Prevention of Transition from Acute Kidney Injury to Chronic Kidney Disease Using Clinical-Grade Perinatal Stem Cells in Non-Clinical Study
Abstract
1. Introduction
2. Results
2.1. Donor Variability
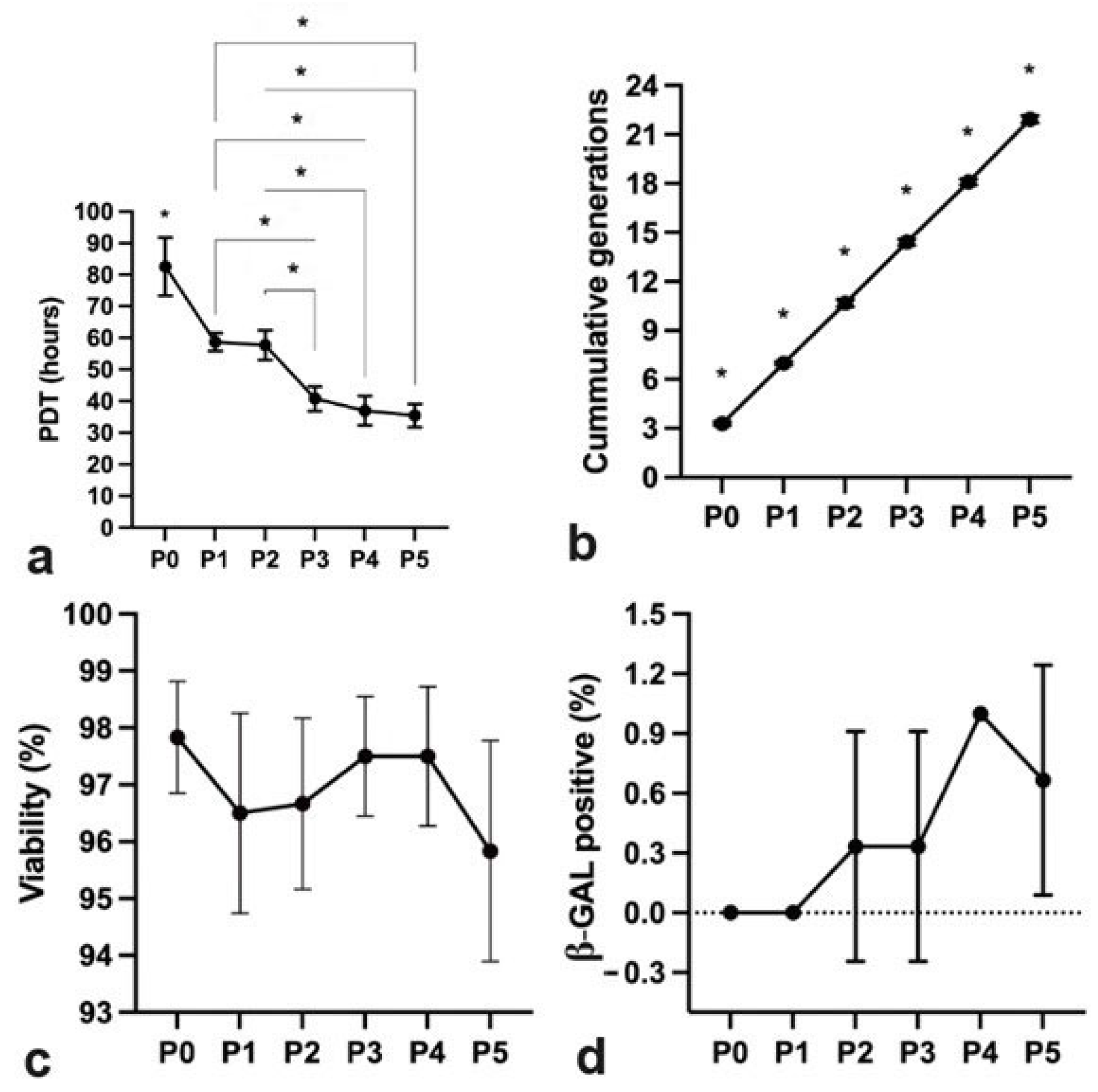
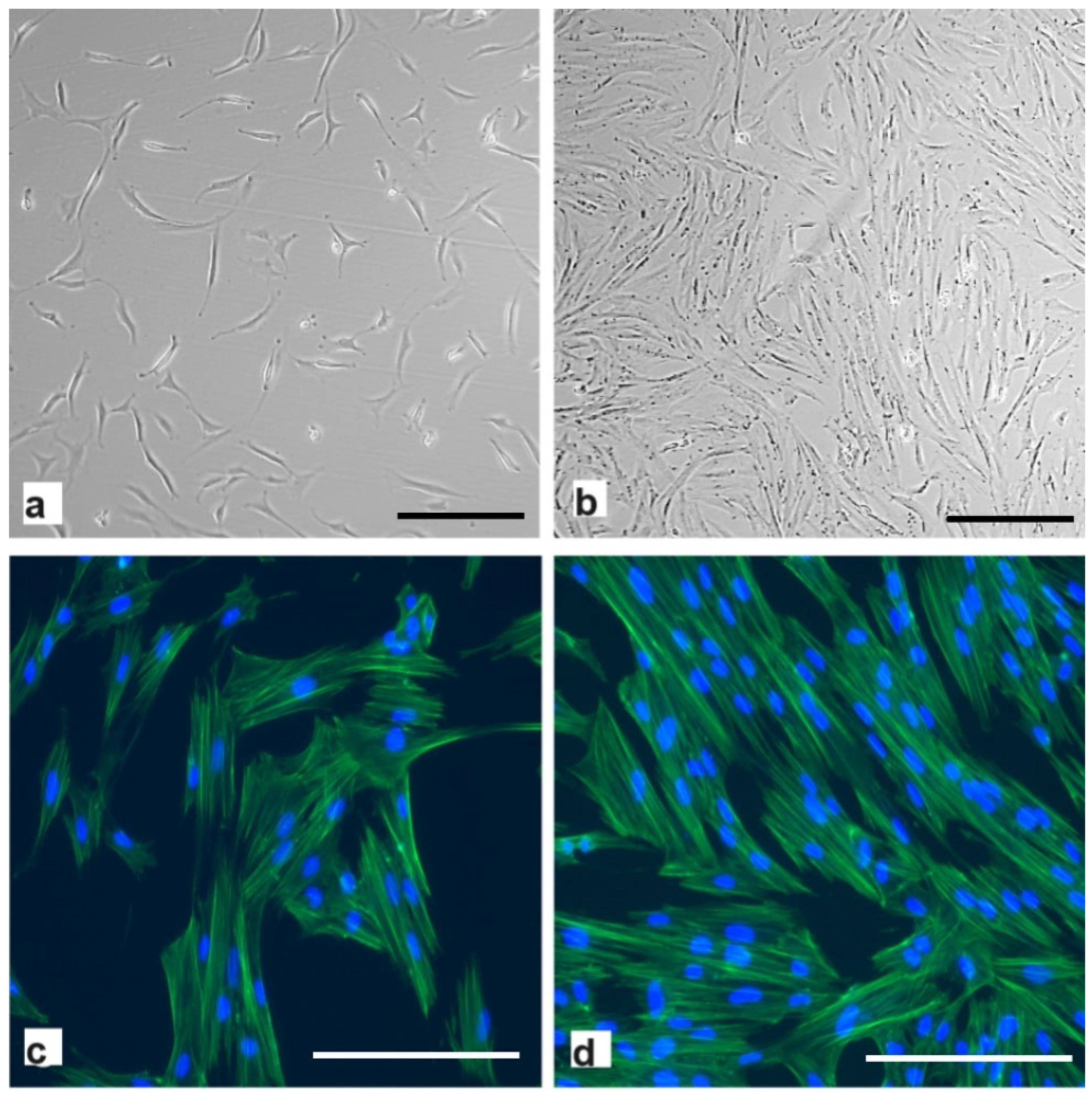
2.2. hpMSCs Exhibit Efficient Proliferation Capacity
2.3. GMP-Grade Production of hpMSCs
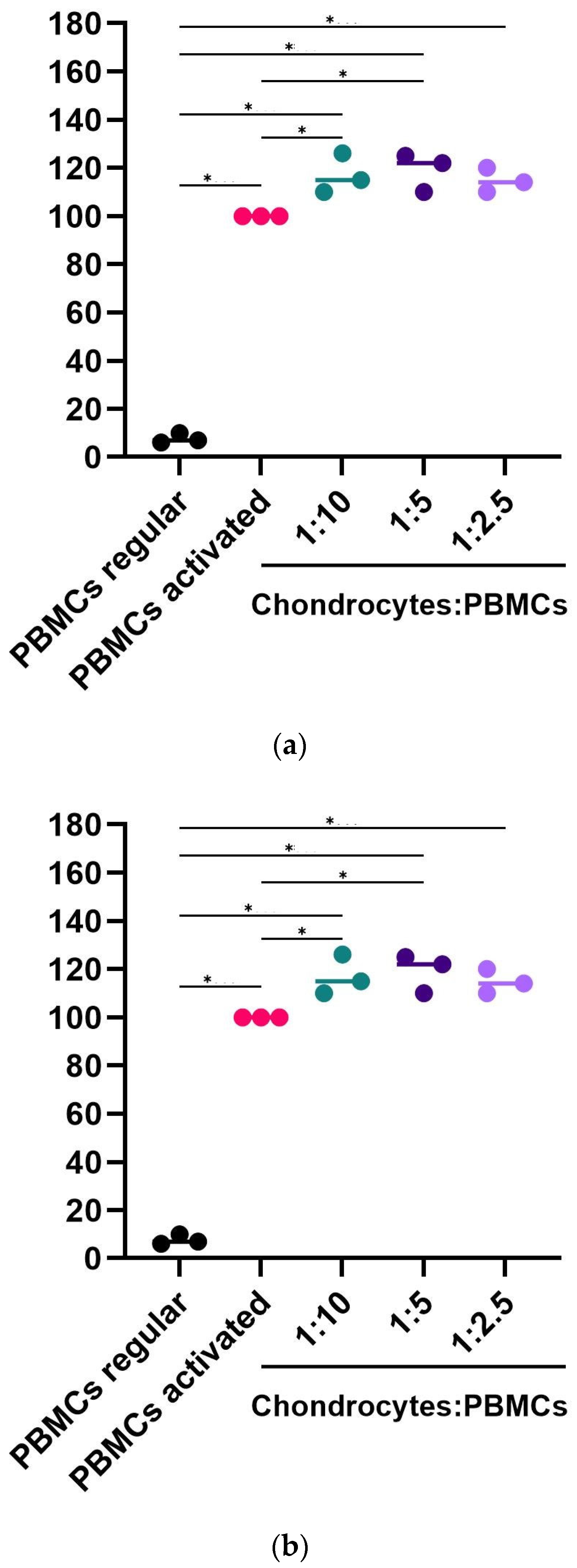
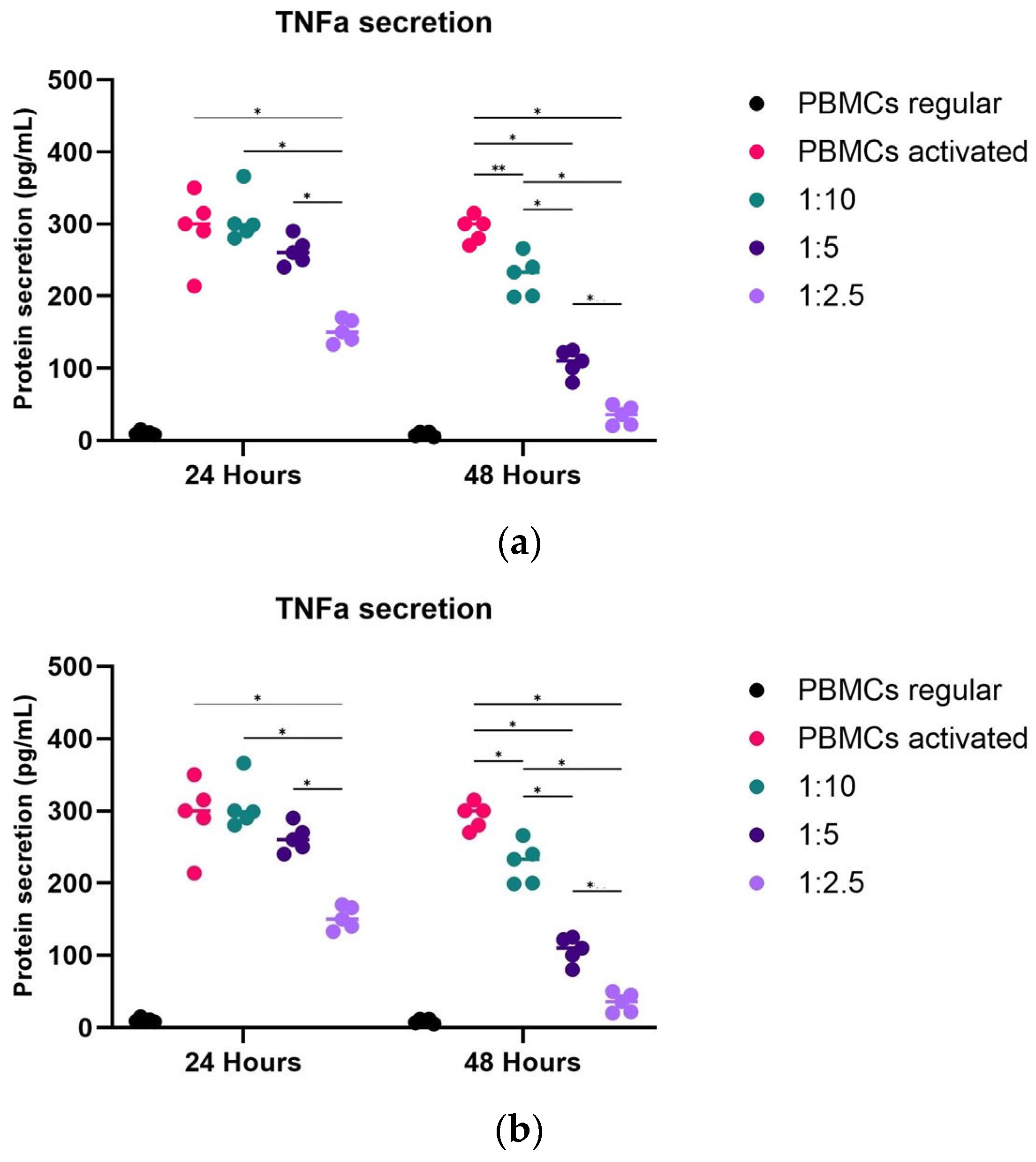
2.4. Potency Mode of Action of hpMSCs
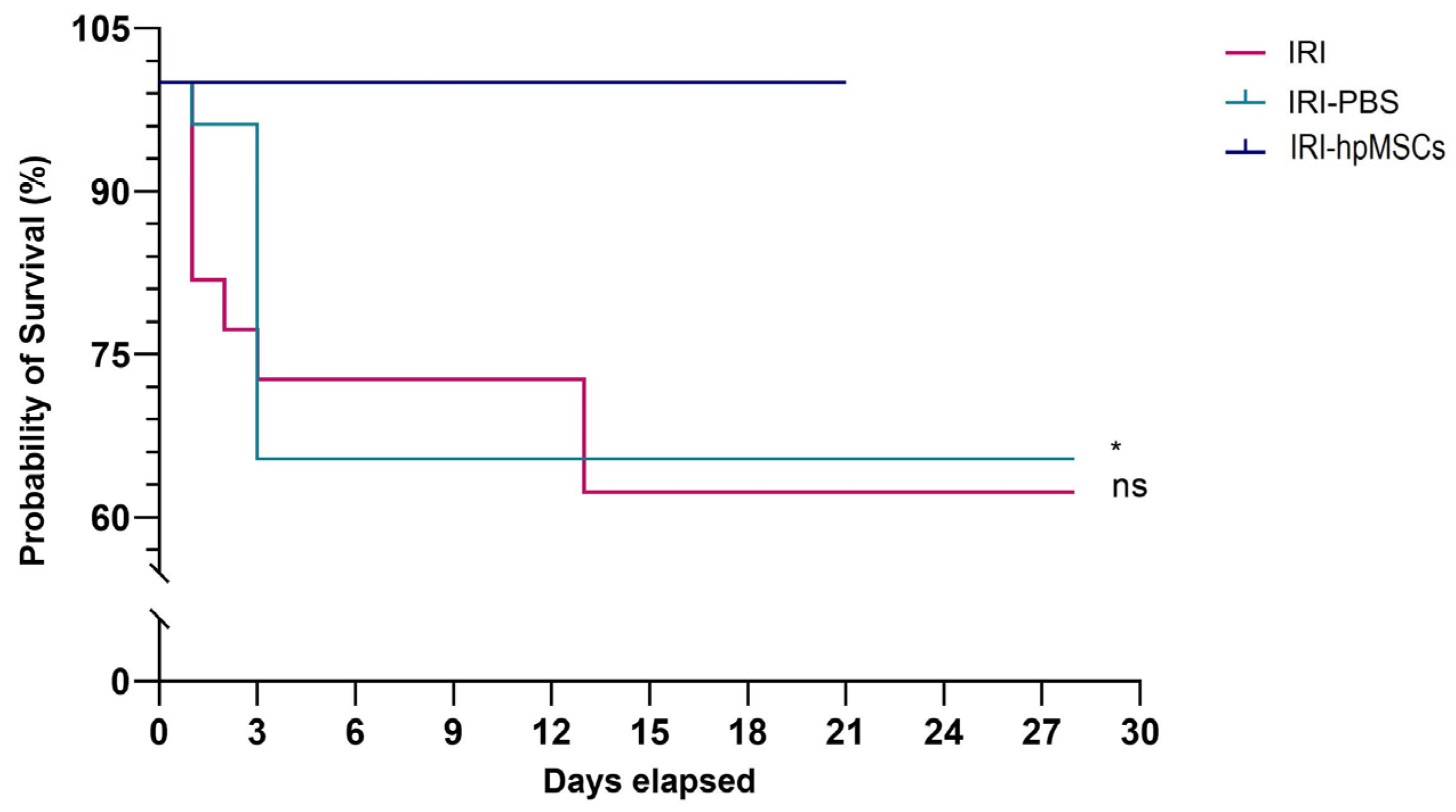
2.5. Survival Rate
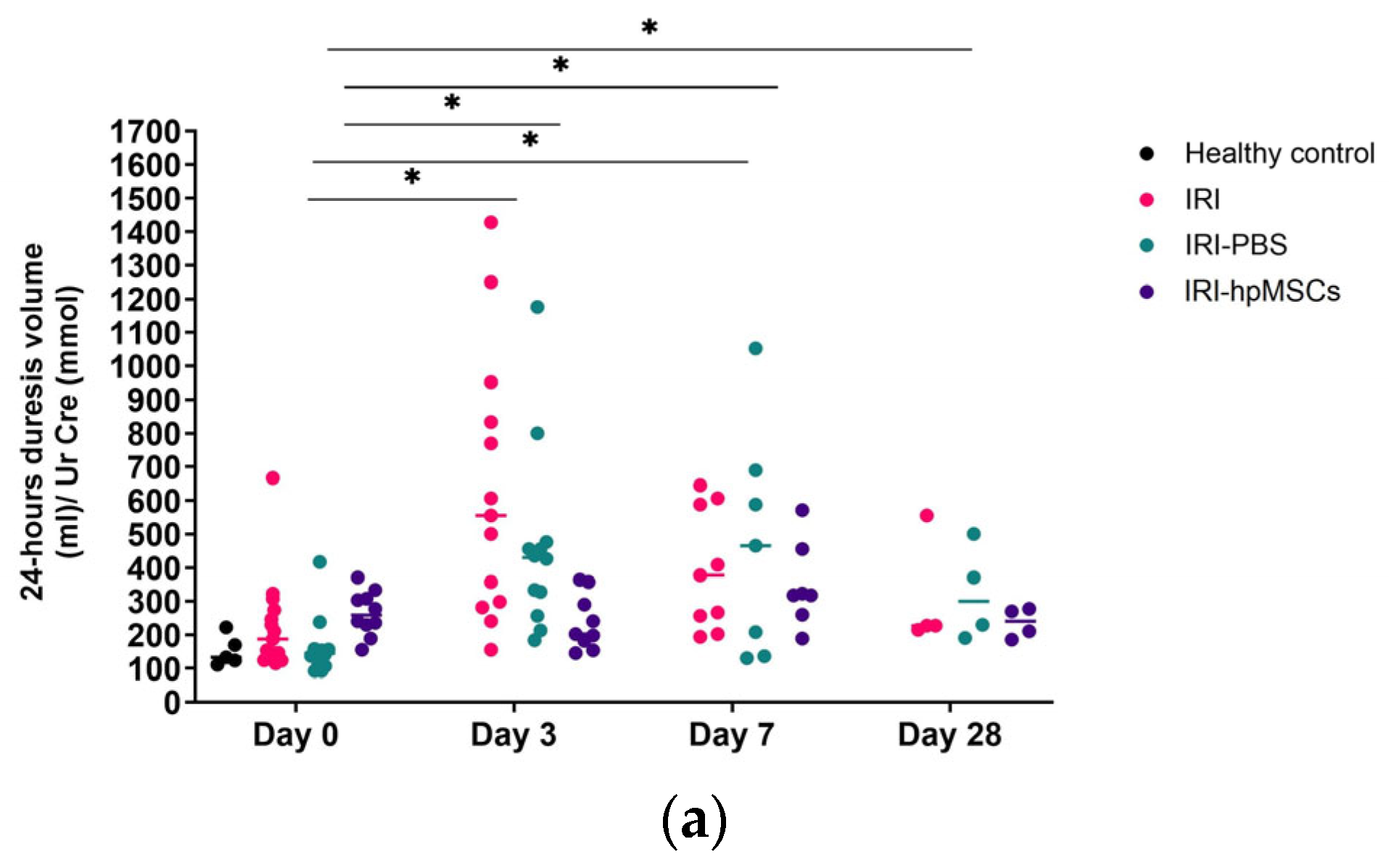
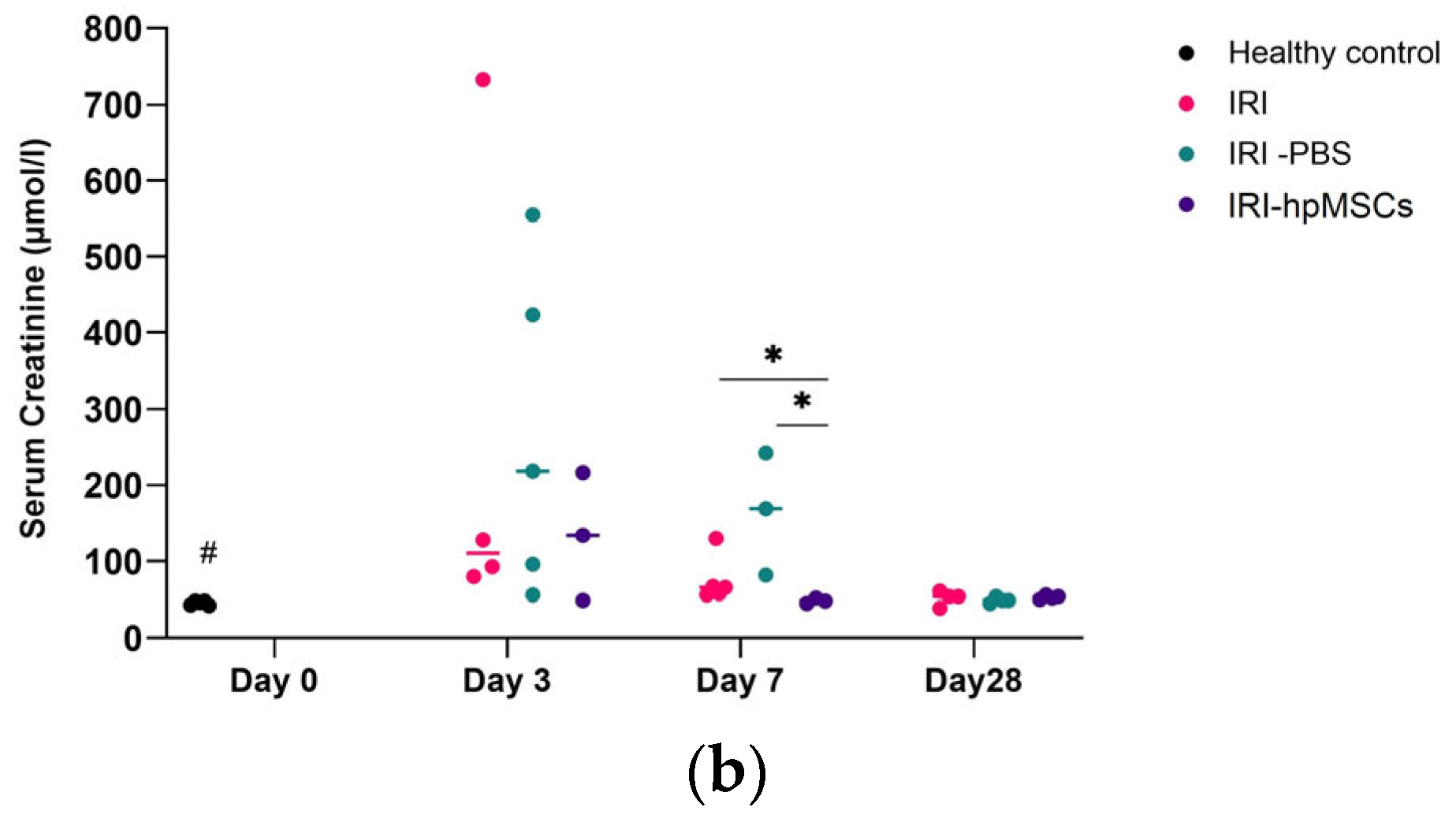
2.6. Kidney Function
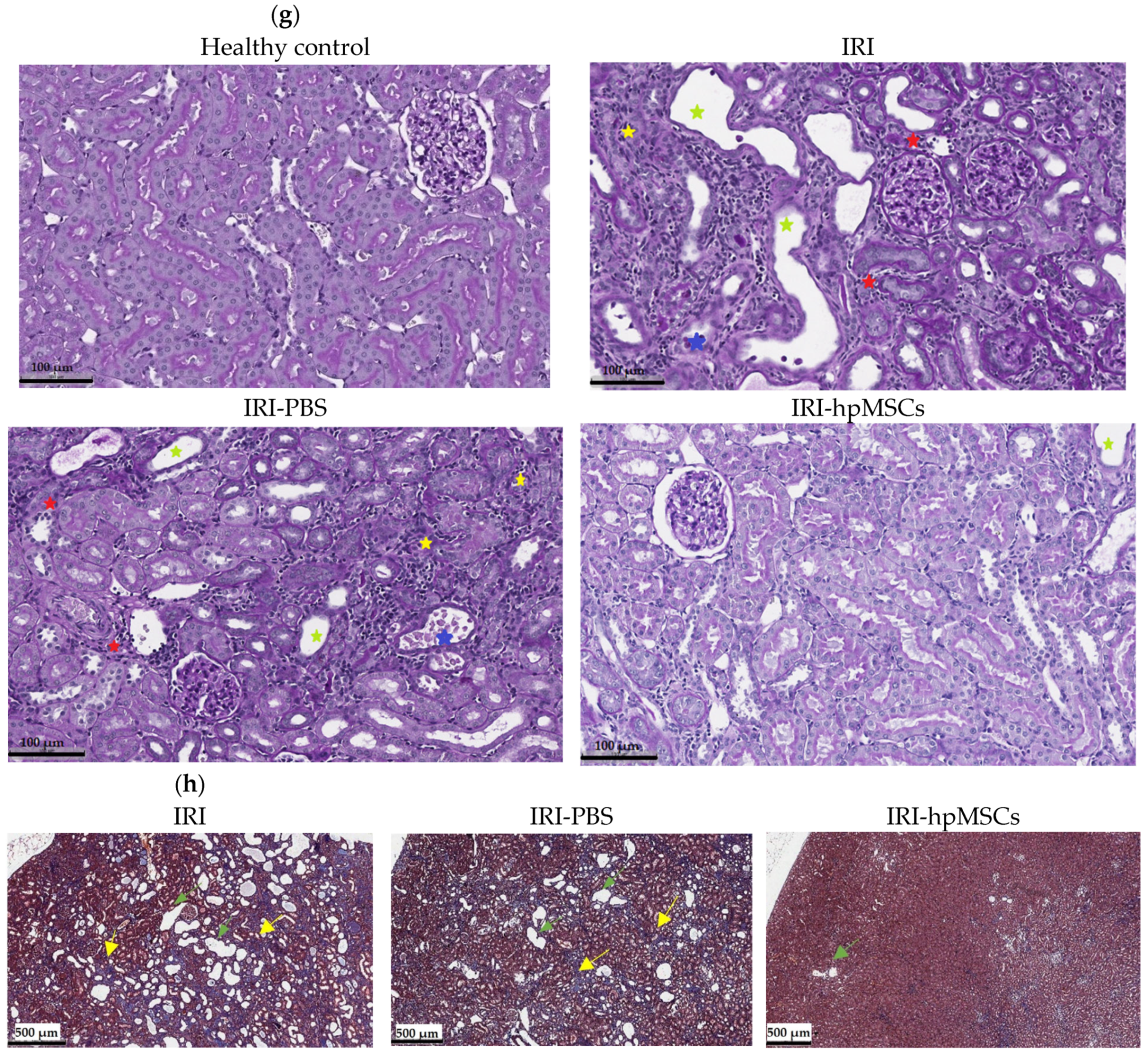
2.7. Kidney Morphology
3. Discussion
4. Methods and Materials
4.1. In Vitro Methods
4.1.1. Donor Eligibility
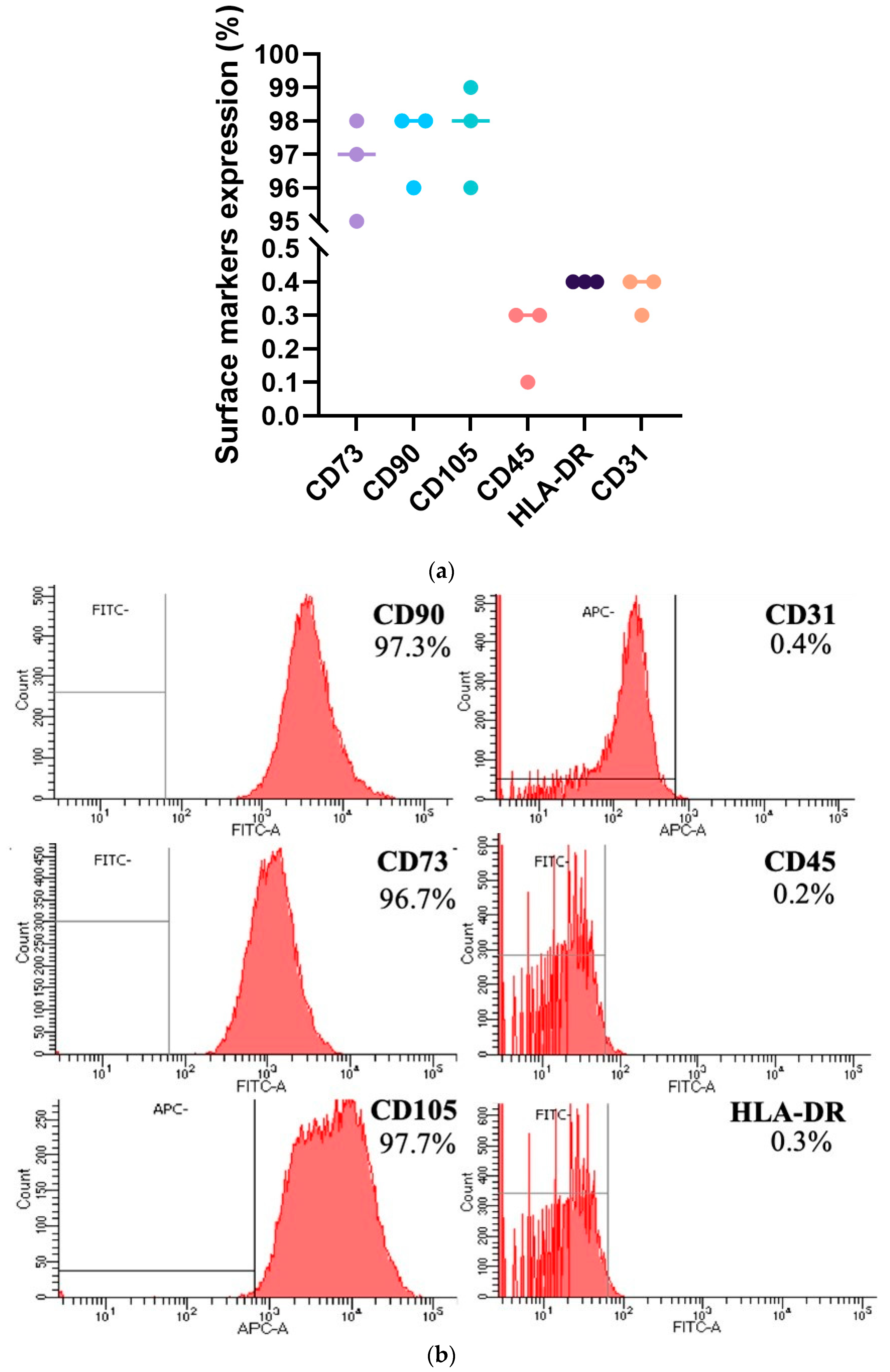
4.1.2. Characterization of hpMSCs
4.2. In Vivo Methods
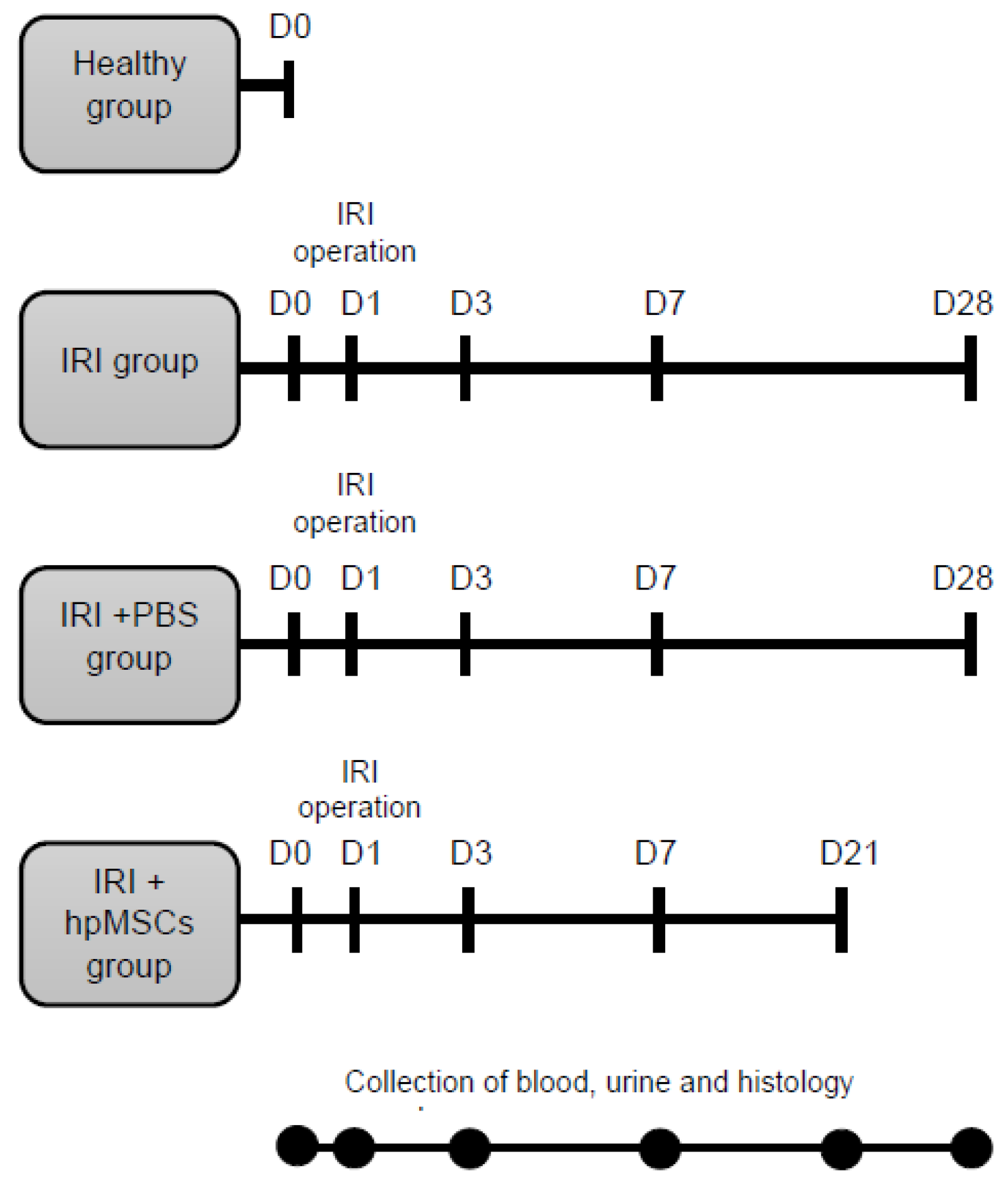
4.2.1. Animals
4.2.2. AKI to CKD Model
4.2.3. Renal Histology
4.2.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Q.; Tian, S.F.; Guo, Y.; Niu, X.; Hu, B.; Guo, S.C.; Wang, N.S.; Wang, Y. Transplantation of Induced Pluripotent Stem Cell-Derived Renal Stem Cells Improved Acute Kidney Injury. Cell Biosci. 2015, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Vives, M.; Hernandez, A.; Parramon, F.; Estanyol, N.; Pardina, B.; Muñoz, A.; Alvarez, P.; Hernandez, C. Acute Kidney Injury after Cardiac Surgery: Prevalence, Impact and Management Challenges. Int. J. Nephrol. Renov. Dis. 2019, 12, 153–166. [Google Scholar] [CrossRef]
- Lei, V.J.; Luong, T.B.; Shan, E.; Chen, X.; Neuman, M.D.; Eneanya, N.D.; Polsky, D.E.; Volpp, K.G.; Fleisher, L.A.; Holmes, J.H.; et al. Risk Stratification for Postoperative Acute Kidney Injury in Major Noncardiac Surgery Using Preoperative and Intraoperative Data. JAMA Netw. Open 2019, 2, e1916921. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, R.; Kellum, J.A.; Ronco, C. Acute Kidney Injury. Lancet 2012, 380, 756–766. [Google Scholar] [CrossRef]
- Susantitaphong, P.; Cruz, D.N.; Cerda, J.; Abulfaraj, M.; Alqahtani, F.; Koulouridis, I.; Jaber, B.L. World Incidence of AKI: A Meta-Analysis. Clin. J. Am. Soc. Nephrol. 2013, 8, 1482–1493. [Google Scholar] [CrossRef]
- Lameire, N.H.; Levin, A.; Kellum, J.A.; Cheung, M.; Jadoul, M.; Winkelmayer, W.C.; Stevens, P.E.; Caskey, F.J.; Farmer, C.K.; Fuentes, A.F.; et al. Harmonizing Acute and Chronic Kidney Disease Definition and Classification: Report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2021, 100, 516–526. [Google Scholar] [CrossRef]
- Kellum, J.A.; Sileanu, F.E.; Bihorac, A.; Hoste, E.A.J.; Chawla, L.S. Recovery after Acute Kidney Injury. Am. J. Respir. Crit. Care Med. 2017, 195, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Pippias, M.; Stel, V.S.; Diez, J.M.A.; Afentakis, N.; Herrero-Calvo, J.A.; Arias, M.; Tomilina, N.; Caamaño, E.B.; Buturovic-Ponikvar, J.; Čala, S.; et al. Renal Replacement Therapy in Europe: A Summary of the 2012 ERA-EDTA Registry Annual Report. Clin. Kidney J. 2015, 8, 248–261. [Google Scholar] [CrossRef]
- Chawla, L.S.; Kimmel, P.L. Acute Kidney Injury and Chronic Kidney Disease: An Integrated Clinical Syndrome. Kidney Int. 2012, 82, 516–524. [Google Scholar] [CrossRef]
