Evaluating the Dermatological Benefits of Snowberry (Symphoricarpos albus): A Comparative Analysis of Extracts and Fermented Products from Different Plant Parts
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Analysis of SE and FSE
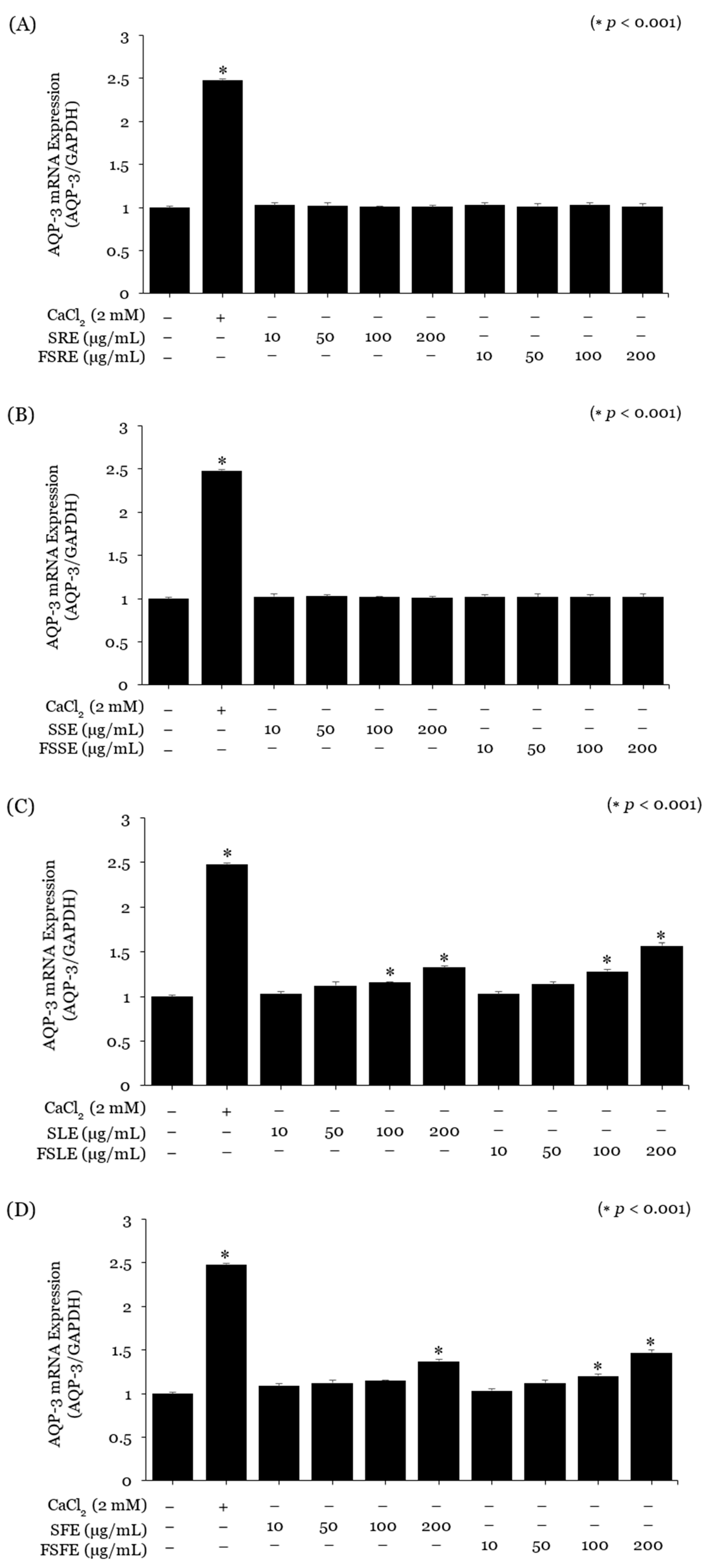
2.2. Evaluation of the Moisturising Properties of the SE and FSE
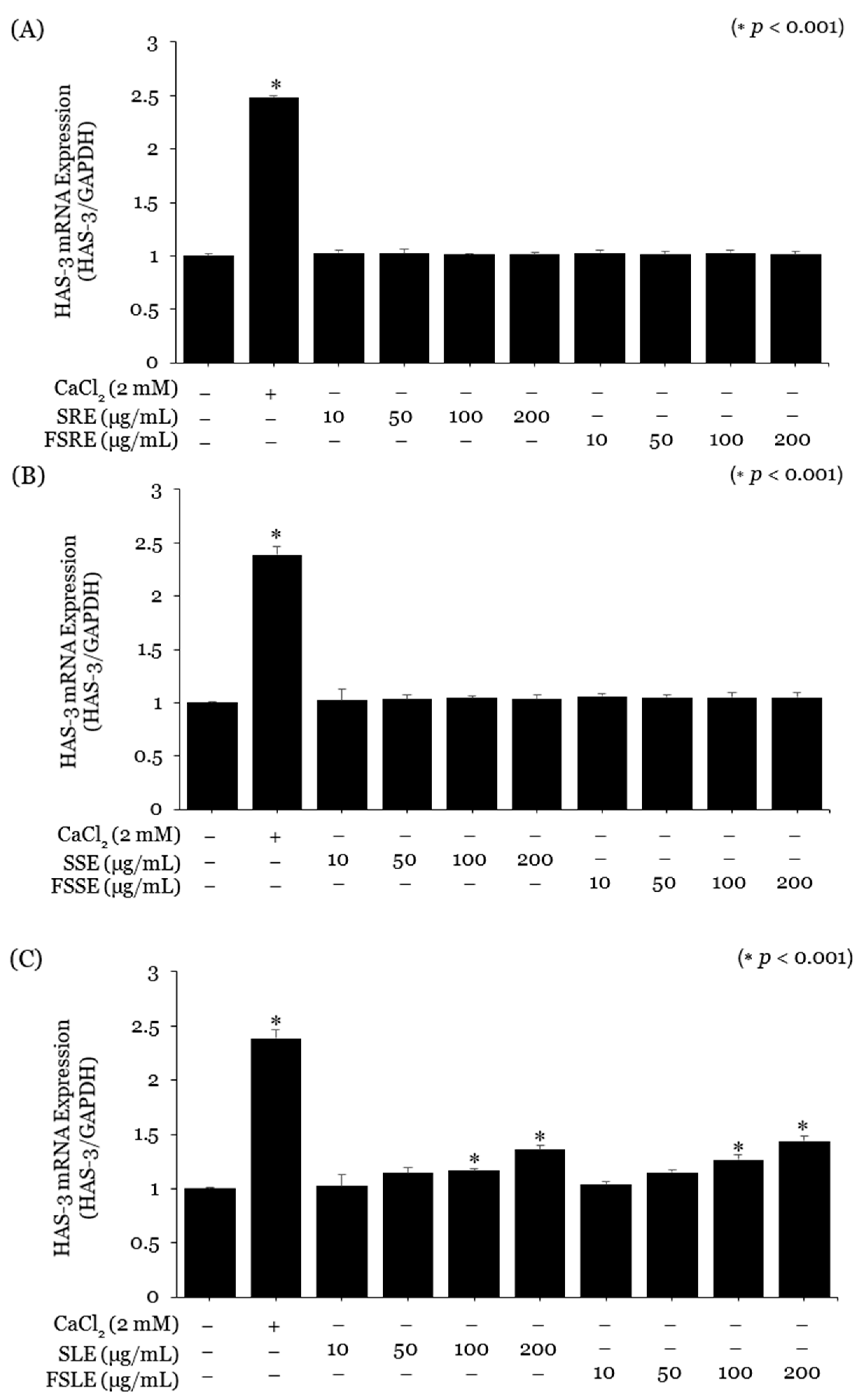
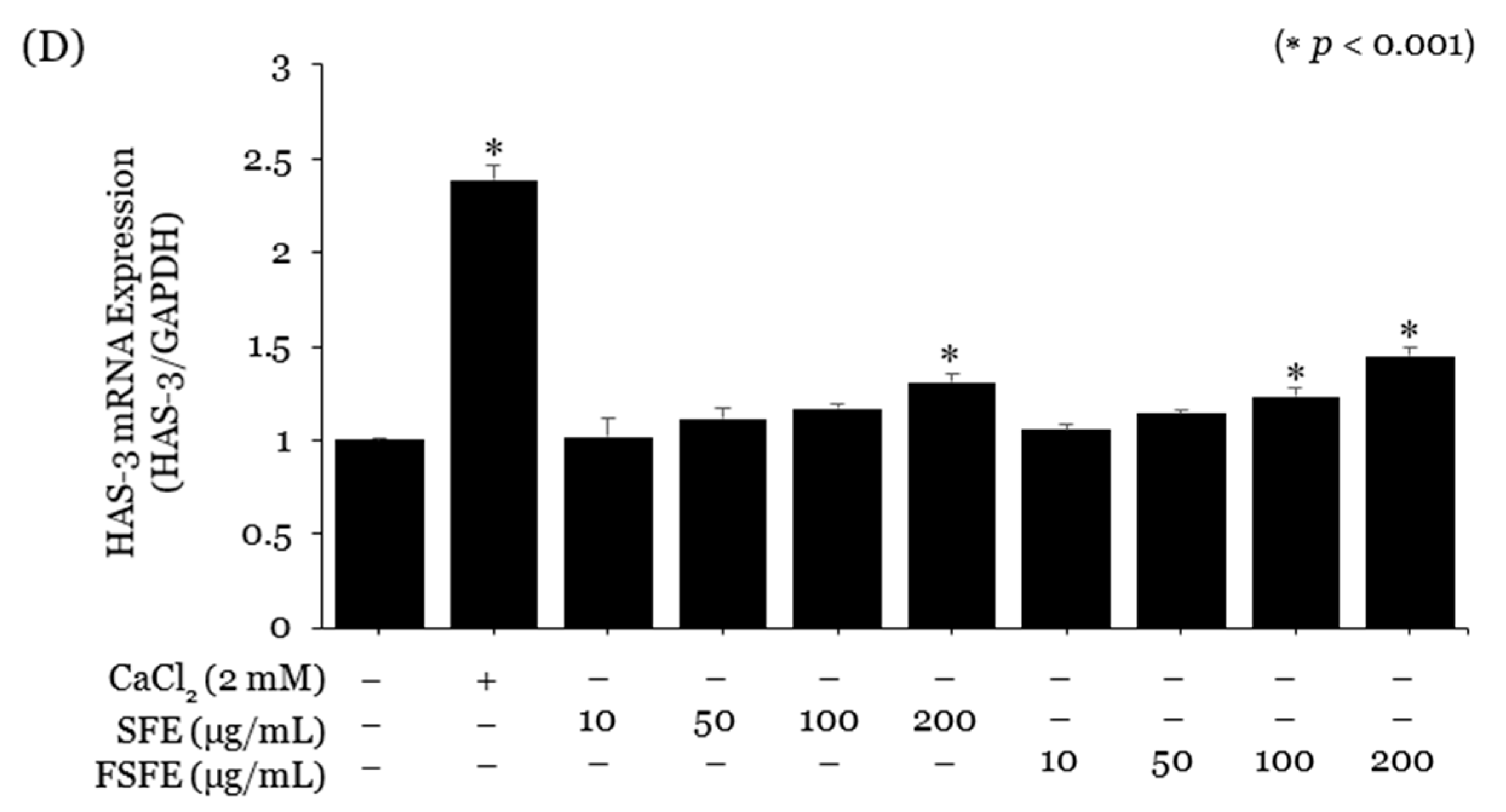
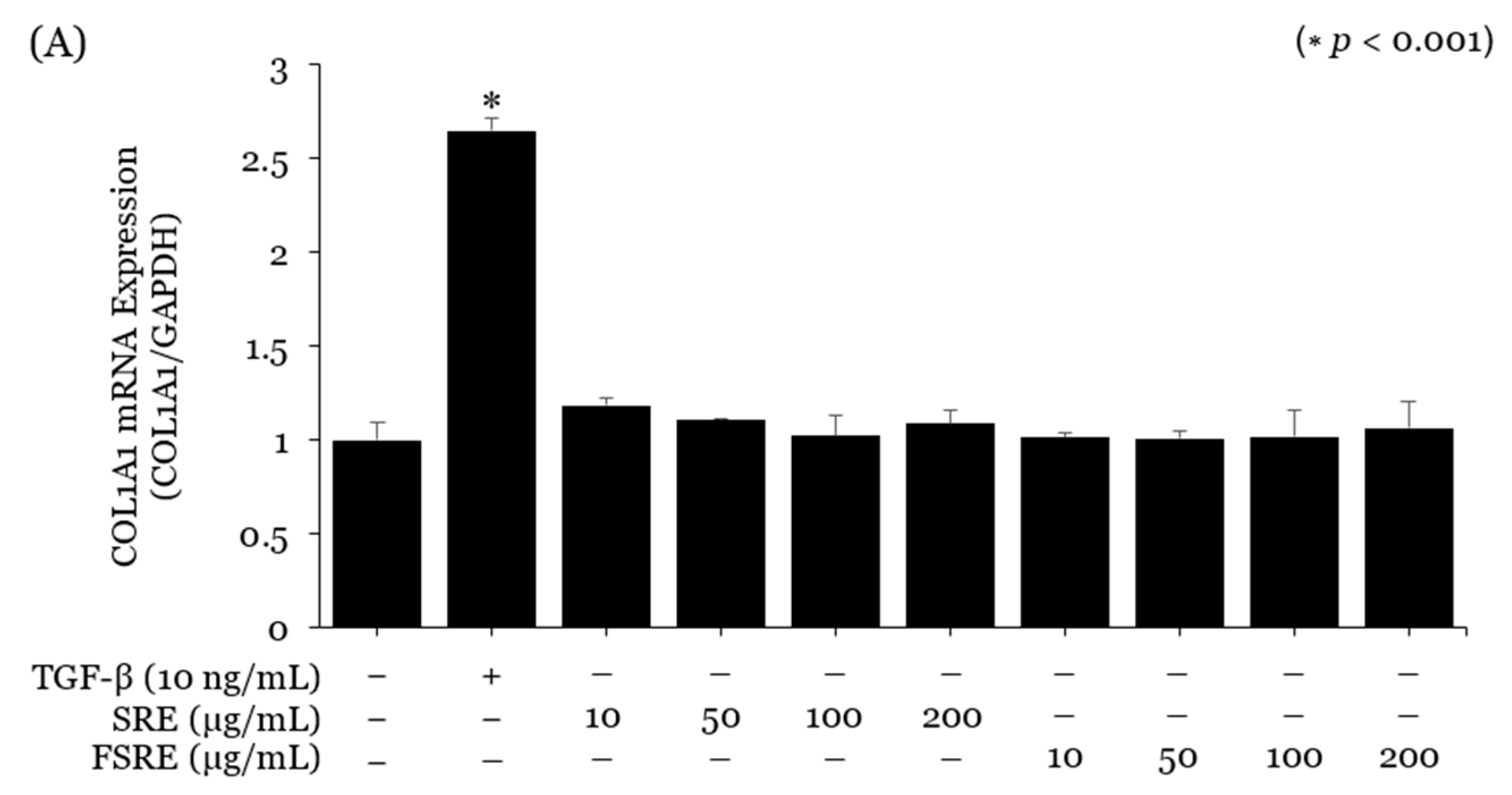
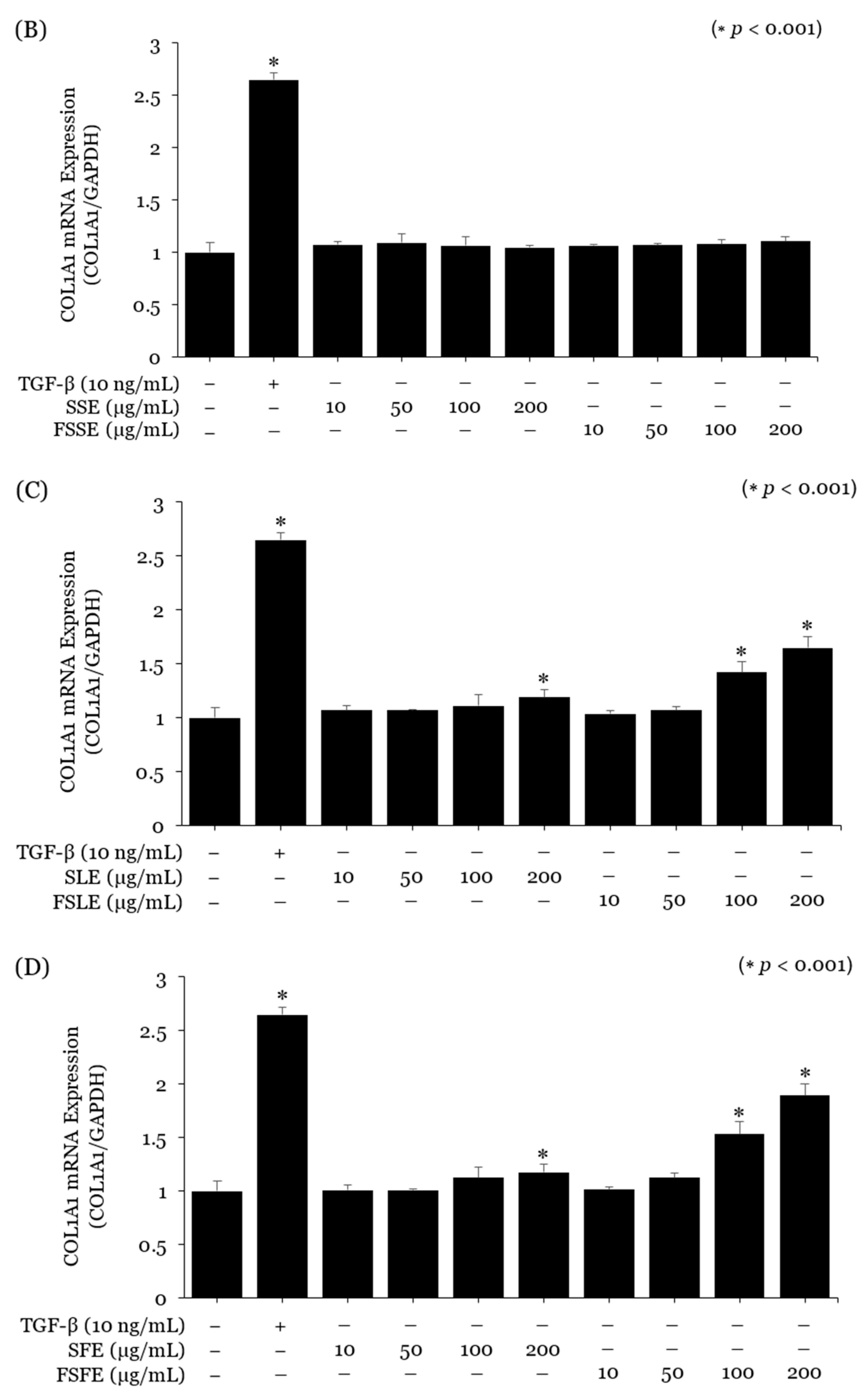
2.3. Evaluation of the SE and FSE for Wrinkle Treatment
3. Discussion
4. Materials and Methods
4.1. Preparation of SE Products
4.2. Preparation of FSE Products
4.3. HPLC Analysis of the SE and FSE
4.4. Real-Time Polymerase Chain Reaction (RT-PCR)
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, H.Y.; Kim, E.-J.; Cho, D.Y.; Jung, J.G.; Kim, M.J.; Lee, J.H.; Kim, W.; Kang, S.S.; Cho, K.M.; Kang, D. Photoprotective Effect of Fermented and Aged Mountain-Cultivated Ginseng Sprout (Panax ginseng) on Ultraviolet Radiation-Induced Skin Aging in a Hairless Mouse Model. Nutrients 2023, 15, 1715. [Google Scholar] [CrossRef] [PubMed]
