Novel Clinical, Immunological, and Metabolic Features Associated with Persistent Post-Acute COVID-19 Syndrome
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
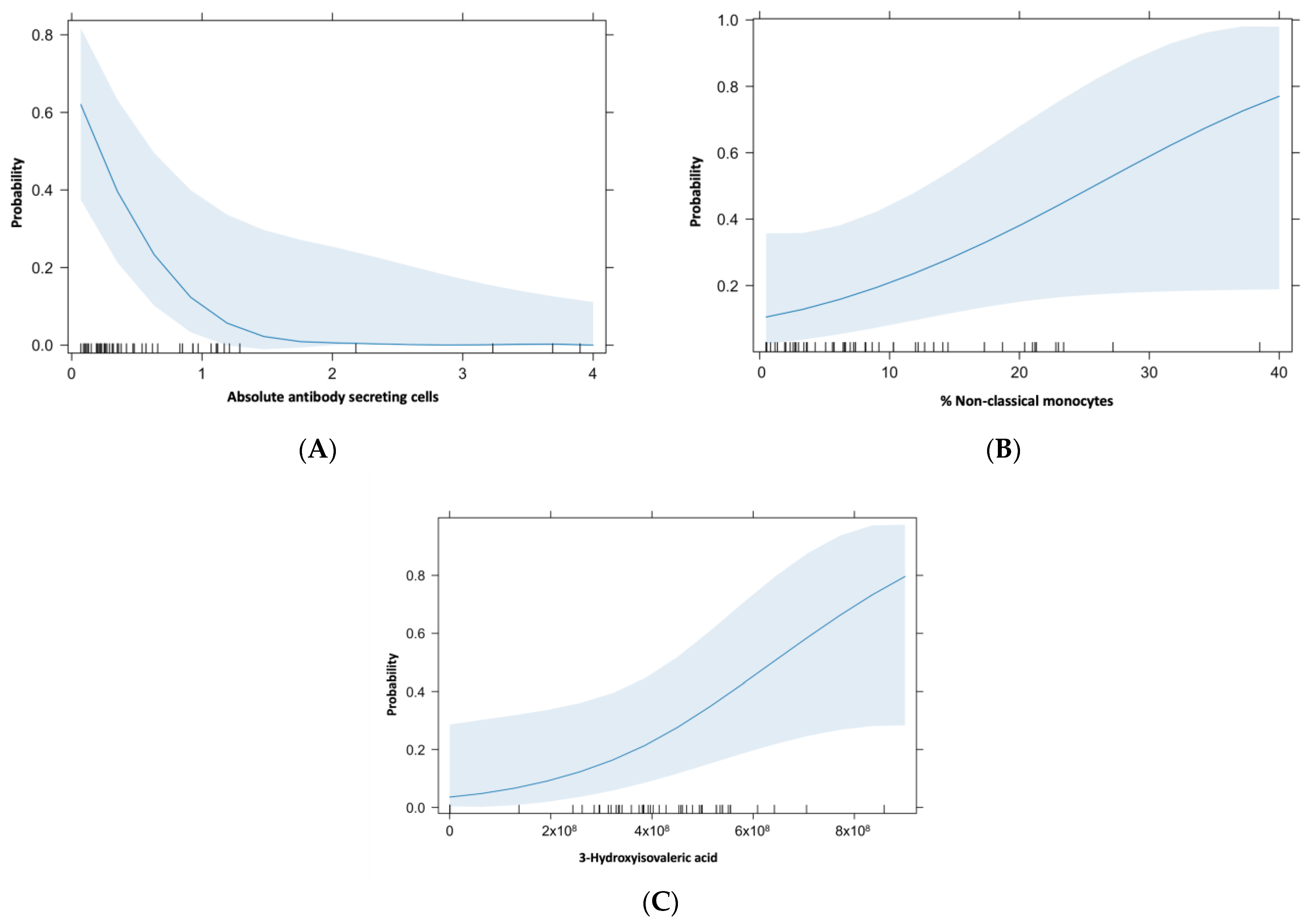
- (A)
- Mild/moderate disease: fever, upper respiratory infection symptoms, with or without pneumonia.
- (B)
- Severe: Any of the following: respiratory failure, respiratory rate >30 breaths per minute, oxygen saturation at rest < 93%, PaO2/FIO2 < 300 mmHg.
