Sex Differences in Expression of Pro-Inflammatory Markers and miRNAs in a Mouse Model of CVB3 Myocarditis
Abstract
:1. Introduction
2. Results
2.1. CVB3-Infected Mice Show Cardiac Hypertrophy, Increased Immune Infiltrates and Fibrosis in Myocardial Tissue
2.2. Sex-Dependent Downregulation of ERK Activity in the Mouse Model of CVB3-Induced Chronic Myocarditis
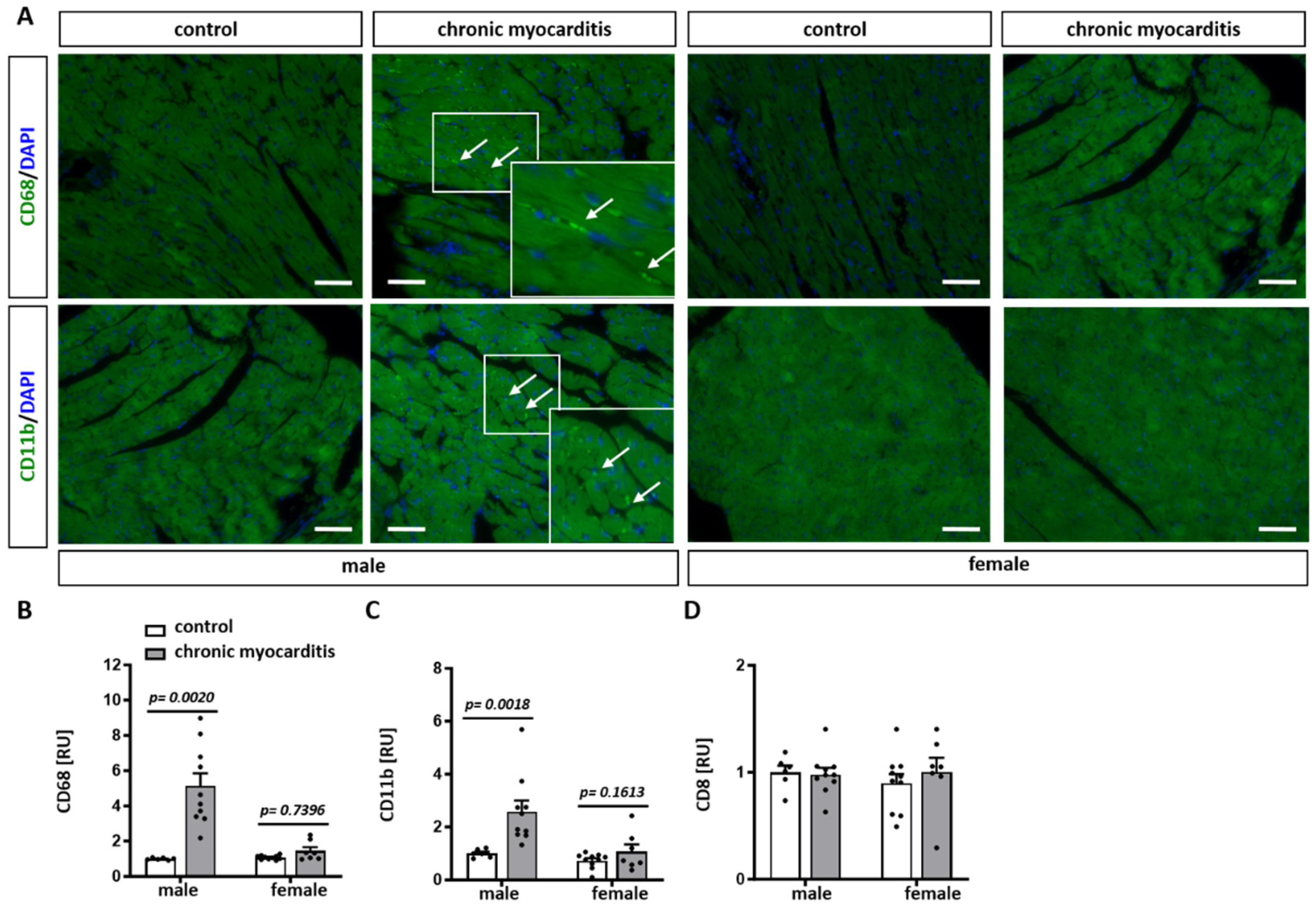
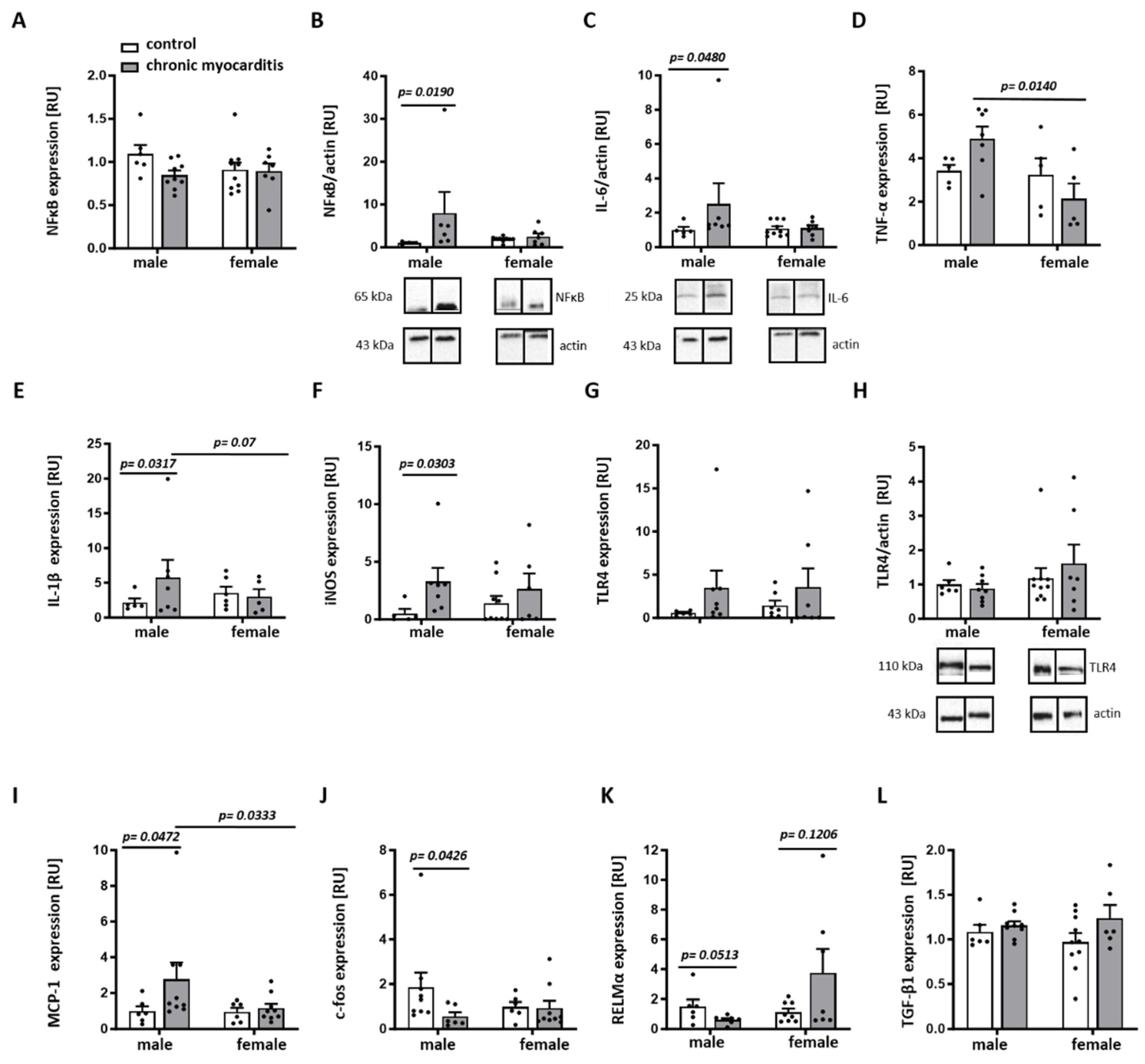
2.3. Sex Differences in the Expression of Pro-Inflammatory Mediators in the Mouse Model of CVB3-Induced Chronic Myocarditis
2.4. Sex Differences in the Inflammatory- and Remodeling-Related miRNA Expression in Mice with CVB3-Induced Chronic Myocarditis
3. Discussion
Limitations of the Study
4. Materials and Methods
4.1. Animals
4.2. Analysis of Heart Weight to Body Weight Ratio
4.3. Analysis of Collagen Content in LV Cardiac Tissue
4.4. Analysis of Immune Cell Infiltrates in LV Cardiac Tissue
4.5. RNA Extraction and Quantitative Real-Time PCR
4.6. Protein Extraction and Immunoblotting
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CVB3 | coxsackievirus B3 |
| E2 | 17β-estradiol |
| ERK | extracellular signal-regulated kinases |
| GM-CSF | granulocyte macrophage colony-stimulating factor |
| MAPK | mitogen-activated protein kinase |
| MCP-1 | monocyte chemotactic protein-1 |
| M-CSF | macrophage colony-stimulating factor |
| miRNA | microRNA |
| NFκB | nuclear factor kappa-light-chain-enhancer of activated B-cells |
| OPN | osteopontin |
| TLR4 | toll-like receptor 4 |
References
- Olejniczak, M.; Schwartz, M.; Webber, E.; Shaffer, A.; Perry, T.E. Viral Myocarditis-Incidence, Diagnosis and Management. J. Cardiothorac. Vasc. Anesth. 2020, 34, 1591–1601. [Google Scholar] [CrossRef]
- Pollack, A.; Kontorovich, A.R.; Fuster, V.; Dec, G.W. Viral myocarditis--diagnosis, treatment options, and current controversies. Nat. Rev. Cardiol. 2015, 12, 670–680. [Google Scholar] [CrossRef]
- Fairweather, D.; Rose, N.R. Coxsackievirus-induced myocarditis in mice: A model of autoimmune disease for studying immunotoxicity. Methods 2007, 41, 118–122. [Google Scholar] [CrossRef]
- Kraft, L.; Erdenesukh, T.; Sauter, M.; Tschope, C.; Klingel, K. Blocking the IL-1beta signalling pathway prevents chronic viral myocarditis and cardiac remodeling. Basic. Res. Cardiol. 2019, 114, 11. [Google Scholar] [CrossRef]