- Eckhart, L.; Zeeuwen, P.L. The skin barrier: Epidermis vs. environment. Exp. Dermatol. 2018, 27, 805–806. [Google Scholar] [CrossRef]
- Shao, L.; Jiang, S.; Li, Y.; Yu, L.; Liu, H.; Ma, L.; Yang, S. Aqueous extract of Cordyceps cicadae (Miq.) promotes hyaluronan synthesis in human skin fibroblasts: A potential moisturizing and anti-aging ingredient. PLoS ONE 2023, 18, e0274479. [Google Scholar] [CrossRef]
- Sun, S.; Jiang, P.; Su, W.; Xiang, Y.; Li, J.; Zeng, L.; Yang, S. Wild chrysanthemum extract prevents UVB radiation-induced acute cell death and photoaging. Cytotechnology 2016, 68, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.-Y.; Chen, J.-Y.; Chen, C.-J.; Huang, C.-Y.; Kuo, W.-W. Protective effects of galangin against H2O2-induced aging via the IGF-1 signaling pathway in human dermal fibroblasts. Environ. Toxicol. 2020, 35, 115–123. [Google Scholar] [CrossRef]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Kim, K.-T.; Kim, Y.-H.; Kim, J.-G.; Han, C.-S.; Park, S.-H.; Lee, B.-Y. Preparation of oligo hyaluronic acid by hydrolysis and its application as a cosmetic ingredient. J. Soc. Cosmet. Sci. Korea 2007, 33, 189–196. [Google Scholar]