- Arias-Cabrales, C.; Rodríguez, E.; Bermejo, S.; Sierra, A.; Burballa, C.; Barrios, C.; Soler, M.J.; Pascual, J. Short- and Long-Term Outcomes after Non-Severe Acute Kidney Injury. Clin. Exp. Nephrol. 2018, 22, 61–67. [Google Scholar] [CrossRef]
- Murugan, R.; Kellum, J.A. Acute Kidney Injury: What’s the Prognosis? Nat. Rev. Nephrol. 2011, 7, 209–217. [Google Scholar] [CrossRef]
- Akbari, A.; Clase, C.M.; Acott, P.; Battistella, M.; Bello, A.; Feltmate, P.; Grill, A.; Karsanji, M.; Komenda, P.; Madore, F.; et al. Canadian Society of Nephrology Commentary on the KDIGO Clinical Practice Guideline for CKD Evaluation and Management. Am. J. Kidney Dis. 2015, 65, 177–205. [Google Scholar] [CrossRef] [PubMed]
- Orieux, A.; Prezelin-Reydit, M.; Prevel, R.; Combe, C.; Gruson, D.; Boyer, A.; Rubin, S. Clinical Trajectories and Impact of Acute Kidney Disease after Acute Kidney Injury in the Intensive Care Unit: A 5-Year Single-Centre Cohort Study. Nephrol. Dial. Transpl. 2023, 38, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, J.; Remuzzi, G.; Saran, R.; Williams, D.E.; Rios-Burrows, N.; Powe, N.; Brück, K.; Wanner, C.; Stel, V.S.; Venuthurupalli, S.K.; et al. Taming the Chronic Kidney Disease Epidemic: A Global View of Surveillance Efforts. Kidney Int. 2014, 86, 246–250. [Google Scholar] [CrossRef]
- Kashani, K.; Shao, M.; Li, G.; Williams, A.W.; Rule, A.D.; Kremers, W.K.; Malinchoc, M.; Gajic, O.; Lieske, J.C. No Increase in the Incidence of Acute Kidney Injury in a Population-Based Annual Temporal Trends Epidemiology Study. Kidney Int. 2017, 92, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Rimes-Stigare, C.; Frumento, P.; Bottai, M.; Mårtensson, J.; Martling, C.R.; Walther, S.M.; Karlström, G.; Bell, M. Evolution of Chronic Renal Impairment and Long-Term Mortality after de Novo Acute Kidney Injury in the Critically Ill; a Swedish Multi-Centre Cohort Study. Crit. Care 2015, 19, 221. [Google Scholar] [CrossRef]
- Noble, R.A.; Lucas, B.J.; Selby, N.M. Long-Term Outcomes in Patients with Acute Kidney Injury. Clin. J. Am. Soc. Nephrol. 2020, 15, 423–429. [Google Scholar] [CrossRef]
- Villanueva, S.; Ewertz, E.; Carrión, F.; Tapia, A.; Vergara, C.; Céspedes, C.; Sáez, P.J.; Luz, P.; Irarrázabal, C.; Carreño, J.E.; et al. Mesenchymal Stem Cell Injection Ameliorates Chronic Renal Failure in a Rat Model. Clin. Sci. 2011, 121, 489–499. [Google Scholar] [CrossRef]
- Imberti, B.; Tomasoni, S.; Ciampi, O.; Pezzotta, A.; Derosas, M.; Xinaris, C.; Rizzo, P.; Papadimou, E.; Novelli, R.; Benigni, A.; et al. Renal Progenitors Derived from Human IPSCs Engraft and Restore Function in a Mouse Model of Acute Kidney Injury. Sci. Rep. 2015, 5, 8826. [Google Scholar] [CrossRef]
- Chamberlain, G.; Fox, J.; Ashton, B.; Middleton, J. Concise Review: Mesenchymal Stem Cells: Their Phenotype, Differentiation Capacity, Immunological Features, and Potential for Homing. Stem Cells 2007, 25, 2739–2749. [Google Scholar] [CrossRef]
- Alfarano, C.; Roubeix, C.; Chaaya, R.; Ceccaldi, C.; Calise, D.; Mias, C.; Cussac, D.; Bascands, J.L.; Parini, A. Intraparenchymal Injection of Bone Marrow Mesenchymal Stem Cells Reduces Kidney Fibrosis after Ischemia-Reperfusion in Cyclosporine-Immunosuppressed Rats. Cell Transpl. 2012, 21, 2009–2019. [Google Scholar] [CrossRef] [PubMed]