- (C)
- Critical: any of the following: requirement of invasive mechanical ventilation, shock, multiple organ failure.
4.1. Peripheral Blood Mononuclear Cells (PBMCs) Immunophenotyping by Flow Cytometry
4.2. Metabolomic Assessment
4.3. Cytokine/Chemokine and Coagulation Profiles Assessment
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, X.; Gu, X.; Zhang, H.; Ren, L.; Guo, L.; Liu, M.; Wang, Y.; Cui, D.; Wang, Y.; et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: A longitudinal cohort study. Lancet Respir. Med. 2022, 10, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Taquet, M.; Sillett, R.; Zhu, L.; Mendel, J.; Camplisson, I.; Dercon, Q.; Harrison, P.J. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: An analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatry 2022, 9, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Hou, C.; Shen, Y.; Zhang, M.; Zhang, K.; Wang, F.; Liu, Y.; Ma, X.; Cheng, L.; Kang, J.; et al. Two-Year Health Outcomes in Hospitalized COVID-19 Survivors in China. JAMA Netw. Open 2022, 5, e2231790. [Google Scholar] [CrossRef]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V.; WHO Clinical Case Definition Working Group on Post-COVID-19 Condition. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, e102–e107. [Google Scholar] [CrossRef]
- Perumal, R.; Shunmugam, L.; Naidoo, K.; Abdool Karim, S.S.; Wilkins, D.; Garzino-Demo, A.; Brechot, C.; Parthasarathy, S.; Vahlne, A.; Nikolich, J. Long COVID: A review and proposed visualization of the complexity of long COVID. Front. Immunol. 2023, 14, 1117464. [Google Scholar] [CrossRef]
- McElvaney, O.J.; McEvoy, N.L.; McElvaney, O.F.; Carroll, T.P.; Murphy, M.P.; Dunlea, D.M.; Choileáin, O.N.; Clarke, J.; O’connor, E.; Hogan, G.; et al. Characterization of the Inflammatory Response to Severe COVID-19 Illness. Am. J. Respir. Crit. Care Med. 2020, 202, 812–821. [Google Scholar] [CrossRef]
- Sungnak, W.; Huang, N.; Becavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Sampaziotis, F.; et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020, 26, 681–687. [Google Scholar] [CrossRef]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and cognitive impairment in Post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef]
- Huang, Y.; Pinto, M.D.; Borelli, J.L.; Asgari Mehrabadi, M.; Abrahim, H.L.; Dutt, N.; Lambert, N.; Nurmi, E.L.; Chakraborty, R.; Rahmani, A.M.; et al. COVID Symptoms, Symptom Clusters, and Predictors for Becoming a Long-Hauler Looking for Clarity in the Haze of the Pandemic. Clin. Nurs. Res. 2022, 31, 1390–1398. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, T.; Knight, M.; A’Court, C.; Buxton, M.; Husain, L. Management of post-acute COVID-19 in primary care. BMJ 2020, 370, m3026. [Google Scholar] [CrossRef] [PubMed]
- Shah, W.; Hillman, T.; Playford, E.D.; Hishmeh, L. Managing the long term effects of COVID-19: Summary of NICE, SIGN, and RCGP rapid guideline. BMJ 2021, 372, n136. [Google Scholar] [CrossRef] [PubMed]