- Kim, S.-H.; Nam, G.-W.; Kang, B.-Y.; Lee, H.-K.; Moon, S.-J.; Chang, I.-S. The effect of kaempferol, guercetin on hyaluronan-synthesis stimulation in human keratinocytes (HaCaT). J. Soc. Cosmet. Sci. Korea 2005, 31, 97–102. [Google Scholar]
- Song, H.J.; Jin, M.H.; Lee, S.H. Effect of ferulic acid isolated from Cnidium officinale on the synthesis of hyaluronic acid. J. Soc. Cosmet. Sci. Korea 2013, 39, 281–288. [Google Scholar] [CrossRef]
- Lee, P.-J.; Kim, H.-T.; Yoon, K.-S.; Park, H.-C.; Ha, H.-Y. The effect of Astragalus membranaceus methanol extract on hyaluronic acid production in HaCaT cells. J. Korean Med. Ophthalmol. Otolaryngol. Dermatol. 2013, 26, 75–81. [Google Scholar] [CrossRef]
- Hara-Chikuma, M.; Verkman, A.S. Roles of aquaporin-3 in the epidermis. J. Investig. Dermatol. 2008, 128, 2145–2151. [Google Scholar] [CrossRef]
- Sougrat, R.; Morand, M.; Gondran, C.; Barré, P.; Gobin, R.; Bonté, F.; Dumas, M.; Verbavatz, J.M. Functional expression of AQP3 in human skin epidermis and reconstructed epidermis. J. Investig. Dermatol. 