- Afanasyeva, M.; Georgakopoulos, D.; Belardi, D.F.; Bedja, D.; Fairweather, D.; Wang, Y.; Kaya, Z.; Gabrielson, K.L.; Rodriguez, E.R.; Caturegli, P.; et al. Impaired up-regulation of CD25 on CD4+ T cells in IFN-gamma knockout mice is associated with progression of myocarditis to heart failure. Proc. Natl. Acad. Sci. USA 2005, 102, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Weinzierl, A.O.; Szalay, G.; Wolburg, H.; Sauter, M.; Rammensee, H.G.; Kandolf, R.; Stevanovic, S.; Klingel, K. Effective chemokine secretion by dendritic cells and expansion of cross-presenting CD4−/CD8+ dendritic cells define a protective phenotype in the mouse model of coxsackievirus myocarditis. J. Virol. 2008, 82, 8149–8160. [Google Scholar] [CrossRef] [PubMed]
- Fung, G.; Luo, H.; Qiu, Y.; Yang, D.; McManus, B. Myocarditis. Circ. Res. 2016, 118, 496–514. [Google Scholar] [CrossRef] [PubMed]
- Coronado, M.J.; Bruno, K.A.; Blauwet, L.A.; Tschope, C.; Cunningham, M.W.; Pankuweit, S.; van Linthout, S.; Jeon, E.S.; McNamara, D.M.; Krejci, J.; et al. Elevated Sera sST2 Is Associated With Heart Failure in Men </= 50 Years Old With Myocarditis. J. Am. Heart Assoc. 2019, 8, e008968. [Google Scholar] [CrossRef]
- Iorga, A.; Cunningham, C.M.; Moazeni, S.; Ruffenach, G.; Umar, S.; Eghbali, M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol. Sex. Differ. 2017, 8, 33. [Google Scholar] [CrossRef]
- Frisancho-Kiss, S.; Nyland, J.F.; Davis, S.E.; Frisancho, J.A.; Barrett, M.A.; Rose, N.R.; Fairweather, D. Sex differences in coxsackievirus B3-induced myocarditis: IL-12Rbeta1 signaling and IFN-gamma increase inflammation in males independent from STAT4. Brain Res. 2006, 1126, 139–147. [Google Scholar] [CrossRef]
- Li, K.; Xu, W.; Guo, Q.; Jiang, Z.; Wang, P.; Yue, Y.; Xiong, S. Differential macrophage polarization in male and female BALB/c mice infected with coxsackievirus B3 defines susceptibility to viral myocarditis. Circ. Res. 2009, 105, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Lasrado, N.; Reddy, J. An overview of the immune mechanisms of viral myocarditis. Rev. Med. Virol. 2020, 30, 1–14. [Google Scholar] [CrossRef]
- Kovats, S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015, 294, 63–69. [Google Scholar] [CrossRef]
- Kumar, R.S.; Goyal, N. Estrogens as regulator of hematopoietic stem cell, immune cells and bone biology. Life Sci. 2021, 269, 119091. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Han, B. Dysregulated CD4+ T Cells and microRNAs in Myocarditis. Front. Immunol. 2020, 11, 539. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.S.; Jin, J.P.; Wang, J.Q.; Zhang, Z.G.; Freedman, J.H.; Zheng, Y.; Cai, L. miRNAS in cardiovascular diseases: Potential biomarkers, therapeutic targets and challenges. Acta Pharmacol. Sin. 2018, 39, 1073–1084. [Google Scholar] [CrossRef]
