Carbon Catalyst. Int. J. Mol. Sci. 2024, 25, 9603. https://doi.org/10.3390/ijms25179603
Villora-Picó J-J, Gil-Muñoz G, Sepúlveda-Escribano A, Pastor-Blas MM. The Facile Production of p-Chloroaniline Facilitated by an Efficient and Chemoselective Metal-Free N/S Co-Doped Carbon Catalyst. International Journal of Molecular Sciences. 2024; 25(17):9603. https://doi.org/10.3390/ijms25179603
Chicago/Turabian StyleVillora-Picó, Juan-José, Gema Gil-Muñoz, Antonio Sepúlveda-Escribano, and M. Mercedes Pastor-Blas. 2024. "The Facile Production of p-Chloroaniline Facilitated by an Efficient and Chemoselective Metal-Free N/S Co-Doped Carbon Catalyst" International Journal of Molecular Sciences 25, no. 17: 9603. https://doi.org/10.3390/ijms25179603
APA StyleVillora-Picó, J.-J., Gil-Muñoz, G., Sepúlveda-Escribano, A., & Pastor-Blas, M. M. (2024). The Facile Production of p-Chloroaniline Facilitated by an Efficient and Chemoselective Metal-Free N/S Co-Doped Carbon Catalyst. International Journal of Molecular Sciences, 25(17), 9603. https://doi.org/10.3390/ijms25179603