- Tögel, F.; Hu, Z.; Weiss, K.; Isaac, J.; Lange, C.; Westenfelder, C. Administered Mesenchymal Stem Cells Protect against Ischemic Acute Renal Failure through Differentiation-Independent Mechanisms. Am. J. Physiol. Ren. Physiol. 2005, 289, F31–F42. [Google Scholar] [CrossRef] [PubMed]
- Morigi, M.; Imberti, B.; Zoja, C.; Corna, D.; Tomasoni, S.; Abbate, M.; Rottoli, D.; Angioletti, S.; Benigni, A.; Perico, N.; et al. Mesenchymal Stem Cells Are Renotropic, Helping to Repair the Kidney and Improve Function in Acute Renal Failure. J. Am. Soc. Nephrol. 2004, 15, 1794–1804. [Google Scholar] [CrossRef] [PubMed]
- Bi, B.; Schmitt, R.; Israilova, M.; Nishio, H.; Cantley, L.G. Stromal Cells Protect against Acute Tubular Injury via an Endocrine Effect. J. Am. Soc. Nephrol. 2007, 18, 2486–2496. [Google Scholar] [CrossRef]
- Lin, F.; Moran, A.; Igarashi, P. Intrarenal Cells, Not Bone Marrow-Derived Cells, Are the Major Source for Regeneration in Postischemic Kidney. J. Clin. Investig. 2005, 115, 1756–1764. [Google Scholar] [CrossRef]
- Villanueva, S.; Carreño, J.E.; Salazar, L.; Vergara, C.; Strodthoff, R.; Fajre, F.; Céspedes, C.; Sáez, P.J.; Irarrázabal, C.; Bartolucci, J.; et al. Human Mesenchymal Stem Cells Derived from Adipose Tissue Reduce Functional and Tissue Damage in a Rat Model of Chronic Renal Failure. Clin. Sci. 2013, 125, 199–210. [Google Scholar] [CrossRef]
- Silini, A.R.; Masserdotti, A.; Papait, A.; Parolini, O. Shaping the Future of Perinatal Cells: Lessons from the Past and Interpretations of the Present. Front. Bioeng. Biotechnol. 2019, 7, 75. [Google Scholar] [CrossRef]
- Wang, H.; Shang, Y.; Chen, X.; Wang, Z.; Zhu, D.; Liu, Y.; Zhang, C.; Chen, P.; Wu, J.; Wu, L.; et al. Delivery of Mscs with a Hybrid β-Sheet Peptide Hydrogel Consisting Igf-1c Domain and d-Form Peptide for Acute Kidney Injury Therapy. Int. J. Nanomed. 2020, 15, 4311–4324. [Google Scholar] [CrossRef]
- Yim, H.E.; Kim, D.S.; Chung, H.C.; Shing, B.; Moon, K.H.; George, S.K.; Kim, M.W.; Atala, Z.; Kim, J.H.; Ko, I.K.; et al. Controlled Delivery of Stem Cell-Derived Trophic Factors Accelerates Kidney Repair After Renal Ischemia-Reperfusion Injury in Rats. Stem Cells Transl. Med. 2019, 8, 959–970. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, J.; Wang, H.; Hezam, K.; Zhao, X.; Huang, H.; Chen, S.; Han, Z.; Han, Z.C.; Guo, Z.; et al. Enhanced Therapeutic Effects of MSC-Derived Extracellular Vesicles with an Injectable Collagen Matrix for Experimental Acute Kidney Injury Treatment. Stem Cell Res. Ther. 2020, 11, 161. [Google Scholar] [CrossRef]
- Meligy, F.Y.; Shigemura, K.; Behnsawy, H.M.; Fujisawa, M.; Kawabata, M.; Shirakawa, T. The Efficiency of in Vitro Isolation and Myogenic Differentiation of MSCs Derived from Adipose Connective Tissue, Bone Marrow, and Skeletal Muscle Tissue. Vitr. Cell Dev. Biol. Anim. 2012, 48, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Monteiro Carvalho Mori Da Cunha, M.G.; Zia, S.; Arcolino, F.O.; Carlon, M.S.; Beckmann, D.V.; Pippi, N.L.; Graça, D.L.; Levtchenko, E.; Deprest, J.; Toelen, J. Amniotic Fluid Derived Stem Cells with a Renal Progenitor Phenotype Inhibit Interstitial Fibrosis in Renal Ischemia and Reperfusion Injury in Rats. PLoS ONE 2015, 10, e0136145. [Google Scholar] [CrossRef][Green Version]