2002, 118, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.P.; Tseng, Y.P.; Liu, C.; Liang, C.H. Fermented pomegranate extracts protect against oxidative stress and aging of skin. J. Cosmet. Dermatol. 2022, 21, 2236–2245. [Google Scholar] [CrossRef]
- Kim, S.Y.; Shin, S.Y.; Kim, J.H.; Lee, H.J.; Kim, K.M. Antioxidant and moisturizing effects of fermented Hordeum vulgare L. extract and heat-killed probiotics by Lactobacillus lactic acid bacteria isolated from kimchi. Korean J. Microbiol. 2021, 57, 30–38. [Google Scholar] [CrossRef]
- Jeon, J.-M.; Choi, S.-K.; Kim, Y.-J.; Jang, S.-J.; Cheon, J.-W.; Lee, H.-S. Antioxidant and antiaging effect of ginseng berry extract fermented by lactic acid bacteria. J. Soc. Cosmet. Sci. Korea 2011, 37, 75–81. [Google Scholar] [CrossRef]
- Jun, D.H.; Cho, W.-A.; Lee, J.B.; Jang, M.J.; You, M.S.; Park, J.Y.; Kim, S.H.; Lee, J.T. Antioxidant Activity of Chestnut (Castanea crenata S.et Z.) bur Fermented by Lactobacillus casei. J. Life Sci. 2014, 24, 1193–1199. [Google Scholar] [CrossRef]
- Kang, H.; Kim, T.; Jhoo, J.; Kim, G. Antioxidant effects of fermented milk added with Enterococcus faecalis EF-2001 heat-killed probiotics. Ann. Anim. Resour. Sci. 2020, 31, 72–81. [Google Scholar] [CrossRef]