- Cihakova, D.; Rose, N.R. Pathogenesis of myocarditis and dilated cardiomyopathy. Adv. Immunol. 2008, 99, 95–114. [Google Scholar] [CrossRef]
- van den Hoogen, P.; van den Akker, F.; Deddens, J.C.; Sluijter, J.P. Heart Failure in Chronic Myocarditis: A Role for microRNAs? Curr. Genom. 2015, 16, 88–94. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, S.; Ding, X.; Lu, C.; Wu, R.; Wu, H.; Shang, Y.; Pang, M. MicroRNA-30a-5p silencing polarizes macrophages toward M2 phenotype to alleviate cardiac injury following viral myocarditis by targeting SOCS1. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H1348–H1360. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Li, X.; Tang, Z.; Wang, X.; Zhong, M.; Suo, Q.; Zhang, Y.; Lv, K. Silencing MicroRNA-155 Attenuates Cardiac Injury and Dysfunction in Viral Myocarditis via Promotion of M2 Phenotype Polarization of Macrophages. Sci. Rep. 2016, 6, 22613. [Google Scholar] [CrossRef]
- Xue, Y.M.; Chen, M.G.; Chen, D.W.; Wu, W.F.; Liu, Y.L.; Lin, F.H. The effect of microRNA-21 on myocardial fibrosis in mice with chronic viral myocarditis. Zhonghua Xin Xue Guan Bing Za Zhi 2018, 46, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Kandolf, R.; Hofschneider, P.H. Molecular cloning of the genome of a cardiotropic Coxsackie B3 virus: Full-length reverse-transcribed recombinant cDNA generates infectious virus in mammalian cells. Proc. Natl. Acad. Sci. USA 1985, 82, 4818–4822. [Google Scholar] [CrossRef] [PubMed]
- Jakel, S.; Kuckelkorn, U.; Szalay, G.; Plotz, M.; Textoris-Taube, K.; Opitz, E.; Klingel, K.; Stevanovic, S.; Kandolf, R.; Kotsch, K.; et al. Differential interferon responses enhance viral epitope generation by myocardial immunoproteasomes in murine enterovirus myocarditis. Am. J. Pathol. 2009, 175, 510–518. [Google Scholar] [CrossRef]
- Barcena, M.L.; Jeuthe, S.; Niehues, M.H.; Pozdniakova, S.; Haritonow, N.; Kuhl, A.A.; Messroghli, D.R.; Regitz-Zagrosek, V. Sex-Specific Differences of the Inflammatory State in Experimental Autoimmune Myocarditis. Front. Immunol. 2021, 12, 686384. [Google Scholar] [CrossRef] [PubMed]
