The Molecular and Immunological Landscape of Meningiomas
Abstract
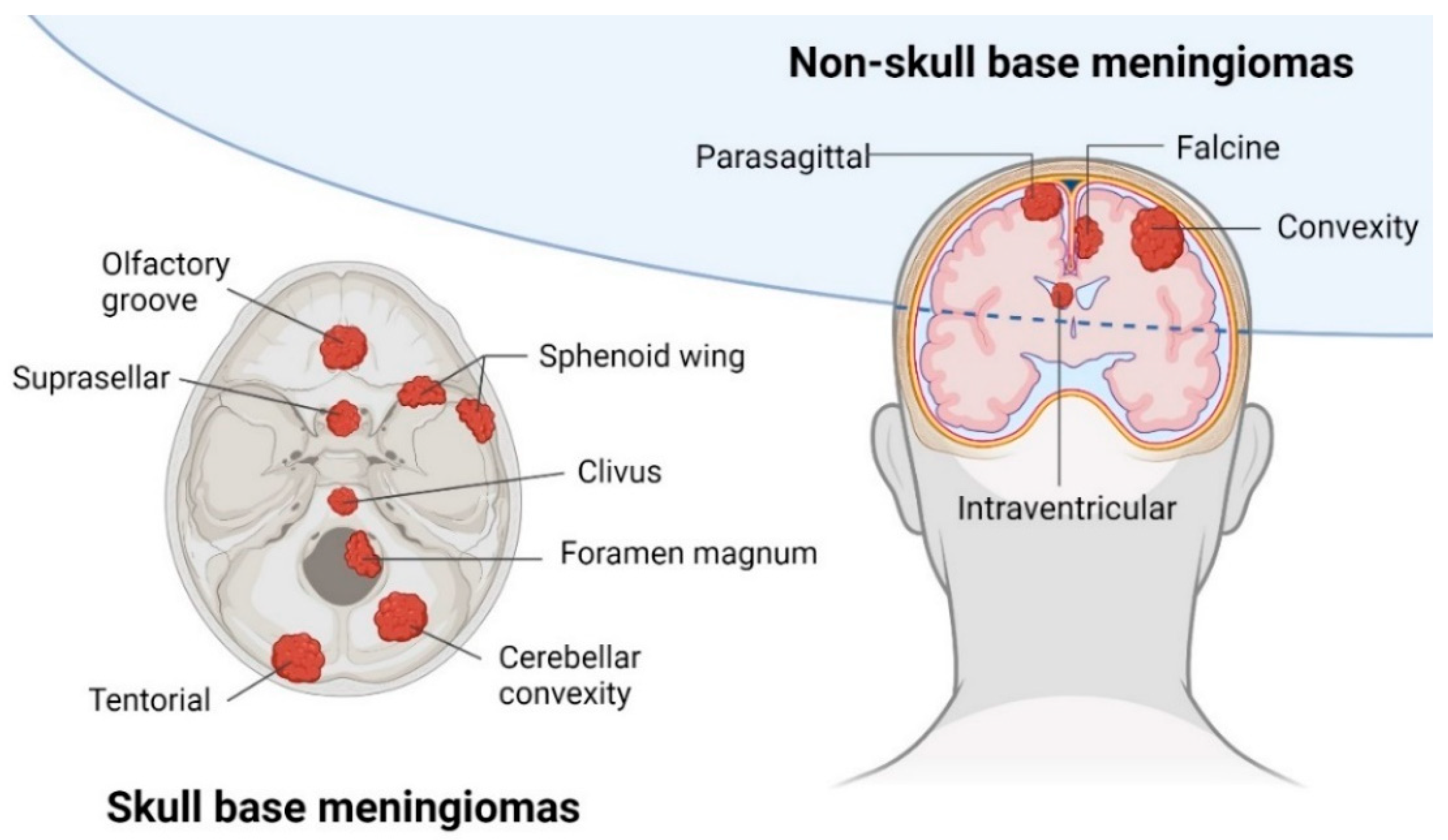
1. Introduction
2. Clinical Presentation
3. Genomic Landscape of Meningiomas
4. Epigenetic Landscape of Meningiomas
5. Immunological Landscape of Meningiomas
6. PD-L1 Expression in Meningiomas
7. Tumor-Infiltrating T Lymphocytes in Meningiomas
8. Tumor-Associated Macrophages in Meningiomas
9. Immunotherapy for Meningiomas
| Clinical Trial Identifier | Design | Treatment | Checkpoint Molecule | N | Status |
|---|---|---|---|---|---|
| NCT02648997 | Two arms, sequential assignment phase 2 | 1. Nivolumab 2. Radiotherapy followed by nivolumab with ipilimumab | 1. PD1 2. PD1/CTLA4 | 50 | Recruiting |
| NCT03016091 | Single arm phase 2 | Pembrolizumab | PD1 | 25 | Unknown |
| NCT03173950 | Basket trial phase 2 | Nivolumab | PD1 | 180 a | Recruiting |
| NCT03267836 | Single arm phase 1b | Proton radiation therapy with avelumab | PD-L1 | 9 | Terminated |
| NCT03279692 | Single arm phase 2 | Pembrolizumab | PD1 | 26 | Active |
| NCT03604978 | Randomized open label phase 1/2 | Nivolumab and multi-fraction stereotactic radiosurgery with or without ipilimumab | PD1/CTLA4 | 38 | Recruiting |
| NCT04659811 | Single arm phase 2 | Pembrolizumab and stereotactic radiosurgery | PD1 | 37 | Recruiting |
10. Immunogenetics and Immunoepigenetics of Meningiomas
11. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Ben | Benign |
| CNA | Copy number alteration |
| CNS | Central nervous system |
| CNV | Copy number variation |
| CpG | Cytosine-guanosine dinucleotide |
| GEMMs | Genetically engineered mouse models |
| ICB | Immune checkpoint blockade |
| IF | Immunofluorescence |
| IHC | Immunohistochemistry |
| Int | Intermediate |
| Mal | Malignant |
| MC | Methylation class |
| MGM | Meningioma |
| NF2 | Neurofibromin-2 |
| NGS | Next-generation sequencing |
| NK cell | Natural killer cell |
| PD-L1 | Programmed death-ligand 1 |
| PFS | Progression-free survival |
| pMGM | Primary meningioma |
| rMGM | Recurrent meningioma |
| SMO | Smoothened |
| TAM | Tumor-associated macrophage |
| TERT | Telomerase reverse transcriptase |
| TERTp | TERT promoter |
| TMA | Tissue microarray |
| TMB | Tumor mutational burden |
| WHO | World Health Organization |
References
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015–2019. Neuro-Oncol. 2022, 24, v1–v95. [Google Scholar] [CrossRef] [PubMed]
- Suppiah, S.; Nassiri, F.; Bi, W.L.; Dunn, I.F.; Hanemann, C.O.; Horbinski, C.M.; Hashizume, R.; James, C.D.; Mawrin, C.; Noushmehr, H.; et al. Molecular and translational advances in meningiomas. Neuro-Oncol. 2019, 21, i4–i17. [Google Scholar] [CrossRef]
- Krischek, B.; Goldbrunner, R. Paradigm Shift in the Treatment of Meningiomas. In Biological and Clinical Landscape of Meningiomas; Zadeh, G., Goldbrunner, R., Krischek, B., Nassiri, F., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–4. [Google Scholar]