- Muizzuddin, N.; Maher, W.; Sullivan, M.; Schnittger, S.; Mammone, T. Physiological effect of a probiotic on skin. J. Cosmet. Sci. 2012, 63, 385–395. [Google Scholar] [PubMed]
- Tsai, Y.-L.; Lin, T.-L.; Chang, C.-J.; Wu, T.R.; Lai, W.F.; Lu, C.C.; Lai, H.C. Probiotics, prebiotics and amelioration of diseases. J. Biomed. Sci. 2019, 26, 3. [Google Scholar] [CrossRef]
- Prakoeswa, C.; Bonita, L.; Karim, A.; Herwanto, N.; Umborowati, M.A.; Setyaningrum, T.; Hidayati, A.N.; Surono, I.S. Beneficial effect of Lactobacillus plantarum IS-10506 supplementation in adults with atopic dermatitis: A randomized controlled trial. J. Dermatolog. Treat. 2022, 33, 1491–1498. [Google Scholar] [CrossRef]
- Szaufer-Hajdrych, M.; Zgórka, G. Phenolic acids from Symphoricarpos albus (L.) Blake (Caprifoliaceae). Acta Pol. Pharm. 2003, 60, 91–95. [Google Scholar] [PubMed]
- Staniforth, V.; Huang, W.-C.; Aravindaram, K.; Yang, N.-S. Ferulic acid, a phenolic phytochemical, inhibits UVB-induced matrix metalloproteinases in mouse skin via posttranslational mechanisms. J. Nutr. Biochem. 