Identification and Analysis of Aluminum-Activated Malate Transporter Gene Family Reveals Functional Diversification in Orchidaceae and the Expression Patterns of Dendrobium catenatum Aluminum-Activated Malate Transporters
Abstract
1. Introduction
2. Results
2.1. Identification and Characterization of the ALMT Gene Family in Orchidaceae
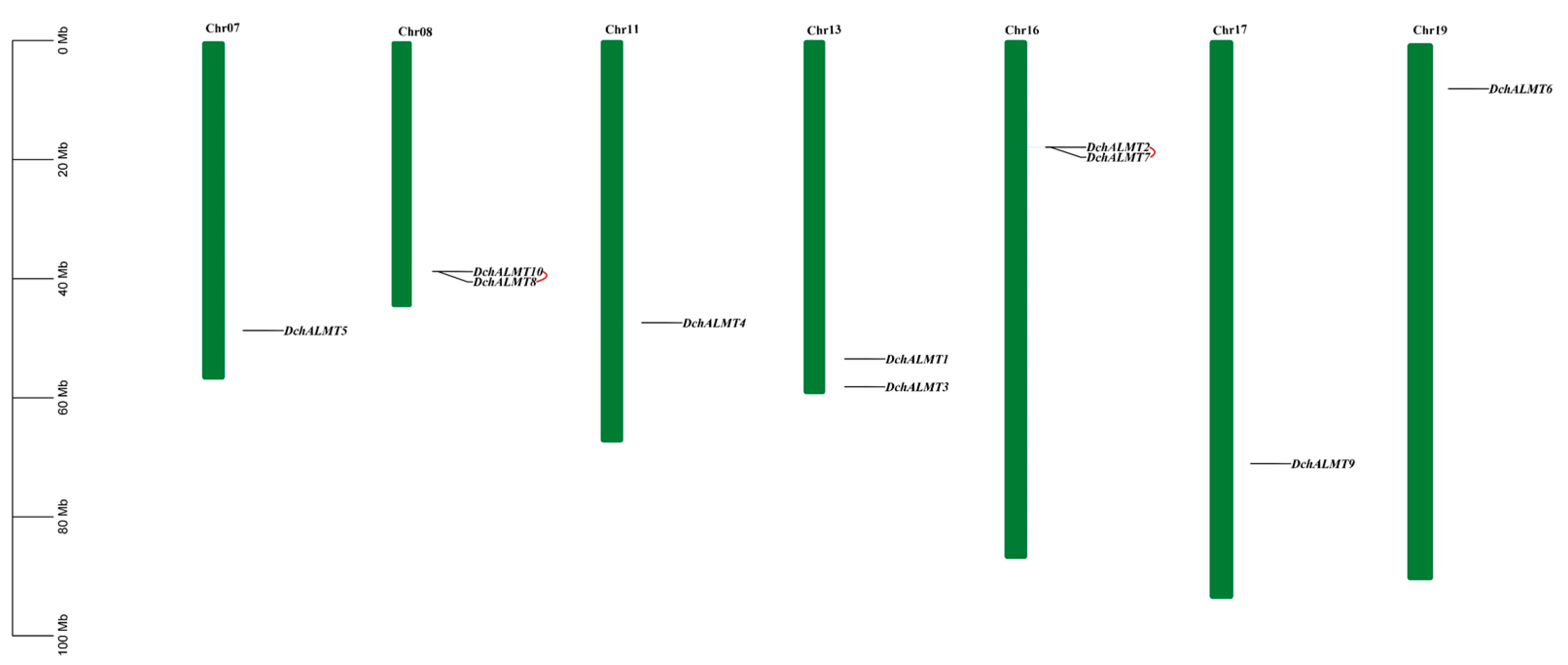
2.2. Chromosomal Localization of DchALMT Genes
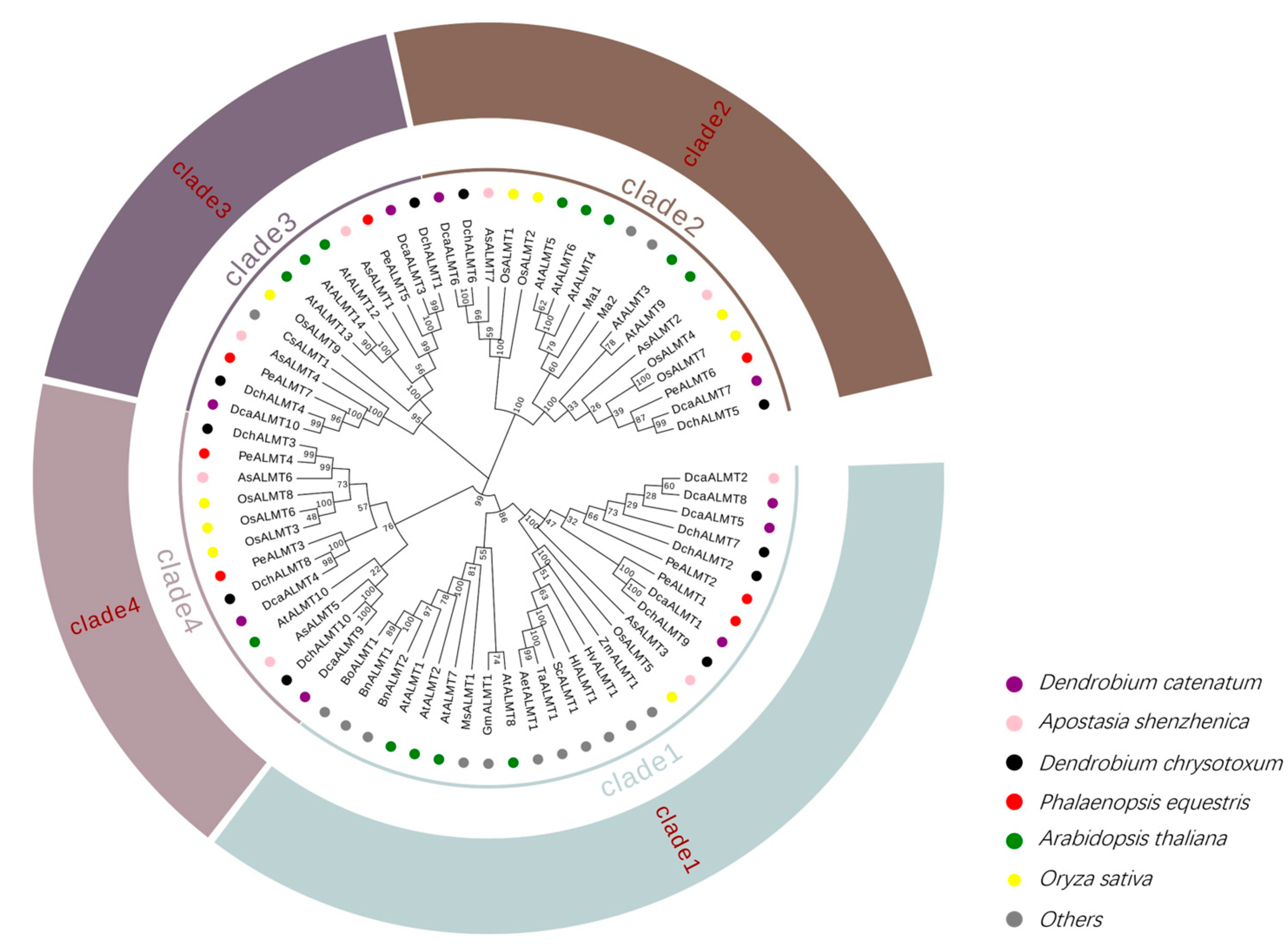
2.3. Multiple Sequence Alignment and Phylogenetic Analysis of ALMT Genes
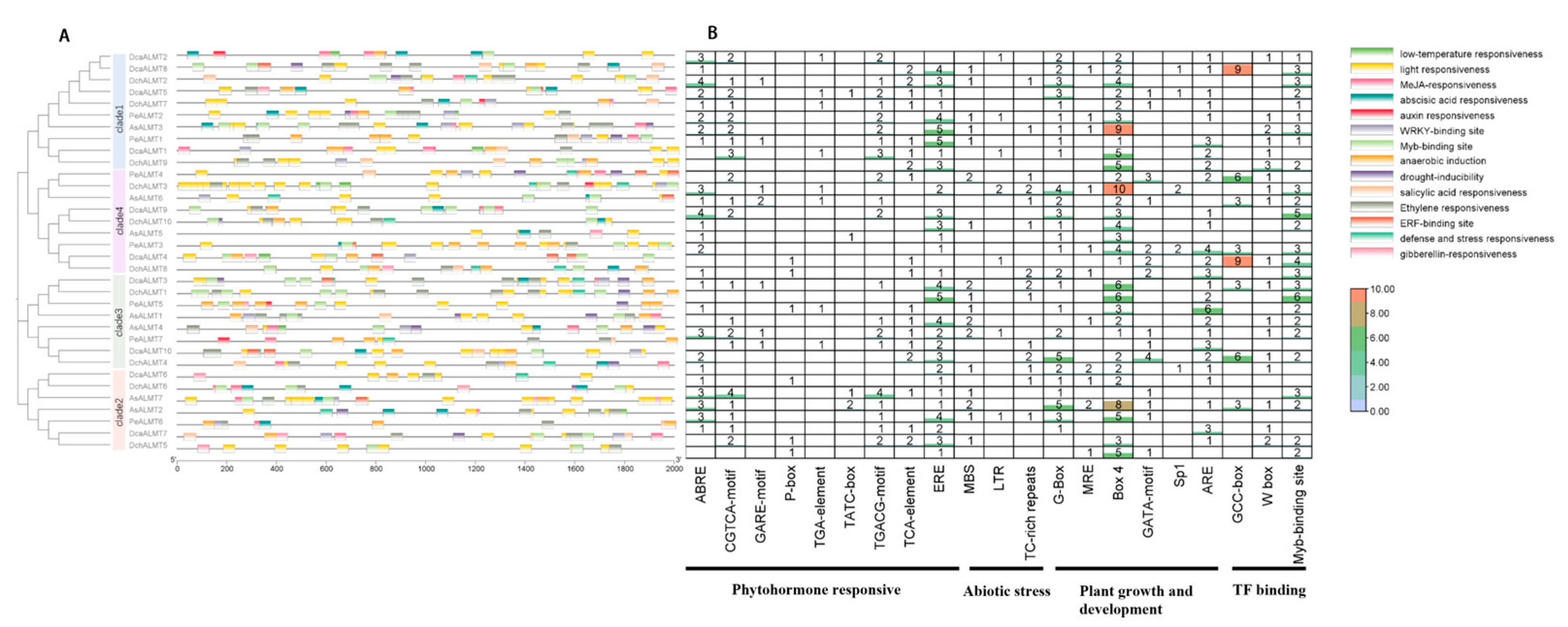
2.4. Gene Structure and Motif Analysis of ALMT Genes
2.5. Cis-Elements in the Promoter Regions of ALMT Genes
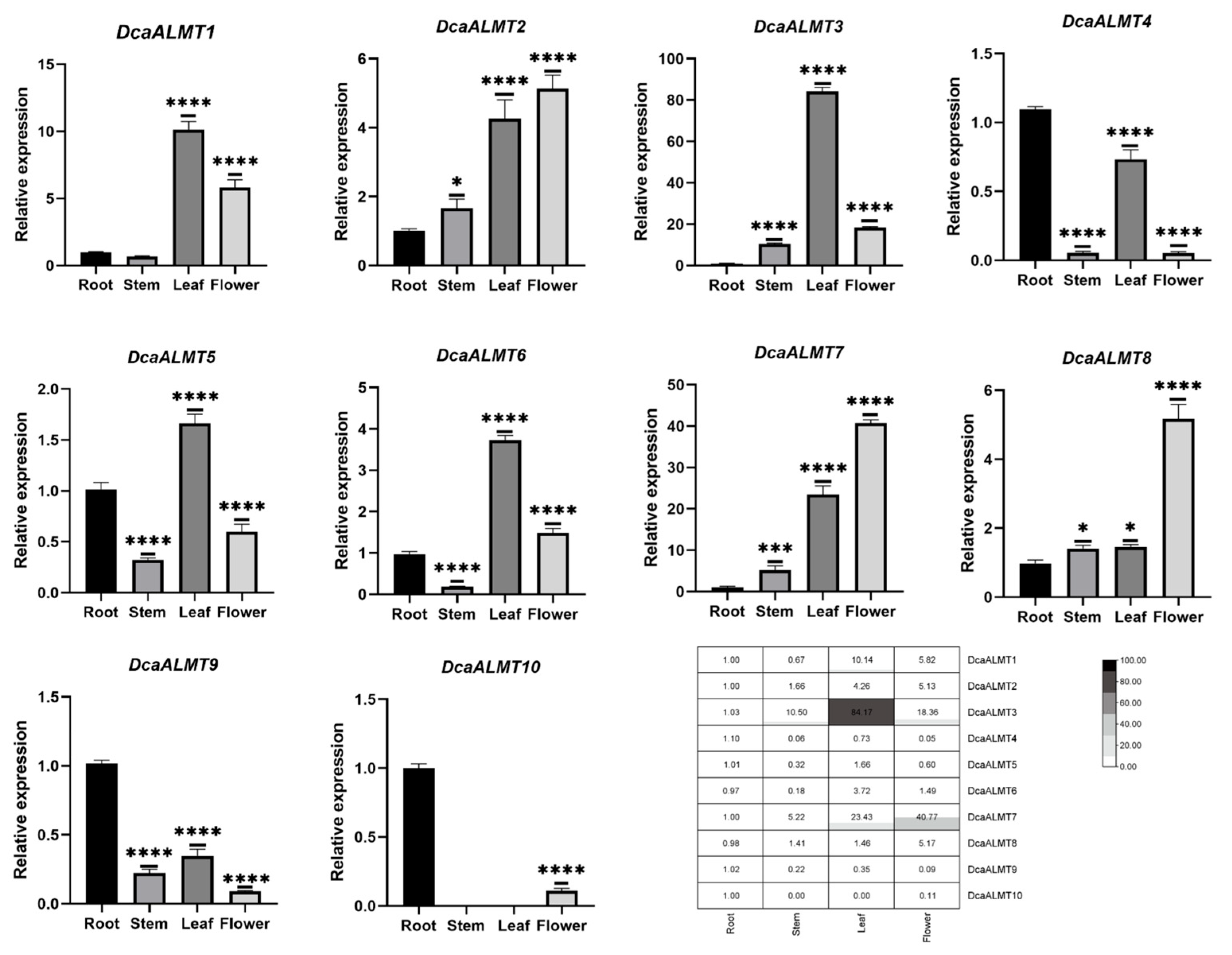
2.6. Tissue-Specific Expression of DcaALMT
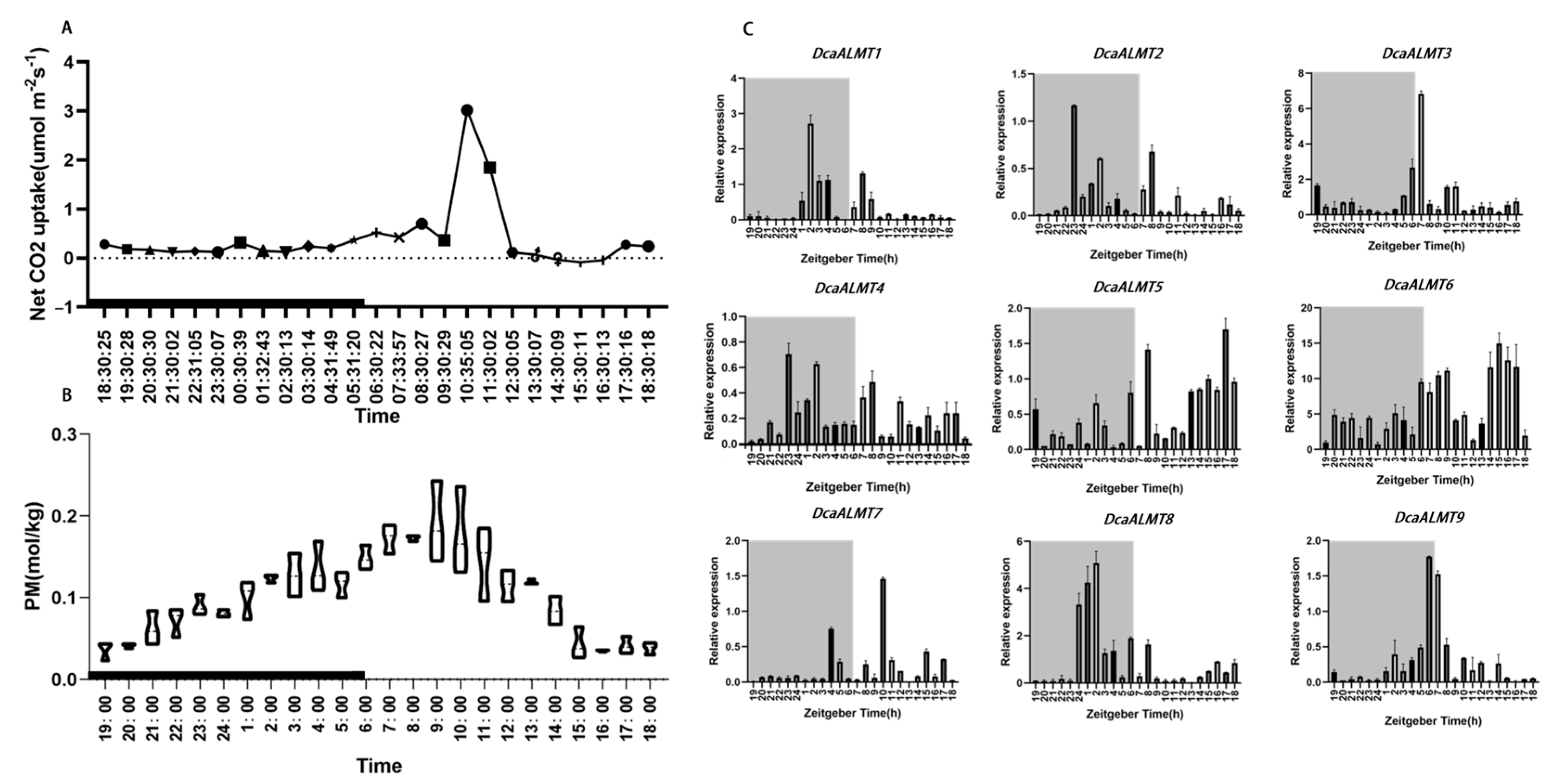
2.7. Diel Expression of DcaALMT
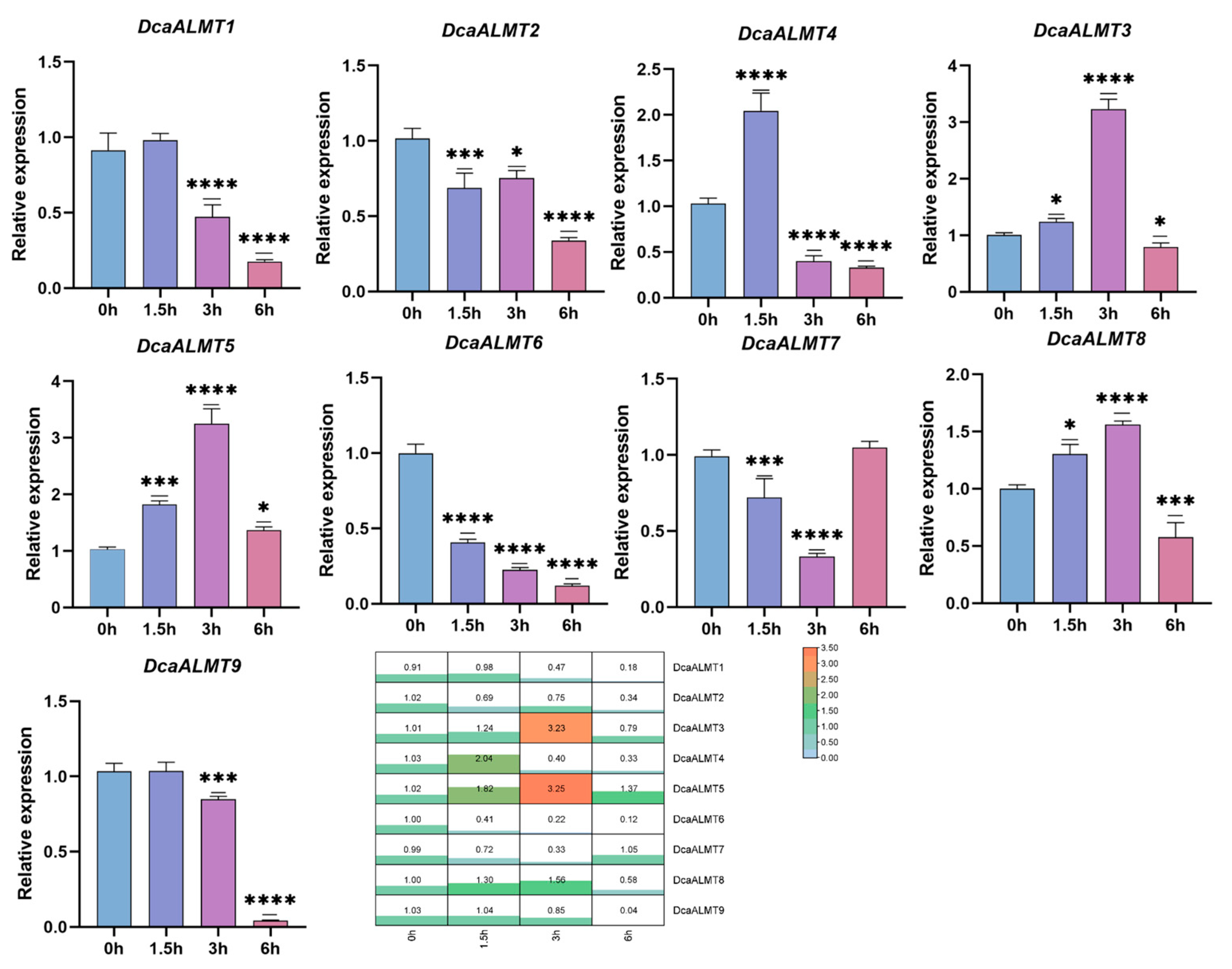
2.8. Expression of DcaALMT under ABA Treatment
3. Discussion
4. Materials and Methods
4.1. Identification and Bioinformatic Analysis of ALMT Genes in Orchid
4.2. Multiple Sequence Alignment and Evolutionary Tree Construction
4.3. Gene Structure and Motif of ALMT Genes
4.4. Prediction of ALMT Genes Cis-Acting Elements
4.5. Chromosome Localization and Collinearity Analysis
4.6. Plant Materials and Growth Conditions
4.7. Physiological Index
4.8. Total RNA Extraction and RT-qPCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Zhou, M. The ALMT Gene Family Performs Multiple Functions in Plants. Agronomy 2018, 8, 20. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Isayenkov, S.V. Recent Updates on ALMT Transporters’ Physiology, Regulation, and Molecular Evolution in Plants. Plants 2023, 12, 3167. [Google Scholar] [CrossRef]
- Medeiros, D.B.; Fernie, A.R.; Araújo, W.L. Discriminating the Function(s) of Guard Cell ALMT Channels. Trends Plant Sci. 2018, 23, 649–651. [Google Scholar] [CrossRef]
- Sharma, T.; Dreyer, I.; Kochian, L.; Piñeros, M.A. The ALMT Family of Organic Acid Transporters in Plants and Their Involvement in Detoxification and Nutrient Security. Front. Plant Sci. 2016, 7, 1488. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, I.; Gomez-Porras, J.L.; Riaño-Pachón, D.M.; Hedrich, R.; Geiger, D. Molecular Evolution of Slow and Quick Anion Channels (SLACs and QUACs/ALMTs). Front. Plant Sci. 2012, 3, 263. [Google Scholar] [CrossRef]
- Eisenach, C.; De Angeli, A. Ion Transport at the Vacuole during Stomatal Movements. Plant Physiol. 2017, 174, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Hoekenga, O.A.; Maron, L.G.; Piñeros, M.A.; Cançado, G.M.A.; Shaff, J.; Kobayashi, Y.; Ryan, P.R.; Dong, B.; Delhaize, E.; Sasaki, T.; et al. AtALMT1, Which Encodes a Malate Transporter, Is Identified as One of Several Genes Critical for Aluminum Tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 9738–9743. [Google Scholar] [CrossRef]
- Wang, J.; Yu, X.; Ding, Z.J.; Zhang, X.; Luo, Y.; Xu, X.; Xie, Y.; Li, X.; Yuan, T.; Zheng, S.J.; et al. Structural Basis of ALMT1-Mediated Aluminum Resistance in Arabidopsis. Cell Res. 2022, 32, 89–98. [Google Scholar] [CrossRef]