- Raghunathan, A.; Giannini, C. Histopathology of Meningiomas. In Biological and Clinical Landscape of Meningiomas; Zadeh, G., Goldbrunner, R., Krischek, B., Nassiri, F., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 35–45. [Google Scholar]
- Brastianos, P.K.; Galanis, E.; Butowski, N.; Chan, J.W.; Dunn, I.F.; Goldbrunner, R.; Herold-Mende, C.; Ippen, F.M.; Mawrin, C.; McDermott, M.W.; et al. Advances in multidisciplinary therapy for meningiomas. Neuro-Oncol. 2019, 21, i18–i31. [Google Scholar] [CrossRef]
- Danish, H.; Brastianos, P. Novel Medical Therapies in Meningiomas. In Biological and Clinical Landscape of Meningiomas; Zadeh, G., Goldbrunner, R., Krischek, B., Nassiri, F., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 213–223. [Google Scholar]
- Millward, C.P.; Keshwara, S.; Islim, A.I.; Zakaria, R.; Jenkinson, M.D. Clinical Presentation and Prognosis. In Biological and Clinical Landscape of Meningiomas; Zadeh, G., Goldbrunner, R., Krischek, B., Nassiri, F., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 5–20. [Google Scholar]
- Susko, M.S.; Raleigh, D.R. Radiotherapy for Meningioma. In Biological and Clinical Landscape of Meningiomas; Zadeh, G., Goldbrunner, R., Krischek, B., Nassiri, F., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 95–106. [Google Scholar]
- Adekanmbi, A.; Youngblood, M.W.; Karras, C.L.; Oyetunji, E.A.; Kalapurakal, J.; Horbinski, C.M.; Najem, H.; Hill, V.B.; Chandler, J.P.; Heimberger, A.B.; et al. Clinical Management of Supratentorial Non-Skull Base Meningiomas. Cancers 2022, 14, 5887. [Google Scholar] [CrossRef] [PubMed]
- Buerki, R.A.; Horbinski, C.M.; Kruser, T.; Horowitz, P.M.; James, C.D.; Lukas, R.V. An overview of meningiomas. Future Oncol. 2018, 14, 2161–2177. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Torp, S.H.; Solheim, O.; Skjulsvik, A.J. The WHO 2021 Classification of Central Nervous System tumours: A practical update on what neurosurgeons need to know—A minireview. Acta Neurochir. 2022, 164, 2453–2464. [Google Scholar] [CrossRef]
- Gritsch, S.; Batchelor, T.T.; Gonzalez Castro, L.N. Diagnostic, therapeutic, and prognostic implications of the 2021 World Health Organization classification of tumors of the central nervous system. Cancer 2022, 128, 47–58. [Google Scholar] [CrossRef]
- Malta, T.M.; Snyder, J.; Noushmehr, H.; Castro, A.V. Advances in Central Nervous System Tumor Classification. In Biological and Clinical Landscape of Meningiomas; Zadeh, G., Goldbrunner, R., Krischek, B., Nassiri, F., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 121–135. [Google Scholar]
- Wang, J.Z.; Nassiri, F.; Mawrin, C.; Zadeh, G. Genomic Landscape of Meningiomas. In Biological and Clinical Landscape of Meningiomas; Zadeh, G., Goldbrunner, R., Krischek, B., Nassiri, F., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 137–158. [Google Scholar]
- Bi, W.L.; Greenwald, N.F.; Abedalthagafi, M.; Wala, J.; Gibson, W.J.; Agarwalla, P.K.; Horowitz, P.; Schumacher, S.E.; Esaulova, E.; Mei, Y.; et al. Genomic landscape of high-grade meningiomas. NPJ Genom. Med. 2017, 2, 15. [Google Scholar] [CrossRef]