- Cóndor, J.M.; Rodrigues, C.E.; de Sousa Moreira, R.; Canale, D.; Volpini, R.A.; Shimizu, M.H.; Camara, N.O.; Noronha, I.D.L.; Andrade, L. Treatment with Human Wharton’s Jelly-Derived Mesenchymal Stem Cells Attenuates Sepsis-Induced Kidney Injury, Liver Injury, and Endothelial Dysfunction. Stem Cells Transl. Med. 2016, 5, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zou, X.; Huang, Y.; Wang, F.; Miao, S.; Liu, G.; Chen, M.; Zhu, Y. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Protect against Acute Kidney Injury through Anti-Oxidation by Enhancing Nrf2/ARE Activation in Rats. Kidney Blood Press. Res. 2016, 41, 119–128. [Google Scholar] [CrossRef]
- Gu, D.; Zou, X.; Ju, G.; Zhang, G.; Bao, E.; Zhu, Y. Mesenchymal Stromal Cells Derived Extracellular Vesicles Ameliorate Acute Renal Ischemia Reperfusion Injury by Inhibition of Mitochondrial Fission through MIR-30. Stem Cells Int. 2016, 2016, 2093940. [Google Scholar] [CrossRef]
- Xu, Q.; Yan, P.; Duan, X.-J.; Wu, X.; Chen, X.-J.; Luo, M.; Peng, J.-C.; Feng, L.-X.; Liu, J.; Zhong, H.-L.; et al. Human Umbilical Cord-derived Mesenchymal Stem Cells and Human Cord Blood Mononuclear Cells Protect against Cisplatin-induced Acute Kidney Injury in Rat Models. Exp. Ther. Med. 2020, 20, 145. [Google Scholar] [CrossRef]
- Zhang, R.; Yin, L.; Zhang, B.; Shi, H.; Sun, Y.; Ji, C.; Chen, J.; Wu, P.; Zhang, L.; Xu, W.; et al. Resveratrol Improves Human Umbilical Cord-Derived Mesenchymal Stem Cells Repair for Cisplatin-Induced Acute Kidney Injury. Cell Death Dis. 2018, 9, 965. [Google Scholar] [CrossRef]
- Tögel, F.E.; Westenfelder, C. Mesenchymal Stem Cells: A New Therapeutic Tool for AKI. Nat. Rev. Nephrol. 2010, 6, 179–183. [Google Scholar] [CrossRef]
- De Chiara, L.; Fagoonee, S.; Ranghino, A.; Bruno, S.; Camussi, G.; Tolosano, E.; Silengo, L.; Altruda, F. Renal Cells from Spermatogonial Germline Stem Cells Protect against Kidney Injury. J. Am. Soc. Nephrol. 2014, 25, 316–328. [Google Scholar] [CrossRef]
- Yao, W.; Hu, Q.; Ma, Y.; Xiong, W.; Wu, T.; Cao, J.; Wu, D. Human Adipose-Derived Mesenchymal Stem Cells Repair Cisplatin-Induced Acute Kidney Injury through Antiapoptotic Pathways. Exp. Ther. Med. 2015, 10, 468–476. [Google Scholar] [CrossRef]
- Chen, Y.T.; Sun, C.K.; Lin, Y.C.; Chang, L.T.; Chen, Y.L.; Tsai, T.H.; Chung, S.Y.; Chua, S.; Kao, Y.H.; Yen, C.H.; et al. Adipose-Derived Mesenchymal Stem Cell Protects Kidneys against Ischemia-Reperfusion Injury through Suppressing Oxidative Stress and Inflammatory Reaction. J. Transl. Med. 2011, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.R.; Park, J.H.; Kwon, G.Y.; Lee, J.E.; Huh, W.; Jin, H.J.; Choi, S.J.; Oh, W.; Oh, H.Y.; Kim, Y.G. Effect of Preemptive Treatment with Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells on the Development of Renal Ischemia-Reperfusion Injury in Mice. Am. J. Physiol. Ren. Physiol. 2014, 307, F1149–F1161. [Google Scholar] [CrossRef]
- Fang, T.C.; Pang, C.Y.; Chiu, S.C.; Ding, D.C.; Tsai, R.K. Renoprotective Effect of Human Umbilical Cord-Derived Mesenchymal Stem Cells in Immunodeficient Mice Suffering from Acute Kidney Injury. PLoS ONE 2012, 7, e46504. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Swaminathan, M.; Stafford-Smith, M.; Chertow, G.M.; Warnock, D.G.; Paragamian, V.; Brenner, R.M.; Lellouche, F.; Fox-Robichaud, A.; Atta, M.G.; Melby, S.; et al. Allogeneic Mesenchymal Stem Cells for Treatment of AKI after Cardiac Surgery. J. Am. Soc. Nephrol. 