2012, 23, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Ghaisas, M.M.; Kshirsagar, S.B.; Sahane, R.S. Evaluation of wound healing activity of ferulic acid in diabetic rats. Int. Wound J. 2014, 11, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-M.; Chiu, J.-H.; Wu, I.-H.; Wang, B.-W.; Pan, C.-M.; Chen, Y.-H. Ferulic acid augments angiogenesis via VEGF, PDGF and HIF-1α. J. Nutr. Biochem. 2010, 21, 627–633. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant properties of ferulic acid and its possible application. Skin Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
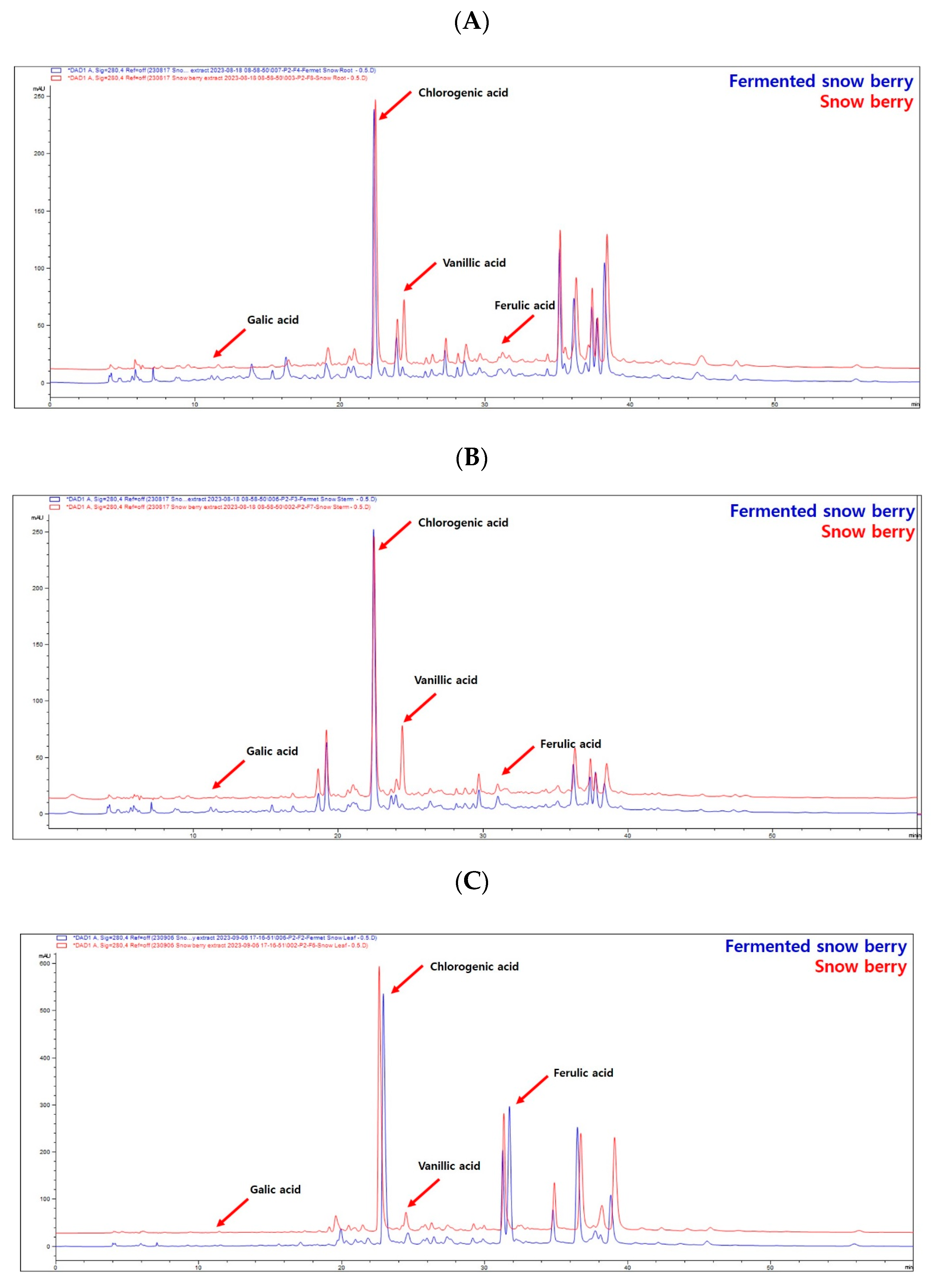
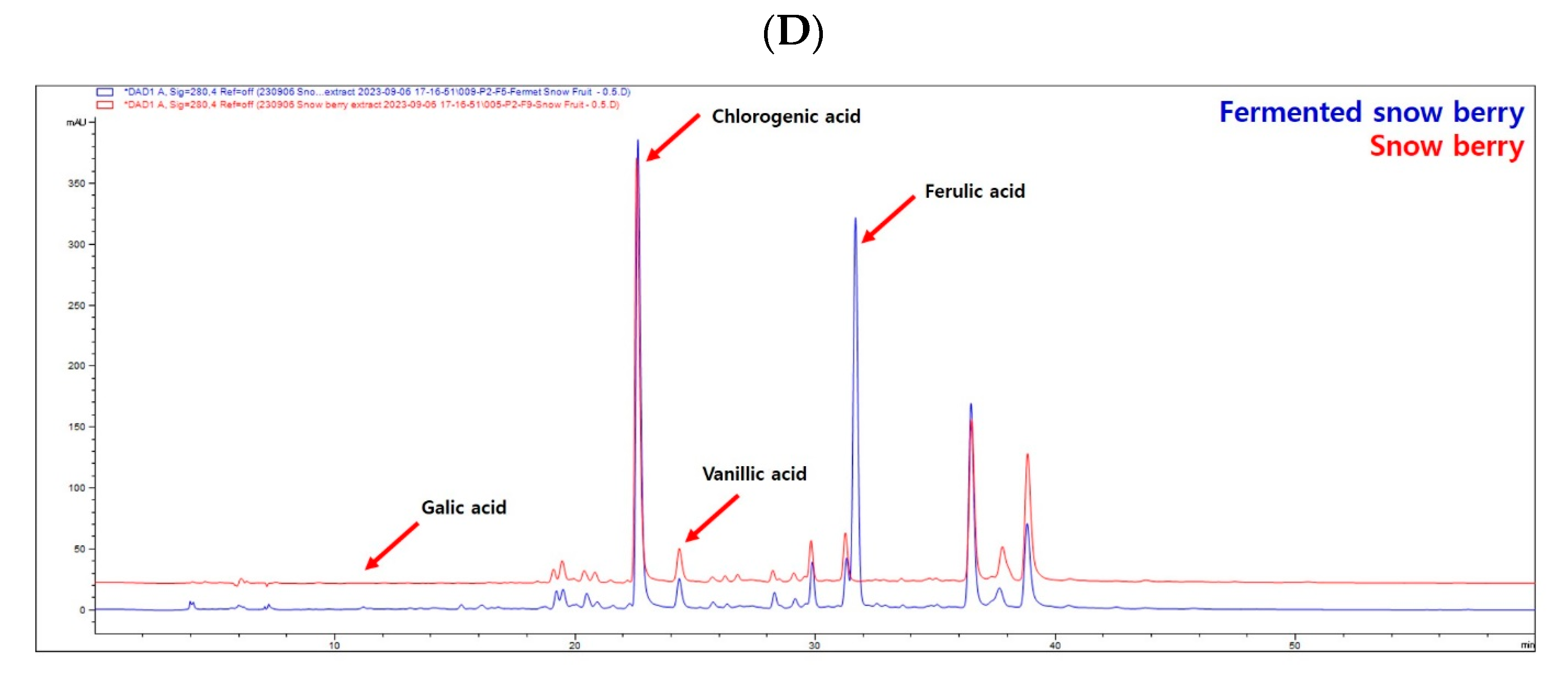
| (Units: mg/g) | Gallic Acid | Chlorogenic Acid | Vanillic Acid | Ferulic Acid |
|---|---|---|---|---|
| Stem | 0.07 ± 0.004 | 16.97 ± 0.43 | 4.10 ± 0.21 | 0.15 ± 0.012 |
| Leaf | 0.10 ± 0.007 | 41.98 ± 0.71 | 2.88 ± 0.19 | 0.00 |
| Root | 0.13 ± 0.01 | 15.02 ± 0.0.57 | 2.26 ± 0.06 | 0.26 ± 0.034 |
| Fruit | 0.00 | 25.43 ± 0.83 | 1.50 ± 0.09 | 0.00 |
| (Units: mg/g) | Gallic Acid | Chlorogenic Acid | Vanillic Acid | Ferulic Acid |