- Amenta, E.M.; Spallone, A.; Rodriguez-Barradas, M.C.; El Sahly, H.M.; Atmar, R.L.; Kulkarni, P.A. Postacute COVID-19: An Overview and Approach to Classification. Open Forum Infect. Dis. 2020, 7, ofaa509. [Google Scholar] [CrossRef]
- Torres-Ruiz, J.; Lomelin-Gascon, J.; Lira-Luna, J.; Perez-Fragoso, A.; Tapia-Conyer, R.; Nunez-Aguirre, M.; Alcalá-Carmona, B.; Absalón-Aguilar, A.; Maravillas-Montero, J.L.; Mejía-Domínguez, N.R.; et al. FANSY POSTCOV: A composite clinical immunological predictive index for post-COVID-19 syndrome unveils distinctive features in a cohort study of mild to critical patients. Clin. Transl. Med. 2021, 11, e623. [Google Scholar] [CrossRef]
- Ravenhill, B.J.; Soday, L.; Houghton, J.; Antrobus, R.; Weekes, M.P. Comprehensive cell surface proteomics defines markers of classical, intermediate and non-classical monocytes. Sci. Rep. 2020, 10, 4560. [Google Scholar] [CrossRef]
- Patterson, B.K.; Francisco, E.B.; Yogendra, R.; Long, E.; Pise, A.; Rodrigues, H.; Hall, E.; Herrera, M.; Parikh, P.; Guevara-Coto, J.; et al. Persistence of SARS CoV-2 S1 Protein in CD16+ Monocytes in Post-Acute Sequelae of COVID-19 (PASC) up to 15 Months Post-Infection. Front. Immunol. 2021, 12, 746021. [Google Scholar] [CrossRef]
- Obermayer, A.; Jakob, L.M.; Haslbauer, J.D.; Matter, M.S.; Tzankov, A.; Stoiber, W. Neutrophil Extracellular Traps in Fatal COVID-19-Associated Lung Injury. Dis. Markers 2021, 2021, 5566826. [Google Scholar] [CrossRef]
- Chevrier, S.; Zurbuchen, Y.; Cervia, C.; Adamo, S.; Raeber, M.E.; de Souza, N.; Sivapatham, S.; Jacobs, A.; Bachli, E.; Rudiger, A.; et al. A distinct innate immune signature marks progression from mild to severe COVID-19. Cell Rep. Med. 2021, 2, 100166. [Google Scholar] [CrossRef]
- Maglietta, G.; Diodati, F.; Puntoni, M.; Lazzarelli, S.; Marcomini, B.; Patrizi, L.; Caminiti, C. Prognostic Factors for Post-COVID-19 Syndrome: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 1541. [Google Scholar] [CrossRef]
- Castanares-Zapatero, D.; Chalon, P.; Kohn, L.; Dauvrin, M.; Detollenaere, J.; Maertens de Noordhout, C.; Jong, C.P.-D.; Cleemput, I.; Heede, K.V.D. Pathophysiology and mechanism of long COVID: A comprehensive review. Ann. Med. 2022, 54, 1473–1487. [Google Scholar] [CrossRef] [PubMed]
- Dean, L.S.; Devendra, G.; Jiyarom, B.; Subia, N.; Tallquist, M.D.; Nerurkar, V.R.; Chang, S.P.; Chow, D.C.; Shikuma, C.M.; Park, J. Phenotypic alteration of low-density granulocytes in people with pulmonary post-acute sequalae of SARS-CoV-2 infection. Front. Immunol. 2022, 13, 1076724. [Google Scholar] [CrossRef]
- Torres-Ruiz, J.; Absalon-Aguilar, A.; Nunez-Aguirre, M.; Perez-Fragoso, A.; Carrillo-Vazquez, D.A.; Maravillas-Montero, J.L.; Mejía-Domínguez, N.R.; Llorente, L.; Alcalá-Carmona, B.; Lira-Luna, J.; et al. Neutrophil Extracellular Traps Contribute to COVID-19 Hyperinflammation and Humoral Autoimmunity. Cells 2021, 10, 2545. [Google Scholar] [CrossRef]