2018, 29, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Arita, M.; Tamura, T.; Saito, M.; Katayama, H.; Kuroda, H.; Suzuki, T.; Kawamata, S. A Novel Approach for Determining the Critical Quality Attributes of Mesenchymal Stem Cells by Specifying Cell Population with Replication Potential. Stem Cells Transl. Med. 2023, 12, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Levy, O.; Kuai, R.; Siren, E.M.J.; Bhere, D.; Milton, Y.; Nissar, N.; de Biasio, M.; Heinelt, M.; Reeve, B.; Abdi, R.; et al. Shattering Barriers toward Clinically Meaningful MSC Therapies. Sci. Adv. 2020, 6, eaba6884. [Google Scholar] [CrossRef]
- European Commission. Annex 15: Qualification and Validation. In EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use; European Commission: Brussels, Belgium, 2015. [Google Scholar]
- Basile, D.P.; Bonventre, J.V.; Mehta, R.; Nangaku, M.; Unwin, R.; Rosner, M.H.; Kellum, J.A.; Ronco, C. Progression after AKI: UnderstanDing Maladaptive Repair Processes to Predict and Identify Therapeutic Treatments. J. Am. Soc. Nephrol. 2016, 27, 687–697. [Google Scholar] [CrossRef]
- Sharifian, R.; Okamura, D.M.; Denisenko, O.; Zager, R.A.; Johnson, A.; Gharib, S.A.; Bomsztyk, K. Distinct Patterns of Transcriptional and Epigenetic Alterations Characterize Acute and Chronic Kidney Injury. Sci. Rep. 2018, 8, 17870. [Google Scholar] [CrossRef]
- Liu, B.C.; Tang, T.T.; Lv, L.L. How Tubular Epithelial Cell Injury Contributes to Renal Fibrosis. In Renal Fibrosis: Mechanisms and Therapies; Advances in Experimental Medicine and Biology; Springer: Singapore, 2019; Volume 1165, pp. 233–252. [Google Scholar] [CrossRef]
- Holderied, A.; Kraft, F.; Marschner, J.A.; Weidenbusch, M.; Anders, H.J. “point of No Return” in Unilateral Renal Ischemia Reperfusion Injury in Mice. J. Biomed. Sci. 2020, 27, 34. [Google Scholar] [CrossRef]
- Taylor, C.T.; Doherty, G.; Fallon, P.G.; Cummins, E.P. Hypoxia-Dependent Regulation of Inflammatory Pathways in Immune Cells. J. Clin. Investig. 2016, 126, 3716–3724. [Google Scholar] [CrossRef]
- Mack, M.; Yanagita, M. Origin of Myofibroblasts and Cellular Events Triggering Fibrosis. Kidney Int. 2015, 87, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Djudjaj, S.; Boor, P. Cellular and Molecular Mechanisms of Kidney Fibrosis. Mol. Asp. Med. 2019, 65, 16–36. [Google Scholar] [CrossRef]
- Venkatachalam, M.A.; Weinberg, J.M.; Kriz, W.; Bidani, A.K. Failed Tubule Recovery, AKI-CKD Transition, and Kidney Disease Progression. J. Am. Soc. Nephrol. 2015, 26, 1765–1776. [Google Scholar] [CrossRef]
- Humphreys, B.D. Mechanisms of Renal Fibrosis. Annu. Rev. Physiol. 2018, 80, 309–326. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, H.; Vanderbrink, B.A.; Campbell, M.T.; Hile, K.L.; Zhang, H.; Meldrum, D.R.; Meldrum, K.K. Arterially Delivered Mesenchymal Stem Cells Prevent Obstruction-Induced Renal Fibrosis. J. Surg. Res. 2011, 168, e51–e59. [Google Scholar] [CrossRef] [PubMed]
- Tögel, F.E.; Westenfelder, C. Kidney Protection and Regeneration Following Acute Injury: Progress through Stem Cell Therapy. Am. J. Kidney Dis. 2012, 60, 1012–1022. [Google Scholar] [CrossRef]
- Morigi, M.; Rota, C.; Montemurro, T.; Montelatici, E.; Lo Cicero, V.; Imberti, B.; Abbate, M.; Zoja, C.; Cassis, P.; Longaretti, L.; et al. Life-Sparing Effect of Human Cord Blood-Mesenchymal Stem Cells in Experimental Acute Kidney Injury. Stem Cells 2010, 28, 513–522. [Google Scholar] [CrossRef]
