A Novel Microbial Dysbiosis Index and Intestinal Microbiota-Associated Markers as Tools of Precision Medicine in Inflammatory Bowel Disease Paediatric Patients
Abstract
:1. Introduction
2. Results
2.1. Study Population
2.2. IBD Faecal Microbiota Compared to Healthy Controls
2.3. Gut Dysbiosis in IBD
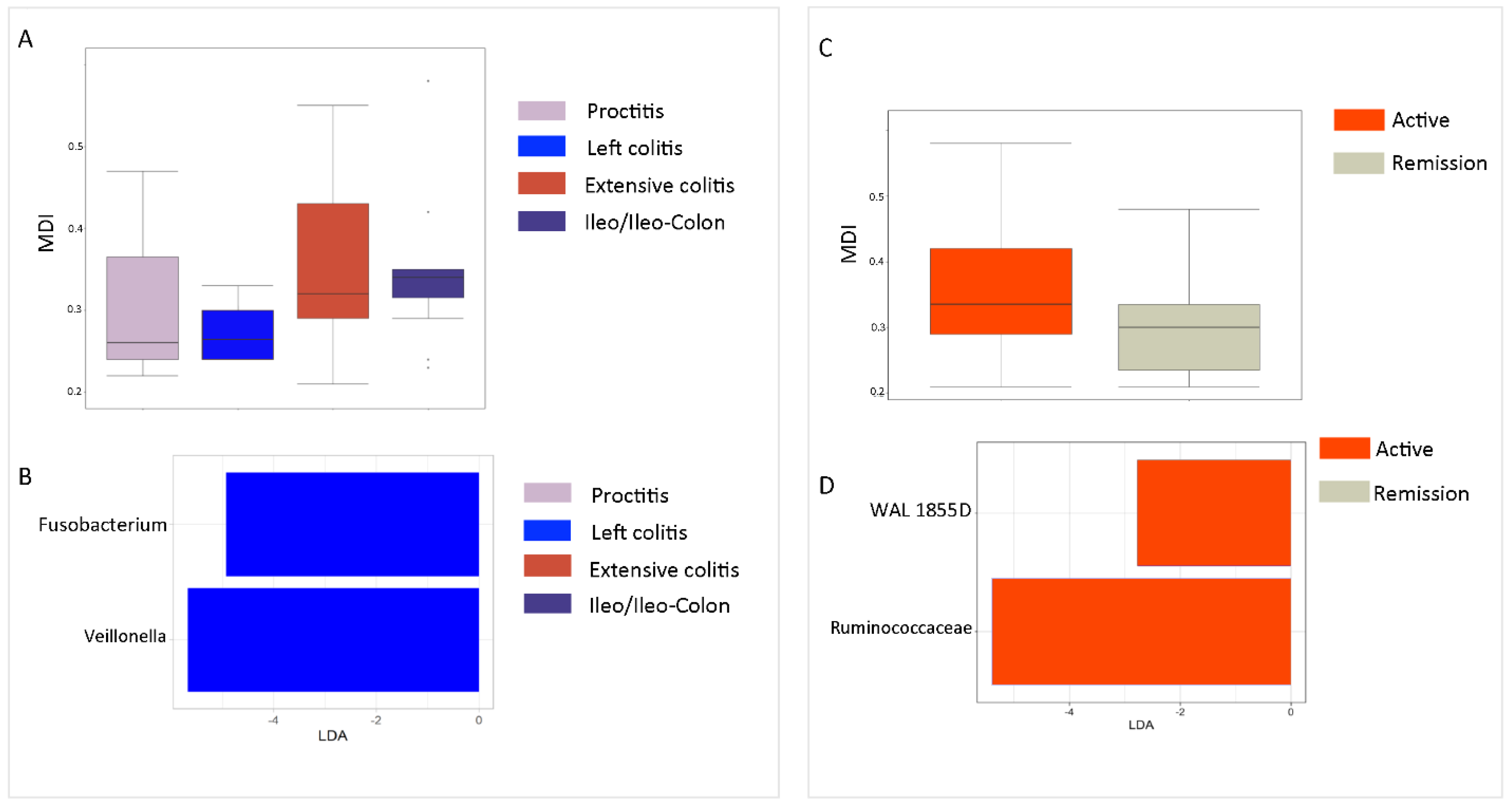
2.4. Correlation of Gut MDI and GM Profile with Disease Site and State
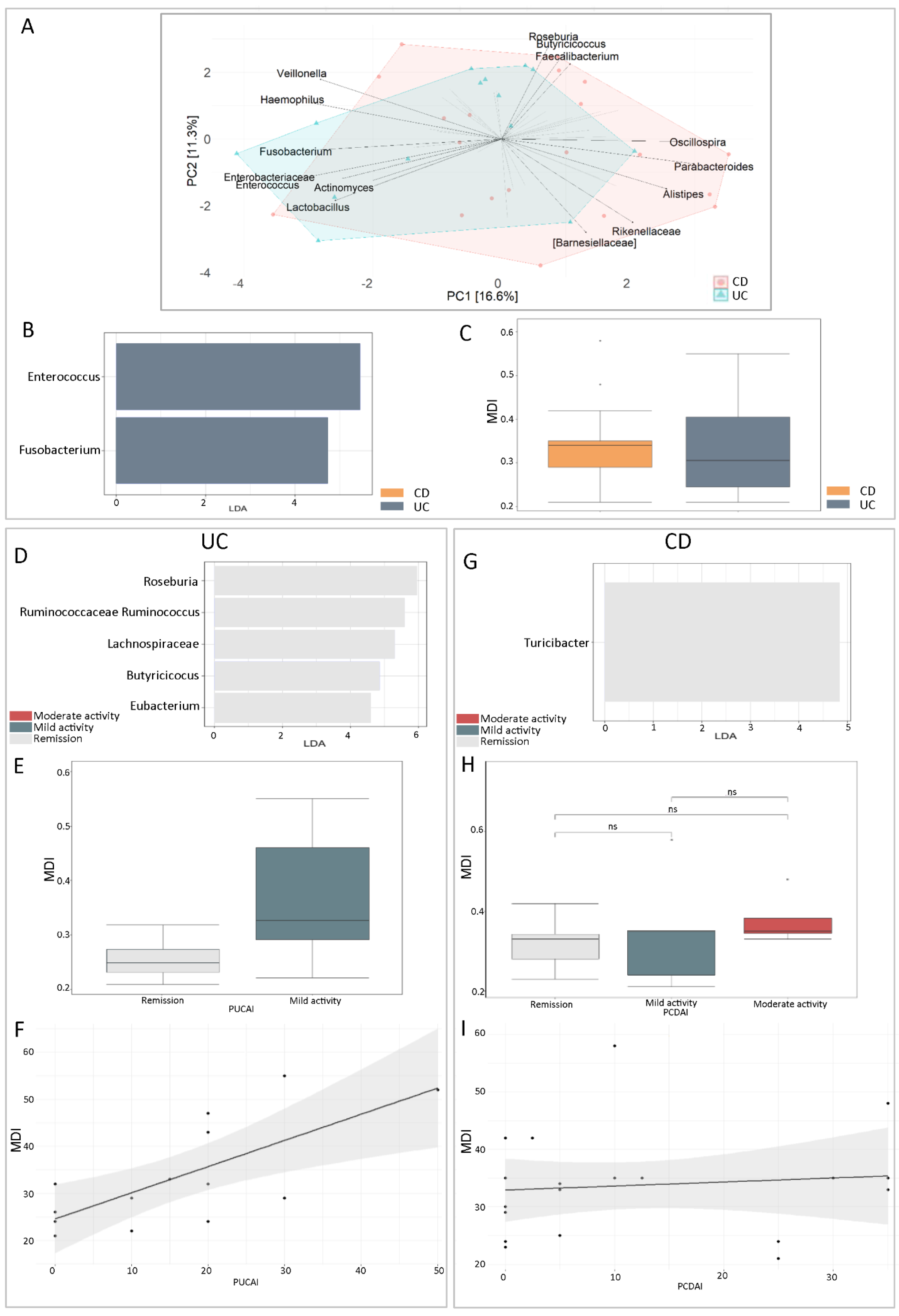
2.5. Gut Dysbiosis in UC and CD
2.6. Metabolic Biomarker Associated with MDI in IBD
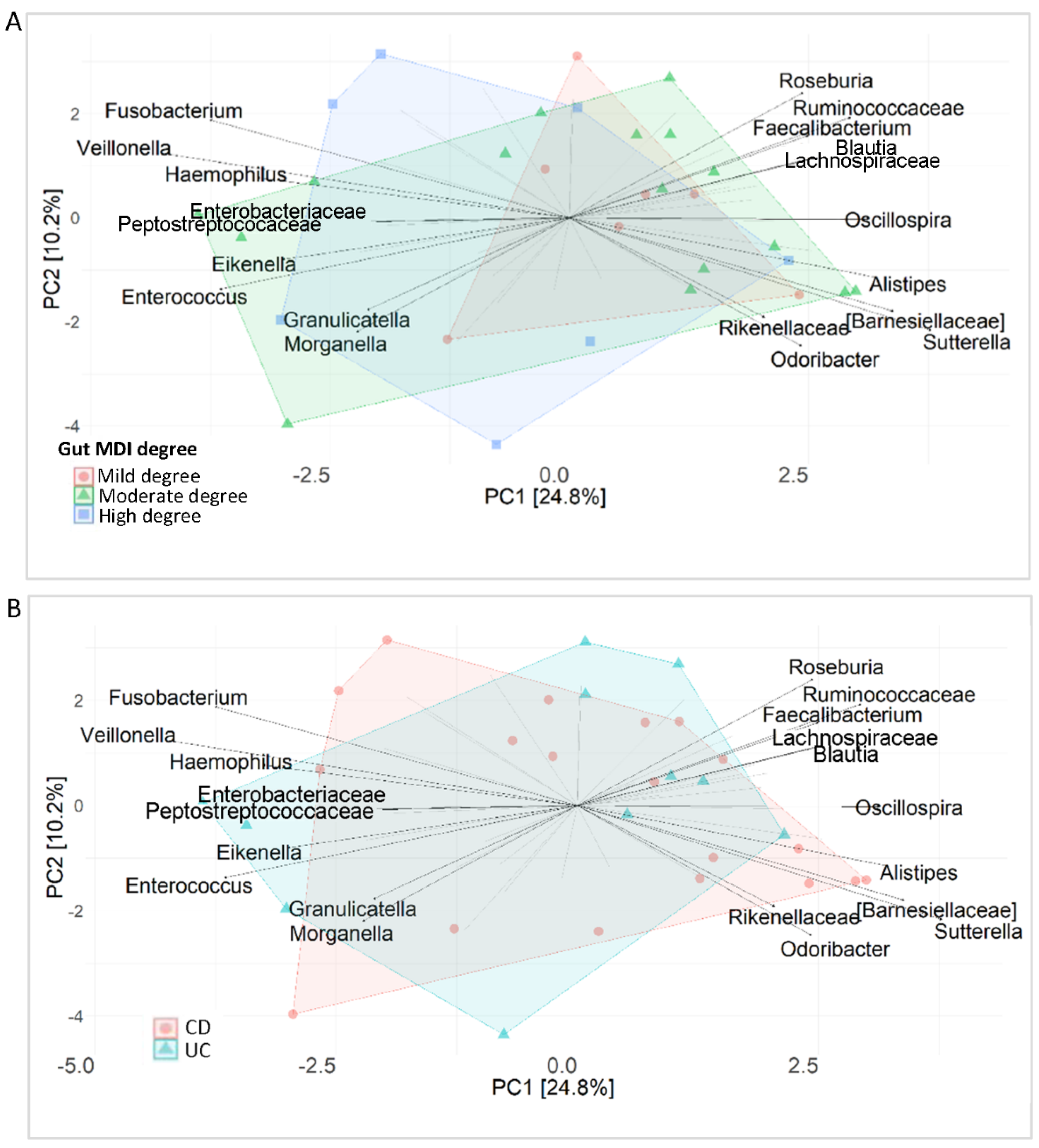
2.7. Ileal Microbiota Fingerprint in IBD
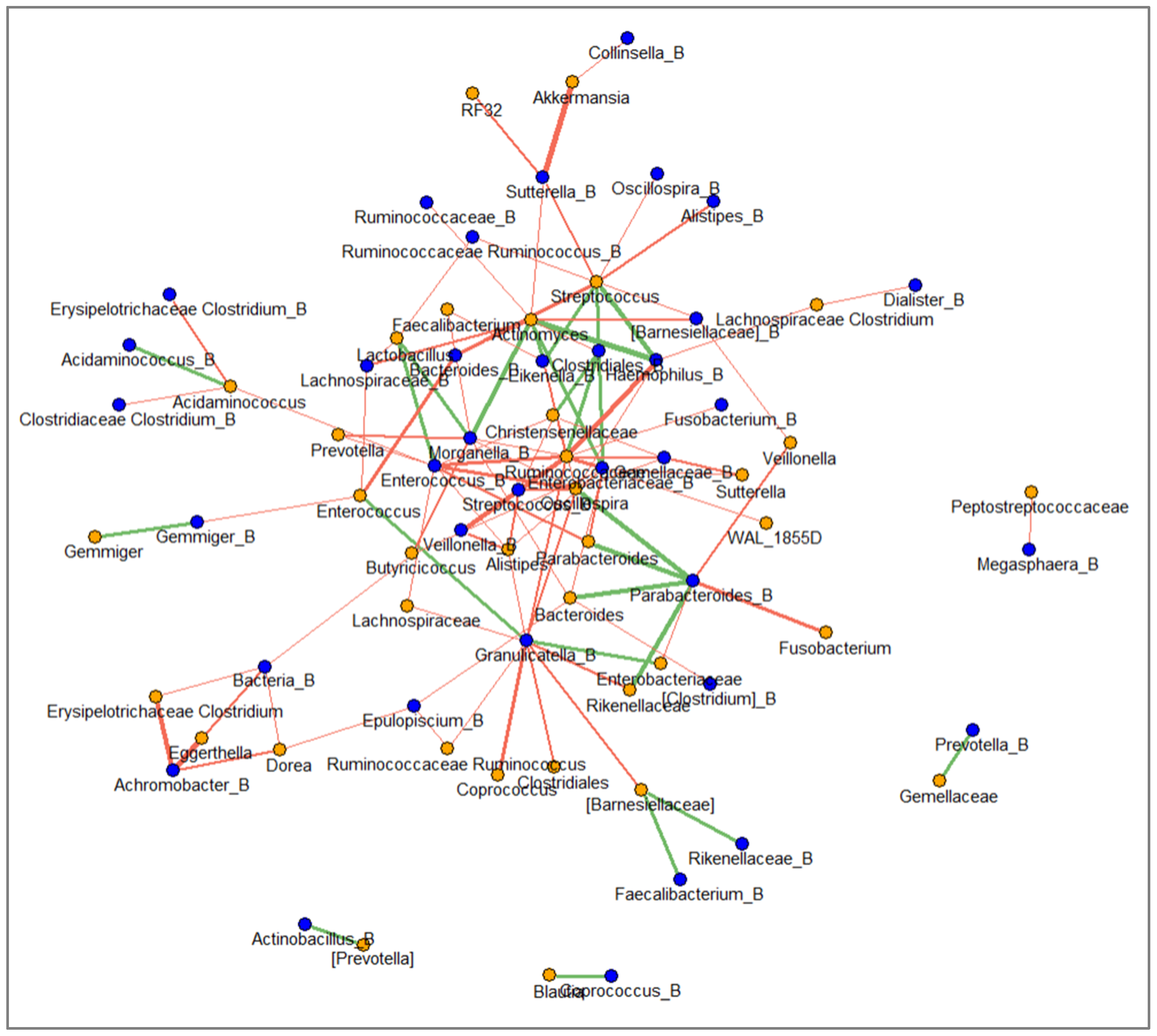
2.8. Network between Faecal and Mucosal Microbiota
2.9. Correlation between Faecal and Mucosal Bacteria and MDI
2.10. Metabolic Dysbiosis, Intestinal Permeability and Mucosal Immune Activation in IBD
3. Discussion
4. Materials and Methods
4.1. Patient Enrolment
4.2. Sample Collection
4.3. Library Preparation and 16S rRNA Sequencing
4.4. Bioinformatic Analysis of 16S Amplicon Sequencing