- Ribeiro, A.P.; Vinecky, F.; Duarte, K.E.; Santiago, T.R.; Das Chagas Noqueli Casari, R.A.; Hell, A.F.; Da Cunha, B.A.D.B.; Martins, P.K.; Da Cruz Centeno, D.; De Oliveira Molinari, P.A.; et al. Enhanced Aluminum Tolerance in Sugarcane: Evaluation of SbMATE Overexpression and Genome-Wide Identification of ALMTs in Saccharum spp. BMC Plant Biol. 2021, 21, 300. [Google Scholar] [CrossRef]
- Sasaki, T.; Yamamoto, Y.; Ezaki, B.; Katsuhara, M.; Ahn, S.J.; Ryan, P.R.; Delhaize, E.; Matsumoto, H. A Wheat Gene Encoding an Aluminum-Activated Malate Transporter. Plant J. 2004, 37, 645–653. [Google Scholar] [CrossRef] [PubMed]
- De Angeli, A.; Zhang, J.; Meyer, S.; Martinoia, E. AtALMT9 Is a Malate-Activated Vacuolar Chloride Channel Required for Stomatal Opening in Arabidopsis. Nat. Commun. 2013, 4, 1804. [Google Scholar] [CrossRef]
- Gruber, B.D.; Ryan, P.R.; Richardson, A.E.; Tyerman, S.D.; Ramesh, S.; Hebb, D.M.; Howitt, S.M.; Delhaize, E. HvALMT1 from Barley Is Involved in the Transport of Organic Anions. J. Exp. Bot. 2010, 61, 1455–1467. [Google Scholar] [CrossRef]
- Kovermann, P.; Meyer, S.; Hörtensteiner, S.; Picco, C.; Scholz-Starke, J.; Ravera, S.; Lee, Y.; Martinoia, E. The Arabidopsis Vacuolar Malate Channel Is a Member of the ALMT Family. Plant J. 2007, 52, 1169–1180. [Google Scholar] [CrossRef]
- Meyer, S.; Mumm, P.; Imes, D.; Endler, A.; Weder, B.; Al-Rasheid, K.A.S.; Geiger, D.; Marten, I.; Martinoia, E.; Hedrich, R. AtALMT12 Represents an R-type Anion Channel Required for Stomatal Movement in Arabidopsis Guard Cells. Plant J. 2010, 63, 1054–1062. [Google Scholar] [CrossRef]
- Meyer, S.; Scholz-Starke, J.; Angeli, A.D.; Kovermann, P.; Burla, B.; Gambale, F.; Martinoia, E. Malate Transport by the Vacuolar AtALMT6 Channel in Guard Cells Is Subject to Multiple Regulation. Plant J. 2011, 67, 247–257. [Google Scholar] [CrossRef]
- Piñeros, M.A.; Cançado, G.M.A.; Maron, L.G.; Lyi, S.M.; Menossi, M.; Kochian, L.V. Not All ALMT1-type Transporters Mediate Aluminum-activated Organic Acid Responses: The Case of ZmALMT1—An Anion-selective Transporter. Plant J. 2007, 53, 352–367. [Google Scholar] [CrossRef]
- Ligaba, A.; Maron, L.; Shaff, J.; Kochian, L.; Piñeros, M. Maize ZmALMT2 Is a Root Anion Transporter That Mediates Constitutive Root Malate Efflux. Plant Cell Environ. 2012, 35, 1185–1200. [Google Scholar] [CrossRef]
- De Angeli, A.; Baetz, U.; Francisco, R.; Zhang, J.; Chaves, M.M.; Regalado, A. The Vacuolar Channel VvALMT9 Mediates Malate and Tartrate Accumulation in Berries of Vitis Vinifera. Planta 2013, 238, 283–291. [Google Scholar] [CrossRef]
- Bai, Y.; Dougherty, L.; Li, M.; Fazio, G.; Cheng, L.; Xu, K. A Natural Mutation-Led Truncation in One of the Two Aluminum-Activated Malate Transporter-like Genes at the Ma Locus Is Associated with Low Fruit Acidity in Apple. Mol. Genet. Genom. 2012, 287, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.D.; Lee, S.; Choi, W.-G.; Yim, W.C.; Cushman, J.C. Laying the Foundation for Crassulacean Acid Metabolism (CAM) Biodesign: Expression of the C4 Metabolism Cycle Genes of CAM in Arabidopsis. Front. Plant Sci. 2019, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Hassan, M.M.; Liu, D.; Lim, S.D.; Yim, W.C.; Cushman, J.C.; Markel, K.; Shih, P.M.; Lu, H.; Weston, D.J.; et al. Biosystems Design to Accelerate C 3 -to-CAM Progression. BioDesign Res. 2020, 2020, 3686791. [Google Scholar] [CrossRef]