- Sievers, P.; Hielscher, T.; Schrimpf, D.; Stichel, D.; Reuss, D.E.; Berghoff, A.S.; Neidert, M.C.; Wirsching, H.-G.; Mawrin, C.; Ketter, R.; et al. CDKN2A/B homozygous deletion is associated with early recurrence in meningiomas. Acta Neuropathol. 2020, 140, 409–413. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Clark, V.E.; Erson-Omay, E.Z.; Serin, A.; Yin, J.; Cotney, J.; Özduman, K.; Avşar, T.; Li, J.; Murray, P.B.; Henegariu, O.; et al. Genomic Analysis of Non- NF2 Meningiomas Reveals Mutations in TRAF7, KLF4, AKT1, and SMO. Science 2013, 339, 1077–1080. [Google Scholar] [CrossRef]
- Nassiri, F.; Liu, J.; Patil, V.; Mamatjan, Y.; Wang, J.Z.; Hugh-White, R.; Macklin, A.M.; Khan, S.; Singh, O.; Karimi, S.; et al. A clinically applicable integrative molecular classification of meningiomas. Nature 2021, 597, 119–125. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Z.; Nassiri, F.; Aldape, K.; von Deimling, A.; Sahm, F. The Epigenetic Landscape of Meningiomas. In Biological and Clinical Landscape of Meningiomas; Zadeh, G., Goldbrunner, R., Krischek, B., Nassiri, F., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 175–188. [Google Scholar]
- Olar, A.; Wani, K.M.; Wilson, C.D.; Zadeh, G.; DeMonte, F.; Jones, D.T.W.; Pfister, S.M.; Sulman, E.P.; Aldape, K.D. Global epigenetic profiling identifies methylation subgroups associated with recurrence-free survival in meningioma. Acta Neuropathol. 2017, 133, 431–444. [Google Scholar] [CrossRef]
- Sahm, F.; Schrimpf, D.; Stichel, D.; Jones, D.T.W.; Hielscher, T.; Schefzyk, S.; Okonechnikov, K.; Koelsche, C.; Reuss, D.E.; Capper, D.; et al. DNA methylation-based classification and grading system for meningioma: A multicentre, retrospective analysis. Lancet Oncol. 2017, 18, 682–694. [Google Scholar] [CrossRef]
- Nassiri, F.; Mamatjan, Y.; Suppiah, S.; Badhiwala, J.H.; Mansouri, S.; Karimi, S.; Saarela, O.; Poisson, L.; Gepfner-Tuma, I.; Schittenhelm, J.; et al. DNA methylation profiling to predict recurrence risk in meningioma: Development and validation of a nomogram to optimize clinical management. Neuro-Oncol. 2019, 21, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Magill, S.T.; Eaton, C.D.; Prager, B.C.; Chen, W.C.; Cady, M.A.; Seo, K.; Lucas, C.-H.G.; Casey-Clyde, T.J.; Vasudevan, H.N.; et al. Meningioma DNA methylation groups identify biological drivers and therapeutic vulnerabilities. Nat. Genet. 2022, 54, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.L.N.; Stichel, D.; Hielscher, T.; Sievers, P.; Berghoff, A.S.; Schrimpf, D.; Sill, M.; Euskirchen, P.; Blume, C.; Patel, A.; et al. Integrated Molecular-Morphologic Meningioma Classification: A Multicenter Retrospective Analysis, Retrospectively and Prospectively Validated. J. Clin. Oncol. 2021, 39, 3839–3852. [Google Scholar] [CrossRef]
- Bayley, J.C.; Hadley, C.C.; Harmanci, A.O.; Harmanci, A.S.; Klisch, T.J.; Patel, A.J. Multiple approaches converge on three biological subtypes of meningioma and extract new insights from published studies. Sci. Adv. 2022, 8, eabm6247. [Google Scholar] [CrossRef]