- Hattori, Y.; Kim, H.; Tsuboi, N.; Yamamoto, A.; Akiyama, S.; Shi, Y.; Katsuno, T.; Kosugi, T.; Ueda, M.; Matsuo, S.; et al. Therapeutic Potential of Stem Cells from Human Exfoliated Deciduous Teeth in Models of Acute Kidney Injury. PLoS ONE 2015, 10, e0140121. [Google Scholar] [CrossRef]
- Pavyde, E.; Maciulaitis, R.; Mauricas, M.; Sudzius, G.; Ivanauskaite Didziokiene, E.; Laurinavicius, A.; Sutkeviciene, N.; Stankevicius, E.; Maciulaitis, J.; Usas, A. Skeletal Muscle-Derived Stem/Progenitor Cells: A Potential Strategy for the Treatment of Acute Kidney Injury. Stem Cells Int. 2016, 2016, 9618480. [Google Scholar] [CrossRef]
- Sávio-Silva, C.; Soinski-Sousa, P.E.; Balby-Rocha, M.T.A.; Lira, Á.D.O.; Rangel, É.B. Mesenchymal Stem Cell Therapy in Acute Kidney Injury (AKI): Review and Perspectives. Rev. Assoc. Med. Bras. (1992) 2020, 66 (Suppl. S1), s45–s54. [Google Scholar] [CrossRef]
- Al-Husseiny, F.; Sobh, M.A.; Ashour, R.H.; Foud, S.; Medhat, T.; El-Gilany, A.H.; Elghannam, D.; Abdel-Ghaffar, H.; Saad, M.A.; Sobh, M. Amniotic Fluid-Derived Mesenchymal Stem Cells Cut Short the Acuteness of Cisplatin-Induced Nephrotoxicity in Sprague-Dawley Rats. Int. J. Stem Cells 2016, 9, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Sadek, E.M.; Afifi, N.M.; Abd Elfattah, L.I.; Abd-El Mohsen, M.A. Histological Study on Effect of Mesenchymal Stem Cell Therapy on Experimental Renal Injury Induced by Ischemia/Reperfusion in Male Albino Rat. Int. J. Stem Cells 2013, 6, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Reis, L.A.; Borges, F.T.; Simões, M.J.; Borges, A.A.; Sinigaglia-Coimbra, R.; Schor, N. Bone Marrow-Derived Mesenchymal Stem Cells Repaired but Did Not Prevent Gentamicin-Induced Acute Kidney Injury through Paracrine Effects in Rats. PLoS ONE 2012, 7, e44092. [Google Scholar] [CrossRef]
- Burgos-Silva, M.; Semedo-Kuriki, P.; Donizetti-Oliveira, C.; Costa, P.B.; Cenedeze, M.A.; Hiyane, M.I.; Pacheco-Silva, A.; Câmara, N.O.S. Adipose Tissue-Derived Stem Cells Reduce Acute and Chronic Kidney Damage in Mice. PLoS ONE 2015, 10, e0142183. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, D.J.; Yun, J.C.; Jung, M.H.; Yeo, H.D.; Kim, H.J.; Kim, D.W.; Yang, J.I.; Lee, G.W.; Jeong, S.H.; et al. Human Adipose Tissue-Derived Mesenchymal Stem Cells Protect Kidneys from Cisplatin Nephrotoxicity in Rats. Am. J. Physiol. Ren. Physiol. 2012, 302, F1141–F1150. [Google Scholar] [CrossRef] [PubMed]
- Gooch, A.; Doty, J.; Flores, J.; Swenson, L.; Toegel, F.E.; Reiss, G.R.; Lange, C.; Zander, A.R.; Hu, Z.; Poole, S.; et al. Initial Report on a Phase I Clinical Trial: Prevention and Treatment of Post-Operative Acute Kidney Injury with Allogeneic Mesenchymal Stem Cells in Patients Who Require on-Pump Cardiac Surgery. Cit. Cell. Ther. Transplant. 2008, 1, 31–35. [Google Scholar] [CrossRef]
- Torrico, S.; Hotter, G.; Játiva, S. Development of Cell Therapies for Renal Disease and Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 15943. [Google Scholar] [CrossRef]
- Perico, N.; Casiraghi, F.; Remuzzi, G. Mesenchymal Stromal Cells for AKI after Cardiac Surgery. J. Am. Soc. Nephrol. 2017, 29, 7–9. [Google Scholar] [CrossRef]