- Meyer, S.; De Angeli, A.; Fernie, A.R.; Martinoia, E. Intra- and Extra-Cellular Excretion of Carboxylates. Trends Plant Sci. 2010, 15, 40–47. [Google Scholar] [CrossRef]
- Liu, J. Biology and Function of the OsALMT1 Gene in Rice (Oryza sativa L.). Ph.D. Thesis, University of Tasmania, Hobart, Australia, 2016. [Google Scholar]
- Ma, B.; Yuan, Y.; Gao, M.; Qi, T.; Li, M.; Ma, F. Genome-Wide Identification, Molecular Evolution, and Expression Divergence of Aluminum-Activated Malate Transporters in Apples. Int. J. Mol. Sci. 2018, 19, 2807. [Google Scholar] [CrossRef]
- Peng, W.; Wu, W.; Peng, J.; Li, J.; Lin, Y.; Wang, Y.; Tian, J.; Sun, L.; Liang, C.; Liao, H. Characterization of the Soybean GmALMT Family Genes and the Function of GmALMT5 in Response to Phosphate Starvation. J. Integr. Plant Biol. 2018, 60, 216–231. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Xin, Y.; Wai, C.M.; Liu, J.; Ming, R. The Role of Cis-Elements in the Evolution of Crassulacean Acid Metabolism Photosynthesis. Hortic. Res. 2020, 7, 5. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Hoekenga, O.A.; Itoh, H.; Nakashima, M.; Saito, S.; Shaff, J.E.; Maron, L.G.; Piñeros, M.A.; Kochian, L.V.; Koyama, H. Characterization of AtALMT1 Expression in Aluminum-Inducible Malate Release and Its Role for Rhizotoxic Stress Tolerance in Arabidopsis. Plant Physiol. 2007, 145, 843–852. [Google Scholar] [CrossRef]
- Zhang, F.; Jiang, S.; Li, Q.; Song, Z.; Yang, Y.; Yu, S.; Nie, Z.; Chu, M.; An, Y. Identification of the ALMT Gene Family in the Potato (Solanum tuberosum L.) and Analysis of the Function of StALMT6/10 in Response to Aluminum Toxicity. Front. Plant Sci. 2023, 14, 1274260. [Google Scholar] [CrossRef]
- Sasaki, T.; Tsuchiya, Y.; Ariyoshi, M.; Nakano, R.; Ushijima, K.; Kubo, Y.; Mori, I.C.; Higashiizumi, E.; Galis, I.; Yamamoto, Y. Two Members of the Aluminum-Activated Malate Transporter Family, SlALMT4 and SlALMT5, Are Expressed during Fruit Development, and the Overexpression of SlALMT5 Alters Organic Acid Contents in Seeds in Tomato (Solanum lycopersicum). Plant Cell Physiol. 2016, 57, 2367–2379. [Google Scholar] [CrossRef]
- Sunagawa, H.; Cushman, J.; Agarie, S. Crassulacean Acid Metabolism May Alleviate Production of Reactive Oxygen Species in a Facultative CAM Plant, the Common Ice Plant Mesembryanthemum crystallinum L. Plant Prod. Sci. 2010, 13, 256–260. [Google Scholar] [CrossRef]
- Borland, A.M.; Griffiths, H.; Hartwell, J.; Smith, J.A.C. Exploiting the Potential of Plants with Crassulacean Acid Metabolism for Bioenergy Production on Marginal Lands. J. Exp. Bot. 2009, 60, 2879–2896. [Google Scholar] [CrossRef]
- Yang, X.; Hu, R.; Yin, H.; Jenkins, J.; Shu, S.; Tang, H.; Liu, D.; Weighill, D.A.; Cheol Yim, W.; Ha, J.; et al. The Kalanchoë Genome Provides Insights into Convergent Evolution and Building Blocks of Crassulacean Acid Metabolism. Nat. Commun. 2017, 8, 1899. [Google Scholar] [CrossRef]
- Silvera, K.; Neubig, K.M.; Whitten, W.M.; Williams, N.H.; Winter, K.; Cushman, J.C. Evolution along the Crassulacean Acid Metabolism Continuum. Funct. Plant Biol. 2010, 37, 995–1010. [Google Scholar] [CrossRef]