4.5. Intestinal MDI Calculation
4.6. Statistical Analyses
4.7. Functional and Network Analyses
4.8. Intestinal Permeability, Mucosal Immunity Activation and Metabolic Dysbiosis Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leylabadlo, H.E.; Ghotaslou, R.; Feizabadi, M.M.; Farajnia, S.; Moaddab, S.Y.; Ganbarov, K.; Khodadadi, E.; Tanomand, A.; Sheykhsaran, E.; Yousefi, B.; et al. The Critical Role of Faecalibacterium Prausnitzii in Human Health: An Overview. Microb. Pathog. 2020, 149, 104344. [Google Scholar] [CrossRef] [PubMed]
- Mah, C.; Jayawardana, T.; Leong, G.; Koentgen, S.; Lemberg, D.; Connor, S.J.; Rokkas, T.; Grimm, M.C.; Leach, S.T.; Hold, G.L. Assessing the Relationship between the Gut Microbiota and Inflammatory Bowel Disease Therapeutics: A Systematic Review. Pathogens 2023, 12, 262. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Zhang, W.; Zhang, T.; He, Q.; Kwok, L.-Y.; Tan, Y.; Zhang, H. Heat-Killed Bifidobacterium Bifidum B1628 May Alleviate Dextran Sulfate Sodium-Induced Colitis in Mice, and the Anti-Inflammatory Effect Is Associated with Gut Microbiota Modulation. Nutrients 2022, 14, 5233. [Google Scholar] [CrossRef]
- Baldelli, V.; Scaldaferri, F.; Putignani, L.; Del Chierico, F. The Role of Enterobacteriaceae in Gut Microbiota Dysbiosis in Inflammatory Bowel Diseases. Microorganisms 2021, 9, 697. [Google Scholar] [CrossRef] [PubMed]
- Cosnes, J.; Gower-Rousseau, C.; Seksik, P.; Cortot, A. Epidemiology and Natural History of Inflammatory Bowel Diseases. Gastroenterology 2011, 140, 1785–1794. [Google Scholar] [CrossRef]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B.; et al. Dysfunction of the Intestinal Microbiome in Inflammatory Bowel Disease and Treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef]
- Histologic Diagnosis of Inflammatory Bowel Diseases: Advances in Anatomic Pathology. Available online: https://journals.lww.com/anatomicpathology/fulltext/2022/01000/histologic_diagnosis_of_inflammatory_bowel.6.aspx (accessed on 22 August 2024).