- Walkowski, B.; Kleibert, M.; Majka, M.; Wojciechowska, M. Insight into the Role of the PI3K/Akt Pathway in Ischemic Injury and Post-Infarct Left Ventricular Remodeling in Normal and Diabetic Heart. Cells 2022, 11, 1553. [Google Scholar] [CrossRef]
- Asrih, M.; Mach, F.; Nencioni, A.; Dallegri, F.; Quercioli, A.; Montecucco, F. Role of mitogen-activated protein kinase pathways in multifactorial adverse cardiac remodeling associated with metabolic syndrome. Mediators Inflamm. 2013, 2013, 367245. [Google Scholar] [CrossRef]
- Opavsky, M.A.; Martino, T.; Rabinovitch, M.; Penninger, J.; Richardson, C.; Petric, M.; Trinidad, C.; Butcher, L.; Chan, J.; Liu, P.P. Enhanced ERK-1/2 activation in mice susceptible to coxsackievirus-induced myocarditis. J. Clin. Investig. 2002, 109, 1561–1569. [Google Scholar] [CrossRef]
- Fu, Q.; Gao, L.; Fu, X.; Meng, Q.; Lu, Z. Scutellaria baicalensis Inhibits Coxsackievirus B3-Induced Myocarditis Via AKT and p38 Pathways. J. Microbiol. Biotechnol. 2019, 29, 1230–1239. [Google Scholar] [CrossRef]
- Kublickiene, K.; Luksha, L. Gender and the endothelium. Pharmacol. Rep. 2008, 60, 49–60. [Google Scholar]
- Piro, M.; Della Bona, R.; Abbate, A.; Biasucci, L.M.; Crea, F. Sex-related differences in myocardial remodeling. J. Am. Coll. Cardiol. 2010, 55, 1057–1065. [Google Scholar] [CrossRef]
- Pinceti, E.; Shults, C.L.; Rao, Y.S.; Pak, T.R. Differential Effects of E2 on MAPK Activity in the Brain and Heart of Aged Female Rats. PLoS ONE 2016, 11, e0160276. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.H.; Feng, X.; Zhang, Y.W.; Lou, X.Y.; Cheng, Y.; Zhou, H.H. Let-7 in cardiovascular diseases, heart development and cardiovascular differentiation from stem cells. Int. J. Mol. Sci. 2013, 14, 23086–23102. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Jun, M.; Ahn, M.R.; Kim, O.Y. Involvement of miR-Let7A in inflammatory response and cell survival/apoptosis regulated by resveratrol in THP-1 macrophage. Nutr. Res. Pract. 2016, 10, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Teng, G.G.; Wang, W.H.; Dai, Y.; Wang, S.J.; Chu, Y.X.; Li, J. Let-7b is involved in the inflammation and immune responses associated with Helicobacter pylori infection by targeting Toll-like receptor 4. PLoS ONE 2013, 8, e56709. [Google Scholar] [CrossRef]