- Park, J.; Dean, L.S.; Jiyarom, B.; Gangcuangco, L.M.; Shah, P.; Awamura, T.; Ching, L.L.; Nerurkar, V.R.; Chow, D.C.; Igno, F.; et al. Elevated circulating monocytes and monocyte activation in COVID-19 convalescent individuals. Front. Immunol. 2023, 14, 1151780. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.R.; Ramelli, S.C.; Grazioli, A.; Chung, J.Y.; Singh, M.; Yinda, C.K.; Winkler, C.W.; Sun, J.; Dickey, J.M.; Ylaya, K.; et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature 2022, 612, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Guntur, V.P.; Nemkov, T.; de Boer, E.; Mohning, M.P.; Baraghoshi, D.; Cendali, F.I.; Winkler, C.W.; Sun, J.; Dickey, J.M.; Ylaya, K.; et al. Signatures of Mitochondrial Dysfunction and Impaired Fatty Acid Metabolism in Plasma of Patients with Post-Acute Sequelae of COVID-19 (PASC). Metabolites 2022, 12, 1026. [Google Scholar] [CrossRef]
- de Boer, E.; Petrache, I.; Goldstein, N.M.; Olin, J.T.; Keith, R.C.; Modena, B.; Mohning, M.P.; Yunt, Z.X.; San-Millán, I.; Swigris, J.J. Decreased Fatty Acid Oxidation and Altered Lactate Production during Exercise in Patients with Post-acute COVID-19 Syndrome. Am. J. Respir. Crit. Care Med. 2022, 205, 126–129. [Google Scholar] [CrossRef]
- Lopez-Hernandez, Y.; Monarrez-Espino, J.; Lopez, D.A.G.; Zheng, J.; Borrego, J.C.; Torres-Calzada, C.; Elizalde-Díaz, J.P.; Mandal, R.; Berjanskii, M.; Martínez-Martínez, E.; et al. The plasma metabolome of long COVID patients two years after infection. Sci. Rep. 2023, 13, 12420. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics by Gas Chromatography-Mass Spectrometry: Combined Targeted and Untargeted Profiling. Curr. Protoc. Mol. Biol. 2016, 114, 21.33.1–21.33.11. [Google Scholar] [CrossRef]

| Variable | Without PACS n = 32 | With PACS n = 19 | p |
|---|---|---|---|
| Demographics | |||
| Age | 49.5 years (25–71) | 48 years (29–67) | NS |
| Female gender | 12 (37.5%) | 7 (36.8%) | NS |
| Acute infection severity | n (%) | n (%) | |
| Mild | 15 (46.9%) | 6 (31.5%) | NS |
| Moderate/severe | 15 (46.9%) | 11 (57.8%) | NS |
| Critical | 2 (6.2%) | 2 (10.5%) | NS |
| Clinical/Comorbidities | n (%) | n (%) | |
| Obesity | 6 (18.75%) | 9 (47.3%) | NS |
| Diabetes mellitus | 5 (15.6%) | 3 (15.78%) | NS |
| Arterial Hypertension | 5 (15.6%) | 8 (42.1%) | NS |
| Cardiopathy | 1 (3.12%) | 0 | NS |
| Dyslipidemia | 0 | 1 (5.26%) | NS |
| Chronic renal disease | 0 | 0 | NS |
| Smoking | 3 (9.37%) | 1 (10.52%) | NS |
| Laboratory | Median (IQR) | Median (IQR) | |
| Leucocytes | 7150 (5225–8375) | 6700 (5200–10,100) | NS |
| Total T lymphocytes | 552 (395–1194) | 858 (620–1263) | NS |
| Ferritin | 645 (203–837) | 541 (317–1026) | NS |
| D Dimer | 859 (380–1113) | 961 (634–1250) | NS |
| Cytokines/Chemokines | Without PACS Median (IQR) | With PACS Median (IQR) | p |
|---|---|---|---|
| Eotaxin (pg/mL) | 104.1 (70.64–111) | 80.06 (73.85–102.1) | NS |