- Rex, D.K.; Petrini, J.L.; Baron, T.H.; Chak, A.; Cohen, J.; Deal, S.E.; Hoffman, B.; Jacobson, B.C.; Mergener, K.; Petersen, B.T.; et al. Quality Indicators for Colonoscopy. Gastrointest. Endosc. 2006, 63, S16–S28. [Google Scholar] [CrossRef]
- Sherid, M.; Samo, S.; Sulaiman, S.; Sherid, M.; Samo, S.; Sulaiman, S. Complications of Colonoscopy. In Colonoscopy and Colorectal Cancer Screening–Future Directions; IntechOpen: London, UK, 2013; ISBN 978-953-51-0949-5. [Google Scholar]
- Yang, C.; Sriranjan, V.; Abou-Setta, A.M.; Poluha, W.; Walker, J.R.; Singh, H. Anxiety Associated with Colonoscopy and Flexible Sigmoidoscopy: A Systematic Review. Am. J. Gastroenterol. 2018, 113, 1810–1818. [Google Scholar] [CrossRef]
- Basha, O.M.; Hafez, R.A.; Salem, S.M.; Anis, R.H.; Hanafy, A.S. Impact of Gut Microbiome Alteration in Ulcerative Colitis Patients on Disease Severity and Outcome. Clin. Exp. Med. 2022, 23, 1763–1772. [Google Scholar] [CrossRef]
- Zois, C.D.; Katsanos, K.H.; Kosmidou, M.; Tsianos, E.V. Neurologic Manifestations in Inflammatory Bowel Diseases: Current Knowledge and Novel Insights. J. Crohn’s Colitis 2010, 4, 115–124. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Zhang, K.; Huang, Y.; Liu, Y.; Lu, X.; Liao, W.; Liu, X.; Zhang, Q.; Pan, W. Gut Microbiota Associated with Cryptococcal Meningitis and Dysbiosis Caused by Anti-Fungal Treatment. Front. Microbiol. 2023, 13, 1086239. [Google Scholar] [CrossRef] [PubMed]
- Hold, G.L.; Smith, M.; Grange, C.; Watt, E.R.; El-Omar, E.M.; Mukhopadhya, I. Role of the Gut Microbiota in Inflammatory Bowel Disease Pathogenesis: What Have We Learnt in the Past 10 Years? World J. Gastroenterol. 2014, 20, 1192–1210. [Google Scholar] [CrossRef]
- Kim, D.H.; Cheon, J.H. Pathogenesis of Inflammatory Bowel Disease and Recent Advances in Biologic Therapies. Immune Netw. 2017, 17, 25–40. [Google Scholar] [CrossRef]
- Putignani, L.; Del Chierico, F.; Vernocchi, P.; Cicala, M.; Cucchiara, S.; Dallapiccola, B. Dysbiotrack Study Group Gut Microbiota Dysbiosis as Risk and Premorbid Factors of IBD and IBS Along the Childhood–Adulthood Transition. Inflamm. Bowel Dis. 2016, 22, 487–504. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, M.; Garner, A.; Vlamakis, H.; Xavier, R.J. Microbial Genes and Pathways in Inflammatory Bowel Disease. Nat. Rev. Microbiol. 2019, 17, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Ye, B.D.; Lee, H.-S.; Baek, J.; Kim, G.; Park, D.; Park, S.H.; Yang, S.-K.; Han, B.; Liu, J.; et al. Identification of Three Novel Susceptibility Loci for Inflammatory Bowel Disease in Koreans in an Extended Genome-Wide Association Study. J. Crohn’s Colitis 2021, 15, 1898–1907. [Google Scholar] [CrossRef]
- Díez-Obrero, V.; Moratalla-Navarro, F.; Ibáñez-Sanz, G.; Guardiola, J.; Rodríguez-Moranta, F.; Obón-Santacana, M.; Díez-Villanueva, A.; Dampier, C.H.; Devall, M.; Carreras-Torres, R.; et al. Transcriptome-Wide Association Study for Inflammatory Bowel Disease Reveals Novel Candidate Susceptibility Genes in Specific Colon Subsites and Tissue Categories. J. Crohns Colitis 2022, 16, 275–285. [Google Scholar] [CrossRef]
- Cordero, R.Y.; Cordero, J.B.; Stiemke, A.B.; Datta, L.W.; Buyske, S.; Kugathasan, S.; McGovern, D.P.B.; Brant, S.R.; Simpson, C.L. Trans-Ancestry, Bayesian Meta-Analysis Discovers 20 Novel Risk Loci for Inflammatory Bowel Disease in an African American, East Asian and European Cohort. Hum. Mol. Genet. 2023, 32, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Scanu, M.; Toto, F.; Petito, V.; Masi, L.; Fidaleo, M.; Puca, P.; Baldelli, V.; Reddel, S.; Vernocchi, P.; Pani, G.; et al. An Integrative Multi-Omic Analysis Defines Gut Microbiota, Mycobiota, and Metabolic Fingerprints in Ulcerative Colitis Patients. Front. Cell Infect. Microbiol. 2024, 14, 1366192. [Google Scholar] [CrossRef]
- Seksik, P.; Rigottier-Gois, L.; Gramet, G.; Sutren, M.; Pochart, P.; Marteau, P.; Jian, R.; Doré, J. Alterations of the Dominant Faecal Bacterial Groups in Patients with Crohn’s Disease of the Colon. Gut 2003, 52, 237–242. [Google Scholar] [CrossRef]
- Sokol, H.; Seksik, P.; Rigottier-Gois, L.; Lay, C.; Lepage, P.; Podglajen, I.; Marteau, P.; Doré, J. Specificities of the Fecal Microbiota in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2006, 12, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Swidsinski, A.; Loening-Baucke, V.; Theissig, F.; Engelhardt, H.; Bengmark, S.; Koch, S.; Lochs, H.; Dörffel, Y. Comparative Study of the Intestinal Mucus Barrier in Normal and Inflamed Colon. Gut 2007, 56, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Sartor, R.B. Microbial Influences in Inflammatory Bowel Diseases. Gastroenterology 2008, 134, 577–594. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humarán, L.G.; Gratadoux, J.-J.; Blugeon, S.; Bridonneau, C.; Furet, J.-P.; Corthier, G.; et al. Faecalibacterium Prausnitzii Is an Anti-Inflammatory Commensal Bacterium Identified by Gut Microbiota Analysis of Crohn Disease Patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef]
- Sokol, H.; Seksik, P.; Furet, J.P.; Firmesse, O.; Nion-Larmurier, I.; Beaugerie, L.; Cosnes, J.; Corthier, G.; Marteau, P.; Doré, J. Low Counts of Faecalibacterium Prausnitzii in Colitis Microbiota. Inflamm. Bowel Dis. 2009, 15, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Putignani, L.; Oliva, S.; Isoldi, S.; Del Chierico, F.; Carissimi, C.; Laudadio, I.; Cucchiara, S.; Stronati, L. Fecal and Mucosal Microbiota Profiling in Pediatric Inflammatory Bowel Diseases. Eur. J. Gastroenterol. Hepatol. 2021, 33, 1376–1386. [Google Scholar] [CrossRef]