- Okoye, I.S.; Coomes, S.M.; Pelly, V.S.; Czieso, S.; Papayannopoulos, V.; Tolmachova, T.; Seabra, M.C.; Wilson, M.S. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity 2014, 41, 89–103. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, J.S.; Yan, Y.; Li, J.; Ni, T.; Shao, W.; Mei, J.H.; Xiong, W.Z.; Wu, H. MiR-199a modulates autophagy and inflammation in rats with cerebral infarction via regulating mTOR expression. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 6338–6345. [Google Scholar] [CrossRef]
- Li, G.; Shao, Y.; Guo, H.C.; Zhi, Y.; Qiao, B.; Ma, K.; Du, J.; Lai, Y.Q.; Li, Y. MicroRNA-27b-3p down-regulates FGF1 and aggravates pathological cardiac remodelling. Cardiovasc. Res. 2022, 118, 2139–2151. [Google Scholar] [CrossRef]
- Fu, Q.; Lu, Z.; Fu, X.; Ma, S.; Lu, X. MicroRNA 27b promotes cardiac fibrosis by targeting the FBW7/Snail pathway. Aging 2019, 11, 11865–11879. [Google Scholar] [CrossRef]
- Asensio-Lopez, M.C.; Sassi, Y.; Soler, F.; Fernandez Del Palacio, M.J.; Pascual-Figal, D.; Lax, A. The miRNA199a/SIRT1/P300/Yy1/sST2 signaling axis regulates adverse cardiac remodeling following MI. Sci. Rep. 2021, 11, 3915. [Google Scholar] [CrossRef]
- Reed, B.G.; Babayev, S.N.; Chen, L.X.; Carr, B.R.; Word, R.A.; Jimenez, P.T. Estrogen-regulated miRNA-27b is altered by bisphenol A in human endometrial stromal cells. Reproduction 2018, 156, 559–567. [Google Scholar] [CrossRef]
- Prabakaran, S.; Schwartz, A.; Lundberg, G. Cardiovascular risk in menopausal women and our evolving understanding of menopausal hormone therapy: Risks, benefits, and current guidelines for use. Ther. Adv. Endocrinol. Metab. 2021, 12, 20420188211013917. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Huang, C. Coxsackieviruses B3 infection of myocardial microvascular endothelial cells activates fractalkine via the ERK1/2 signaling pathway. Mol. Med. Rep. 2017, 16, 7548–7552. [Google Scholar] [CrossRef]
- Luo, H.; Yanagawa, B.; Zhang, J.; Luo, Z.; Zhang, M.; Esfandiarei, M.; Carthy, C.; Wilson, J.E.; Yang, D.; McManus, B.M. Coxsackievirus B3 replication is reduced by inhibition of the extracellular signal-regulated kinase (ERK) signaling pathway. J. Virol. 2002, 76, 3365–3373. [Google Scholar] [CrossRef]
- Wang, L.; Xie, W.; Zhang, L.; Li, D.; Yu, H.; Xiong, J.; Peng, J.; Qiu, J.; Sheng, H.; He, X.; et al. CVB3 Nonstructural 2A Protein Modulates SREBP1a Signaling via the MEK/ERK Pathway. J. Virol. 2018, 92, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Dama, A.; Baggio, C.; Boscaro, C.; Albiero, M.; Cignarella, A. Estrogen Receptor Functions and Pathways at the Vascular Immune Interface. Int. J. Mol. Sci. 2021, 22, 4254. [Google Scholar] [CrossRef]