| TGF-β1 (pg/mL) | 90,435 (70,347–101,814) | 92,251 (79,717–110,959) | NS |
| TGF-β2 (pg/mL) | 2849 (845.3–3435) | 1005 (476.1–2775) | NS |
| TGF-β3 (pg/mL) | 6753 (68.24–7444) | 716.3 (68.24–5839) | NS |
| G-CSF (pg/mL) | 49.9 (24.93–91.69) | 23.39 (9.25–91.69) | NS |
| IFN-α2 (pg/mL) | 27.82 (10.9–44.32) | 36.02 (14.81–58.99) | NS |
| IFN-γ (pg/mL) | 11.58 (5.693–20.77) | 9.11 (3.05–15) | NS |
| IL-10 (pg/mL) | 15.82 (9.99–23) | 17.51 (7.823–24.55) | NS |
| GM-CSF (pg/mL) | 6.79 (4.03–9.19) | 7.755 (1.023–10.99) | NS |
| VEGF (pg/mL) | 80.27 (56.33–129.1) | 96.3 (58.99–122) | NS |
| TNF-β (pg/mL) | 6.365 (0.7–24.06) | 1.945 (0.7–5.04) | NS |
| TNF-α (pg/mL) | 16.88 (12.38–43.71) | 33.2 (23.67–134.13) | NS |
| MIP-1B (pg/mL) | 34.45 (22.38–46.78) | 43.98 (26.12–66.02) | NS |
| MIP-1A (pg/mL) | 3.88 (0.76–8.513) | 2.085 (0.7–11.59) | NS |
| MCP-1 (pg/mL) | 377.9 (222–509.1) | 215.8 (94.15–520.8) | NS |
| IP-10 (pg/mL) | 686.7 (421.6–1189) | 1344 (498.4–1780) | NS |
| IL-8 (pg/mL) | 17.31 (9.97–31.38) | 38.28 (25.84–98.47) | <0.01 |
| IL-7 (pg/mL) | 14.02 (7.61–34.62) | 13.31 (3.49–54.87) | NS |
| IL-6 (pg/mL) | 15.88 (11.96–26.38) | 19.43 (6.505–26.97) | NS |
| IL-5 (pg/mL) | 3.47 (1.91–16.91) | 6.26 (0.7275–141.3) | NS |
| IL-4 (pg/mL) | 42.26 (11.9–164.9) | 11.9 (4.4–34.67) | NS |
| IL-3 (pg/mL) | 0.705 (0.205–0.745) | 0.19 (0.145–0.595) | NS |
| IL-2 (pg/mL) | 1.74 (1.09–8.1) | 1.89 (0.445–36.29) | NS |
| IL-1β (pg/mL) | 2.93 (1.91–4.05) | 4.54 (0.98–6.305) | NS |
| IL-1A (pg/mL) | 11.44 (7.643–36.52) | 4.905 (0.98–11.51) | NS |
| IL-1RA (pg/mL) | 36.63 (16.91–53.24) | 70.84 (24.18–132.4) | NS |
| IL-17A (pg/mL) | 5.92 (1.833–10.47) | 4.62 (1.705–16.89) | NS |
| IL-15 (pg/mL) | 4.78 (3.558–6.805) | 3.93 (1.348–6.138) | NS |
| IL-13 (pg/mL) | 5.43 (3.24–9.62) | 3.74 (0.56–6.315) | NS |
| IL-12p70 (pg/mL) | 4.63 (1.82–7.333) | 4.905 (0.655–14.18) | NS |
| IL-12p40 (pg/mL) | 15.61 (2.95–30.31) | 9.095 (2.95–27.9) | NS |
| IL-18 (pg/mL) | 576.2 (333.6–768.8) | 693.9 (475.7–1070) | NS |
| Immune cell subsets | Median (IQR) | Median (IQR) | |
| B Lymphocytes (cells/μL) | 4.24 (2.07–114.8) | 54.16 (6.928–107.8) | NS |
| Memory B cells (cells/μL) | 7.965 (0.345–17.99) | 7.955 (0.7975–22.25) | NS |
| Unswitched memory B cells (cells/μL) | 0.585 (0.0825–2.98) | 1.065 (0.0725–5.583) | NS |
| Switched memory B cells (cells/μL) | 5.975 (0.2625–16) | 5.235 (0.395–15.69) | NS |
| Antibody secreting cells (cells/μL) | 0.945 (0.0225–1.453) | 0.72 (0.0725–1.518) | NS |
| CD27- B cells (cells/μL) | 43.04 (1.643–73.63) | 44.47 (4.36–83.76) | NS |
| Transitional1 B cells (cells/μL) | 0.385 (0.0225–1.978) | 0.27 (0.0325–3.348) | NS |
| Transitional 2 B cells (cells/μL) | 0.335 (0.0075–1.723) | 1.07 (0.255–4.245) | NS |
| Mature B cells (cells/μL) | 26.52 (1.02–49.47) | 29.52 (2.873–61.65) | NS |
| Double negative B cells (cells/μL) | 8.04 (0.27–19.75) | 5.275 (0.6875–21.25) | NS |