- Zhuang, X.; Liu, C.; Zhan, S.; Tian, Z.; Li, N.; Mao, R.; Zeng, Z.; Chen, M. Gut Microbiota Profile in Pediatric Patients With Inflammatory Bowel Disease: A Systematic Review. Front. Pediatr. 2021, 9, 626232. [Google Scholar] [CrossRef]
- Imai, T.; Inoue, R.; Kawada, Y.; Morita, Y.; Inatomi, O.; Nishida, A.; Bamba, S.; Kawahara, M.; Andoh, A. Characterization of Fungal Dysbiosis in Japanese Patients with Inflammatory Bowel Disease. J. Gastroenterol. 2019, 54, 149–159. [Google Scholar] [CrossRef]
- Nishino, K.; Nishida, A.; Inoue, R.; Kawada, Y.; Ohno, M.; Sakai, S.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Kawahara, M.; et al. Analysis of Endoscopic Brush Samples Identified Mucosa-Associated Dysbiosis in Inflammatory Bowel Disease. J. Gastroenterol. 2018, 53, 95–106. [Google Scholar] [CrossRef]
- Sartor, R.B.; Wu, G.D. Roles for Intestinal Bacteria, Viruses, and Fungi in Pathogenesis of Inflammatory Bowel Diseases and Therapeutic Approaches. Gastroenterology 2017, 152, 327–339. [Google Scholar] [CrossRef]
- Olesen, S.W.; Alm, E.J. Dysbiosis Is Not an Answer. Nat. Microbiol. 2016, 1, 16228. [Google Scholar] [CrossRef]
- Wei, S.; Bahl, M.I.; Baunwall, S.M.D.; Hvas, C.L.; Licht, T.R. Determining Gut Microbial Dysbiosis: A Review of Applied Indexes for Assessment of Intestinal Microbiota Imbalances. Appl. Environ. Microbiol. 2021, 87, e00395-21. [Google Scholar] [CrossRef] [PubMed]
- Lord, R.S.; Bralley, J.A. Clinical Applications of Urinary Organic Acids. Part 2. Dysbiosis Markers. Altern. Med. Rev. 2008, 13, 292–306. [Google Scholar] [PubMed]
- Fasano, A.; Not, T.; Wang, W.; Uzzau, S.; Berti, I.; Tommasini, A.; Goldblum, S.E. Zonulin, a Newly Discovered Modulator of Intestinal Permeability, and Its Expression in Coeliac Disease. Lancet 2000, 355, 1518–1519. [Google Scholar] [CrossRef]
- Fasano, A. Zonulin and Its Regulation of Intestinal Barrier Function: The Biological Door to Inflammation, Autoimmunity, and Cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef]
- Szymanska, E.; Wierzbicka, A.; Dadalski, M.; Kierkus, J. Fecal Zonulin as a Noninvasive Biomarker of Intestinal Permeability in Pediatric Patients with Inflammatory Bowel Diseases—Correlation with Disease Activity and Fecal Calprotectin. J. Clin. Med. 2021, 10, 3905. [Google Scholar] [CrossRef]
- Cassani, E.; Barichella, M.; Cancello, R.; Cavanna, F.; Iorio, L.; Cereda, E.; Bolliri, C.; Zampella Maria, P.; Bianchi, F.; Cestaro, B.; et al. Increased Urinary Indoxyl Sulfate (Indican): New Insights into Gut Dysbiosis in Parkinson’s Disease. Park. Relat. Disord. 2015, 21, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Malíčková, K.; Francová, I.; Lukáš, M.; Kolář, M.; Králíková, E.; Bortlík, M.; Ďuricová, D.; Štěpánková, L.; Zvolská, K.; Pánková, A.; et al. Fecal Zonulin Is Elevated in Crohn’s Disease and in Cigarette Smokers. Pract. Lab. Med. 2017, 9, 39–44. [Google Scholar] [CrossRef]
- Jendraszak, M.; Gałęcka, M.; Kotwicka, M.; Schwiertz, A.; Regdos, A.; Pazgrat-Patan, M.; Andrusiewicz, M. Impact of Biometric Patient Data, Probiotic Supplementation, and Selected Gut Microorganisms on Calprotectin, Zonulin, and sIgA Concentrations in the Stool of Adults Aged 18-74 Years. Biomolecules 2022, 12, 1781. [Google Scholar] [CrossRef]
- Caenepeel, C.; Sadat Seyed Tabib, N.; Vieira-Silva, S.; Vermeire, S. Review Article: How the Intestinal Microbiota May Reflect Disease Activity and Influence Therapeutic Outcome in Inflammatory Bowel Disease. Aliment. Pharmacol. Ther. 2020, 52, 1453–1468. [Google Scholar] [CrossRef]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The Treatment-Naïve Microbiome in New-Onset Crohn’s Disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef]
- Braun, T.; Di Segni, A.; BenShoshan, M.; Neuman, S.; Levhar, N.; Bubis, M.; Picard, O.; Sosnovski, K.; Efroni, G.; Farage Barhom, S.; et al. Individualized Dynamics in the Gut Microbiota Precede Crohn’s Disease Flares. Off. J. Am. Coll. Gastroenterol.|ACG 2019, 114, 1142. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, O.; Castellana, S.; Biscaglia, G.; Panza, A.; Latiano, A.; Fontana, R.; Guerra, M.; Corritore, G.; Latiano, T.; Martino, G.; et al. Microbiome Analysis of Mucosal Ileoanal Pouch in Ulcerative Colitis Patients Revealed Impairment of the Pouches Immunometabolites. Cells 2021, 10, 3243. [Google Scholar] [CrossRef]
- Lemons, J.M.S.; Conrad, M.; Tanes, C.; Chen, J.; Friedman, E.S.; Roggiani, M.; Curry, D.; Chau, L.; Hecht, A.L.; Harling, L.; et al. Enterobacteriaceae Growth Promotion by Intestinal Acylcarnitines, a Biomarker of Dysbiosis in Inflammatory Bowel Disease. Cell Mol. Gastroenterol. Hepatol. 2023, 17, 131–148. [Google Scholar] [CrossRef]
- Scaldaferri, F.; D’Onofrio, A.M.; Calia, R.; Di Vincenzo, F.; Ferrajoli, G.F.; Petito, V.; Maggio, E.; Pafundi, P.C.; Napolitano, D.; Masi, L.; et al. Gut Microbiota Signatures Are Associated With Psychopathological Profiles in Patients With Ulcerative Colitis: Results From an Italian Tertiary IBD Center. Inflamm. Bowel Dis. 2023, 29, 1805–1818. [Google Scholar] [CrossRef]
- Duvallet, C.; Gibbons, S.M.; Gurry, T.; Irizarry, R.A.; Alm, E.J. Meta-Analysis of Gut Microbiome Studies Identifies Disease-Specific and Shared Responses. Nat. Commun. 2017, 8, 1784. [Google Scholar] [CrossRef]