- Seyhan, A.A. Lost in Translation: The Valley of Death across Preclinical and Clinical Divide—Identification of Problems and Overcoming Obstacles. Transl. Med. Commun. 2019, 4, 18. [Google Scholar] [CrossRef]
- Nangaku, M.; Kitching, A.R.; Boor, P.; Fornoni, A.; Rgen Floege, J.; Coates, P.T.; Himmelfarb, J.; Lennon, R.; Anders, H.-J.; Humphreys, B.D.; et al. International Society of Nephrology First Consensus Guidance for Preclinical Animal Studies in Translational Nephrology. Kidney Int. 2023, 104, 36–45. [Google Scholar] [CrossRef]
- Calcat-i-Cervera, S.; Rendra, E.; Scaccia, E.; Amadeo, F.; Hanson, V.; Wilm, B.; Murray, P.; O’Brien, T.; Taylor, A.; Bieback, K. Harmonised Culture Procedures Minimise but Do Not Eliminate Mesenchymal Stromal Cell Donor and Tissue Variability in a Decentralised Multicentre Manufacturing Approach. Stem Cell Res. Ther. 2023, 14, 120. [Google Scholar] [CrossRef]
- Ashour, R.H.; Saad, M.A.; Sobh, M.A.; Al-Husseiny, F.; Abouelkheir, M.; Awad, A.; Elghannam, D.; Abdel-Ghaffar, H.; Sobh, M. Comparative Study of Allogenic and Xenogeneic Mesenchymal Stem Cells on Cisplatin-Induced Acute Kidney Injury in Sprague-Dawley Rats. Stem Cell Res. Ther. 2016, 7, 126. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.K.; Mačiulaitis, R. Challenges with Advanced Therapy Medicinal Products and How to Meet Them. Nat. Rev. Drug. Discov. 2010, 9, 195–201. [Google Scholar] [CrossRef]
- Chinnadurai, R.; Viswanathan, S.; Moll, G. Editorial: Next Generation MSC Therapy Manufacturing, Potency and Mechanism of Action Analysis. Front. Immunol. 2023, 14, 1192636. [Google Scholar] [CrossRef] [PubMed]
- Chinnadurai, R.; Rajan, D.; Qayed, M.; Anderson, L.J.; Gibson, G.; Correspondence, J.G. Potency Analysis of Mesenchymal Stromal Cells Using a Combinatorial Assay Matrix Approach. Cell Rep. 2018, 22, 2504–2517. [Google Scholar] [CrossRef]
- Rota, C.; Imberti, B.; Pozzobon, M.; Piccoli, M.; De Coppi, P.; Atala, A.; Gagliardini, E.; Xinaris, C.; Benedetti, V.; Fabricio, A.S.C.; et al. Human Amniotic Fluid Stem Cell Preconditioning Improves Their Regenerative Potential. Stem Cells Dev. 2012, 21, 1911–1923. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Wu, D. Bone Marrow-Derived Mesenchymal Stem Cells Protect against Cisplatin-Induced Acute Kidney Injury in Rats by Inhibiting Cell Apoptosis. Int. J. Mol. Med. 2013, 32, 1262–1272. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Rai, P.; Plagov, A.; Lan, X.; Kumar, D.; Salhan, D.; Rehman, S.; Malhotra, A.; Bhargava, K.; Palestro, C.J.; et al. Transplantation of Bone Marrow-Derived MSCs Improves Cisplatinum-Induced Renal Injury through Paracrine Mechanisms. Exp. Mol. Pathol. 2013, 94, 466–473. [Google Scholar] [CrossRef]
- Tsuda, H.; Yamahara, K.; Otani, K.; Okumi, M.; Yazawa, K.; Kaimori, J.Y.; Taguchi, A.; Kangawa, K.; Ikeda, T.; Takahara, S.; et al. Transplantation of Allogenic Fetal Membrane-Derived Mesenchymal Stem Cells Protects against Ischemia/Reperfusion-Induced Acute Kidney Injury. Cell Transpl. 2014, 23, 889–899. [Google Scholar] [CrossRef]
- Neugarten, J.; Golestaneh, L. Female Sex Reduces the Risk of Hospital-Associated Acute Kidney Injury: A Meta-Analysis. BMC Nephrol. 2018, 19, 314. [Google Scholar] [CrossRef]
- Ozturk, H.; Firat, T.; Tekce, B.K.; Yilmaz, F.; Ozturk, H. Effects of Nicorandil on Renal Function and Histopathology in Rats with Partial Unilateral Ureteral Obstruction. Kaohsiung J. Med. Sci. 2017, 33, 236–245. [Google Scholar] [CrossRef]