| Double negative 1 B cells (cells/μL) | 1.24 (0.0375–5.235) | 1.87 (0.145–8.72) | NS |
| Double negative 2 B cells (cells/μL) | 0.07 (0–0.605) | 0.06 (0–0.81) | NS |
| Double negative 3 B cells (cells/μL) | 4.385 (0.0375–12.5) | 2.705 (0.4525–11.25) | NS |
| Double negative 4 B cells (cells/μL) | 0 (0–0.0225) | 0 (0–0.0175) | NS |
| IgD+ B cells (cells/μL) | 1.06 (0.1125–3.915) | 2.08 (0.14–4.135) | NS |
| IgD− B cells (cells/μL) | 3.74 (0.2025–10.61) | 3.065 (0.2975–5.968) | NS |
| Naïve B cells (cells/μL) | 16.89 (0.72–36.38) | 20.32 (1.505–41.36) | NS |
| Resting naïve B cells (cells/μL) | 16.6 (0.72–35.93) | 19.98 (1.505–41.13) | NS |
| Activated Naïve B cells (cells/μL) | 0.12 (0–0.44) | 0.17 (0.005–0.3725) | NS |
| T1+T2 B cells (cells/μL) | 1.885 (0.1125–5.72) | 1.535 (0.29–8.4) | NS |
| CD24+CD38lo- (cells/μL) | 6.365 (0.33–14.4) | 5.195 (0.765–9.09) | NS |
| CD4+ T cells (cells/μL) | 245.5 (139.8–577.2) | 274.8 (135.9–312.3) | NS |
| CD4+ regulatory T cells(cells/μL) | 132.7 (50.94–211.3) | 99.75 (64.01–201.4) | NS |
| CD4+ memory T cells(cells/μL) | 52.47 (26.9–82.91) | 68.11 (38.37–79.54) | NS |
| CD4+ central memory T cells(cells/μL) | 6.995 (1.66–11.68) | 4.14 (1.078–7.883) | NS |
| CD4+ Naïve T cells(cells/μL) | 193 (86.22–308.5) | 160.6 (73.43–216.4) | NS |
| CD8+ T cells (cells/μL) | 268.4 (93.48–401.2) | 175.3 (81.43–375) | NS |
| MFI of CD57 on CD8+ T cells (cells/μL) | 42,308 (11,669–58,972) | 55,609 (33,741–85,811) | NS |
| CD8+ memory T cells (cells/μL) | 32.38 (15.43–56.46) | 31.25 (19.13–52.1) | NS |
| CD8+ effector memory T cells (cells/μL) | 14.71 (8.048–33.88) | 18.98 (9.748–33.37) | NS |
| CD8+ central memory T cells (cells/μL) | 1.51 (0.55–5.755) | 2.115 (0.515–2.408) | NS |
| Naïve CD8+ T cells (cells/μL) | 196.4 (73.71–233.6) | 115.2 (61.23–267.3) | NS |
| Th1 cells (cells/μL) | 65.2 (23.7–165.9) | 109.1 (63.39–180.7) | NS |
| Th2 cells (cells/μL) | 10.26 (4.555–25.44) | 10.72 (3.608–21) | NS |
| Th17 cells (cells/μL) | 1.66 (0.3625–6.973) | 2.395 (0.8125–4.913) | NS |
| Tc1 cells (cells/μL) | 78.01 (25.47–266.8) | 143.8 (53.98–251.6) | NS |
| Tc2 cells (cells/μL) | 3.66 (2.265–9.193) | 3.76 (1.713–8.678) | NS |
| Tc17 cells (cells/μL) | 3.66 (2.265–9.193) | 3.76 (1.713–8.678) | NS |
| Classical monocytes (cells/μL) | 306.1 (251.4–400.5) | 338.2 (189.9–526.6) | NS |
| Intermediate monocytes (cells/μL) | 53.04 (19.75–127.6) | 60.13 (29.06–125.7) | NS |
| Non-classical monocytes (cells/μL) | 21.73 (12.77–46.74) | 37.9 (20.8–99.57) | NS |
| Immature low density granulocytes (%) | 0.09 (0.035–0.3025) | 0.12 (0.01–0.965) | NS |
| Immature low density granulocytes (cells/μL) | 0.055 (0.01–0.5775) | 0.26 (0–1.11) | NS |
| Mature low density granulocytes (%) | 0.55 (0.32–1.133) | 1.555 (0.5725–4.268) | <0.02 |
| Mature low density granulocytes (cells/μL) | 0.225 (0.0675–1.403) | 1.85 (0.17–13.43) | NS |
| NK cells (cells/μL) | 19.65 (10.35–29.88) | 20.35 (7.195–31.73) | NS |
| CD56high cells (cells/μL) | 7.725 (4.653–12.63) | 5 (2.25–8.573) | NS |