- Garzon-Muvdi, T.; Bailey, D.D.; Pernik, M.N.; Pan, E. Basis for Immunotherapy for Treatment of Meningiomas. Front. Neurol. 2020, 11, 945. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Z.; Nassiri, F.; Bi, L.; Zadeh, G. Immune Profiling of Meningiomas. In Biological and Clinical Landscape of Meningiomas; Zadeh, G., Goldbrunner, R., Krischek, B., Nassiri, F., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 189–198. [Google Scholar]
- Wirsching, H.-G.; Weller, M. Immunotherapy for Meningiomas. In Biological and Clinical Landscape of Meningiomas; Zadeh, G., Goldbrunner, R., Krischek, B., Nassiri, F., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 225–234. [Google Scholar]
- Giammalva, G.R.; Brunasso, L.; Paolini, F.; Costanzo, R.; Bonosi, L.; Benigno, U.E.; Ferini, G.; Sava, S.; Colarossi, C.; Umana, G.E.; et al. The Long and Winding Road: An Overview of the Immunological Landscape of Intracranial Meningiomas. Cancers 2022, 14, 3639. [Google Scholar] [CrossRef] [PubMed]
- Rapp, C.; Dettling, S.; Liu, F.; Ull, A.T.; Warta, R.; Jungk, C.; Roesch, S.; Mock, A.; Sahm, F.; Schmidt, M.; et al. Cytotoxic T Cells and their Activation Status are Independent Prognostic Markers in Meningiomas. Clin. Cancer Res. 2019, 25, 5260–5270. [Google Scholar] [CrossRef] [PubMed]
- Domingues, P.H.; Teodósio, C.; Ortiz, J.; Sousa, P.; Otero, Á.; Maillo, A.; Bárcena, P.; García-Macias, M.C.; Lopes, M.C.; de Oliveira, C.; et al. Immunophenotypic Identification and Characterization of Tumor Cells and Infiltrating Cell Populations in Meningiomas. Am. J. Pathol. 2012, 181, 1749–1761. [Google Scholar] [CrossRef]
- Proctor, D.T.; Huang, J.; Lama, S.; Albakr, A.; Van Marle, G.; Sutherland, G.R. Tumor-associated macrophage infiltration in meningioma. Neuro-Oncol. Adv. 2019, 1, vdz018. [Google Scholar] [CrossRef]
- Pinton, L.; Solito, S.; Masetto, E.; Vettore, M.; Canè, S.; Della Puppa, A.; Mandruzzato, S. Immunosuppressive activity of tumor-infiltrating myeloid cells in patients with meningioma. OncoImmunology 2018, 7, e1440931. [Google Scholar] [CrossRef]
- Li, Y.D.; Veliceasa, D.; Lamano, J.B.; Kaur, G.; Biyashev, D.; Horbinski, C.M.; Kruser, T.J.; Bloch, O. Systemic and local immunosuppression in patients with high-grade meningiomas. Cancer Immunol. Immunother. 2019, 68, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Lowther, D.E.; Meizlish, M.L.; Anderson, R.C.E.; Bruce, J.N.; Devine, L.; Huttner, A.J.; Kleinstein, S.H.; Lee, J.-Y.; Stern, J.N.H.; et al. The immune cell infiltrate populating meningiomas is composed of mature, antigen-experienced T and B cells. Neuro-Oncol. 2013, 15, 1479–1490. [Google Scholar] [CrossRef]
- Polyzoidis, S.; Koletsa, T.; Panagiotidou, S.; Ashkan, K.; Theoharides, T.C. Mast cells in meningiomas and brain inflammation. J. Neuroinflamm. 2015, 12, 1–8. [Google Scholar] [CrossRef]
- Du, Z.; Abedalthagafi, M.; Aizer, A.A.; McHenry, A.R.; Sun, H.H.; Bray, M.-A.; Viramontes, O.; Machaidze, R.; Brastianos, P.K.; Reardon, D.A.; et al. Increased expression of the immune modulatory molecule PD-L1 (CD274) in anaplastic meningioma. Oncotarget 2014, 6, 4704–4716. [Google Scholar] [CrossRef]