- Gamisch, A.; Winter, K.; Fischer, G.A.; Comes, H.P. Evolution of Crassulacean Acid Metabolism (CAM) as an Escape from Ecological Niche Conservatism in Malagasy Bulbophyllum (Orchidaceae). New Phytol. 2021, 231, 1236–1248. [Google Scholar] [CrossRef]
- Bolaños-Villegas, P.; Chen, F.-C. Advances and Perspectives for Polyploidy Breeding in Orchids. Plants 2022, 11, 1421. [Google Scholar] [CrossRef]
- Zhang, G.-Q.; Liu, K.-W.; Li, Z.; Lohaus, R.; Hsiao, Y.-Y.; Niu, S.-C.; Wang, J.-Y.; Lin, Y.-C.; Xu, Q.; Chen, L.-J.; et al. The Apostasia Genome and the Evolution of Orchids. Nature 2017, 549, 379–383. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, G.-Q.; Zhang, D.; Liu, X.-D.; Xu, X.-Y.; Sun, W.-H.; Yu, X.; Zhu, X.; Wang, Z.-W.; Zhao, X.; et al. Chromosome-Scale Assembly of the Dendrobium Chrysotoxum Genome Enhances the Understanding of Orchid Evolution. Hortic. Res. 2021, 8, 183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-Q.; Xu, Q.; Bian, C.; Tsai, W.-C.; Yeh, C.-M.; Liu, K.-W.; Yoshida, K.; Zhang, L.-S.; Chang, S.-B.; Chen, F.; et al. The Dendrobium Catenatum Lindl. Genome Sequence Provides Insights into Polysaccharide Synthase, Floral Development and Adaptive Evolution. Sci. Rep. 2016, 6, 19029. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Liu, X.; Vanneste, K.; Proost, S.; Tsai, W.-C.; Liu, K.-W.; Chen, L.-J.; He, Y.; Xu, Q.; Bian, C.; et al. The Genome Sequence of the Orchid Phalaenopsis Equestris. Nat. Genet. 2015, 47, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Tang, L.; Xu, J.; Zhang, X.; Zhu, Y.; Zhang, C.; Wang, M.; Liu, X.; Li, F.; Sun, F.; et al. Cryo-EM Structure and Electrophysiological Characterization of ALMT from Glycine Max Reveal a Previously Uncharacterized Class of Anion Channels. Sci. Adv. 2022, 8, eabm3238. [Google Scholar] [CrossRef]
- Xu, B.; Long, Y.; Feng, X.; Zhu, X.; Sai, N.; Chirkova, L.; Betts, A.; Herrmann, J.; Edwards, E.J.; Okamoto, M.; et al. GABA Signalling Modulates Stomatal Opening to Enhance Plant Water Use Efficiency and Drought Resilience. Nat. Commun. 2021, 12, 1952. [Google Scholar] [CrossRef]
- Din, I.; Ullah, I.; Wang, W.; Zhang, H.; Shi, L. Genome-Wide Analysis, Evolutionary History and Response of ALMT Family to Phosphate Starvation in Brassica napus. Int. J. Mol. Sci. 2021, 22, 4625. [Google Scholar] [CrossRef] [PubMed]
- Mumm, P.; Imes, D.; Martinoia, E.; Al-Rasheid, K.A.S.; Geiger, D.; Marten, I.; Hedrich, R. C-Terminus-Mediated Voltage Gating of Arabidopsis Guard Cell Anion Channel QUAC1. Mol. Plant 2013, 6, 1550–1563. [Google Scholar] [CrossRef]
- Ligaba, A.; Dreyer, I.; Margaryan, A.; Schneider, D.J.; Kochian, L.; Piñeros, M. Functional, Structural and Phylogenetic Analysis of Domains Underlying the A l Sensitivity of the Aluminum-activated Malate/Anion Transporter, T a ALMT 1. Plant J. 2013, 76, 766–780. [Google Scholar] [CrossRef] [PubMed]
- Domingos, P.; Dias, P.N.; Tavares, B.; Portes, M.T.; Wudick, M.M.; Konrad, K.R.; Gilliham, M.; Bicho, A.; Feijó, J.A. Molecular and Electrophysiological Characterization of Anion Transport in Arabidopsis thaliana Pollen Reveals Regulatory Roles for PH, Ca2+ and GABA. New Phytol. 2019, 223, 1353–1371. [Google Scholar] [CrossRef]