| CD56lo cells (cells/μL) | 92 (87–95.3) | 95 (91.4–97.75) | NS |
| Metabolites | Without PACS Median (IQR) | With PACS Median (IQR) | p |
|---|---|---|---|
| Pyruvate | 1.844 (0.6563–2.701) | 3.114 (2.193–5.362) | <0.02 |
| Glycolic acid | 0.4015 (0.368–0.5598) | 0.3815 (0.3462–0.5491) | NS |
| 2-keto-3-methylvaleric acid | 3.652 (3.191–4.3) | 3.528 (2.981–4.513) | NS |
| Alpha-hydroybutyric acid | 28.14 (18.52–48.82) | 42.28 (24.31–51.76) | NS |
| 3-hydroxybutyric acid | 8.195 (4.863–18.34) | 6.419 (4.195–10.72) | NS |
| Alpha-hydroxyisovaleric acid | 4.568 (3.899–8.757) | 6.99 (5.424–21.35) | NS |
| Beta-alanine | 1.724 (1.403–2.418) | 1.482 (1.272–2.127) | NS |
| 3-hydroxyisovaleric acid | 1.741 (1.413–2.201) | 1.967 (1.453–3.456) | NS |
| Valine 2TMS | 33.76 (30.54–37.76) | 34.87 (28.52–37.73) | NS |
| Leucine 2TMS | 18.87 (15.09–20.17) | 19.54 (15.33–20.74) | NS |
| Glycerol | 19.02 (12.98–29.34) | 15.59 (12.62–26.14) | NS |
| Isoleucine 2TMS | 58.57 (48.28–69.39) | 60.33 (36.31–80.24) | NS |
| Proline 2TMS | 24.04 (16.87–29.08) | 20.31 (15.51–25.4) | NS |
| Pipecolinic acid | 2.066 (1.595–2.792) | 1.152 (0.9851–2.096) | NS |
| Glyceric acid | 0.1139 (0.0891–0.1349) | 0.1008 (0.07092–0.1594) | NS |
| 2,3-dihydroxybutanoic acid | 1.194 (0.8146–2.5) | 0.8958 (0.6935–1.022) | NS |
| Serine 3TMS | 70.22 (64.25–79.05) | 71.28 (61.78–81.82) | NS |
| Threonine 3TMS | 54.97 (48.77–73.67) | 50.47 (43.86–75.71) | NS |
| 3,4-dihydroxybutanoic acid | 2.01 (1.692–2.523) | 2.921 (1.839–3.793) | NS |
| Malic acid | 2.98 (2.399–4.069) | 4.309 (2.325–5.804) | NS |
| Methionine 2TMS | 2.321 (2.097–2.515) | 2.557 (1.805–2.652) | NS |
| 5-oxoproline | 62.35 (54.03–65.8) | 51.65 (51–76.96) | NS |
| Cysteine 3TMS | 6.729 (5.233–8.265) | 8.384 (6.411–12.62) | NS |
| Threonic acid | 6.675 (3.999–8.895) | 8.187 (4.092–12.45) | NS |
| Alpha-ketoglutarate | 4.363 (3.34–5.842) | 5.434 (3.878–7.548) | NS |
| Ornithine | 3.672 (2.549–4.483) | 3.941 (3.299–6.685) | NS |
| Glutamic acid 3TMS | 43.78 (30.03–60.35) | 56.74 (40.73–71.63) | NS |
| Phenylalanine 2TMS | 40.87 (32.91–48.38) | 40.6 (37.75–45.18) | NS |
| Lysine 3TMS | 26.42 (17.57–36.95) | 32.54 (21.06–44.83) | NS |
| Glutamine 3TMS | 74.46 (66.08–85.97) | 69.38 (38.27–83.38) | NS |
| Azelaic acid | 8.829 (5.808–12.17) | 8.512 (3.258–11.68) | NS |
| hypoxanthine | 4.466 (3.74–9.376) | 4.081 (3.211–4.76) | NS |
| Ornithine | 15.91 (11.71–25.67) | 21.73 (13.56–23.67) | NS |
| Citric acid | 7.113 (4.643–9.788) | 4.943 (4.249–6.16) | NS |
| Myristic acid | 3.323 (2.426–4.9) | 2.853 (2.376–4.483) | NS |
| 1,5-anhydro-D-sorbitol | 62.49 (52.08–82.1) | 56.85 (29.94–93.65) | NS |
| Tyrosine 3TMS | 59.48 (51.92–76.6) | 60.75 (49.37–71.57) | NS |
| Palmitic acid | 68.83 (56.72–85.56) | 71.35 (62.74–90.55) | NS |
| Myo-inositol | 12.78 (10.59–17.97) | 14.37 (11.34–16.28) | NS |
| Heptadecanoic acid | 1.147 (0.9246–1.288) | 1.018 (0.6353–1.086) | NS |