- Gaifem, J.; Mendes-Frias, A.; Wolter, M.; Steimle, A.; Garzón, M.J.; Ubeda, C.; Nobre, C.; González, A.; Pinho, S.S.; Cunha, C.; et al. Akkermansia Muciniphila and Parabacteroides Distasonis Synergistically Protect from Colitis by Promoting ILC3 in the Gut. mBio 2024, 15, e0007824. [Google Scholar] [CrossRef] [PubMed]
- Kverka, M.; Zakostelska, Z.; Klimesova, K.; Sokol, D.; Hudcovic, T.; Hrncir, T.; Rossmann, P.; Mrazek, J.; Kopecny, J.; Verdu, E.F.; et al. Oral Administration of Parabacteroides Distasonis Antigens Attenuates Experimental Murine Colitis through Modulation of Immunity and Microbiota Composition. Clin. Exp. Immunol. 2011, 163, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Teofani, A.; Marafini, I.; Laudisi, F.; Pietrucci, D.; Salvatori, S.; Unida, V.; Biocca, S.; Monteleone, G.; Desideri, A. Intestinal Taxa Abundance and Diversity in Inflammatory Bowel Disease Patients: An Analysis Including Covariates and Confounders. Nutrients 2022, 14, 260. [Google Scholar] [CrossRef] [PubMed]
- Altomare, A.; Putignani, L.; Del Chierico, F.; Cocca, S.; Angeletti, S.; Ciccozzi, M.; Tripiciano, C.; Dalla Piccola, B.; Cicala, M.; Guarino, M.P.L. Gut Mucosal-Associated Microbiota Better Discloses Inflammatory Bowel Disease Differential Patterns than Faecal Microbiota. Dig. Liver Dis. 2019, 51, 648–656. [Google Scholar] [CrossRef]
- Lett, B.; Costello, S.; Roberts-Thomson, I. Analyzing the Intestinal Microbiome in Inflammatory Bowel Disease: From RNA to Multiomics. JGH Open 2020, 4, 779–781. [Google Scholar] [CrossRef]
- Ma, X.; Lu, X.; Zhang, W.; Yang, L.; Wang, D.; Xu, J.; Jia, Y.; Wang, X.; Xie, H.; Li, S.; et al. Gut Microbiota in the Early Stage of Crohn’s Disease Has Unique Characteristics. Gut Pathog. 2022, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Chiodini, R.J.; Dowd, S.E.; Galandiuk, S.; Davis, B.; Glassing, A. The Predominant Site of Bacterial Translocation across the Intestinal Mucosal Barrier Occurs at the Advancing Disease Margin in Crohn’s Disease. Microbiology 2016, 162, 1608–1619. [Google Scholar] [CrossRef]
- Walujkar, S.A.; Kumbhare, S.V.; Marathe, N.P.; Patangia, D.V.; Lawate, P.S.; Bharadwaj, R.S.; Shouche, Y.S. Molecular Profiling of Mucosal Tissue Associated Microbiota in Patients Manifesting Acute Exacerbations and Remission Stage of Ulcerative Colitis. World J. Microbiol. Biotechnol. 2018, 34, 76. [Google Scholar] [CrossRef] [PubMed]
- Hirano, A.; Umeno, J.; Okamoto, Y.; Shibata, H.; Ogura, Y.; Moriyama, T.; Torisu, T.; Fujioka, S.; Fuyuno, Y.; Kawarabayasi, Y.; et al. Comparison of the Microbial Community Structure between Inflamed and Non-Inflamed Sites in Patients with Ulcerative Colitis. J. Gastroenterol. Hepatol. 2018, 33, 1590–1597. [Google Scholar] [CrossRef]
- Shen, Y.; Yu, X.; Wang, Q.; Yao, X.; Lu, D.; Zhou, D.; Wang, X. Association between Primary Sjögren’s Syndrome and Gut Microbiota Disruption: A Systematic Review and Meta-Analysis. Clin. Rheumatol. 2023, 43, 603–619. [Google Scholar] [CrossRef]
- Leung, H.K.M.; Lo, E.K.K.; Chen, C.; Zhang, F.; Felicianna; Ismaiah, M.J.; El-Nezami, H. Zearalenone Attenuates Colitis Associated Colorectal Tumorigenesis through Ras/Raf/ERK Pathway Suppression and SCFA-Producing Bacteria Promotion. Biomed. Pharmacother. 2023, 164, 114973. [Google Scholar] [CrossRef] [PubMed]
- Jean Wilson, E.; Sirpu Natesh, N.; Ghadermazi, P.; Pothuraju, R.; Shanmugam, M.; Prajapati, D.R.; Pandey, S.; Kaifi, J.T.; Dodam, J.R.; Bryan, J.; et al. Red Cabbage Juice-Mediated Gut Microbiota Modulation Improves Intestinal Epithelial Homeostasis and Ameliorates Colitis. bioRxiv 2023, 25, 539. [Google Scholar] [CrossRef]
- Miquel, S.; Martín, R.; Rossi, O.; Bermúdez-Humarán, L.G.; Chatel, J.M.; Sokol, H.; Thomas, M.; Wells, J.M.; Langella, P. Faecalibacterium Prausnitzii and Human Intestinal Health. Curr. Opin. Microbiol. 2013, 16, 255–261. [Google Scholar] [CrossRef]
- Steppe, M.; Van Nieuwerburgh, F.; Vercauteren, G.; Boyen, F.; Eeckhaut, V.; Deforce, D.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F. Safety Assessment of the Butyrate-Producing Butyricicoccus Pullicaecorum Strain 25-3(T), a Potential Probiotic for Patients with Inflammatory Bowel Disease, Based on Oral Toxicity Tests and Whole Genome Sequencing. Food Chem. Toxicol. 2014, 72, 129–137. [Google Scholar] [CrossRef]
- Sohn, J.; Li, L.; Zhang, L.; Genco, R.J.; Falkner, K.L.; Tettelin, H.; Rowsam, A.M.; Smiraglia, D.J.; Novak, J.M.; Diaz, P.I.; et al. Periodontal Disease Is Associated with Increased Gut Colonization of Pathogenic Haemophilus Parainfluenzae in Patients with Crohn’s Disease. Cell Rep. 2023, 42, 112120. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, J.; Ta, A.; Ollberding, N.J.; Bezold, R.; Lake, K.; Jackson, K.; Dirksing, K.; Bonkowski, E.; Haslam, D.B.; Denson, L.A. Patient-Reported Outcomes Correlate With Microbial Community Composition Independent of Mucosal Inflammation in Pediatric Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2023, 29, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Tang, P.; Li, C.; Yang, Q.; Xu, Y.; Su, C.; Li, L. Fusobacterium Nucleatum and Its Associated Systemic Diseases: Epidemiologic Studies and Possible Mechanisms. J. Oral. Microbiol. 2023, 15, 2145729. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Arriaga, B.; Sandoval-Jiménez, S.; Rodríguez-Silverio, J.; Lizeth Alcaráz-Estrada, S.; Cortés-Espinosa, T.; Pérez-Cabeza de Vaca, R.; Licona-Cassani, C.; Gámez-Valdez, J.S.; Shaw, J.; Mondragón-Terán, P.; et al. Gut Dysbiosis and Clinical Phases of Pancolitis in Patients with Ulcerative Colitis. Microbiologyopen 2021, 10, e1181. [Google Scholar] [CrossRef]