- Santen, R.J.; Song, R.X.; McPherson, R.; Kumar, R.; Adam, L.; Jeng, M.H.; Yue, W. The role of mitogen-activated protein (MAP) kinase in breast cancer. J. Steroid Biochem. Mol. Biol. 2002, 80, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, J.X.; Fry, A.C.; Mosier, E.M. Sex-based differences in resting MAPK, androgen, and glucocorticoid receptor phosphorylation in human skeletal muscle. Steroids 2019, 141, 23–29. [Google Scholar] [CrossRef]
- Mendell, A.L.; Chung, B.Y.T.; Creighton, C.E.; Kalisch, B.E.; Bailey, C.D.C.; MacLusky, N.J. Neurosteroid metabolites of testosterone and progesterone differentially inhibit ERK phosphorylation induced by amyloid beta in SH-SY5Y cells and primary cortical neurons. Brain Res. 2018, 1686, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Mendell, A.L.; MacLusky, N.J. The testosterone metabolite 3alpha-androstanediol inhibits oxidative stress-induced ERK phosphorylation and neurotoxicity in SH-SY5Y cells through an MKP3/DUSP6-dependent mechanism. Neurosci. Lett. 2019, 696, 60–66. [Google Scholar] [CrossRef]
- Zhang, S.; He, B.; Goldstein, S.; Ge, J.; Wang, Z.; Ruiz, G. The expression and significance of proto-oncogene c-fos in viral myocarditis. Virol. J. 2010, 7, 285. [Google Scholar] [CrossRef]
- Ray, N.; Kuwahara, M.; Takada, Y.; Maruyama, K.; Kawaguchi, T.; Tsubone, H.; Ishikawa, H.; Matsuo, K. c-Fos suppresses systemic inflammatory response to endotoxin. Int. Immunol. 2006, 18, 671–677. [Google Scholar] [CrossRef]
- Tang, X.; Liao, Y.; Chen, Z.; Gao, X.; Dong, J. Expression of IL-1beta and TNF-alpha in MCMV myocarditis and its role. J. Huazhong Univ. Sci. Technol. Med. Sci. 2005, 25, 254–256, 259. [Google Scholar] [CrossRef]
- Klingel, K.; Kandolf, R. Osteopontin: A biomarker to predict the outcome of inflammatory heart disease. Semin. Thromb. Hemost. 2010, 36, 195–202. [Google Scholar] [CrossRef]
- Hober, D.; Andreoletti, L.; Shen, L.; Copin, M.C.; Desmidt, A.; Wattre, P. Coxsackievirus B3-induced chronic myocarditis in mouse: Use of whole blood culture to study the activation of TNF alpha-producing cells. Microbiol. Immunol. 1996, 40, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yu, J.; Sun, H.; Zhao, Y.; Wang, J.; Zhang, J.; Meng, B. Effects of ubiquitin-proteasome inhibitor on the expression levels of TNF-alpha and TGF-beta1 in mice with viral myocarditis. Exp. Ther. Med. 2019, 18, 2799–2804. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, C.; Mao, Y.; Cui, J.; Wang, X.; Dang, J.; Wang, S. The expression of STAT3 inhibited the NF-KappaB signalling pathway and reduced inflammatory responses in mice with viral myocarditis. Int. Immunopharmacol. 2021, 95, 107534. [Google Scholar] [CrossRef]