- Karimi, S.; Mansouri, S.; Mamatjan, Y.; Liu, J.; Nassiri, F.; Suppiah, S.; Singh, O.; Aldape, K.; Zadeh, G. Programmed death ligand-1 (PD-L1) expression in meningioma; prognostic significance and its association with hypoxia and NFKB2 expression. Sci. Rep. 2020, 10, 14115. [Google Scholar] [CrossRef]
- Han, S.J.; Reis, G.; Kohanbash, G.; Shrivastav, S.; Magill, S.T.; Molinaro, A.M.; McDermott, M.W.; Theodosopoulos, P.V.; Aghi, M.K.; Berger, M.S.; et al. Expression and prognostic impact of immune modulatory molecule PD-L1 in meningioma. J. Neuro-Oncol. 2016, 130, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Yeung, J.; Yaghoobi, V.; Aung, T.N.; Vesely, M.D.; Zhang, T.; Gaule, P.; Gunel, M.; Rimm, D.L.; Chen, L. Spatially Resolved and Quantitative Analysis of the Immunological Landscape in Human Meningiomas. J. Neuropathol. Exp. Neurol. 2021, 80, 150–159. [Google Scholar] [CrossRef]
- Domingues, P.H.; Teodosio, C.; Otero, A.; Sousa, P.; Ortiz, J.; Macias, M.D.C.G.; Gonçalves, J.M.; Nieto, A.B.; Lopes, M.C.; de Oliveira, C.; et al. Association between Inflammatory Infiltrates and Isolated Monosomy 22/del(22q) in Meningiomas. PLoS ONE 2013, 8, e74798. [Google Scholar] [CrossRef]
- Wang, A.Z.; Bowman-Kirigin, J.A.; Desai, R.; Kang, L.-I.; Patel, P.R.; Patel, B.; Khan, S.M.; Bender, D.; Marlin, M.C.; Liu, J.; et al. Single-cell profiling of human dura and meningioma reveals cellular meningeal landscape and insights into meningioma immune response. Genome Med. 2022, 14, 49. [Google Scholar] [CrossRef]
- Ott, M.; Prins, R.M.; Heimberger, A.B. The immune landscape of common CNS malignancies: Implications for immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 729–744. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. The Microenvironmental Landscape of Brain Tumors. Cancer Cell 2017, 31, 326–341. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.L.; Ercolano, E.; Ferluga, S.; Sofela, A.; Dave, F.; Negroni, C.; Kurian, K.M.; Hilton, D.A.; Hanemann, C.O. A Rapid Robust Method for Subgrouping Non-NF2 Meningiomas According to Genotype and Detection of Lower Levels of M2 Macrophages in AKT1 E17K Mutated Tumours. Int. J. Mol. Sci. 2020, 21, 1273. [Google Scholar] [CrossRef] [PubMed]
- Yeung, J.; Yaghoobi, V.; Miyagishima, D.; Vesely, M.D.; Zhang, T.; Badri, T.; Nassar, A.; Han, X.; Sanmamed, M.F.; Youngblood, M.; et al. Targeting the CSF1/CSF1R axis is a potential treatment strategy for malignant meningiomas. Neuro-Oncol. 2021, 23, 1922–1935. [Google Scholar] [CrossRef]
- Topalian, S.L.; Taube, J.M.; Anders, R.A.; Pardoll, D.M. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat. Rev. Cancer 2016, 16, 275–287. [Google Scholar] [CrossRef]
- Jungwirth, G.; Hanemann, C.O.; Dunn, I.F.; Herold-Mende, C. Preclinical Models of Meningioma. In Biological and Clinical Landscape of Meningiomas; Zadeh, G., Goldbrunner, R., Krischek, B., Nassiri, F., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 199–211. [Google Scholar]