| Oleic acid | 3.75 (2.69–6.138) | 4.872 (3.724–9.967) | NS |
| Stearic acid | 51.3 (46.13–56.93) | 51.46 (41.07–54.04) | NS |
| Cystine | 9.655 (3.803–14.16) | 19.13 (17.18–21.08) | <0.01 |
| Pseudouridine | 1.575 (1.44–1.929) | 1.729 (1.441–2.419) | <0.04 |
| Alpha-tocopherol | 4.663 (3.536–7.073) | 3.499 (2.674–3.674) | NS |
| Cholesterol | 25 (22.4–27.19) | 23.31 (18.9–26.24) | NS |
| Variable | OR | 95% CI | p Value |
|---|---|---|---|
| Body mass index | 1.15 | 1.00–1.35 | 0.04 |
| ALT | 0.99 | 0.98–0.99 | 0.01 |
| Pyruvate | 1.83 | 1.09–3.64 | 0.01 |
| Cystine | 1.15 | 1.02–1.36 | 0.01 |
| Alpha tocopherol | 0.59 | 0.30–0.98 | 0.04 |
| Albumine | 0.42 | 0.12–1.13 | 0.09 |
| MIP 1b | 1.02 | 0.99–1.06 | 0.09 |
| IL-8 | 1.00 | 0.99–1.01 | 0.09 |
| Pipecolinic acid | 0.30 | 0.65–1.04 | 0.058 |
| 2,3 dyhidroxybutanoic acid | 0.43 | 0.11–1.05 | 0.06 |
| Malic acid | 1.71 | 0.97–3.80 | 0.06 |
| Cysteine 3 TMS | 1.36 | 0.98–2.05 | 0.059 |
| Heptadecanoic acid | 0.05 | 0.001–1.40 | 0.08 |
| Absolute B lymphocytes TR2+ | 1.30 | 0.96–2.16 | 0.09 |
| NK lymphocytes CD56low | 1.15 | 0.97–1.41 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santana-de Anda, K.; Torres-Ruiz, J.; Mejía-Domínguez, N.R.; Alcalá-Carmona, B.; Maravillas-Montero, J.L.; Páez-Franco, J.C.; Vargas-Castro, A.S.; Lira-Luna, J.; Camacho-Morán, E.A.; Juarez-Vega, G.; et al. Novel Clinical, Immunological, and Metabolic Features Associated with Persistent Post-Acute COVID-19 Syndrome. Int. J. Mol. Sci. 2024, 25, 9661. https://doi.org/10.3390/ijms25179661
Santana-de Anda K, Torres-Ruiz J, Mejía-Domínguez NR, Alcalá-Carmona B, Maravillas-Montero JL, Páez-Franco JC, Vargas-Castro AS, Lira-Luna J, Camacho-Morán EA, Juarez-Vega G, et al. Novel Clinical, Immunological, and Metabolic Features Associated with Persistent Post-Acute COVID-19 Syndrome. International Journal of Molecular Sciences. 2024; 25(17):9661. https://doi.org/10.3390/ijms25179661
Chicago/Turabian StyleSantana-de Anda, Karina, Jiram Torres-Ruiz, Nancy R. Mejía-Domínguez, Beatriz Alcalá-Carmona, José L. Maravillas-Montero, José Carlos Páez-Franco, Ana Sofía Vargas-Castro, Jaquelin Lira-Luna, Emmanuel A. Camacho-Morán, Guillermo Juarez-Vega, and et al. 2024. "Novel Clinical, Immunological, and Metabolic Features Associated with Persistent Post-Acute COVID-19 Syndrome" International Journal of Molecular Sciences 25, no. 17: 9661. https://doi.org/10.3390/ijms25179661
APA StyleSantana-de Anda, K., Torres-Ruiz, J., Mejía-Domínguez, N. R., Alcalá-Carmona, B., Maravillas-Montero, J. L., Páez-Franco, J. C., Vargas-Castro, A. S., Lira-Luna, J., Camacho-Morán, E. A., Juarez-Vega, G., Meza-Sánchez, D., Núñez-Álvarez, C., Rull-Gabayet, M., & Gómez-Martín, D. (2024). Novel Clinical, Immunological, and Metabolic Features Associated with Persistent Post-Acute COVID-19 Syndrome. International Journal of Molecular Sciences, 25(17), 9661. https://doi.org/10.3390/ijms25179661