- Kostic, A.D.; Gevers, D.; Pedamallu, C.S.; Michaud, M.; Duke, F.; Earl, A.M.; Ojesina, A.I.; Jung, J.; Bass, A.J.; Tabernero, J.; et al. Genomic Analysis Identifies Association of Fusobacterium with Colorectal Carcinoma. Genome Res. 2012, 22, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium Nucleatum Potentiates Intestinal Tumorigenesis and Modulates the Tumor Immune Microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef]
- Zepeda-Rivera, M.; Minot, S.S.; Bouzek, H.; Wu, H.; Blanco-Míguez, A.; Manghi, P.; Jones, D.S.; LaCourse, K.D.; Wu, Y.; McMahon, E.F.; et al. A Distinct Fusobacterium Nucleatum Clade Dominates the Colorectal Cancer Niche. Nature 2024, 628, 424–432. [Google Scholar] [CrossRef]
- Zhu, H.; Li, M.; Bi, D.; Yang, H.; Gao, Y.; Song, F.; Zheng, J.; Xie, R.; Zhang, Y.; Liu, H.; et al. Fusobacterium Nucleatum Promotes Tumor Progression in KRAS p.G12D-Mutant Colorectal Cancer by Binding to DHX15. Nat. Commun. 2024, 15, 1688. [Google Scholar] [CrossRef]
- Ma, K.L.; Kei, N.; Yang, F.; Lauw, S.; Chan, P.L.; Chen, L.; Cheung, P.C.K. In Vitro Fermentation Characteristics of Fungal Polysaccharides Derived from Wolfiporia Cocos and Their Effect on Human Fecal Microbiota. Foods 2023, 12, 4014. [Google Scholar] [CrossRef]
- Bálint, A.; Farkas, K.; Méhi, O.; Kintses, B.; Vásárhelyi, B.M.; Ari, E.; Pál, C.; Madácsy, T.; Maléth, J.; Szántó, K.J.; et al. Functional Anatomical Changes in Ulcerative Colitis Patients Determine Their Gut Microbiota Composition and Consequently the Possible Treatment Outcome. Pharmaceuticals 2020, 13, 346. [Google Scholar] [CrossRef] [PubMed]
- Del Chierico, F.; Cardile, S.; Baldelli, V.; Alterio, T.; Reddel, S.; Bramuzzo, M.; Knafelz, D.; Lega, S.; Bracci, F.; Torre, G.; et al. Characterization of the Gut Microbiota and Mycobiota in Italian Pediatric Patients With Primary Sclerosing Cholangitis and Ulcerative Colitis. Inflamm. Bowel Dis. 2023, 30, 529–537. [Google Scholar] [CrossRef]
- Shaw, K.A.; Bertha, M.; Hofmekler, T.; Chopra, P.; Vatanen, T.; Srivatsa, A.; Prince, J.; Kumar, A.; Sauer, C.; Zwick, M.E.; et al. Dysbiosis, Inflammation, and Response to Treatment: A Longitudinal Study of Pediatric Subjects with Newly Diagnosed Inflammatory Bowel Disease. Genome Med. 2016, 8, 75. [Google Scholar] [CrossRef] [PubMed]
- Curzon, G.; Walsh, J. A Method for the Determination of Urinary Indoxyl Sulphate (Indican). Clin. Chim. Acta 1962, 7, 657–663. [Google Scholar] [CrossRef]
- Dion, C.; Montagne, P.; Bene, M.C.; Faure, G. Measurement of Faecal Immunoglobulin a Levels in Young Children. J. Clin. Lab. Anal. 2004, 18, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Meillet, D.; Raichvarg, D.; Tallet, F.; Savel, J.; Yonger, J.; Gobert, J.G. Measurement of Total, Monomeric and Polymeric IgA in Human Faeces by Electroimmunodiffusion. Clin. Exp. Immunol. 1987, 69, 142–147. [Google Scholar] [PubMed]
- Corthésy, B. Role of Secretory IgA in Infection and Maintenance of Homeostasis. Autoimmun. Rev. 2013, 12, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.-R.; Shen, S.-Y.; Shen, H.-Q.; Yi, P.-F.; Fu, B.-D.; Peng, L.-Y. The Role of Endoplasmic Reticulum Stress in Regulation of Intestinal Barrier and Inflammatory Bowel Disease. Exp. Cell Res. 2023, 424, 113472. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Y.; Yang, Y.; Dai, Z.; Kim, I.H.; Wu, G.; Wu, Z. Dietary Supplementation with Glycine Enhances Intestinal Mucosal Integrity and Ameliorates Inflammation in C57BL/6J Mice with High-Fat Diet-Induced Obesity. J. Nutr. 2021, 151, 1769–1778. [Google Scholar] [CrossRef]
- Alves, A.; Bassot, A.; Bulteau, A.-L.; Pirola, L.; Morio, B. Glycine Metabolism and Its Alterations in Obesity and Metabolic Diseases. Nutrients 2019, 11, 1356. [Google Scholar] [CrossRef]
- Dai, Z.-L.; Wu, G.; Zhu, W.-Y. Amino Acid Metabolism in Intestinal Bacteria: Links between Gut Ecology and Host Health. Front. Biosci. 2011, 16, 1768–1786. [Google Scholar] [CrossRef]
- Yi, X.; Huang, C.; Huang, C.; Zhao, M.; Lu, Q. Fecal Microbiota from MRL/Lpr Mice Exacerbates Pristane-Induced Lupus. Arthritis Res. Ther. 2023, 25, 42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yin, X.; Wang, H.; Wu, X.; Li, X.; Li, Y.; Zhang, X.; Fu, C.; Li, H.; Qiu, Y. Fecal Metabolomics and Potential Biomarkers for Systemic Lupus Erythematosus. Front. Immunol. 2019, 10, 976. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Jiang, H.; Gu, S.; Feng, N.; Zhang, N.; Lv, L.; Liu, F. Fecal Metabolites Were Altered, Identified as Biomarkers and Correlated With Disease Activity in Patients With Systemic Lupus Erythematosus in a GC-MS-Based Metabolomics Study. Front. Immunol. 2020, 11, 2138. [Google Scholar] [CrossRef]
- Ruseler-van Embden, J.G.H.; van Lieshout, L.M.C. Increased Proteolysis and Leucine Aminopeptidase Activity in Faeces of Patients with Crohn’s Disease. Digestion 2009, 40, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, J.M.; Gallimore, R.; Elias, E.; Allan, R.N.; Kennedy, J.F. Faecal Mucus Degrading Glycosidases in Ulcerative Colitis and Crohn’s Disease. Gut 1985, 26, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.-J.; Ding, L.; Yang, T.; Yang, Y.-F.; Jin, Y.-P.; Zhang, X.-P.; Ma, A.-H.; Qin, Y.-H. The Proteolytic Activity in Inflammatory Bowel Disease: Insight from Gut Microbiota. Microb. Pathog. 2024, 188, 106560. [Google Scholar] [CrossRef]