- Ramesh, S.A.; Kamran, M.; Sullivan, W.; Chirkova, L.; Okamoto, M.; Degryse, F.; McLaughlin, M.; Gilliham, M.; Tyerman, S.D. Aluminum-Activated Malate Transporters Can Facilitate GABA Transport. Plant Cell 2018, 30, 1147–1164. [Google Scholar] [CrossRef] [PubMed]
- Jo, B.-S.; Choi, S.S. Introns: The Functional Benefits of Introns in Genomes. Genom. Inform. 2015, 13, 112–118. [Google Scholar] [CrossRef]
- Fontecha, G.; Silva-Navas, J.; Benito, C.; Mestres, M.A.; Espino, F.J.; Hernández-Riquer, M.V.; Gallego, F.J. Candidate Gene Identification of an Aluminum-Activated Organic Acid Transporter Gene at the Alt4 Locus for Aluminum Tolerance in Rye (Secale cereale L.). Theor. Appl. Genet. 2006, 114, 249–260. [Google Scholar] [CrossRef]
- Ryan, P.R.; Raman, H.; Gupta, S.; Sasaki, T.; Yamamoto, Y.; Delhaize, E. The Multiple Origins of Aluminium Resistance in Hexaploid Wheat Include Aegilops Tauschii and More Recent Cis Mutations to TaALMT1: Evolution of Aluminium Resistance in Wheat. Plant J. 2010, 64, 446–455. [Google Scholar] [CrossRef]
- Linlin, X.; Xin, Q.; Mingyue, Z.; Shaoling, Z. Genome-Wide Analysis of Aluminum-Activated Malate Transporter Family Genes in Six Rosaceae Species, and Expression Analysis and Functional Characterization on Malate Accumulation in Chinese White Pear. Plant Sci. 2018, 274, 451–465. [Google Scholar] [CrossRef]
- Liang, C.; Piñeros, M.A.; Tian, J.; Yao, Z.; Sun, L.; Liu, J.; Shaff, J.; Coluccio, A.; Kochian, L.V.; Liao, H. Low pH, Aluminum, and Phosphorus Coordinately Regulate Malate Exudation through GmALMT1 to Improve Soybean Adaptation to Acid Soils. Plant Physiol. 2013, 161, 1347–1361. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, X.D.; Wang, S.S.; Wang, Q.F.; Wang, G.Q.; Nian, H.J.; Li, K.Z.; Yu, Y.X.; Chen, L.M. Transcriptional and Physiological Changes of Alfalfa in Response to Aluminium Stress. J. Agric. Sci. 2011, 149, 737–751. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, W.; Zhang, L.; Li, D.-Z.; Qi, J.; Ma, H. Phylogenomic Profiles of Whole-Genome Duplications in Poaceae and Landscape of Differential Duplicate Retention and Losses among Major Poaceae Lineages. Nat. Commun. 2024, 15, 3305. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, J.; He, J.; Liu, C.; Yi, W.; Xie, J.; Wu, Y.; Xie, T.; Ma, J.; Zhong, Z.; et al. AcMYB266, a Key Regulator of the Red Coloration in Pineapple Peel: A Case of Subfunctionalization in Tandem Duplicated Genes. Hortic. Res. 2024, 11, uhae116. [Google Scholar] [CrossRef]
- Xun, H.; Zhang, Z.; Zhou, Y.; Qian, X.; Dong, Y.; Feng, X.; Pang, J.; Wang, S.; Liu, B. Identification and Functional Characterization of R3 MYB Transcription Factor Genes in Soybean. J. Plant Biol. 2018, 61, 85–96. [Google Scholar] [CrossRef]
- Ma, X.; An, F.; Wang, L.; Guo, D.; Xie, G.; Liu, Z. Genome-Wide Identification of Aluminum-Activated Malate Transporter (ALMT) Gene Family in Rubber Trees (Hevea brasiliensis) Highlights Their Involvement in Aluminum Detoxification. Forests 2020, 11, 142. [Google Scholar] [CrossRef]