- Bianconi, V.; Sahebkar, A.; Atkin, S.L.; Pirro, M. The regulation and importance of monocyte chemoattractant protein-1. Curr. Opin. Hematol. 2018, 25, 44–51. [Google Scholar] [CrossRef]
- Fuse, K.; Kodama, M.; Hanawa, H.; Okura, Y.; Ito, M.; Shiono, T.; Maruyama, S.; Hirono, S.; Kato, K.; Watanabe, K.; et al. Enhanced expression and production of monocyte chemoattractant protein-1 in myocarditis. Clin. Exp. Immunol. 2001, 124, 346–352. [Google Scholar] [CrossRef]
- Cihakova, D.; Sharma, R.B.; Fairweather, D.; Afanasyeva, M.; Rose, N.R. Animal models for autoimmune myocarditis and autoimmune thyroiditis. Methods Mol. Med. 2004, 102, 175–193. [Google Scholar] [CrossRef]
- Lam, C.S.P.; Arnott, C.; Beale, A.L.; Chandramouli, C.; Hilfiker-Kleiner, D.; Kaye, D.M.; Ky, B.; Santema, B.T.; Sliwa, K.; Voors, A.A. Sex differences in heart failure. Eur. Heart J. 2019, 40, 3859–3868. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Kararigas, G. Mechanistic Pathways of Sex Differences in Cardiovascular Disease. Physiol. Rev. 2017, 97, 1–37. [Google Scholar] [CrossRef]
- Pardo, A.; Gibson, K.; Cisneros, J.; Richards, T.J.; Yang, Y.; Becerril, C.; Yousem, S.; Herrera, I.; Ruiz, V.; Selman, M.; et al. Up-regulation and profibrotic role of osteopontin in human idiopathic pulmonary fibrosis. PLoS Med. 2005, 2, e251. [Google Scholar] [CrossRef]
- Szalay, G.; Sauter, M.; Haberland, M.; Zuegel, U.; Steinmeyer, A.; Kandolf, R.; Klingel, K. Osteopontin: A fibrosis-related marker molecule in cardiac remodeling of enterovirus myocarditis in the susceptible host. Circ. Res. 2009, 104, 851–859. [Google Scholar] [CrossRef]
- Abdelaziz Mohamed, I.; Gadeau, A.P.; Hasan, A.; Abdulrahman, N.; Mraiche, F. Osteopontin: A Promising Therapeutic Target in Cardiac Fibrosis. Cells 2019, 8, 1558. [Google Scholar] [CrossRef] [PubMed]
- Ti, D.; Hao, H.; Tong, C.; Liu, J.; Dong, L.; Zheng, J.; Zhao, Y.; Liu, H.; Fu, X.; Han, W. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J. Transl. Med. 2015, 13, 308. [Google Scholar] [CrossRef]