- Boetto, J.; Peyre, M.; Kalamarides, M. Mouse Models in Meningioma Research: A Systematic Review. Cancers 2021, 13, 3712. [Google Scholar] [CrossRef]
- Pyonteck, S.M.; Akkari, L.; Schuhmacher, A.J.; Bowman, R.L.; Sevenich, L.; Quail, D.F.; Olson, O.C.; Quick, M.L.; Huse, J.T.; Teijeiro, V.; et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 2013, 19, 1264–1272. [Google Scholar] [CrossRef]
- Yan, D.; Kowal, J.; Akkari, L.; Schuhmacher, A.J.; Huse, J.T.; West, B.L.; Joyce, J.A. Inhibition of colony stimulating factor-1 receptor abrogates microenvironment-mediated therapeutic resistance in gliomas. Oncogene 2017, 36, 6049–6058. [Google Scholar] [CrossRef] [PubMed]
- Fermi, V.; Warta, R.; Wöllner, A.; Lotsch, C.; Jassowicz, L.; Rapp, C.; Knoll, M.; Jungwirth, G.; Jungk, C.; Trong, P.D.; et al. Effective Reprogramming of Patient-Derived M2-Polarized Glioblastoma-Associated Microglia/Macrophages by Treatment with GW2580. Clin. Cancer Res. 2023, 29, 4685–4697. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://clinicaltrials.gov/search?cond=meningioma (accessed on 9 July 2024).
- Zador, Z.; Landry, A.P.; Balas, M.; Cusimano, M.D. Landscape of immune cell gene expression is unique in predominantly WHO grade 1 skull base meningiomas when compared to convexity. Sci. Rep. 2020, 10, 9065. [Google Scholar] [CrossRef]
- Wang, J.Z.; Nassiri, F.; Landry, A.P.; Patil, V.; Liu, J.; Aldape, K.; Gao, A.; Zadeh, G. The multiomic landscape of meningiomas: A review and update. J. Neuro-Oncol. 2023, 161, 405–414. [Google Scholar] [CrossRef] [PubMed]
| WHO Grade | Histological Subtype | Molecular Markers |
|---|---|---|
| 1 | Meningothelial Fibrous Transitional Psammomatous Angiomatous (vascular) Microcystic Secretory Lymphoplasmacyte-rich Metaplastic | Mutation: TRAF7, KLF4, AKT1, SMO, PIK3CA Mutation: NF2 Mutation: NF2, TRAF7, KLF4, AKT1, SMO, PIK3CA Mutation: TRAF7, KLF4, AKT1, SMO |
| 2 | Clear cell Chordoid Atypical | Mutation: NF2, SMARCE1 Mutation: NF2 Mutation: NF2 |
| 3 | Rhabdoid Papillary Anaplastic | Mutation: BAP1 Mutation: NF2, BAP1 Mutation: NF2, TERTp; Chrom alt: CDKN2A/B loss |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lotsch, C.; Warta, R.; Herold-Mende, C. The Molecular and Immunological Landscape of Meningiomas. Int. J. Mol. Sci. 2024, 25, 9631. https://doi.org/10.3390/ijms25179631
Lotsch C, Warta R, Herold-Mende C. The Molecular and Immunological Landscape of Meningiomas. International Journal of Molecular Sciences. 2024; 25(17):9631. https://doi.org/10.3390/ijms25179631
Chicago/Turabian StyleLotsch, Catharina, Rolf Warta, and Christel Herold-Mende. 2024. "The Molecular and Immunological Landscape of Meningiomas" International Journal of Molecular Sciences 25, no. 17: 9631. https://doi.org/10.3390/ijms25179631
APA StyleLotsch, C., Warta, R., & Herold-Mende, C. (2024). The Molecular and Immunological Landscape of Meningiomas. International Journal of Molecular Sciences, 25(17), 9631. https://doi.org/10.3390/ijms25179631