- Hoffman, S.; Aviv Cohen, N.; Carroll, I.M.; Tulchinsky, H.; Borovok, I.; Dotan, I.; Maharshak, N. Faecal Proteases from Pouchitis Patients Activate Protease Activating Receptor-2 to Disrupt the Epithelial Barrier. J. Crohn’s Colitis 2019, 13, 1558–1568. [Google Scholar] [CrossRef]
- MacDonald, E.M.S.; Powell, G.K.; Regier, D.A.; Glass, N.L.; Roberto, F.; Kosuge, T.; Morris, R.O. Secretion of Zeatin, Ribosylzeatin, and Ribosyl-1″ -Methylzeatin by Pseudomonas Savastanoi 1: Plasmid-Coded Cytokinin Biosynthesis. Plant Physiol. 1986, 82, 742–747. [Google Scholar] [CrossRef]
- Levine, A.; Koletzko, S.; Turner, D.; Escher, J.C.; Cucchiara, S.; de Ridder, L.; Kolho, K.-L.; Veres, G.; Russell, R.K.; Paerregaard, A.; et al. ESPGHAN Revised Porto Criteria for the Diagnosis of Inflammatory Bowel Disease in Children and Adolescents. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 795–806. [Google Scholar] [CrossRef]
- Irvine, E.J. Development and Subsequent Refinement of the Inflammatory Bowel Disease Questionnaire: A Quality-of-Life Instrument for Adult Patients with Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 1999, 28, S23–S27. [Google Scholar] [CrossRef]
- Lo Presti, A.; Zorzi, F.; Del Chierico, F.; Altomare, A.; Cocca, S.; Avola, A.; De Biasio, F.; Russo, A.; Cella, E.; Reddel, S.; et al. Fecal and Mucosal Microbiota Profiling in Irritable Bowel Syndrome and Inflammatory Bowel Disease. Front. Microbiol. 2019, 10, 1655. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree: Computing Large Minimum Evolution Trees with Profiles Instead of a Distance Matrix. Mol. Biol. Evol. 2009, 26, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences with QIIME 2′s Q2-Feature-Classifier Plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Leti, G. Statistica Descrittiva; il Mulino: Bologna, Italy, 2001; ISBN 978-88-15-00278-5. [Google Scholar]
- Cao, Y.; Dong, Q.; Wang, D.; Zhang, P.; Liu, Y.; Niu, C. microbiomeMarker: An R/Bioconductor Package for Microbiome Marker Identification and Visualization. Bioinformatics 2022, 38, 4027–4029. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic Biomarker Discovery and Explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Paulson, J.N.; Stine, O.C.; Bravo, H.C.; Pop, M. Robust Methods for Differential Abundance Analysis in Marker Gene Surveys. Nat. Methods 2013, 10, 1200–1202. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Ratner, B. The Correlation Coefficient: Its Values Range between +1/−1, or Do They? J. Target. Meas. Anal. Mark. 2009, 17, 139–142. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for Prediction of Metagenome Functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]



| Clinical Features | N (%) | |
|---|---|---|
| Gender, male/female (%) | 18/17 (51.4/48.6) | |
| Age, mean (years) | 14.4 | |
| Treatment | ||
| 5-ASA 1 | 16 (45.71) | |
| Antibiotics | 3 (8.57) | |
| Immunosuppressants | 10 (28.57) | |
| Biological therapies | 11 (31.42) | |
| Disease activity | ||
| Active | 11 (31.4) | |
| Remission | 24 (68.6) | |
| Disease severity | ||
| Remission | 16 (45.7) | |
| Mild | 14 (40) | |
| Moderate | 5 (14.3) | |
| Disease localisation | ||
| Absence | 4 (11.43) | |
| Proctitis | 3 (8.57) | |
| Left colitis | 4 (11.43) | |
| Extensive colitis | 9 (25.71) | |
| Ileo/Ileocolon | 15 (42.8) | |
| IBD conditions | ||
| UC (n = 14) | CD (n = 21) | |
| Activity index | PUCAI N (%) | PCDAI N (%) |
| Remission (<10) | 4 (28.6) | 12 (57.1) |
| Mild (10–34) | 10 (71.4) | 6 (28.6) |
| Moderate (35–64) | NA | 3 (14.3) |
| Severe (>65) | -- | -- |
| Faecal Microbiota | Linear Correlation Coefficient | p-Value | q-Value |
|---|---|---|---|
| Enterobacteriaceae | 0.634 | 0.00004 | 0.00217 |
| Fusobacterium | 0.435 | 0.00908 | 0.44492 |
| Haemophilus | 0.399 | 0.01745 | 0.80292 |
| WAL_1855D | 0.337 | 0.04738 | 1.00000 |
| Lachnospiraceae_Clostridium | −0.422 | 0.01152 | 0.55273 |
| Bacteroides | −0.409 | 0.01474 | 0.69299 |
| Butyricicoccus | −0.392 | 0.02000 | 0.89989 |
| Faecalibacterium | −0.537 | 0.00087 | 0.04341 |
| Ileal Microbiota | |||
| Achromobacter | 0.4017 | 0.02778 | 1.00000 |
| Actinobacillus | 0.4547 | 0.01159 | 0.59126 |
| Cloacibacterium | 0.4832 | 0.00684 | 0.35559 |
| Haemophilus | 0.4507 | 0.01244 | 0.62188 |
| Prevotella | 0.4257 | 0.01901 | 0.93166 |
| Pseudomonadaceae | 0.4230 | 0.01985 | 0.95267 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toto, F.; Marangelo, C.; Scanu, M.; De Angelis, P.; Isoldi, S.; Abreu, M.T.; Cucchiara, S.; Stronati, L.; Del Chierico, F.; Putignani, L. A Novel Microbial Dysbiosis Index and Intestinal Microbiota-Associated Markers as Tools of Precision Medicine in Inflammatory Bowel Disease Paediatric Patients. Int. J. Mol. Sci. 2024, 25, 9618. https://doi.org/10.3390/ijms25179618
Toto F, Marangelo C, Scanu M, De Angelis P, Isoldi S, Abreu MT, Cucchiara S, Stronati L, Del Chierico F, Putignani L. A Novel Microbial Dysbiosis Index and Intestinal Microbiota-Associated Markers as Tools of Precision Medicine in Inflammatory Bowel Disease Paediatric Patients. International Journal of Molecular Sciences. 2024; 25(17):9618. https://doi.org/10.3390/ijms25179618
Chicago/Turabian StyleToto, Francesca, Chiara Marangelo, Matteo Scanu, Paola De Angelis, Sara Isoldi, Maria Teresa Abreu, Salvatore Cucchiara, Laura Stronati, Federica Del Chierico, and Lorenza Putignani. 2024. "A Novel Microbial Dysbiosis Index and Intestinal Microbiota-Associated Markers as Tools of Precision Medicine in Inflammatory Bowel Disease Paediatric Patients" International Journal of Molecular Sciences 25, no. 17: 9618. https://doi.org/10.3390/ijms25179618