- Fairweather, D.; Beetler, D.J.; Musigk, N.; Heidecker, B.; Lyle, M.A.; Cooper, L.T., Jr.; Bruno, K.A. Sex and gender differences in myocarditis and dilated cardiomyopathy: An update. Front. Cardiovasc. Med. 2023, 10, 1129348. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.P.; Huber, S.A.; Moussawi, M.; Roberts, B.; Teuscher, C.; Watkins, R.; Arnold, A.P.; Klein, S.L. Sex chromosome complement contributes to sex differences in coxsackievirus B3 but not influenza A virus pathogenesis. Biol. Sex. Differ. 2011, 2, 8. [Google Scholar] [CrossRef]
- Case, L.K.; Toussaint, L.; Moussawi, M.; Roberts, B.; Saligrama, N.; Brossay, L.; Huber, S.A.; Teuscher, C. Chromosome y regulates survival following murine coxsackievirus b3 infection. Genes Genomes Genet. 2012, 2, 115–121. [Google Scholar] [CrossRef]
- Fang, M.; Zhang, A.; Du, Y.; Lu, W.; Wang, J.; Minze, L.J.; Cox, T.C.; Li, X.C.; Xing, J.; Zhang, Z. TRIM18 is a critical regulator of viral myocarditis and organ inflammation. J. Biomed. Sci. 2022, 29, 55. [Google Scholar] [CrossRef]
- Souyris, M.; Mejia, J.E.; Chaumeil, J.; Guery, J.C. Female predisposition to TLR7-driven autoimmunity: Gene dosage and the escape from X chromosome inactivation. Semin. Immunopathol. 2019, 41, 153–164. [Google Scholar] [CrossRef]
- Umiker, B.R.; Andersson, S.; Fernandez, L.; Korgaokar, P.; Larbi, A.; Pilichowska, M.; Weinkauf, C.C.; Wortis, H.H.; Kearney, J.F.; Imanishi-Kari, T. Dosage of X-linked Toll-like receptor 8 determines gender differences in the development of systemic lupus erythematosus. Eur. J. Immunol. 2014, 44, 1503–1516. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estepa, M.; Niehues, M.H.; Vakhrusheva, O.; Haritonow, N.; Ladilov, Y.; Barcena, M.L.; Regitz-Zagrosek, V. Sex Differences in Expression of Pro-Inflammatory Markers and miRNAs in a Mouse Model of CVB3 Myocarditis. Int. J. Mol. Sci. 2024, 25, 9666. https://doi.org/10.3390/ijms25179666
Estepa M, Niehues MH, Vakhrusheva O, Haritonow N, Ladilov Y, Barcena ML, Regitz-Zagrosek V. Sex Differences in Expression of Pro-Inflammatory Markers and miRNAs in a Mouse Model of CVB3 Myocarditis. International Journal of Molecular Sciences. 2024; 25(17):9666. https://doi.org/10.3390/ijms25179666
Chicago/Turabian StyleEstepa, Misael, Maximilian H. Niehues, Olesya Vakhrusheva, Natalie Haritonow, Yury Ladilov, Maria Luisa Barcena, and Vera Regitz-Zagrosek. 2024. "Sex Differences in Expression of Pro-Inflammatory Markers and miRNAs in a Mouse Model of CVB3 Myocarditis" International Journal of Molecular Sciences 25, no. 17: 9666. https://doi.org/10.3390/ijms25179666