|---|---|---|---|---|
| Stem | 0.10 ± 0.014 | 16.18 ± 0.31 | 0.97 ± 0.072 | 0.21 ± 0.012 |
| Leaf | 0.10 ± 0.025 | 38.21 ± 0.92 | 1.58 ± 0.049 | 11.45 ± 0.16 |
| Root | 0.14 ± 0.019 | 14.61 ± 0.45 | 2.35 ± 0.067 | 0.42 ± 0.037 |
| Fruit | 0.00 | 28.01 ± 0.71 | 0.86 ± 0.019 | 13.21 ± 0.092 |
| Components | Ratio of Contents (%) |
|---|---|
| Glucose | 1% |
| Soy peptone (soy protein) | 1% |
| Yeast extract | 0.5% |
| Sodium acetate | 0.25% |
| Dipotassium phosphate | 0.1% |
| Ammonium citrate | 0.1% |
| Tween 80 | 0.05% |
| Magnesium sulphate | 0.005% |
| Manganese sulphate | 0.0025% |
| Lyophilised snowberry extract powder | 1% |
| Column | Capcell pak C18 4.6 mm × 250 mm, 5 µL | |
| Flow rate | 1.0 mL/min | |
| Detection | UV 280 nm | |
| Temperature | 30 °C | |
| Injection vol | 10 μL | |
| Mobile phase (min) | 0.2% Acetic acid in water | 0.2% Acetic acid in ACN |
| 0 | 95 | 5 |
| 30 | 70 | 30 |
| 55 | 65 | 35 |
| 58 | 95 | 5 |
| 60 | 95 | 5 |
| Target Gene | Forward Primer | Reverse Primer |
|---|---|---|
| GAPDH | ACCACAGTCCATGCCATCAC | TCCACCACCCTGTTGCTGTA |
| COL1A1 | AGGGCCAAGACGAAGACATC | AGATCACGTCATCGCACAACA |