- Zhang, J.; Baetz, U.; Krügel, U.; Martinoia, E.; De Angeli, A. Identification of a Probable Pore-Forming Domain in the Multimeric Vacuolar Anion Channel AtALMT9. Plant Physiol. 2013, 163, 830–843. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Dougherty, L.; Coluccio, A.E.; Meng, D.; El-Sharkawy, I.; Borejsza-Wysocka, E.; Liang, D.; Piñeros, M.A.; Xu, K.; Cheng, L. Apple ALMT9 Requires a Conserved C-Terminal Domain for Malate Transport Underlying Fruit Acidity. Plant Physiol. 2020, 182, 992–1006. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Zhang, X.; Ramesh, S.; Gilliham, M.; Tyerman, S.D.; Zhang, W.-H. Ethylene Negatively Regulates Aluminium-Induced Malate Efflux from Wheat Roots and Tobacco Cells Transformed with TaALMT1. J. Exp. Bot. 2014, 65, 2415–2426. [Google Scholar] [CrossRef]
- Zheng, L.; Ma, W.; Liu, P.; Song, S.; Wang, L.; Yang, W.; Ren, H.; Wei, X.; Zhu, L.; Peng, J.; et al. Transcriptional Factor MdESE3 Controls Fruit Acidity by Activating Genes Regulating Malic Acid Content in Apple. Plant Physiol. 2024, 196, kiae282. [Google Scholar] [CrossRef]
- Eisenach, C.; Baetz, U.; Huck, N.V.; Zhang, J.; De Angeli, A.; Beckers, G.J.M.; Martinoia, E. ABA-Induced Stomatal Closure Involves ALMT4, a Phosphorylation-Dependent Vacuolar Anion Channel of Arabidopsis. Plant Cell 2017, 29, 2552–2569. [Google Scholar] [CrossRef]
- Hu, D.; Li, Y.; Zhang, Q.; Li, M.; Sun, C.; Yu, J.; Hao, Y. The R2R3-MYB Transcription Factor Md MYB 73 Is Involved in Malate Accumulation and Vacuolar Acidification in Apple. Plant J. 2017, 91, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.N.; Griffiths, H. Competing Carboxylases: Circadian and Metabolic Regulation of Rubisco in C3 and CAM Mesembryanthemum crystallinum L. Plant Cell Environ. 2012, 35, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Armarego-Marriott, T. Stop the Clock: Optimized Carbon Fixation and Circadian Rhythm in a CAM Plant. Plant Cell 2017, 29, 2314–2315. [Google Scholar] [CrossRef]
- Merilo, E.; Yarmolinsky, D.; Jalakas, P.; Parik, H.; Tulva, I.; Rasulov, B.; Kilk, K.; Kollist, H. Stomatal VPD Response: There Is More to the Story Than ABA. Plant Physiol. 2018, 176, 851–864. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Chen, G.; Wang, Y.; Huang, Y.; Marchant, D.B.; Wang, Y.; Yang, Q.; Dai, F.; Hills, A.; Franks, P.J.; et al. Evolutionary Conservation of ABA Signaling for Stomatal Closure. Plant Physiol. 2017, 174, 732–747. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Song, Y.; Gong, Z. Integrative Regulatory Mechanisms of Stomatal Movements under Changing Climate. J. Integr. Plant Biol. 2024, 66, 368–393. [Google Scholar] [CrossRef]
- Geiger, D.; Scherzer, S.; Mumm, P.; Stange, A.; Marten, I.; Bauer, H.; Ache, P.; Matschi, S.; Liese, A.; Al-Rasheid, K.A.S.; et al. Activity of Guard Cell Anion Channel SLAC1 Is Controlled by Drought-Stress Signaling Kinase-Phosphatase Pair. Proc. Natl. Acad. Sci. USA 2009, 106, 21425–21430. [Google Scholar] [CrossRef]
- Malcheska, F.; Ahmad, A.; Batool, S.; Müller, H.M.; Ludwig-Müller, J.; Kreuzwieser, J.; Randewig, D.; Hänsch, R.; Mendel, R.R.; Hell, R.; et al. Drought-Enhanced Xylem Sap Sulfate Closes Stomata by Affecting ALMT12 and Guard Cell ABA Synthesis. Plant Physiol. 2017, 174, 798–814. [Google Scholar] [CrossRef]