- Kim, M.G.; Kim, S.C.; Ko, Y.S.; Lee, H.Y.; Jo, S.K.; Cho, W. The Role of M2 Macrophages in the Progression of Chronic Kidney Disease Following Acute Kidney Injury. PLoS ONE 2015, 10, e0143961. [Google Scholar] [CrossRef] [PubMed]
- Iran-Nejad, A.; Nematbakhsh, M.; Eshraghi-Jazi, F.; Talebi, A. Preventive Role of Estradiol on Kidney Injury Induced by Renal Ischemia-Reperfusion in Male and Female Rats. Int. J. Prev. Med. 2015, 6, 22. [Google Scholar] [CrossRef]
- Lima-Posada, I.; Portas-Cortés, C.; Pérez-Villalva, R.; Fontana, F.; Rodríguez-Romo, R.; Prieto, R.; Sánchez-Navarro, A.; Rodríguez-González, G.L.; Gamba, G.; Zambrano, E.; et al. Gender Differences in the Acute Kidney Injury to Chronic Kidney Disease Transition. Sci. Rep. 2017, 7, 12270. [Google Scholar] [CrossRef] [PubMed]
- James, M.T.; Pannu, N.; Hemmelgarn, B.R.; Austin, P.C.; Tan, Z.; McArthur, E.; Manns, B.J.; Tonelli, M.; Wald, R.; Quinn, R.R.; et al. Derivation and External Validation of Prediction Models for Advanced Chronic Kidney Disease Following Acute Kidney Injury. JAMA 2017, 318, 1787–1797. [Google Scholar] [CrossRef]
- Kim, M.-J.; Moon, D.; Jung, S.; Lee, J.; Kim, J. Cisplatin Nephrotoxicity Is Induced via Poly(ADP-Ribose) Polymerase Activation in Adult Zebrafish and Mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R843–R854. [Google Scholar] [CrossRef] [PubMed]
- Melnikov, V.Y.; Faubel, S.; Siegmund, B.; Lucia, M.S.; Ljubanovic, D.; Edelstein, C.L. Neutrophil-Independent Mechanisms of Caspase-1- and IL-18-Mediated Ischemic Acute Tubular Necrosis in Mice. J. Clin. Investig. 2002, 110, 1083–1091. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gryguc, A.; Maciulaitis, J.; Mickevicius, L.; Laurinavicius, A.; Sutkeviciene, N.; Grigaleviciute, R.; Zigmantaite, V.; Maciulaitis, R.; Bumblyte, I.A. Prevention of Transition from Acute Kidney Injury to Chronic Kidney Disease Using Clinical-Grade Perinatal Stem Cells in Non-Clinical Study. Int. J. Mol. Sci. 2024, 25, 9647. https://doi.org/10.3390/ijms25179647
Gryguc A, Maciulaitis J, Mickevicius L, Laurinavicius A, Sutkeviciene N, Grigaleviciute R, Zigmantaite V, Maciulaitis R, Bumblyte IA. Prevention of Transition from Acute Kidney Injury to Chronic Kidney Disease Using Clinical-Grade Perinatal Stem Cells in Non-Clinical Study. International Journal of Molecular Sciences. 2024; 25(17):9647. https://doi.org/10.3390/ijms25179647
Chicago/Turabian StyleGryguc, Agne, Justinas Maciulaitis, Lukas Mickevicius, Arvydas Laurinavicius, Neringa Sutkeviciene, Ramune Grigaleviciute, Vilma Zigmantaite, Romaldas Maciulaitis, and Inga Arune Bumblyte. 2024. "Prevention of Transition from Acute Kidney Injury to Chronic Kidney Disease Using Clinical-Grade Perinatal Stem Cells in Non-Clinical Study" International Journal of Molecular Sciences 25, no. 17: 9647. https://doi.org/10.3390/ijms25179647
APA StyleGryguc, A., Maciulaitis, J., Mickevicius, L., Laurinavicius, A., Sutkeviciene, N., Grigaleviciute, R., Zigmantaite, V., Maciulaitis, R., & Bumblyte, I. A. (2024). Prevention of Transition from Acute Kidney Injury to Chronic Kidney Disease Using Clinical-Grade Perinatal Stem Cells in Non-Clinical Study. International Journal of Molecular Sciences, 25(17), 9647. https://doi.org/10.3390/ijms25179647