| HAS-3 | CCCAGCCAGATTTGTTGATG | AGTGGTCACGGGTTTCTTCC |
| AQP3 | AGACAGCCCCTTCAGGATTT | TCCCTTGCCCTGAATATCTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.; Cho, H.; Kim, M.; Kim, B.; Jang, Y.-P.; Park, J. Evaluating the Dermatological Benefits of Snowberry (Symphoricarpos albus): A Comparative Analysis of Extracts and Fermented Products from Different Plant Parts. Int. J. Mol. Sci. 2024, 25, 9660. https://doi.org/10.3390/ijms25179660
Lee C, Cho H, Kim M, Kim B, Jang Y-P, Park J. Evaluating the Dermatological Benefits of Snowberry (Symphoricarpos albus): A Comparative Analysis of Extracts and Fermented Products from Different Plant Parts. International Journal of Molecular Sciences. 2024; 25(17):9660. https://doi.org/10.3390/ijms25179660
Chicago/Turabian StyleLee, Chanwoo, Hana Cho, Myunsoo Kim, Boae Kim, Young-Pyo Jang, and Junseong Park. 2024. "Evaluating the Dermatological Benefits of Snowberry (Symphoricarpos albus): A Comparative Analysis of Extracts and Fermented Products from Different Plant Parts" International Journal of Molecular Sciences 25, no. 17: 9660. https://doi.org/10.3390/ijms25179660