- Ward’, J.M. Calcium-Activated K+ Channels and Calcium-Lnduced Calcium Release by Slow Vacuolar Lon Channels in Guard Cell Vacuoles Lmplicated in the Control of Stomatal Closure. Plant Cell 1994, 6, 669–683. [Google Scholar] [CrossRef]
- Xu, B.; Sai, N.; Gilliham, M. The Emerging Role of GABA as a Transport Regulator and Physiological Signal. Plant Physiol. 2021, 187, 2005–2016. [Google Scholar] [CrossRef]
- Han, Y.; Wang, W.; Sun, J.; Ding, M.; Zhao, R.; Deng, S.; Wang, F.; Hu, Y.; Wang, Y.; Lu, Y.; et al. Populus Eu-phratica XTH Overexpression Enhances Salinity Tolerance by the Development of Leaf Succulence in Transgenic Tobacco Plants. J. Exp. Bot. 2013, 64, 4225–4238. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Troshin, P.V.; Procter, J.B.; Barton, G.J. Java Bioinformatics Analysis Web Services for Multiple Sequence Alignment—JABAWS: MSA. Bioinformatics 2011, 27, 2001–2002. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, H.; Gao, S.; Lercher, M.J.; Chen, W.-H.; Hu, S. Evolview v2: An Online Visualization and Management Tool for Customized and Annotated Phylogenetic Trees. Nucleic Acids Res. 2016, 44, W236–W241. [Google Scholar] [CrossRef]
- Lescot, M. PlantCARE, a Database of Plant Cis-Acting Regulatory Elements and a Portal to Tools for in Silico Analysis of Promoter Sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A Toolkit for Detection and Evolutionary Analysis of Gene Synteny and Collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Zhang, Z.; Zhu, J.; Yu, J. KaKs_Calculator 2.0: A Toolkit Incorporating Gamma-Series Methods and Sliding Window Strategies. Genom. Proteom. Bioinform. 2010, 8, 77–80. [Google Scholar] [CrossRef]


| Species | Name | Gene ID | AA(aa) | MW (Da) | pI | II | AI | GRAVY | TMD | Localization |
|---|---|---|---|---|---|---|---|---|---|---|
| D. catenatum | DcaALMT1 | Dca007047 | 463 | 50,348.46 | 9.02 | 38.15 | 99.33 | 0.179 | 6 | PM |
| DcaALMT2 | Dca006647 | 482 | 52,841.68 | 8.42 | 37 | 101.2 | 0.235 | 6 | PM | |
| DcaALMT3 | Dca024716 | 527 | 59,656.09 | 8.85 | 33.3 | 96.76 | 0.04 | 6 | PM | |
| DcaALMT4 | Dca000405 | 486 | 54,431.84 | 5.77 | 40.58 | 102.94 | 0.019 | 6 | PM | |
| DcaALMT5 | Dca006649 | 287 | 49,094.16 | 8.6 | 31.2 | 97.58 | 0.236 | 5 | PM | |
| DcaALMT6 | DcaN03602 | 527 | 59,414.26 | 6.3 | 36.79 | 90.34 | 0.086 | 5 | Vacuole | |
| DcaALMT7 | Dca000739 | 549 | 61,017.74 | 6.23 | 38.94 | 103.33 | 0.16 | 6 | ER | |
| DcaALMT8 | Dca006648 | 463 | 50,712.19 | 8.9 | 33.78 | 99.05 | 0.19 | 5 | PM | |
| DcaALMT9 | Dca000404 | 435 | 48,375.72 | 8.99 | 32.78 | 106.92 | 0.254 | 7 | PM | |
| DcaALMT10 | Dca013272 | 441 | 48,376.82 | 8.45 | 34.65 | 114.72 | 0.329 | 5 | PM | |
| D. chrysotoxum | DchALMT1 | Maker66807 | 527 | 59,567.04 | 8.85 | 35.88 | 96.56 | −0.04 | 6 | PM |
| DchALMT2 | Maker53533 | 445 | 48,443.5 | 8.44 | 37.5 | 98.27 | 0.226 | 6 | PM | |
| DchALMT3 | Maker67094 | 463 | 50,479.82 | 6.76 | 44.6 | 94.16 | 0.199 | 6 | PM | |
| DchALMT4 | Maker95167 | 395 | 42,849.9 | 9.39 | 42.92 | 100.23 | 0.145 | 6 | PM | |
| DchALMT5 | Maker80236 | 368 | 40,776.73 | 8.34 | 25 | 103.32 | 0.375 | 6 | PM | |
| DchALMT6 | Maker66167 | 528 | 59,246.11 | 6.26 | 37.56 | 89.03 | −0.093 | 7 | ER | |
| DchALMT7 | Maker53596 | 887 | 96,227.92 | 8.47 | 33.41 | 97.63 | 0.23 | 12 | Extracellular | |
| DchALMT8 | Maker58155 | 493 | 54,475.68 | 5.7 | 39.93 | 98.56 | 0.015 | 5 | PM | |
| DchALMT9 | Maker39596 | 454 | 49,362.17 | 8.84 | 40.56 | 96.58 | 0.114 | 6 | PM | |
| DchALMT10 | Maker58172 | 433 | 48,090.46 | 9.2 | 32.62 | 109 | 0.242 | 5 | PM | |
| P. equestris | PeALMT1 | XM_020717192.1 | 455 | 49,782.37 | 7.63 | 44.22 | 93.37 | 0.072 | 6 | PM |
| PeALMT2 | XM_020720250.1 | 456 | 49,218.33 | 9.13 | 35.64 | 100.81 | 0.238 | 6 | PM | |
| PeALMT3 | XM_020726555.1 | 479 | 52,949.23 | 6.12 | 45.14 | 98.35 | 0.04 | 6 | PM | |
| PeALMT4 | XM_020728361.1 | 466 | 50,732.38 | 8.23 | 36.38 | 96.9 | 0.231 | 7 | PM | |
| PeALMT5 | XM_020727176.1 | 523 | 59,234.72 | 8.9 | 33.83 | 95.63 | −0.046 | 6 | PM | |
| PeALMT6 | XM_020730006.1 | 590 | 65,819.3 | 6.42 | 36.63 | 100.15 | 0.069 | 6 | ER | |
| PeALMT7 | XM_020743275.1 | 282 | 30,544.02 | 8.82 | 26.07 | 123.81 | 0.533 | 6 | PM | |
| A. shenzhenica | AsALMT1 | Ash009758 | 513 | 58,212.41 | 8.64 | 33.54 | 97.29 | −0.083 | 6 | PM |
| AsALMT2 | Ash018925 | 532 | 59,665.42 | 6.37 | 48.49 | 93.07 | −0.147 | 4 | ER | |
| AsALMT3 | Ash011418 | 447 | 48,104.86 | 8.09 | 41.7 | 101.79 | 0.233 | 6 | PM | |
| AsALMT4 | Ash007723 | 253 | 27,270.69 | 8.24 | 37.28 | 98.73 | 0.32 | 5 | PM | |
| AsALMT5 | Ash020233 | 431 | 48,267.47 | 9.12 | 37.49 | 105.23 | 0.169 | 7 | PM | |
| AsALMT6 | Ash011252 | 457 | 49,292.44 | 8.37 | 33.41 | 102.98 | 0.256 | 6 | PM | |
| AsALMT7 | Ash005263 | 520 | 58,377.16 | 6.52 | 40.54 | 91.93 | −0.056 | 6 | ER |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, F.-C.; Yuan, M.; Zhou, L.; Zheng, B.-Q.; Wang, Y. Identification and Analysis of Aluminum-Activated Malate Transporter Gene Family Reveals Functional Diversification in Orchidaceae and the Expression Patterns of Dendrobium catenatum Aluminum-Activated Malate Transporters. Int. J. Mol. Sci. 2024, 25, 9662. https://doi.org/10.3390/ijms25179662
Peng F-C, Yuan M, Zhou L, Zheng B-Q, Wang Y. Identification and Analysis of Aluminum-Activated Malate Transporter Gene Family Reveals Functional Diversification in Orchidaceae and the Expression Patterns of Dendrobium catenatum Aluminum-Activated Malate Transporters. International Journal of Molecular Sciences. 2024; 25(17):9662. https://doi.org/10.3390/ijms25179662
Chicago/Turabian StylePeng, Fu-Cheng, Meng Yuan, Lin Zhou, Bao-Qiang Zheng, and Yan Wang. 2024. "Identification and Analysis of Aluminum-Activated Malate Transporter Gene Family Reveals Functional Diversification in Orchidaceae and the Expression Patterns of Dendrobium catenatum Aluminum-Activated Malate Transporters" International Journal of Molecular Sciences 25, no. 17: 9662. https://doi.org/10.3390/ijms25179662
APA StylePeng, F.-C., Yuan, M., Zhou, L., Zheng, B.-Q., & Wang, Y. (2024). Identification and Analysis of Aluminum-Activated Malate Transporter Gene Family Reveals Functional Diversification in Orchidaceae and the Expression Patterns of Dendrobium catenatum Aluminum-Activated Malate Transporters. International Journal of Molecular Sciences, 25(17), 9662. https://doi.org/10.3390/ijms25179662





